13.3
Impact Factor
Theranostics 2023; 13(3):1109-1129. doi:10.7150/thno.81403 This issue Cite
Review
Exploring neurotransmitters and their receptors for breast cancer prevention and treatment
1. General Surgery Department, Third Hospital of Shanxi Medical University, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Taiyuan, Shanxi, China.
2. School of Biomedical Sciences and Pharmacy, The University of Newcastle, New South Wales, Australia.
3. Children's Cancer Institute Australia for Medical Research, The University of New South Wales, Sydney, NSW, Australia.
4. Translational Research Institute, Henan Provincial and Zhengzhou City Key laboratory of Non-coding RNA and Cancer Metabolism, Henan International Joint Laboratory of Non-coding RNA and Metabolism in Cancer, Zhengzhou University People's Hospital and Henan Provincial People's Hospital, Academy of Medical Sciences, Zhengzhou University, Henan, China.
5. These authors contributed equally to this work.
Received 2022-11-30; Accepted 2023-1-18; Published 2023-1-31
Abstract
While psychological factors have long been linked to breast cancer pathogenesis and outcomes, accumulating evidence is revealing how the nervous system contributes to breast cancer development, progression, and treatment resistance. Central to the psychological-neurological nexus are interactions between neurotransmitters and their receptors expressed on breast cancer cells and other types of cells in the tumor microenvironment, which activate various intracellular signaling pathways. Importantly, the manipulation of these interactions is emerging as a potential avenue for breast cancer prevention and treatment. However, an important caveat is that the same neurotransmitter can exert multiple and sometimes opposing effects. In addition, certain neurotransmitters can be produced and secreted by non-neuronal cells including breast cancer cells that similarly activate intracellular signaling upon binding to their receptors. In this review we dissect the evidence for the emerging paradigm linking neurotransmitters and their receptors with breast cancer. Foremost, we explore the intricacies of such neurotransmitter-receptor interactions, including those that impinge on other cellular components of the tumor microenvironment, such as endothelial cells and immune cells. Moreover, we discuss findings where clinical agents used to treat neurological and/or psychological disorders have exhibited preventive/therapeutic effects against breast cancer in either associative or pre-clinical studies. Further, we elaborate on the current progress to identify druggable components of the psychological-neurological nexus that can be exploited for the prevention and treatment of breast cancer as well as other tumor types. We also provide our perspectives regarding future challenges in this field where multidisciplinary cooperation is a paramount requirement.
Keywords: Neurotransmitters, Neurotransmitter receptors, Nerves, Tumor microenvironments, Breast cancer
Introduction
Breast cancer (BC) is the most common cancer in women worldwide [1]. It accounts for approximately 30% of female malignancies with a mortality-to-incidence ratio of 15% [2]. Early BCs that commonly refer to those that are confined to the breast or spread only to the axillary lymph nodes are considered curable [3]. However, despite recent advances in endocrine therapy, targeted therapy and immunotherapy that have improved the prognosis and quality of life of late-stage BC patients, there is currently no curative treatment once the disease has spread to distant sites.
BC cells are highly heterogenous with extensive inter- and intra-tumoral variations in their genetic and molecular makeup. A relatively small portion of BCs (approximately 10% of all cases) have a clear familial association, with germline mutations of the tumor suppressor genes BRCA1 and BRCA2 being the most common genetic predisposition [4]. The major risk factors of sporadic BCs are estrogen exposure and genomic amplification of ERBB2, the gene encoding epidermal growth factor receptor 2 (HER2) [3]. These varying characteristics are fundamental to contemporary biology-centered approaches in classification and systemic treatment of BCs [5-7]: selective estrogen receptor modulators (SERMs), such as tamoxifen and toremifene, and humanized anti-HER2 antibodies, such as trastuzumab and pertuzumab, are the standard of care for patients with estrogen receptor (ER)- and HER2-positive BCs, respectively; whereas the poly(ADP-ribose) polymerase (PARP) inhibitors olaparib and talazoparib are used in BRCA mutation carriers with metastatic HER2-negative BCs based respectively on the results of the OlympiAD and EMBRACA trials [6, 7]. Nevertheless, BC cells may undergo molecular evolution, especially at metastatic sites and under treatment pressure, which necessitates dynamic optimization of treatment strategies [8].
A large number of novel targeted therapeutics have recently entered clinical application or are being evaluated in clinical trials [9-12]. For example, the cyclin-dependent kinases 4 and 6 (CDK4/6) inhibitors, palbociclib, ribociclib, as well as abeamciclib, and the allosteric inhibitor of mTOR complex 1 (mTORC1) everolimus are now available for the treatment of ER-positive HER2-negative advanced BCs [9-12]. Similarly, the selective estrogen receptor degrader (SERD) elacestrant is being investigated in clinical trials for the treatment of ER-positive HER2-negative advanced BCs [ClinicalTrials.gov Identifier: NCT03778931], and the humanized monoclonal antibody against trophoblast cell-surface antigen 2 (TROP2), for the treatment of triple-negative BCs (TNBCs) [ClinicalTrials.gov Identifier: NCT04230109; NCT04927884; NCT02574455]. Noticeably, complementary and alternative medicines (CAMs) such as natural products and mineral supplements have attracted increasing attention from BC patients [13]. However, while the frequency of CAMs use and its impact on therapeutic adherence in BC patients undergoing standard treatments are being evaluated [ClinicalTrials.gov Identifier: NCT01823549; NCT04740697], the definitive effects of CAMs on BC patient outcomes remain to be determined in rigorously implemented clinical studies [14].
BCs develop and progress in a complex microenvironment with diverse cellular components including fibroblasts, endothelial cells, as well as innate and adaptive immune cells [3]. The immune system plays an important role in preventing carcinogenesis at the initiating stage through surveillance to eliminate transforming/transformed cells [15]. Nevertheless, according to the immune editing principle, the eventual outgrowth of malignant cells capable of evading immune cell-mediated killing results in tumor development and progression [16], with infiltrating immune cells disabled or even reprogrammed to support BC cells [17]. Immunotherapy to reactivate cytotoxic T cells using immune checkpoint antibodies such as anti-programmed cell death protein 1(PD1)/programmed death-ligand 1 (PD-L1) is now clinically available for the treatment of unresectable locally advanced or metastatic TNBCs expressing PD-L1 [18]. Another important composition of the BC microenvironment involves aberrant angiogenesis triggered by factors such as vascular endothelial growth factor (VEGF) produced by BC cells and other types of infiltrating cells [19]. Such reorganization of blood vessels supports rapid tumor growth by supplying sufficient oxygen and nutrients to cancer cells [20]. On this basis, anti-angiogenesis therapy is emerging as a promising strategy for BC treatment [21].
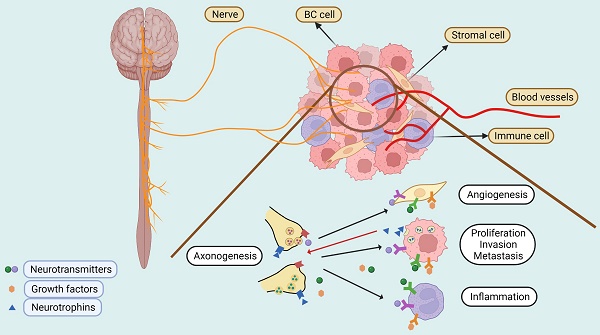
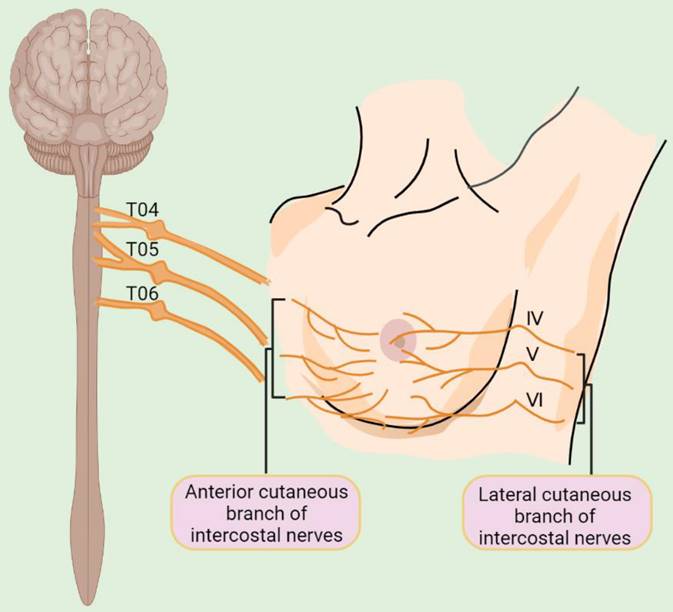
Despite these advances in our understanding of the cellular components of the BC microenvironment, the presence and the role of nerves that represent an essential constituent of cellular microenvironments of nearly all human tissues have largely been overlooked [22]. The breast is primarily innervated by the anterior and lateral cutaneous branches of the 4th to 6th intercostal nerves, which contain both sensory and autonomic nerve fibers (Figure 1) [23]. Intriguingly, BCs are more strongly innervated compared with corresponding normal compartments [24]. Moreover, BC cells frequently express neurotransmitter receptors, implicating the potential effect of the nervous system on BC cells [25]. The nervous system was traditionally regarded as a passive bystander of cancer pathogenesis [26], with perineural invasion (PNI), a process in which cancer cells grow around existing nerves and/or invade the perineural space, being thought to represent the only association between the nervous system and BC cells [27]. Nevertheless, increasing epidemiological and clinical evidence has pointed to an important role of neurotransmitters and their receptors in regulating BC development and progression [28-30]. For instance, population-based studies have demonstrated that the use of beta blockers, competitive antagonists that block the interactions of epinephrine and norepinephrine with β-adrenergic receptors (ARs), reduce BC progression and improve patient outcomes [28, 29]. Moreover, perioperative inhibition of β-adrenergic signaling inhibits multiple cellular and molecular pathways related to metastasis and disease recurrence in early-stage BCs [30].
Nerves - active players in the BC microenvironment
The active crosstalk between the nervous system and BC cells as well as other cell types in the tumor microenvironment has not been appreciated until recently [26]. On the one hand, neurotransmitters and growth factors secreted by nerves activate signal pathways that regulate BC cell proliferation, invasion, metastasis and resistance to treatment [31]. On the other hand, BC cells produce neurotrophins such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) that stimulate axonogenesis (the outgrowth of nerves) [24, 32]. Chronic neuronal activity can be recorded within the BC masses [33], whereas denervation causes regression of established BCs in mouse models [34], providing direct evidence that nerve supply is necessary for BC growth.
Signals generated by nerves can also modulate the tumor microenvironment through regulating immune cell activity and angiogenesis directly and/or indirectly [34-39]. This is mediated by various neurotransmitter receptors expressed on the surface of BC cells, immune cells, and/or endothelial cells [34-36]. For example, both α- and β-ARs are expressed on the surface of monocytes and dendritic cells (DCs), which, upon stimulation, promote an immunosuppressive phenotype, whereas blockade of β-AR signaling results in the downregulation of PD-1 and increased production of interferon (IFN)-γ in BC-infiltrating CD4+ and CD8+ T cells [34]. On the other hand, the high expression of β2-AR is associated with high surface PD-L1 expression on BC cells that plays an important role in BC cell-mediated suppression of T cell activation [35]. Activation of β2-AR on the surface of BC cells also stimulates the production of VEGF and thus angiogenesis [36]. Similarly, activation of serotonin receptors expressed in blood vessels also promotes angiogenesis in the BC microenvironment [40].
Schematic illustration of innervation of the breast. T04: the fourth thoracic nerve; T05: the fifth thoracic nerve; T06: the sixth thoracic nerve; IV: the fourth intercostal nerve; V: the fifth intercostal nerve; VI: the sixth intercostal nerve.
The central nervous system can also interact with metastatic BC cells to regulate disease progression [41, 42]. In the brain, metastatic BC cells can form gap junctions with astrocytes, the most abundant cell type in the brain, facilitating transfer of the second messenger cyclic GMP-AMP (cGAMP) to astrocytes. In turn, this activates the cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) pathway, invoking the production of inflammatory cytokines IFNα and tumor necrosis factor α (TNFα) that subsequently activates the signal transducer and activator of transcription 1 (STAT1) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathways in BC cells, thereby supporting metastatic tumor growth and chemoresistance [41]. Moreover, brain metastatic BC cells can hijack neuronal signals through formation of pseudo-tripartite synapses with glutamatergic neurons to promote their colonization [42]. Therefore, cellular components of the central nervous system can serve as “soil” for the brain-selective metastasis of BC cell “seeds”.
Innervation - the link between cancer neuroscience and psycho-oncology in BC?
While the roadmap for the emerging field of cancer neuroscience is being rapidly pieced together, the field of psycho-oncology has also gained increasing appreciation [43]. The notion that psychological factors impinge on cancer pathogenesis dates back to pre-Christian times when it was noted that cancer was more frequently seen in melancholic (depressed) than in sanguine (cheerful) women [44]. Indeed, there is now a substantial body of epidemiological and clinical evidence showing that adverse psychosocial factors, such as acute life events, work stress, and physical stress, are associated with a higher BC incidence and poorer survival of patients [45]. However, the cellular and molecular mechanisms that link psychological factors to BC pathogenesis remain largely missing, although the hypothalamic-pituitary-adrenal (HPA) axis has been implicated [46]. Indeed, flatter daytime cortisol patterns are associated with early mortality of BC patients [47].
Of interest, while physical exercise and stress are inversely related [48], exercise protects against cancer through mechanisms potentially associated with alterations in the levels of catecholamines, the neurotransmitters produced by sympathetic nerves [49]. Moreover, chronic stress promotes primary BC growth and enhances distant metastasis in mouse models [50-52], which recapitulates the effect of pharmacological activation of β-adrenergic signaling and is inhibited by treatment with the β-antagonist, propranolol, substantiating the involvement of the sympathetic nervous system in stress-associated BC metastasis [52]. Importantly, it has been recently demonstrated that selective activation of dopaminergic neurons in the ventral tegmental area (VTA) projecting to the medial prefrontal cortex (mPFC) of the brain reverses BC growth in mice subjected to unpredictable chronic mild stress (UCMS) [50], shedding new light on understanding how the central nervous system regulates BC pathogenesis. Noticeably, chronic stress leads to elevated serum levels of norepinephrine, implicating the role of norepinephrine in promoting BC progression [50].
Given that all nerves are connected to the central nerve system and ultimately the brain through direct and indirect neuronal networks [31], it is conceivable that BCs, like other systemic diseases such as cardiovascular or endocrine disorders, are subjected to brain-mediated regulation [53]. Should this be experimentally and clinically verified, innervation would prove to be the fundamental link of psychosocial factors to BC pathogenesis.
Neurotransmitters and their receptors - what determine their effects on BC pathogenesis?
With cancer neuroscience still at an early stage in the BC field, many questions are eagerly waiting for answers. For example, how are the effects of nerves on BC cells regulated? Can the local nervous system in the BC microenvironment be manipulated for BC treatment? Can the nervous system be exploited for BC prevention in those suffering stress? Moreover, although it is known that both sympathetic and parasympathetic nerves play roles in modulating immune activity [54], whether they can be explored for the development of novel immunotherapeutic approaches in the treatment of BC remains to be clarified.
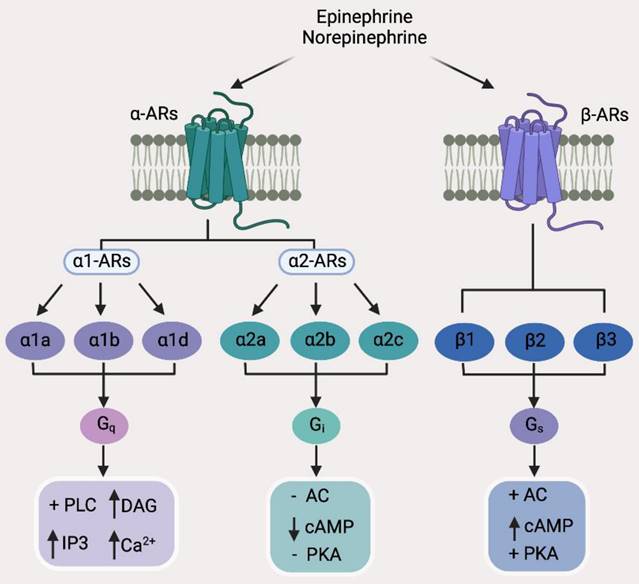
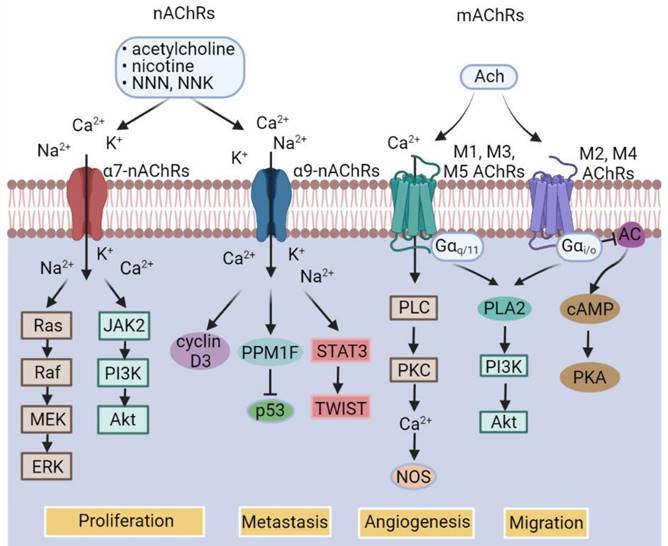
While catecholamine neurotransmitters released by sympathetic nerves bind to ARs, which are a group of G protein-coupled receptors (GPCRs), consisting of two main groups divided into 9 subtypes (Figure 2) [55], acetylcholine, the neurotransmitter released by parasympathetic nerves, binds to acetylcholine receptors (AChRs), which are further classified into nicotinic acetylcholine receptors (nAChRs) that are ligand-gated ion channels and muscarinic acetylcholine receptors (mAChRs) that belong to the GPCR superfamily (Figure 3) [56]. As engagement of different receptors triggers activation of different intracellular signaling pathways (Figure 3), it is conceivable that the effect of nerves on BC pathogenesis is dependent on the varying expression patterns of diverse types of corresponding receptors. In the following sections, we summarize the current knowledge about the roles of common neurotransmitters and their receptors in regulation of BC development, progression, and response to treatment. The practical implications of the interaction between neurotransmitters and BC cells and other types of cells in the BC microenvironment will also be deliberated.
Classification of adrenergic receptors (ARs) and the major downstream effectors of individual AR subtypes. AC, Adenylyl cyclase; cAMP, cyclic adenosine monophosphate; DAG, diacyl glycerol; IP3, inositol 1,4,5-trisphosphate; PKA, protein kinase A; PLC, phospholipase C.
Expression of ARs in BC cell lines and tissue sections
| Subtypes of ARs | BC cell lines | BC tissue sections | References |
|---|---|---|---|
| α1a-AR | MDA-MB-231, BT-483, T-47D, MDA-MB-415, HCC1806, HCC1397 | [58] | |
| α1b-AR | MDA-MB-231, HCC1397, HCC38, T47D | Basal-like BC HER2 positive BC Luminal BC | [59] |
| α1d-AR | BT-483, T47D, Hs578T, BT549, HCC1937 | [58] | |
| α2a-AR | T47D, HS578T, IBH-4, IBH-6, MDA-MB-231, HCC1187, HCC1428 | Basal-like BC HER2 positive BC Luminal BC | [60, 61] |
| α2b-AR | T47D, MCF-7, IBH-4, IBH-6 | Luminal A BC | [60, 62] |
| α2c-AR | T47D, MCF-7, IBH-4, IBH-6 | Luminal A BC HER2 positive BC Basal-like breast BC | [59, 60, 62] |
| β1-AR | MCF-7, BT-549, MDA-MB-648, MDA-MB-231HM | [63] | |
| β2-AR | MCF-7, ZR-75, BT474, SKBR3, MDA-MB-453, MDA-MB-231, MDA-MB-468, | Luminal BC Basal-like BC HER2 positive BC | [36, 61, 63-66, 68] |
| β3-AR | MCF-7 | Basal-like BC Non-Basal-like BC | [67] |
Abbreviations: ARs, adrenergic receptors; BC, breast cancer; HER2, human epidermal growth factor receptor 2.
Norepinephrine and epinephrine
Norepinephrine and epinephrine released respectively by peripheral sympathetic nerves and the adrenal gland are arguably the most studied neurotransmitters in relation to BC pathogenesis [57]. The expression of a variety of ARs have been documented in BC cell lines and tissue sections (Table 1) [36, 58-68]. Among them, β2-AR expression appears a potential poor prognostic factor in ER-negative BCs, although its relationship with ER-positive BC patient outcomes varies in different studies [68]. High β2-AR expression is also associated with increased lymph node metastasis and poor survival of patients with HER2-positive BCs [65]. On the other hand, high α1b-AR expression is related to poor prognosis of TNBC patients [59], whereas high α2c-AR expression is differentially associated with BC patient prognosis depending on responses to systemic treatment [59]. Of significance, various ARs are expressed by BC-infiltrating immune cells, consistent with a role of norepinephrine and epinephrine in regulating the immune response against BC [69].
Classification of acetylcholine (ACh) receptors (AChRs) and the major downstream signal pathways of individual AChR subtypes. AC, Adenylyl cyclase; cAMP, cyclic adenosine monophosphate; mAChRs, muscarinic AChRs; nAChRs, nicotinic AChRs; NNK, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; NNN, N′- nitrosonornicotine; NOS, nitric oxide synthase; PKA, protein kinase A; PLA2, phospholipase A2; PLC, Phospholipase C; PPM1F, protein phosphatase 1F.
The biological consequences of the engagement of norepinephrine or epinephrine with ARs expressed on the BC cell surface appear multifaceted, varying widely in different studies and with different experimental models. In particular, the effects of these neurotransmitters on BC cell proliferation seem to be concentration- and context-dependent. For example, low levels of epinephrine promote BC cell proliferation [70], but strikingly, stimulation with β2-AR agonists decelerates the proliferation of BC cells [70]. Whether this is related to the expression of other ARs on the cell surface remains to be determined, but it is known that epinephrine binds to different ARs with varying affinities in a concentration-dependent manner [71]. Promotion of BC cell proliferation by epinephrine at low concentrations can be abrogated by an α2c-AR antagonist [62], indicating that α2c-AR signaling enhances BC cell division. The effect of norepinephrine and epinephrine on BC cell proliferation is mediated, at least in part, by the DNA damage response, which leads to alterations in cell cycle progression and responses to chemotherapy [58]. Moreover, the MEK/ERK pathway may play a role in β2-AR signaling-mediated regulation of BC cell survival and proliferation [66]. Phosphorylation (deactivation) of the pro-apoptotic protein B-cell lymphoma 2 (BCL2)-associated agonist of cell death (BAD) through the 3′ 5′-cyclic adenosine monophosphate (cAMP)-dependent protein kinase, protein kinase A (PKA), has also been demonstrated to be involved in supporting BC cell survival by epinephrine [72].
AR signaling also plays a role in regulating BC metastasis, as demonstrated by the finding that sympathetic denervation of primary BC tumors reduces metastatic lesions [34]. This is primarily mediated by β-AR signaling, as pharmacologic activation of β-AR promotes, whereas treatment with a β-AR antagonist inhibits BC metastasis in tumor-bearing mice under stress [73]. Indeed, β-AR signaling regulates the epithelial-to-mesenchymal transition (EMT) in BC cells [74]. Moreover, increased calcium mobilization resulting from the accumulation of intracellular cAMP upon β-AR activation is also involved in regulating BC cell invasion [64]. Additional mechanisms include induction of macrophage-derived pro-metastatic molecules and preparation of distant metastatic niches [52]. Importantly, clinical studies substantiate the role of β-AR in regulating BC metastasis, as preoperative application of pharmacological β-blockers reduces metastatic biomarkers expressed by BC and promotes immune cell infiltration into tumor tissues [30].
Another important role of norepinephrine and epinephrine in BC pathogenesis is the regulation of angiogenesis [57]. This has been mainly demonstrated in studies on β2-AR signaling, which stimulates the production of VEGF by BC cells, although β2-AR-independent mechanisms have also been implicated [36]. Moreover, norepinephrine promotes cell-cell contact between BC cells and endothelial cells, thus potentiating formation of capillary structures [36]. Indeed, treatment with the β-antagonist propranolol potentiates the anti-angiogenic effect of chemotherapeutic drugs [75]. Various downstream effectors contribute to β2-AR signaling-mediated angiogenesis, including activation of the mTOR pathway, downregulation of peroxisome proliferator-activated receptor γ (PPARγ), and upregulation of the Notch ligand Jagged-1 in BC cells [36]. In support of the role of norepinephrine and epinephrine signaling in BC angiogenesis, chronic stress increases VEGF production in BC cells, resulting in enhanced angiogenesis that can be diminished by treatment with propranolol in mouse models [76].
The engagement of norepinephrine or epinephrine with ARs on the BC cell surface is also involved in regulating the interaction between the immune system and BC cells [57]. High β2-AR expression is associated with high expression of PD-L1 and reduced tumor-infiltrating lymphocyte grade in ER-negative BCs [68]. Moreover, treatment with pharmacological α- or β-AR blockers downregulates the expression of PD-L1 and forkhead box P3 (FOXP3), albeit to a lesser extent compared with tumor-specific sympathetic denervation, in human BC xenografts and in rats with chemically induced BCs [34]. Studies with human BC tissues also revealed that increased sympathetic nerve density is correlated with higher expression of immune checkpoint molecules [34]. Therefore, norepinephrine and epinephrine signaling may play a role in the regulation of BC responses to immunotherapy using checkpoint inhibitors [77]. The downstream mechanisms remain to be clarified, but roles for metabolic pathways have been proposed [78]. It would be of interest to test whether pharmacological interference with AR signaling alters BC sensitivity to immune checkpoint inhibitors in clinical settings.
Norepinephrine and epinephrine are known to regulate immune cell activity. For example, β2-AR signaling regulates activation of cytotoxic T cells, polarization of macrophages and antigen presentation by DCs [79-81]. Furthermore, α- and β-ARs expressed on the surface of monocytes and DCs promote immunosuppressive phenotype upon stimulation [35]. Indeed, β2-AR signaling in tumor-associated macrophages triggers differentiation towards the M2 phenotype and production of factors that promote BC progression [81]. Consistently, chronic stress causes differentiation of tumor-associated macrophages towards the M2 phenotype [78]. Moreover, chronic stress induces the infiltration of regulatory CD4+ T cells and myeloid-derived immunosuppressive cells into the BC microenvironment [35]. It has been demonstrated that approximately 30-40% of CD4+ and CD8+ BC-infiltrating T cells express β2-AR and that blockade of β-AR signaling results in downregulation of PD-1 and increased production of IFN-γ in these cells [35]. Reduction in the density of T cells infiltrating into the BC microenvironment in stressed compared to non-stressed mice has also been reported [82]. Therefore, norepinephrine and epinephrine play a profound immunosuppressive role in the BC microenvironment, supporting the notion of interference with AR signaling to sensitize BC to immunotherapy.
Dopamine (DA)
DA is a catecholamine that functions as a neurotransmitter in the brain and as a hormone in peripheral tissues [83, 84]. In the brain, DA is mainly synthesized by dopaminergic neurons and primarily functions as a motivational component of reward-motivated behavior [83]. As DA does not cross the blood-brain barrier, its synthesis and functions in peripheral tissues are brain independent [84]. DA in peripheral tissues is mainly synthesized and released by adrenal medulla, the mesentery, and sympathetic nerves [85]. Of note, circulating DA exists predominantly in the form of DA-sulfate that does not bind to DA receptors (DARs) and is thus biologically inactive [86].
DA functions through binding to five receptors of the GPCR superfamily, which are classified according to structure, function and pharmacology into DA type-1 receptor (D1R)-like DARs including D1R and D5R, and D2R-like DARs consisting of D2R, D3R and D4R (Figure 4) [87]. Engagement of DARs with DA regulates activation of signaling pathways such as the adenylate cyclase (AC)/cAMP/PKA and guanylate cyclase (GC)/cGMP/protein kinase G (PKG) pathways [88]. D1R is the most abundant DARs expressed in the nervous system, followed by D2R, with D3, D4, and D5 receptors being present at markedly lower levels [89], while the expression status of DARs in other peripheral tissues remains less understood.
Classification of dopamine (DA) receptors (DARs) and the major downstream signal pathways of individual DARs. AC, Adenylyl cyclase; cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosine monophosphate; D1R-like receptors, DA type1-like receptors; D2R-like receptors, DA type 2-like receptors; GC, guanylate cyclase; PKA, protein kinase A; PKG, protein kinase G; VEGF, vascular endothelial growth factor; VPF, vascular permeability factor.
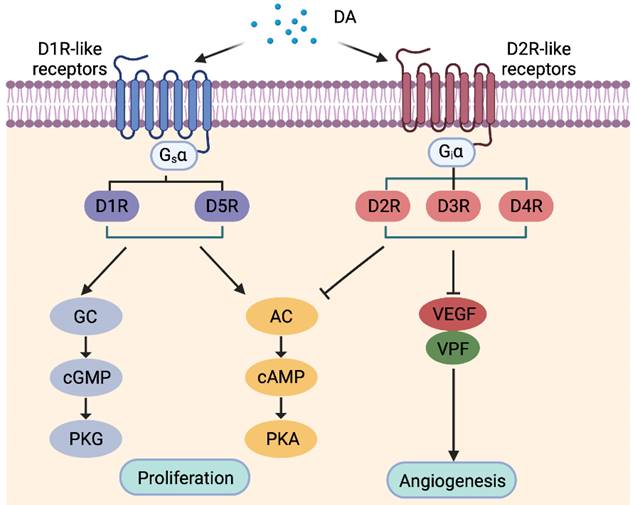
Intriguingly, DA is detected in BC tissues but not in most non-malignant breast samples, suggesting that DARs expressed by BC cells may be activated [90]. However, DA can hardly be detected in the culture media of BC cells [91], proposing that the source of DA in BC tissues is likely other tumor-resident cells such as immune and/or endothelial cells. Both D1R and D2R are expressed by BC cells in vivo [88, 90], but they appear to exert differential effects on BC progression [88, 90]. Although D1R expression is associated with larger tumors, higher tumor grades, lymph node metastasis and shorter patient survival, stimulation of D1R with an agonist paradoxically induces cell death and suppresses BC growth [88]. Moreover, activation of D1R reduces the frequency of cancer stem-like cells (CSCs) and inhibits BC metastasis [92]. On the other hand, stimulation of D2R with a specific agonist promotes self-renewal of BC CSCs, whereas blockade of D2R signaling inhibits CSC-like activity, reduces proliferation and triggers apoptosis in BC cells [90]. However, the expression status of D2R in BC cell lines does not appear to be associated with the sensitivity to D2R blockade [93], suggesting that the expression of other DARs is involved. More detailed characterization of the expression patterns of varying DARs in BC cells is therefore warranted. As DA is known to inhibit angiogenesis and is immunosuppressive through inhibiting lymphocyte activity [94], it is likely that DA may exert a role in regulating angiogenesis and immune activity in the BC microenvironment, which conceivably contributes to DA-mediated regulation of BC pathogenesis. Of note, the expression of genes encoding D1R and D2R-like DARs are increased in peripheral blood mononuclear cells from BC patients compared to normal individuals [95], supporting an immune regulatory role of the dopaminergic system. Chronic stress also causes increased peripheral DA production that contributes to the immunosuppressive phenotype frequently observed in BC patients [96].
Dopaminergic drugs are commonly used for the treatment of diseases associated with the central nervous systems, such as Parkinson's disease, addiction, and schizophrenia, and are also applied to management of severe episodes of hypertension [87]. For example, thioridazine, an antagonist of D2R, is used as an antipsychotic for the treatment of schizophrenia and psychosis [97], whereas the anti-hypertension drug fenoldopam is a selective peripheral D1R agonist [98]. Given the role of the dopaminergic system in regulating BC pathogenesis, there is increasing interest in repurposing of dopaminergic drugs for BC treatment. Indeed, both thioridazine and fenoldopam exhibit beneficials in pre-clinical BC models [93, 99]. Moreover, novel dopaminergic drugs, such as the D1R agonist A77636, are emerging [100]. Optimistically, dopaminergic drugs, alone or in combination with existing drugs, would represent a new avenue for BC treatment.
Serotonin
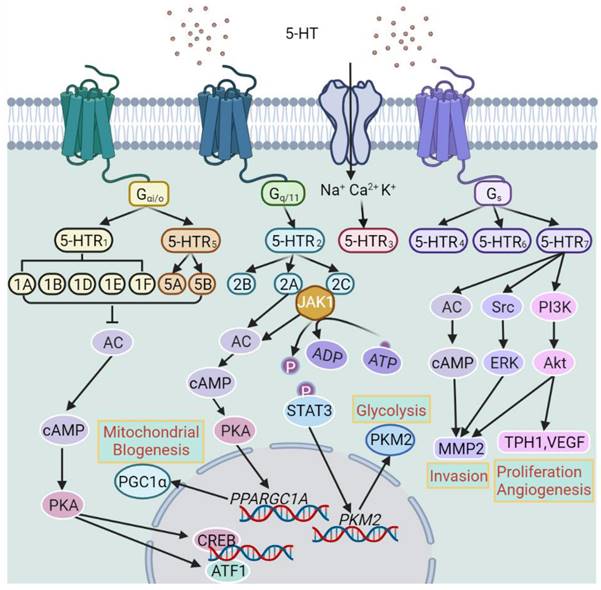
Serotonin, also known as 5-hydroxytryptamine (5-HT), is a monoamine that functions as a neurotransmitter in the central nervous system and as a signaling molecule in the circulation and peripheral tissues [101]. It is synthesized from the essential amino acid tryptophan with tryptophan hydroxylase (TPH) being the rate-limiting enzyme [102]. There are two forms of TPH: TPH1 exists in peripheral tissues whereas TPH2 is preferentially expressed in the central nervous system [102]. In the brain, 5-HT is involved in the regulation of mood, cognition, and reward upon corresponding stimulation. Nevertheless, most 5-HT occurs in the body periphery, where it is mainly synthesized and stored in intestinal enterochromaffin cells that play an essential role in regulating gastrointestinal motility [103]. A portion of peripheral 5-HT is taken up by platelets through the serotonin transporter (SERT) with these stores later released as a vasoconstrictor or vasodilator regulating hemostasis and blood clotting [104]. 5-HT is also involved in many other biological processes, such as organ development and wound healing [104]. Moreover, 5-HT acts as a mitogen for a range of normal cells including vascular smooth muscle cells and fibroblasts [105]. There are multiple types 5-HT receptors (5-HTRs) that are grouped into 7 classes (5-HTR1-7) including a total of 14 receptors (5-HTR1A, 1B, 1D-F, 5-HTR2A-C, 5-HTR3, 5-HTR4, 5-HTR5A, B, 5-HTR6, and 5-HTR7) (Figure 5). Apart from 5-HT3 that is a ligand-gated ion channel, all 5-HT receptors belong to the GPCR superfamily [106]. The multiple and sometimes opposing functions that 5-HT exerts are conceivably associated with the expression patterns of 5-HTRs [107].
5-HT plays an important role in regulating breast development and homeostasis in an autocrine-paracrine fashion [108]. This was initially demonstrated by the increased expression of TPH1 in mouse breasts stimulated with prolactin, which in turn elicits the production of 5-HT by the breast epithelium that is also detected in milk [108]. Subsequent studies revealed that 5-HT plays a critical role in the lactation-to-involution switch through binding to 5-HTR7 and thus reduces proliferation and induces apoptosis in breast epithelial cells [109]. The effect of the serotonergic system on breast homeostasis and involution appears to be biphasic, with transient, modest activation of 5-HTR7 promoting breast epithelial tight junction integrity, whereas sustained 5-HTR7 activation resulting in disruption of tight junctions that marks early stage of breast involution [110]. While both phases are mediated by cAMP, PKA signaling is responsible for promoting tight junctions and activation of the p38 mitogen-activated protein kinase (MAPK) pathway that is involved in the induction of apoptosis [111].
Strikingly, the serotonergic system exerts a contrasting effect on BC cells, as 5-HT promotes cell proliferation to enhance BC growth [112]. Indirect evidence in support of the role of 5-HT in BC pathogenesis comes from the finding that TPH1 protein levels are increased in tumors metastasized to lymph nodes [112]. Furthermore, plasma-free 5-HT levels are elevated in patients with advanced BC [113]. Indeed, TPH1 and many 5-HTRs are found in BC cell lines and tissues, including 5-HTR1A, 5-HTR1B, 5-HTR2A-C, 5-HTR3, 5-HTR4 and 5-HTR7 [114]. However, the effect of 5-HT on BC cells has mostly been shown to be mediated by 5-HTR2A and/or 5-HTR2C signaling and therefore the potential involvement of other 5-HTRs remains to be clarified [115]. Noticeably, 5-HTR1B and 5-HTR2B are predominantly located to the cytoplasm of BC cells, whereas 5-HTR4 exhibits nuclear localization [40]. Moreover, the expression levels of 5-HTR2B and 5-HTR4 correlate with ER-α and progesterone receptor expression levels, respectively [40], suggesting potential regulatory and/or functional relationships between these receptors. Mechanistically, 5-HTR2A and/or 5-HTR2C signaling promotes BC pathogenesis through two metabolic pathways: the JAK1/STAT3 pathway that promotes glycolysis through upregulation of pyruvate kinase M2 (PKM2) and the cAMP/PKA pathway that promotes the expression of peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC1α) leading to enhanced mitochondrial biogenesis [115]. Interestingly, TPH1 and 5-HTR7 have been reported to be preferentially expressed in TNBC [114]. In addition, the serotonergic system has also been demonstrated to promote CSC-like activity in BC [116].
Promotion of angiogenesis by the serotonergic system also plays a role in BC pathogenesis [40, 117]. This is largely mediated by activation of the G beta-gamma complex (Gβγ)/Src/PI3K pathway. In accordance, 5-HTR1A along with 5-HTR2B are expressed in blood vessels of BC and non-malignant breast tissues [40]. Nevertheless, 5-HT has also other effects on blood vessels including vasoconstriction and vasodilation depending on which 5-HTR is engaged [117]. Moreover, since varying 5-HTRs are expressed by immune cells, an additional mechanism linking 5-HT to BC pathogenesis may involve the effect of 5-HT on the immune system [118]. It would be of interest to test whether immune cells infiltrating into the BC microenvironment express 5-HTRs and how these cells respond to manipulation of the serotonergic system.
Many patients with BC experience depression, who are commonly treated with antidepressants such as 5-HT uptake inhibitors that increase 5-HT in the brain and conceivably in the blood plasma [119]. It has been shown in animal models that antidepressant medications may increase BC risk and promote BC growth [120], although evidence in support of a significant association between antidepressant use and BC development and progression in human is currently lacking [121]. Regardless, given the tumor-promoting effect of 5-HT, inhibition of the serotonergic system may represent an approach for BC treatment. For example, the TPH inhibitor telotristat is currently in clinical evaluation for the treatment of neuroendocrine and other solid cancers [ClinicalTrials.gov Identifier: NCT04810091; NCT03453489] [122]. Whether similar strategies are useful for BC treatment is awaiting investigation.
Classification of 5-hydroxytryptamine (5-HT) receptors (5-HTRs) and the major downstream signal pathways of individual 5-HTRs. AC, Adenylyl cyclase; ATF1, activating transcription factor 1; cAMP, cyclic adenosine monophosphate; CREB, cAMP response element-binding protein; JAK1, Janus kinase 1; MMP2, matrix metalloproteinase-2; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; PI3K, phosphoinositide 3-kinase; PKA, protein kinase A; PKM2, pyruvate kinase M2; STAT3, signal transducer and activator of transcription 3; TPH1, tryptophan hydroxylase 1; VEGF, Vascular endothelial growth factor.
Acetylcholine
Although acetylcholine (ACh) is best known as the primary neurotransmitter of the parasympathetic nervous system, it can also be synthesized and released by many types of non-neuronal cells, such as immune cells and epithelial cells [123]. In particular, various types of cancer cells produce and secrete ACh to create a cholinergic autocrine loop that regulates cell survival and proliferation [124]. ACh is synthesized from choline and acetyl-CoA, which is catalyzed by choline acetyltransferase (ChAT), the only known enzyme that produces ACh [125]. The expression of ChAT is thus believed to signify the production of ACh [125]. On the other hand, the enzyme acetylcholinesterase catalyzes the conversion of ACh to the inactive metabolites, choline and acetate, leading to signaling termination [125]. Inhibitors of acetylcholinesterase are currently in clinical use for the treatment of Alzheimer's disease and other diseases involving in reductions in the cellular levels of ACh [126]. In the cancer neuroscience field, it is paradoxical that while acetylcholinergic signaling is cancer-promoting in certain cancer types such as prostate cancer and colorectal cancer [127], its effects on BC are largely inhibitory as elegantly demonstrated in a recent study using genetic, surgical, and pharmacological approaches [128]. In accordance, the activity of the Vagus nerve, the main component of the parasympathetic nervous system, is associated with reduced adrenal metastasis of BC and better outcome of patients with metastatic or recurrent BCs [129]. Although these observations suggest that the acetylcholinergic system primary exerts an inhibitory effect on BC cell proliferation and metastasis, experimental stimulation of different types of AChRs often generate complex and sometimes opposing effects on BC cells [130].
There are two major classes of AChRs, nAChRs and mAChRs. As ligand-gated ion channels, nAChRs are pentameric transmembrane protein complexes permeable to sodium, potassium, and calcium ions upon activation [131]. There are at least 16 human nAChR subunits, including α1-α7, α9, α10, β1- β4, δ, γ and ε (Table 2) [132-141]. Among them, α7 and α9 can form homopentamers, whereas heteropentamers are formed by various combinations of α, β, γ and δ subunits [142]. Apart from ACh, nicotine, the major addictive substance in tobacco smoke, and nicotine-derived carcinogenic nitrosamine, such as, N′-nitrosonornicotine (NNN) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), can bind to and activate nAChRs [143]. Indeed, the interaction of nicotine and its derivatives with nAChRs has been well linked to the development of many types of cancers [144]. In particular, a number of studies have shown that exposure to nicotine or NNK induces malignant transformation of normal breast epithelial cells and promotes proliferation and metastasis of BC cells [145]. This is supported by epidemiological findings that cigarette smoking is associated with increased BC risk.
While multiple nAChRs, including α4-, α5-, α7- and α9-nAChRs are expressed at higher levels in BC cells than normal breast epithelial cells, α7-nAChR and α9-nAChR are by far the two best characterized nAChRs in terms of their roles in BC pathogenesis. In particular, α9-nAChR has been well demonstrated to be upregulated in BC tissues compared with normal breast tissues and is associated with BC progression and poor progression-free survival (PFS) of patients [146]. Knockdown of α9-nAChR inhibits BC cell proliferation and BC xenograft in mice, whereas overexpression of α9-nAChR promotes division of BC cells [147]. Moreover, α9-nAChR signaling has been shown to contribute to lung metastasis in TNBC [148]. Interestingly, the increased expression of α9-nAChR in BC cells has been linked to cigarette smoking [149], implicating the potential of smoking in induction of α9-nAChR in breast epithelial cells. Mechanistically, α9-nAChR signaling upregulates the expression of protein phosphatase 1F (PPM1F) that suppresses the activity of the tumor suppressor p53 [150]. Moreover, α9-nAChR signaling activates the STAT3/TWIST pathway to promote BC progression and metastasis [151]. Upregulation of cyclin D3 has also been implicated in α9-nAChR-mediated BC cell proliferation [152]. Similar to α9-nAChR, α7-nAChR plays a role in promoting BC cell proliferation and migration [153], although is mediated by distinct mechanisms, including activation of the MEK/ERK and the JAK2/PI3K pathways [154].
mAChRs are members of the GPCR superfamily consisting of 5 distinct yet highly homologous subtypes, namely, M1-M5 AChRs (Figure 3) [155]. Upon activation, M1AChR, M3AChR and M5 AChR interact with the Gq/G11 family of heterotrimeric G proteins, stimulating phosphatidylinositol metabolism, arachidonic acid release, tyrosine kinase, resulting in calcium influx that regulates activation of calcium-dependent enzymes including nitric oxide synthase (NOS) [156]. The M2 and M4 subtypes of mAChRs couple to Gi/Go family proteins, attenuating AC activity, leading to reduction in intracellular levels of cAMP and consequent inhibition of PKA activation [157]. In addition, mAChRs can activate several other signaling pathways through non-canonical mechanisms. For instance, M1AChR, M3AChR and M5AChR stimulate phospholipase A2 and phospholipase D, whereas M2AChR and M4AChR can also activate phospholipase A2 as a second messenger [158]. Moreover, M3AChR signaling can activate the RAS/RAF pathway leading to activation of ERK1/2 and Akt [159].
Strikingly, while stimulation of mAChRs with the agonist carbachol at low concentrations for short period promotes BC cell proliferation and migration, exposure to carbachol at relatively high concentrations or longer periods induces BC cell death [160]. Similarly, stimulation with carbachol at low concentrations for prolonged periods reduces the viability of BC cells [160]. It is thus postulated that low-concentration and long-term treatment with mAChR agonists may be a useful approach for BC treatment [161]. In support, mAChRs are commonly detected in BC cell lines and tissues, whereas they are not found in normal breast epithelial cells and benign breast tumors [162]. Nonetheless, normal breast epithelial cells become responsive to mAChR agonist treatment once mAChRs are experimentally introduced [154]. Of interest, the inhibitory effects of some commonly used chemotherapeutic drugs on BC cell proliferation have been linked to their ability to bind to and activate mAChRs [162, 163]. For example, the inhibitory effect of paclitaxel can be prevented by pre-treatment with a muscarinic antagonist, suggesting that the effect of paclitaxel is mediated by mAChRs [162]. Similarly, doxorubicin can bind to mAChRs and exerts its inhibitory effect on TNBC cell proliferation, recapitulating the effect of carbachol [163]. Noticeably, while all 5 mAChRs may contribute to the regulation of survival and proliferation of BC cells, most previous studies indicated that M1AChR and M3AChR play a predominant role through regulating NOS and its downstream enzymes [156].
It is well known that immune cells synthesize and release ACh, and AChRs are widely expressed in many types of immune cells, such as T cells and macrophages [164]. Indeed, immune cell-derived ACh is involved in the regulation of immune responses to viral infection [164]. However, the potential role of ACh and its receptors in the interaction between the immune system and BC cells remains undefined. Nevertheless, simulation of parasympathetic innervation inhibits PD-1 and PD-L1 expression in experimental BC models, whereas decreased parasympathetic nerve density in human BCs is associated with poor clinical outcomes and correlates with higher expression of immune checkpoint molecules [34]. It seems therefore that acetylcholinergic signaling promotes immune activation against BC, although the involvement of individual AChRs remains undefined. Regardless, pharmacological manipulation of the immune regulatory effect of acetylcholinergic signaling, alone or in combination with immune checkpoint inhibitors, e.g., anti-PD1/PD-L1 antibodies, is potentially useful for the treatment of BC and other types of cancers.
Classification and characteristics of human nAChRs
| nAChR types | Subunits | nAChR subtypes | Major functions | References |
|---|---|---|---|---|
| Muscle-type | α1, β1, γ, δ, ε | α1β1γδ α1β1δε | Mediate neuromuscular transmission and involved in fast synaptic transmission throughout the peripheral and central nervous system during development or in adult separately | [132] |
| Neuronal-type (αBgtx-sensitive receptors) | α5, α7, α8, α9, α10, β2-β4 | α7 | Regulate immune pathways, inflammatory responses, neurite outgrowth and is essential for the plasticity of the airway epithelium | [133] |
| α7α8 | Participate in retina neurogenesis | [134] | ||
| α7β2 | Involved in the regulation of brain cognitive function and the pathogenesis of Alzheimer's disease | [135] | ||
| α9 | Involved in the development of synaptic connections in cochlear outer hair cells as well as in cochlear responses and control of a specific pars tuberalis endocrime system | [136] | ||
| α9α10 | Involved in cochlea hair cell development, cochlear response, keratinocyte adhesion | [136] | ||
| Neuronal-type (αBgtx-insensitive receptors) | α2-α6, β2- β4 | α2β2 | Involved in the physiology of motor control | [137] |
| α2α5β2, α4α5α6β2, α5α7β2, α5α7β4 | The signaling of α5-containing receptors can alter the expression of the components of cell-cell and/or cell-matrix adhesion complexes | [133] | ||
| α3α5β4 | Involved in working memory and impulsivity | [138] | ||
| α3α6β2, α3α6β4 | Mediate the addictive response to nicotine | [139] | ||
| α3β3β4 | Facilitate ACh release in the Hb-IPn system | [140] | ||
| α3β4 | Affect reward circuits and addiction in brain areas | [141] | ||
| α4β2 | Involved in neuronal survival, neuroprotection, synaptic plasticity, memory, learning, cognition, and analgesia. | [133, 141] |
Abbreviations: ACh, acetylcholine; Hb-IPn, habenulo-interpeduncular; nAChRs, nicotinic ACh receptors.
Glutamate
As one of the most abundant amino acids in the human body, the non-essential amino acid glutamate (Glu) is the major excitatory neurotransmitter in the central nervous system and is involved in neuronal differentiation, migration, and survival of neuronal progenitor cells and immature neurons [165]. It also plays a role in the brain on cognitive functions such as learning and memory [166]. Glu acts as an extracellular signal mediator in non-neuronal peripheral tissues, regulating cellular processes such as cell survival and proliferation [167]. Moreover, Glu participates in regulation of the development of organs such as bone and lung, and cell lineages including lymphocytes, and platelets [167]. Glu is produced from glutamine by the enzyme glutaminase and can also be synthesized by the enzyme glutamate dehydrogenase from α-ketoglutarate that is primarily generated as part of the citric acid cycle [168]. As Glu cannot cross the blood-brain barrier without active transportation, the levels of Glu in the serum are markedly higher than the levels in the brain [169]. Pathologically high levels of Glu triggers excessive activation of its receptors, causing excitotoxicity, a toxic process leading to the loss of neural function and cell death [170].
Classification of glutamate (Glu) receptors (GluRs) and the major downstream signal pathways of individual GluRs. AC, Adenylyl cyclase; AMPAR, amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; cAMP, cyclic adenosine monophosphate; DAG, diacylglycerol; iGluRs, ionotropic Glu receptors; IP3, Inositol trisphosphate; KR, kainate receptor; mGluRs, metabotropic Glu receptors; NMDAR, N-methyl-D-aspartate receptor; PKC, Protein kinase C; PLC, Phospholipase C.
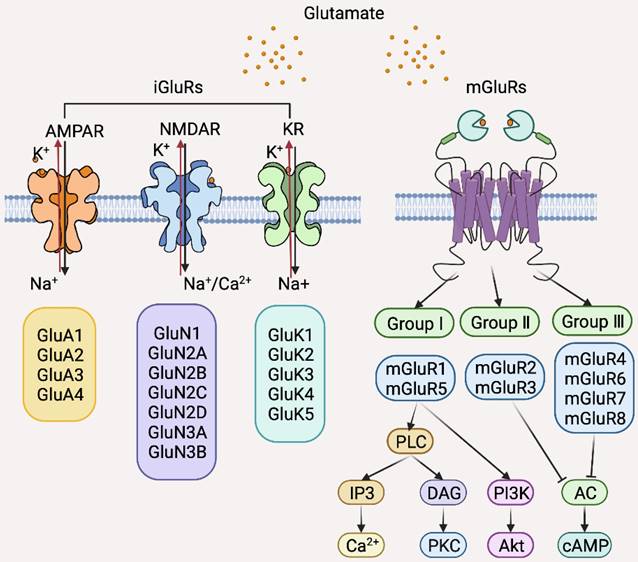
There are two classes of Glu receptors (GluRs) that are categorized based on their differential intracellular signal transduction mechanisms and molecular homologies: ionotropic GluRs (iGluRs) that are ligand-gated ion channels permeable to sodium, potassium, and calcium ions upon Glu binding and metabotropic GluRs (mGluRs), members of the GPCR superfamily that activate second messenger pathways upon stimulation (Figure 6) [171]. iGluRs are further divided into three subclasses according to ligand binding: amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR), N-methyl-D-aspartate receptor (NMDAR), and kainate receptor (KR) (Figure 6) [168]. mGluRs are also further classified into three subgroups according to their differential downstream effectors: Group I is comprised of mGluR1 and 5, which couple to a excitatory Gaq-like protein resulting in activation of the phospholipase C (PLC)-Inositol 1,4,5-triphosphate-diacylglycerol (PLC-IP3-DAG) pathway and subsequent activation of PI3K/Akt and MAPK signaling; group II contains mGluR2 and 3, and group III, mGluR4, 6, 7, 8, which all activate the inhibitory Gi proteins leading to reduction in cAMP levels [171].
Although several GluRs are expressed by BC cells, mGluR1 is the most extensively studied GluR in the context of BC pathogenesis [172-176]. The expression of mGluR1 is increased in BC cells compared to normal breast epithelial cells in vitro and is upregulated in BC tissues compared with normal breast tissues in vivo [172]. Moreover, mGluR1 is expressed at high levels in premalignant cells as shown in isogenic BC cell lines genetically constructed to echo BC initiation and progression [173]. Indeed, knockdown of mGluR1 or pharmacological inhibition of mGluR1 reduces BC cell proliferation and retards BC xenograft growth [174]. However, overexpression of mGluR1 did not impinge on the proliferation of MCF10A premalignant breast epithelial cells [173], suggesting that mGluR1 is involved in BC progression at later stages. In accordance, mGluR1 signaling promotes BC cell invasion and metastasis while high mGluR1 expression is associated with poor distant metastasis-free survival of BC patients [175]. Since overexpression of mGluR1 in MCF10AT1 cells that mirror atypical ductal hyperplasia results in increased proliferation, it is postulated that mGluR1 may interact with other oncogenic factors to promote cell proliferation [173]. Intriguingly, ER signaling upregulates mGluR1, as exposure of ER-positive but not ER-negative BC cells to 17β-estradiol increases mGluR1 expression [176]. Consistently, mGluR1 is more frequently expressed in ER-positive compared to ER-negative BCs [176]. Therefore, ER signaling plays a role in regulating mGluR1 in BC cells, which may in turn affect the response of BC to endocrine therapy. In support, high expression of GRM1, the gene encoding mGluR1, correlates with poor distant metastasis-free survival in tamoxifen-treated patients [176]. Regardless, many studies have clearly demonstrated that mGluR1 promotes TNBC pathogenesis, suggesting that targeting mGluR1 may be useful for the treatment of BCs with varying molecular subtypes. Of note, polymorphisms of GRM1 have been identified in BC cells with the rs6923492 and rs362962 variants associating with BC diagnostic age, the rs362962 TT genotype, and risk of ER or progesterone receptor positivity [176]. Whether these properties can be exploited as biomarkers for BC diagnosis and treatment await further investigation. Interestingly, riluzole, a glutamate release inhibitor in clinical use for the treatment of amyotrophic lateral sclerosis, has shown potent therapeutic effects in BC, in particular, against TNBC, in preclinical models [174]. Thus, repurposing of riluzole appears a promising strategy for BC treatment.
Glu/mGluR1 signaling has also been demonstrated to promote angiogenesis in BC [177, 178]. Endothelial cells expressing high levels of mGluR1 are more sensitive to the inhibition of angiogenesis caused by pharmacological blockade of Glu/mGluR1 signaling [177]. Consistently, Glu/mGluR1 blockade reduces neovascular density in mouse BC models [178]. Therefore, targeting Glu/mGluR1 may be a useful anti-angiogenesis approach in BC treatment. Another potential pathogenic mechanism involving Glu/mGluR1 signaling in BC involves inflammation [179]. Notably, knockdown of mGluR1 upregulates genes encoding chemokines important for chemoattraction of proinflammatory immune cells, such as CXCL1, IL6 and IL8 [179]. In accordance, mGluR1 knockdown results in increased endothelial adhesion and neutrophil transmigration in mixed cell culture systems and in mouse BC models [179]. Manipulation of Glu/mGluR1 signaling may therefore improve the response of BC to immunotherapy.
While the significance of mGluR1 in regulating BC pathogenesis has been well demonstrated, the potential roles of other GluRs cannot be underestimated. For example, NMDARs on the surface of brain metastatic BC cells mediate a neuronal signaling pathway through pseudo-tripartite synapses formed between BC cells and glutamatergic neurons, thus promoting metastatic colonization and invasive growth [42]. In contrast, mGluR4 signaling inhibits BC cell proliferation, migration and invasion, and its expression is correlated with better prognosis [180]. Clearly, more studies are needed to define the biological and clinical significance of the glutamatergic system in BC.
Gamma-aminobutyric acid (GABA)
In contrast to glutamate, GABA is the major inhibitory neurotransmitter, functioning to reduce neuronal excitability in the central nervous system [181]. Endogenous GABA is mainly synthesized from glutamate by the enzyme glutamate decarboxylase (GAD) with pyridoxal phosphate as a cofactor [165]. In the nervous system, GABA regulates the differentiation, proliferation, and migration of neuronal progenitor cells, the elongation of neurites, and the formation of synapses [182]. A lack of GABA in the brain results in defective cognition and is associated with diseases such as depression and schizophrenia [183]. Indeed, GABA has long been used as dietary supplements for the purpose of relieving stress and improving relaxation and sleep, although it is generally believed that GABA cannot cross the blood-brain barrier [184]. GABA also exerts biological functions in many other types of cells outside the nervous system under physiological conditions. For example, pancreatic insulin-producing β-cells secrete GABA to support their survival and proliferation while promoting transdifferentiation of α-cells to β-cells [185].
There are two major types of GABA receptors (GABARs): ionotropic GABAA receptors (GABAARs) that are ligand-gated ion channels allowing the flow of chloride ions, and the metabotropic GABAB receptors (GABABRs) that are GPCRs [181]. GABAARs have at least 19 subunits in the human including α1-α6, β1-β3, γ1-γ3, δ, ε, θ, π, and ρ1-ρ3 that form pentamers comprised of various subunits, whereas GABABRs are heterodimers composed of GABAB1 and GABAB2 subunits (Table 3) [186-190]. GABAB1 has at least 14 isoforms (GABAB1a-n) with GABAB1a and GABAB1b being the most abundant isoforms primarily expressed in the nervous system [191]. GABARs are expressed in many peripheral tissues [192]. For instance, immune cells express GABARs, which, upon activation, regulate secretion of cytokines that suppress inflammatory immune responses [193].
Expression and Role of GABAR subunits in breast cancer cells
| Major GABAR types | Cell type | GABAR Subunits | Effect of action | Molecular targets | References |
|---|---|---|---|---|---|
| GABAA receptors (ligand-gated ion channels) | HCC70 | GABAAR π | Migration↑ | ERK1/2, KRT6B, KRT14, and KRT17 | [186] |
| HCC1187 | GABAAR π | Migration↑ Secondary tumorsphere formation↑ | ERK1/2, KRT5, KRT6B, KRT14, and KRT17 | [186] | |
| MCF-7 MDA-MB-436 | GABAAR α3 | Migration ↑ Invasion↑ | pAKT | [187] | |
| HCC1806 HCC1937 | GABAAR π | Proliferation↑ | EGFR | [188] | |
| GABAB receptors (G-protein coupled receptors) | 4T1 | GABAB1 | Invasion↑ Migration↑ | ERK1/2, MMP2 | [189] |
| MCF-7 T-47D | GABAB1e | Proliferation↑ | PTPN12, EGFR | [190] | |
| MDA-MB-231 BT-549 | GABAB1e | Proliferation↑ Invasion↑ Migration↑ | PTPN12, EGFR | [190] |
Abbreviations: EGFR, epidermal growth factor receptor; GABA, Gamma-aminobutyric acid; GABAR, GABA receptor; KRT, Keratin; MMP2, matrix metalloproteinase-2; pAKT, phosphorylated AKT; PTPN12, protein tyrosine phosphatase non-receptor type 12.
Although both GABA and GAD are detectable in normal breast tissues, they are expressed at increased levels in BC samples [194]. Similarly, both GABAARs and GABABRs are expressed at higher levels in BC compared to normal breast tissues [189, 195]. These observations strongly suggest that the GABAergic system may be involved in the regulation of BC biology. Consistently, GABA expression is correlated with BC pathological stage and its levels are of significant prognostic value when E-cadherin is concurrently lost [194]. Indeed, the GABAergic system plays an important role in regulating BC cell invasion and metastasis [196]. Moreover, it has been demonstrated that BC cells acquire neural characteristics after metastasizing to the brain, expressing high levels of GABAergic proteins, including the GABAAR, GABA transporter, GABA transaminase, parvalbumin, and reelin [197]. This remarkably enables such BC cells to take up and catabolize GABA into succinate, enhancing NADH biosynthesis to promote their proliferation [197]. Both ERK1/2- and Akt-signaling pathways mediate GABA-induced invasion and metastasis of BC cells, although the GABAR subunits involved vary widely in different studies [186, 187]. For example, GABAAR π subunit signaling promotes TNBC cell migration through activation of ERK1/2, whereas the GABAAR α3 subunit mediates Akt activation to support BC cell migration, invasion and metastasis [186]. Interestingly, an A-to-I RNA-edited form of GABAAR α3 has only been identified in non-invasive BC cells, which suppresses activation of Akt required for BC cell migration and invasion [187]. Since the enzyme adenosine deaminase acting on RNA (ADAR) is responsible for GABAAR α3 RNA editing in BC cells, ADAR is conceivably involved in regulation of BC metastasis [187]. Given these well-demonstrated roles of the GABAergic system in BC cells, it is not surprising that exposure to GABA or GABA mimetics causes activation of ERK1/2 and Akt, resulting in invasion, migration, and metastasis of BC cells [186]. Noticeably, the GABA mimetic gabapentin is clinically used to relieve chemotherapy-associated peripheral nerve pain [198]. The potential impact of such interventions on BC invasion and metastasis warrants further investigation.
The GABAergic system has also been reported to regulate CSC characteristics in BC [188]. For example, the GABAAR π subunit is enriched in TNBC CSCs, which interacts with epidermal growth factor receptor (EGFR) to sustain its expression, leading to stemness maintenance and resistance to chemotherapy [188]. The GABABR subunit GABAB1e can also promote EGFR signaling through binding to protein tyrosine phosphatase non-receptor type 2 (PTPN2) to disrupt its interaction with EGFR [190]. Moreover, exposure to a GABABR agonist inhibits BC growth, suggesting that the GABAergic system also plays a role in regulating BC cell survival and proliferation [189]. Of interest, increased GABA expression and GAD activity in BC tissues have been linked to local anti-tumor immune responses [199], but further investigation is needed to substantiate this phenomenon.
Conclusions and perspectives
This review summarizes the current knowledge about the roles and mechanisms of many common neurotransmitters and their receptors in BC pathogenesis and discusses their potential usefulness as biomarkers and therapeutic targets in BC diagnosis, prognosis and treatment. Notwithstanding this knowledge, there are many other neurotransmitters and neuropeptides that are potentially released within the BC microenvironment, either being produced by nerves, BC cells themselves or other tumor-infiltrating cells. Each of these factors has the potential to impact BC pathogenesis in either an autocrine or paracrine manner [53]. For example, substance P (SP), a member of the tachykinin neuropeptide family, is expressed at increased levels in BC cells and BC patient sera, promoting BC cell proliferation and regulating the immune response against BC [200], whereas neuropeptide Y (NPY), a class of highly conserved neuropeptides, also promotes BC cell proliferation and metastasis and is involved in regulation of angiogenesis in BC tissues. Further investigation is clearly required to define the mechanism of action of individual neurotransmitters/neuropeptides, and importantly, to determine the extent to which each contributes to BC development, progression and treatment responses.
Nevertheless, with the emergence of cancer neuroscience, our understanding of the interaction between the nervous system and BC cells is rapidly gaining momentum. It is now clear that there is not only direct crosstalk, but also indirect interactions through other biological processes, such as the immune response and angiogenesis, between the nervous system and BC cells. These advances also bear fundamental impact on the field of psycho-oncology through addressing the long-standing question as to what the biological basis is for the links between stress and BC. However, translation from the laboratory to the clinical setting will require the identification of druggable components of the nerve-BC interaction. Notably, these efforts require new types of collaborations among researchers and clinicians from the historically different fields of neuroscience and oncology, bringing together cancer researchers, psychologists, pharmacologists, and others. The impetus for these collaborations remarkably already exists: several clinical agents used to treat neurological and/or psychological disorders through interfering with neurotransmitter-receptor interactions have been shown to have preventive/therapeutic effects against BC [28, 30, 99, 161, 162]. Further exploration of repurposing of these drugs for BC prevention/treatment is warranted.
Abbreviations
AC: adenylate cyclase; ACh: acetylcholine; AChRs: acetylcholine receptors; ADAR: adenosine deaminase acting on RNA; AMPAR: amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; ARs: adrenergic receptors; ATF1: activating transcription factor 1; BAD: B-cell lymphoma 2 (BCL2)-associated agonist of cell death; BC: breast cancer; BDNF: brain-derived neurotrophic factor; CAM: complementary and alternative medicines; cAMP: cyclic adenosine monophosphate; CDK4/6: cyclin-dependent kinases 4 and 6; cGMP: cyclic guanosine monophosphate; cGAS: cGMP-AMP synthase; cGAMP: cyclic GMP-AMP; ChAT: choline acetyltransferase; CREB: cAMP response element-binding protein; CSCs: cancer stem-like cells; DA: dopamine; DAG: diacylglycerol; DAR: DA receptors; DCs: dendritic cells; EGFR: epidermal growth factor receptor; EMT: epithelial-to-mesenchymal transition; ER: estrogen receptor; FOXP3: forkhead box P3; GABA: Gamma-aminobutyric acid; GABARs: GABA receptors; GAD: glutamate decarboxylase; GC: guanylate cyclase; Glu: glutamate; GluRs: glutamate receptors; GPCRs: G protein-coupled receptors; Gβγ: G beta-gamma complex; Hb-IPn: habenulo-interpeduncular; HER2: epidermal growth factor receptor 2; HPA: hypothalamic-pituitary-adrenal; iGluRs: ionotropic GluRs; IP3: Inositol 1,4,5-triphosphate; JAK1: Janus kinase 1; KR: kainate receptor; KRT: Keratin; mAChRs: muscarinic acetylcholine receptors; MAPK: p38 mitogen-activated protein kinase; mGluRs: metabotropic GluRs; MMP2: matrix metalloproteinase-2; mPFC: medial prefrontal cortex; mTORC1: mTOR complex 1; nAChRs: nicotinic acetylcholine receptors; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; NGF: nerve growth factor; NMDAR: N-methyl-D-aspartate receptor; NNK: N′- nitrosonornicotine (NNN) and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; NOS: nitric oxide synthase; NPY: neuropeptide Y; pAKT: phosphorylated AKT; PARP: poly(ADP-ribose) polymerase; PD1: programmed cell death protein 1; PD-L1: programmed death-ligand 1; PFS: progression-free survival; PGC1α: peroxisome proliferator-activated receptor gamma coactivator 1-α; PI3K: phosphoinositide 3-kinase; PKA: protein kinase A; PKC: Protein kinase C; PKG: protein kinase G; PKM2: pyruvate kinase M2; PLA2: phospholipase A2; PLC: phospholipase C; PNI: perineural invasion; PPARγ: peroxisome proliferator-activated receptor γ; PPM1F: protein phosphatase 1F; PTPN2: phosphatase non-receptor type 2; SERD: selective estrogen receptor degrader; SERMs: selective estrogen receptor modulators; SERT: serotonin transporter; SP: substance P; STAT1: signal transducer and activator of transcription 1; STING: stimulator of interferon genes; TNBC: triple-negative breast cancer; TPH: tryptophan hydroxylase; UCMS: unpredictable chronic mild stress; VEGF: vascular endothelial growth factor; VPF: vascular permeability factor; VTA: ventral tegmental area; 5-HT: 5-hydroxytryptamine; 5-HTRs: 5-HT receptors.
Acknowledgements
This work was supported by Shanxi Province 136 Revitalization Medical Project Construction Fund (2021YZ10) and the Key Research and Development Plan of Shanxi Province (through Shanxi Science and Technology Department) (201903D421025), China, and Cancer Council NSW Project Grants (RG20-01 and RG21-10), Australia. The figures were created with BioRender.com under a paid subscription.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Loibl S, Poortmans P, Morrow M, Denkert C, Curigliano G. Breast cancer. Lancet. 2021;397:1750-69
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30
3. Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P. et al. Breast cancer. Nat Rev Dis Primers. 2019;5:66
4. Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ. et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317:2402-16
5. Korde LA, Somerfield MR, Carey LA, Crews JR, Denduluri N, Hwang ES. et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol. 2021;39:1485-505
6. Robson ME, Tung N, Conte P, Im SA, Senkus E, Xu B. et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician's choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol. 2019;30:558-66
7. Litton JK, Hurvitz SA, Mina LA, Rugo HS, Lee KH, Gonçalves A. et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: final overall survival results from the EMBRACA trial. Ann Oncol. 2020;31:1526-35
8. Jiang YZ, Liu Y, Xiao Y, Hu X, Jiang L, Zuo WJ. et al. Molecular subtyping and genomic profiling expand precision medicine in refractory metastatic triple-negative breast cancer: the FUTURE trial. Cell Res. 2021;31:178-86
9. Bidard FC, Hardy-Bessard AC, Dalenc F, Bachelot T, Pierga JY, de la Motte Rouge T. et al. Switch to fulvestrant and palbociclib versus no switch in advanced breast cancer with rising ESR1 mutation during aromatase inhibitor and palbociclib therapy (PADA-1): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2022;23:1367-77
10. Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Hart L. et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N Engl J Med. 2022;386:942-50
11. Harbeck N, Rastogi P, Martin M, Tolaney SM, Shao ZM, Fasching PA. et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol. 2021;32:1571-81
12. Bachelot T, Cottu P, Chabaud S, Dalenc F, Allouache D, Delaloge S. et al. Everolimus added to adjuvant endocrine therapy in patients with high-risk hormone receptor-positive, human epidermal growth factor receptor 2-negative primary breast cancer. J Clin Oncol. 2022;40:3699-708
13. Greenlee H, Neugut AI, Falci L, Hillyer GC, Buono D, Mandelblatt JS. et al. Association between complementary and alternative medicine use and breast cancer chemotherapy initiation: The breast cancer quality of care (BQUAL) study. JAMA Oncol. 2016;2:1170-6
14. Lee RT, Kwon N, Wu J, To C, To S, Szmulewitz R. et al. Prevalence of potential interactions of medications, including herbs and supplements, before, during, and after chemotherapy in patients with breast and prostate cancer. Cancer. 2021;127:1827-35
15. Baxevanis CN, Fortis SP, Perez SA. The balance between breast cancer and the immune system: Challenges for prognosis and clinical benefit from immunotherapies. Semin Cancer Biol. 2021;72:76-89
16. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565-70
17. Clark NM, Martinez LM, Murdock S, deLigio JT, Olex AL, Effi C. et al. Regulatory T cells support breast cancer progression by opposing IFN-γ-dependent functional reprogramming of myeloid cells. Cell Rep. 2020;33:108482
18. Li Z, Cai H, Li Z, Ren L, Ma X, Zhu H. et al. A tumor cell membrane-coated self-amplified nanosystem as a nanovaccine to boost the therapeutic effect of anti-PD-L1 antibody. Bioact Mater. 2023;21:299-312
19. Feng Q, Zhang C, Lum D, Druso JE, Blank B, Wilson KF. et al. A class of extracellular vesicles from breast cancer cells activates VEGF receptors and tumour angiogenesis. Nat Commun. 2017;8:14450
20. Shashni B, Nishikawa Y, Nagasaki Y. Management of tumor growth and angiogenesis in triple-negative breast cancer by using redox nanoparticles. Biomaterials. 2021;269:120645
21. Wu SY, Xu Y, Chen L, Fan L, Ma XY, Zhao S. et al. Combined angiogenesis and PD-1 inhibition for immunomodulatory TNBC: Concept exploration and biomarker analysis in the FUTURE-C-Plus trial. Mol Cancer. 2022;21:84
22. Marchesi F, Piemonti L, Mantovani A, Allavena P. Molecular mechanisms of perineural invasion, a forgotten pathway of dissemination and metastasis. Cytokine Growth Factor Rev. 2010;21:77-82
23. Gupta M, Goyal N. Applied anatomy of breast cancer. In: Bose MS, Mazumdar A, Kaushik R, Ed. Breast cancer. 1st ed. Singapore: Springer Singapore. 2022:23-25
24. Pundavela J, Roselli S, Faulkner S, Attia J, Scott RJ, Thorne RF. et al. Nerve fibers infiltrate the tumor microenvironment and are associated with nerve growth factor production and lymph node invasion in breast cancer. Mol Oncol. 2015;9:1626-35
25. Entschladen F, Drell TLt, Lang K, Joseph J, Zaenker KS. Tumour-cell migration, invasion, and metastasis: navigation by neurotransmitters. Lancet Oncol. 2004;5:254-8
26. Boilly B, Faulkner S, Jobling P, Hondermarck H. Nerve dependence: From regeneration to cancer. Cancer cell. 2017;31:342-54
27. Narayan P, Flynn J, Zhang Z, Gillespie EF, Mueller B, Xu AJ. et al. Perineural invasion as a risk factor for locoregional recurrence of invasive breast cancer. Sci Rep. 2021;11:12781
28. Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population- based study. J Clin Oncol. 2011;29:2635-44
29. Melhem-Bertrandt A, Chavez-Macgregor M, Lei X, Brown EN, Lee RT, Meric-Bernstam F. et al. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol. 2011;29:2645-52
30. Shaashua L, Shabat-Simon M, Haldar R, Matzner P, Zmora O, Shabtai M. et al. Perioperative COX-2 and β-adrenergic blockade improves metastatic biomarkers in breast cancer patients in a phase-II randomized trial. Clin Cancer Res. 2017;23:4651-61
31. Faulkner S, Jobling P, March B, Jiang CC, Hondermarck H. Tumor neurobiology and the war of nerves in cancer. Cancer Discov. 2019;9:702-10
32. Hondermarck H. Neurotrophins and their receptors in breast cancer. Cytokine Growth Factor Rev. 2012;23:357-65
33. McCallum GA, Shiralkar J, Suciu D, Covarrubias G, Yu JS, Karathanasis E. et al. Chronic neural activity recorded within breast tumors. Sci Rep. 2020;10:14824
34. Kamiya A, Hayama Y, Kato S, Shimomura A, Shimomura T, Irie K. et al. Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nat Neurosci. 2019;22:1289-305
35. Mohammadpour H, MacDonald CR, Qiao G, Chen M, Dong B, Hylander BL. et al. β2 adrenergic receptor-mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells. J Clin Invest. 2019;129:5537-52
36. Chen H, Liu D, Yang Z, Sun L, Deng Q, Yang S. et al. Adrenergic signaling promotes angiogenesis through endothelial cell-tumor cell crosstalk. Endocrine-related cancer. 2014;21:783-95
37. Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer. 2015;15:563-72
38. Mulcrone PL, Campbell JP, Clément-Demange L, Anbinder AL, Merkel AR, Brekken RA. et al. Skeletal colonization by breast cancer cells is stimulated by an osteoblast and β2AR-dependent neo-angiogenic switch. J Bone Miner Res. 2017;32:1442-54
39. Le CP, Nowell CJ, Kim-Fuchs C, Botteri E, Hiller JG, Ismail H. et al. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat Commun. 2016;7:10634
40. Kopparapu PK, Tinzl M, Anagnostaki L, Persson JL, Dizeyi N. Expression and localization of serotonin receptors in human breast cancer. Anticancer Res. 2013;33:363-70
41. Chen Q, Boire A, Jin X, Valiente M, Er EE, Lopez-Soto A. et al. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature. 2016;533:493-8
42. Zeng Q, Michael IP, Zhang P, Saghafinia S, Knott G, Jiao W. et al. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature. 2019;573:526-31
43. Monje M, Borniger JC, D'Silva NJ, Deneen B, Dirks PB, Fattahi F. et al. Roadmap for the emerging field of cancer neuroscience. Cell. 2020;181:219-22
44. Bleiker EM, van der Ploeg HM. Psychosocial factors in the etiology of breast cancer: review of a popular link. Patient Educ Couns. 1999;37:201-14
45. Rosenberg SM, Dominici LS, Gelber S, Poorvu PD, Ruddy KJ, Wong JS. et al. Association of breast cancer surgery with quality of life and psychosocial well-being in young breast cancer survivors. JAMA Surg. 2020;155:1035-42
46. Saxton JM, Scott EJ, Daley AJ, Woodroofe M, Mutrie N, Crank H. et al. Effects of an exercise and hypocaloric healthy eating intervention on indices of psychological health status, hypothalamic-pituitary-adrenal axis regulation and immune function after early-stage breast cancer: A randomised controlled trial. Breast Cancer Res. 2014;16:R39
47. Abercrombie HC, Giese-Davis J, Sephton S, Epel ES, Turner-Cobb JM, Spiegel D. Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology. 2004;29:1082-92
48. Stults-Kolehmainen MA, Sinha R. The effects of stress on physical activity and exercise. Sports Med. 2014;44:81-121
49. Dethlefsen C, Hansen LS, Lillelund C, Andersen C, Gehl J, Christensen JF. et al. Exercise-induced catecholamines activate the hippo tumor suppressor pathway to reduce risks of breast cancer development. Cancer Res. 2017;77:4894-904
50. Xu XR, Xiao Q, Hong YC, Liu YH, Liu Y, Tu J. Activation of dopaminergic VTA inputs to the mPFC ameliorates chronic stress-induced breast tumor progression. CNS Neurosci Ther. 2021;27:206-19
51. Cui B, Luo Y, Tian P, Peng F, Lu J, Yang Y. et al. Stress-induced epinephrine enhances lactate dehydrogenase A and promotes breast cancer stem-like cells. J Clin Invest. 2019;129:1030-46
52. Chen H, Liu D, Guo L, Cheng X, Guo N, Shi M. Chronic psychological stress promotes lung metastatic colonization of circulating breast cancer cells by decorating a pre-metastatic niche through activating β-adrenergic signaling. J Pathol. 2018;244:49-60
53. Mancino M, Ametller E, Gascón P, Almendro V. The neuronal influence on tumor progression. Biochim Biophys Acta. 2011;1816:105-18
54. Schiller M, Ben-Shaanan TL, Rolls A. Neuronal regulation of immunity: why, how and where? Nat Rev Immunol. 2021;21:20-36
55. Tank AW, Lee Wong D. Peripheral and central effects of circulating catecholamines. Compr Physiol. 2015;5:1-15
56. Sun Z, Zhangsun M, Dong S, Liu Y, Qian J, Zhangsun D. et al. Differential expression of nicotine acetylcholine receptors associates with human breast cancer and mediates antitumor activity of αo-conotoxin gexiva. Mar Drugs. 2020 18
57. Conceição F, Sousa DM, Paredes J, Lamghari M. Sympathetic activity in breast cancer and metastasis: partners in crime. Bone Res. 2021;9:9
58. Reeder A, Attar M, Nazario L, Bathula C, Zhang A, Hochbaum D. et al. Stress hormones reduce the efficacy of paclitaxel in triple negative breast cancer through induction of DNA damage. Br J Cancer. 2015;112:1461-70
59. Powe DG, Voss MJ, Habashy HO, Zänker KS, Green AR, Ellis IO. et al. Alpha- and beta-adrenergic receptor (AR) protein expression is associated with poor clinical outcome in breast cancer: an immunohistochemical study. Breast Cancer Res Treat. 2011;130:457-63
60. Castillo LF, Rivero EM, Goffin V, Lüthy IA. Alpha(2)-adrenoceptor agonists trigger prolactin signaling in breast cancer cells. Cell Signal. 2017;34:76-85
61. Du Y, Zhou L, Wang Y, Yan T, Jiang Y, Shao Z. et al. Association of alpha2a and beta2 adrenoceptor expression with clinical outcome in breast cancer. Curr Med Res Opin. 2014;30:1337-44
62. Bruzzone A, Piñero CP, Rojas P, Romanato M, Gass H, Lanari C. et al. α(2)-adrenoceptors enhance cell proliferation and mammary tumor growth acting through both the stroma and the tumor cells. Curr Cancer Drug Targets. 2011;11:763-74
63. Gruet M, Cotton D, Coveney C, Boocock DJ, Wagner S, Komorowski L. et al. β2-adrenergic signalling promotes cell migration by upregulating expression of the metastasis-associated molecule LYPD3. Biology (Basel). 2020 9
64. Pon CK, Lane JR, Sloan EK, Halls ML. The β2-adrenoceptor activates a positive cAMP-calcium feedforward loop to drive breast cancer cell invasion. FASEB J. 2016;30:1144-54
65. Liu D, Deng Q, Sun L, Wang T, Yang Z, Chen H. et al. A Her2-let-7-β2-ar circuit affects prognosis in patients with Her2-positive breast cancer. BMC Cancer. 2015;15:832
66. Shi M, Liu D, Duan H, Qian L, Wang L, Niu L. et al. The β2-adrenergic receptor and Her2 comprise a positive feedback loop in human breast cancer cells. Breast Cancer Res Treat. 2011;125:351-62
67. Zhou Z, Zhan J, Luo Q, Hou X, Wang S, Xiao D. et al. ADRB3 induces mobilization and inhibits differentiation of both breast cancer cells and myeloid-derived suppressor cells. Cell Death Dis. 2022;13:141
68. Kurozumi S, Kaira K, Matsumoto H, Hirakata T, Yokobori T, Inoue K. et al. β(2)-adrenergic receptor expression is associated with biomarkers of tumor immunity and predicts poor prognosis in estrogen receptor-negative breast cancer. Breast Cancer Res Treat. 2019;177:603-10
69. An J, Feng L, Ren J, Li Y, Li G, Liu C. et al. Chronic stress promotes breast carcinoma metastasis by accumulating myeloid-derived suppressor cells through activating β-adrenergic signaling. Oncoimmunology. 2021;10:2004659
70. Pérez Piñero C, Bruzzone A, Sarappa MG, Castillo LF, Lüthy IA. Involvement of α2- and β2-adrenoceptors on breast cancer cell proliferation and tumour growth regulation. Br J Pharmacol. 2012;166:721-36
71. Jiao X, Gonzalez-Cabrera PJ, Xiao L, Bradley ME, Abel PW, Jeffries WB. Tonic inhibitory role for cAMP in alpha(1a)-adrenergic receptor coupling to extracellular signal-regulated kinases 1/2. J Pharmacol Exp Ther. 2002;303:247-56
72. Sastry KS, Karpova Y, Prokopovich S, Smith AJ, Essau B, Gersappe A. et al. Epinephrine protects cancer cells from apoptosis via activation of cAMP-dependent protein kinase and BAD phosphorylation. J Biol Chem. 2007;282:14094-100
73. Chang A, Le CP, Walker AK, Creed SJ, Pon CK, Albold S. et al. β2-adrenoceptors on tumor cells play a critical role in stress-enhanced metastasis in a mouse model of breast cancer. Brain Behav Immun. 2016;57:106-15
74. Du P, Zeng H, Xiao Y, Zhao Y, Zheng B, Deng Y. et al. Chronic stress promotes EMT-mediated metastasis through activation of STAT3 signaling pathway by miR-337-3p in breast cancer. Cell Death Dis. 2020;11:761
75. Pasquier E, Ciccolini J, Carre M, Giacometti S, Fanciullino R, Pouchy C. et al. Propranolol potentiates the anti-angiogenic effects and anti-tumor efficacy of chemotherapy agents: implication in breast cancer treatment. Oncotarget. 2011;2:797-809
76. Zhou J, Liu Z, Zhang L, Hu X, Wang Z, Ni H. et al. Activation of β2-adrenergic receptor promotes growth and angiogenesis in breast cancer by down-regulating PPARγ. Cancer Res Treat. 2020;52:830-47
77. Bucsek MJ, Qiao G, MacDonald CR, Giridharan T, Evans L, Niedzwecki B. et al. β-adrenergic signaling in mice housed at standard temperatures suppresses an effector phenotype in CD8(+) T cells and undermines checkpoint inhibitor therapy. Cancer Res. 2017;77:5639-51
78. Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V. et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70:7042-52
79. Hervé J, Dubreil L, Tardif V, Terme M, Pogu S, Anegon I. et al. β2-adrenoreceptor agonist inhibits antigen cross-presentation by dendritic cells. J Immunol. 2013;190:3163-71
80. Zalli A, Bosch JA, Goodyear O, Riddell N, McGettrick HM, Moss P. et al. Targeting β2 adrenergic receptors regulate human T cell function directly and indirectly. Brain Behav Immun. 2015;45:211-8
81. Qin JF, Jin FJ, Li N, Guan HT, Lan L, Ni H. et al. Adrenergic receptor β2 activation by stress promotes breast cancer progression through macrophages M2 polarization in tumor microenvironment. BMB Rep. 2015;48:295-300
82. Qiao G, Chen M, Mohammadpour H, MacDonald CR, Bucsek MJ, Hylander BL. et al. Chronic adrenergic stress contributes to metabolic dysfunction and an exhausted phenotype in T cells in the tumor microenvironment. Cancer Immunol Res. 2021;9:651-64
83. Ferenczi EA, Zalocusky KA, Liston C, Grosenick L, Warden MR, Amatya D. et al. Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science. 2016;351:aac9698
84. Obray JD, Small CA, Baldwin EK, Jang EY, Lee JG, Yang CH. et al. Dopamine D2-subtype receptors outside the blood-brain barrier mediate enhancement of mesolimbic dopamine release and conditioned place preference by intravenous dopamine. Front Cell Neurosci. 2022;16:944243
85. Villanueva I, Piñón M, Quevedo-Corona L, Martínez-Olivares R, Racotta R. Epinephrine and dopamine colocalization with norepinephrine in various peripheral tissues: Guanethidine effects. Life Sci. 2003;73:1645-53
86. Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: A contemporary view with implications for physiology and medicine. Pharmacol Rev. 2004;56:331-49
87. Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182-217
88. Borcherding DC, Tong W, Hugo ER, Barnard DF, Fox S, LaSance K. et al. Expression and therapeutic targeting of dopamine receptor-1 (D1R) in breast cancer. Oncogene. 2016;35:3103-13
89. Rangel-Barajas C, Coronel I, Florán B. Dopamine receptors and neurodegeneration. Aging Dis. 2015;6:349-68
90. Tegowski M, Fan C, Baldwin AS. Selective effects of thioridazine on self-renewal of basal-like breast cancer cells. Sci Rep. 2019;9:18695
91. Pornour M, Ahangari G, Hejazi SH, Deezagi A. New perspective therapy of breast cancer based on selective dopamine receptor D2 agonist and antagonist effects on MCF-7 cell line. Recent Pat Anticancer Drug Discov. 2015;10:214-23
92. Wang S, Mou Z, Ma Y, Li J, Li J, Ji X. et al. Dopamine enhances the response of sunitinib in the treatment of drug-resistant breast cancer: Involvement of eradicating cancer stem-like cells. Biochem Pharmacol. 2015;95:98-109
93. Tegowski M, Fan C, Baldwin AS. Thioridazine inhibits self-renewal in breast cancer cells via DRD2-dependent STAT3 inhibition, but induces a G1 arrest independent of DRD2. J Biol Chem. 2018;293:15977-90
94. Peters MA, Walenkamp AM, Kema IP, Meijer C, de Vries EG, Oosting SF. Dopamine and serotonin regulate tumor behavior by affecting angiogenesis. Drug Resist Updat. 2014;17:96-104
95. Pornour M, Ahangari G, Hejazi SH, Ahmadkhaniha HR, Akbari ME. Dopamine receptor gene (DRD1-DRD5) expression changes as stress factors associated with breast cancer. Asian Pac J Cancer Prev. 2014;15:10339-43
96. Borgi M, Collacchi B, Ortona E, Cirulli F. Stress and coping in women with breast cancer:unravelling the mechanisms to improve resilience. Neurosci Biobehav Rev. 2020;119:406-21
97. Fenton M, Rathbone J, Reilly J, Sultana A. Thioridazine for schizophrenia. Cochrane Database Syst Rev. 2007;2007:Cd001944
98. Murphy MB, Murray C, Shorten GD. Fenoldopam: A selective peripheral dopamine-receptor agonist for the treatment of severe hypertension. N Engl J Med. 2001;345:1548-57
99. Yang L, Yao Y, Yong L, Feng Y, Su H, Yao Q. et al. Dopamine D1 receptor agonists inhibit lung metastasis of breast cancer reducing cancer stemness. Eur J Pharmacol. 2019;859:172499
100. Treseder SA, Smith LA, Jenner P. Endogenous dopaminergic tone and dopamine agonist action. Mov Disord. 2000;15:804-12
101. Yabut JM, Crane JD, Green AE, Keating DJ, Khan WI, Steinberg GR. Emerging roles for serotonin in regulating metabolism: New implications for an ancient molecule. Endocr Rev. 2019;40:1092-107
102. Bader M. Inhibition of serotonin synthesis: A novel therapeutic paradigm. Pharmacol Ther. 2020;205:107423
103. Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355-66
104. Maroteaux L, Kilic F. Frontiers of serotonin beyond the brain. Pharmacol Res. 2019;140:1-6
105. Shah PA, Park CJ, Shaughnessy MP, Cowles RA. Serotonin as a mitogen in the gastrointestinal tract: Revisiting a familiar molecule in a new role. Cell Mol Gastroenterol Hepatol. 2021;12:1093-104
106. McCorvy JD, Roth BL. Structure and function of serotonin g protein-coupled receptors. Pharmacol Ther. 2015;150:129-42
107. Karmakar S, Lal G. Role of serotonin receptor signaling in cancer cells and anti-tumor immunity. Theranostics. 2021;11:5296-312
108. Matsuda M, Imaoka T, Vomachka AJ, Gudelsky GA, Hou Z, Mistry M. et al. Serotonin regulates mammary gland development via an autocrine-paracrine loop. Dev Cell. 2004;6:193-203
109. Liu HM, Ma LL, Li C, Cao B, Jiang Y, Han L. et al. The molecular mechanism of chronic stress affecting the occurrence and development of breast cancer and potential drug therapy. Transl Oncol. 2022;15:101281
110. Stull MA, Pai V, Vomachka AJ, Marshall AM, Jacob GA, Horseman ND. Mammary gland homeostasis employs serotonergic regulation of epithelial tight junctions. Proc Natl Acad Sci U S A. 2007;104:16708-13
111. Pai VP, Horseman ND. Biphasic regulation of mammary epithelial resistance by serotonin through activation of multiple pathways. J Biol Chem. 2008;283:30901-10
112. Pai VP, Marshall AM, Hernandez LL, Buckley AR, Horseman ND. Altered serotonin physiology in human breast cancers favors paradoxical growth and cell survival. Breast Cancer Res. 2009;11:R81
113. Fröbe A, Čičin-Šain L, Jones G, Soldič Ž, Lukač J, Bolanča A. et al. Plasma free serotonin as a marker for early detection of breast cancer recurrence. Anticancer Res. 2014;34:1167-9
114. Gautam J, Banskota S, Regmi SC, Ahn S, Jeon YH, Jeong H. et al. Tryptophan hydroxylase 1 and 5-HT(7) receptor preferentially expressed in triple-negative breast cancer promote cancer progression through autocrine serotonin signaling. Mol Cancer. 2016;15:75
115. Sola-Penna M, Paixão LP, Branco JR, Ochioni AC, Albanese JM, Mundim DM. et al. Serotonin activates glycolysis and mitochondria biogenesis in human breast cancer cells through activation of the Jak1/STAT3/ERK1/2 and adenylate cyclase/PKA, respectively. Br J Cancer. 2020;122:194-208
116. Gwynne WD, Shakeel MS, Girgis-Gabardo A, Hassell JA. The role of serotonin in breast cancer stem cells. Molecules. 2021 26
117. Griffith SG, Burnstock G. Immunohistochemical demonstration of serotonin in nerves supplying human cerebral and mesenteric blood-vessels. Some speculations about their involvement in vascular disorders. Lancet. 1983;1:561-2
118. Herr N, Bode C, Duerschmied D. The effects of serotonin in immune cells. Front Cardiovasc Med. 2017;4:48
119. Fischer A, Rennert HS, Rennert G. Selective serotonin reuptake inhibitors associated with increased mortality risk in breast cancer patients in northern israel. Int J Epidemiol. 2022;51:807-16
120. Shapovalov Y, Zettel M, Spielman SC, Amico-Ruvio SA, Kelly EA, Sipe GO. et al. Fluoxetine modulates breast cancer metastasis to the brain in a murine model. BMC Cancer. 2014;14:598
121. Haque R, Shi J, Schottinger JE, Ahmed SA, Cheetham TC, Chung J. et al. Tamoxifen and antidepressant drug interaction in a cohort of 16,887 breast cancer survivors. J Natl Cancer Inst. 2016 108
122. Herrera-Martínez AD, Feelders RA, Van den Dungen R, Dogan-Oruc F, van Koetsveld PM, Castaño JP. et al. Effect of the tryptophan hydroxylase inhibitor telotristat on growth and serotonin secretion in 2D and 3D cultured pancreatic neuroendocrine tumor cells. Neuroendocrinology. 2020;110:351-63
123. Cox MA, Bassi C, Saunders ME, Nechanitzky R, Morgado-Palacin I, Zheng C. et al. Beyond neurotransmission: acetylcholine in immunity and inflammation. J Intern Med. 2020;287:120-33
124. Kamiya A, Hiyama T, Fujimura A, Yoshikawa S. Sympathetic and parasympathetic innervation in cancer: therapeutic implications. Clin Auton Res. 2021;31:165-78
125. Soreq H. Checks and balances on cholinergic signaling in brain and body function. Trends Neurosci. 2015;38:448-58
126. Xiao S, Wang T, Ma X, Qin Y, Li X, Zhao Z. et al. Efficacy and safety of a novel acetylcholinesterase inhibitor octohydroaminoacridine in mild-to-moderate alzheimer's disease: A phase II multicenter randomised controlled trial. Age Ageing. 2017;46:767-73
127. Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ. et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341:1236361
128. Calaf GM. Role of organophosphorous pesticides and acetylcholine in breast carcinogenesis. Semin Cancer Biol. 2021;76:206-17
129. Giese-Davis J, Wilhelm FH, Tamagawa R, Palesh O, Neri E, Taylor CB. et al. Higher vagal activity as related to survival in patients with advanced breast cancer: an analysis of autonomic dysregulation. Psychosom Med. 2015;77:346-55
130. Español AJ, Sales ME. Different muscarinc receptors are involved in the proliferation of murine mammary adenocarcinoma cell lines. Int J Mol Med. 2004;13:311-7
131. Hurst R, Rollema H, Bertrand D. Nicotinic acetylcholine receptors: from basic science to therapeutics. Pharmacol Ther. 2013;137:22-54
132. Hogg RC, Raggenbass M, Bertrand D. Nicotinic acetylcholine receptors: from structure to brain function. Rev Physiol Biochem Pharmacol. 2003;147:1-46
133. Zoli M, Pucci S, Vilella A, Gotti C. Neuronal and extraneuronal nicotinic acetylcholine receptors. Curr Neuropharmacol. 2018;16:338-49
134. Gotti C, Moretti M, Maggi R, Longhi R, Hanke W, Klinke N. et al. Alpha7 and alpha8 nicotinic receptor subtypes immunopurified from chick retina have different immunological, pharmacological and functional properties. Eur J Neurosci. 1997;9:1201-11
135. Wu J, Liu Q, Tang P, Mikkelsen JD, Shen J, Whiteaker P. et al. Heteromeric α7β2 nicotinic acetylcholine receptors in the brain. Trends Pharmacol Sci. 2016;37:562-74
136. Sgard F, Charpantier E, Bertrand S, Walker N, Caput D, Graham D. et al. A novel human nicotinic receptor subunit, alpha10, that confers functionality to the alpha9-subunit. Mol Pharmacol. 2002;61:150-9
137. García-Colunga J, Miledi R. Modulation of nicotinic acetylcholine receptors by strychnine. Proc Natl Acad Sci U S A. 1999;96:4113-8
138. Yu CR, Role LW. Functional contribution of the alpha5 subunit to neuronal nicotinic channels expressed by chick sympathetic ganglion neurones. J Physiol. 1998;509( Pt 3):667-81
139. Kuryatov A, Olale F, Cooper J, Choi C, Lindstrom J. Human alpha6 AChR subtypes: subunit composition, assembly, and pharmacological responses. Neuropharmacology. 2000;39:2570-90
140. Grady SR, Moretti M, Zoli M, Marks MJ, Zanardi A, Pucci L. et al. Rodent habenulo-interpeduncular pathway expresses a large variety of uncommon nAChR subtypes, but only the alpha3beta4* and alpha3beta3beta4* subtypes mediate acetylcholine release. J Neurosci. 2009;29:2272-82
141. Gharpure A, Teng J, Zhuang Y, Noviello CM, Walsh RM Jr, Cabuco R. et al. Agonist selectivity and ion permeation in the α3β4 ganglionic nicotinic receptor. Neuron. 2019;104:501-11.e6
142. Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB. et al. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269-76
143. Brusco S, Ambrosi P, Meneghini S, Becchetti A. Agonist and antagonist effects of tobacco-related nitrosamines on human α4β2 nicotinic acetylcholine receptors. Front Pharmacol. 2015;6:201
144. Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol. 2009;49:57-71
145. Fararjeh AS, Tu SH, Chen LC, Cheng TC, Liu YR, Chang HL. et al. Long-term exposure to extremely low-dose of nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) induce non-malignant breast epithelial cell transformation through activation of the a9-nicotinic acetylcholine receptor-mediated signaling pathway. Environ Toxicol. 2019;34:73-82
146. Lee CH, Chang YC, Chen CS, Tu SH, Wang YJ, Chen LC. et al. Crosstalk between nicotine and estrogen-induced estrogen receptor activation induces α9-nicotinic acetylcholine receptor expression in human breast cancer cells. Breast Cancer Res Treat. 2011;129:331-45
147. Lee CH, Huang CS, Chen CS, Tu SH, Wang YJ, Chang YJ. et al. Overexpression and activation of the alpha9-nicotinic receptor during tumorigenesis in human breast epithelial cells. J Natl Cancer Inst. 2010;102:1322-35
148. Huang LC, Lin CL, Qiu JZ, Lin CY, Hsu KW, Tam KW. et al. Nicotinic acetylcholine receptor subtype alpha-9 mediates triple-negative breast cancers based on a spontaneous pulmonary metastasis mouse model. Front Cell Neurosci. 2017;11:336
149. Kumari K, Das B, Adhya A, Chaudhary S, Senapati S, Mishra SK. Nicotine associated breast cancer in smokers is mediated through high level of EZH2 expression which can be reversed by methyltransferase inhibitor DZNepA. Cell Death Dis. 2018;9:152
150. Tu SH, Lin YC, Huang CC, Yang PS, Chang HW, Chang CH. et al. Protein phosphatase Mg2+/Mn2+ dependent 1F promotes smoking-induced breast cancer by inactivating phosphorylated-p53-induced signals. Oncotarget. 2016;7:77516-31
151. Guha P, Bandyopadhyaya G, Polumuri SK, Chumsri S, Gade P, Kalvakolanu DV. et al. Nicotine promotes apoptosis resistance of breast cancer cells and enrichment of side population cells with cancer stem cell-like properties via a signaling cascade involving galectin-3, α9 nicotinic acetylcholine receptor and STAT3. Breast Cancer Res Treat. 2014;145:5-22
152. Chen CS, Lee CH, Hsieh CD, Ho CT, Pan MH, Huang CS. et al. Nicotine-induced human breast cancer cell proliferation attenuated by garcinol through down-regulation of the nicotinic receptor and cyclin D3 proteins. Breast Cancer Res Treat. 2011;125:73-87
153. Mei D, Lin Z, Fu J, He B, Gao W, Ma L. et al. The use of α-conotoxin ImI to actualize the targeted delivery of paclitaxel micelles to α7 nAChR-overexpressing breast cancer. Biomaterials. 2015;42:52-65
154. Kalantari-Dehaghi M, Parnell EA, Armand T, Bernard HU, Grando SA. The nicotinic acetylcholine receptor-mediated reciprocal effects of the tobacco nitrosamine NNK and SLURP-1 on human mammary epithelial cells. Int Immunopharmacol. 2015;29:99-104
155. Eglen RM. Muscarinic receptor subtypes in neuronal and non-neuronal cholinergic function. Auton Autacoid Pharmacol. 2006;26:219-33
156. Fiszman GL, Middonno MC, de la Torre E, Farina M, Español AJ, Sales ME. Activation of muscarinic cholinergic receptors induces MCF-7 cells proliferation and angiogenesis by stimulating nitric oxide synthase activity. Cancer Biol Ther. 2007;6:1106-13
157. Boczek T, Mackiewicz J, Sobolczyk M, Wawrzyniak J, Lisek M, Ferenc B. et al. The role of G protein-coupled receptors (GPCRs) and calcium signaling in schizophrenia. Focus on GPCRs activated by neurotransmitters and chemokines. Cells. 2021;10:1228
158. Lanzafame AA, Christopoulos A, Mitchelson F. Cellular signaling mechanisms for muscarinic acetylcholine receptors. Recept Channels. 2003;9:241-60
159. Luo J, Busillo JM, Benovic JL. M3 muscarinic acetylcholine receptor-mediated signaling is regulated by distinct mechanisms. Mol Pharmacol. 2008;74:338-47
160. Español A, Eiján AM, Mazzoni E, Davel L, Jasnis MA, Sacerdote De Lustig E. et al. Nitric oxide synthase, arginase and cyclooxygenase are involved in muscarinic receptor activation in different murine mammary adenocarcinoma cell lines. Int J Mol Med. 2002;9:651-7
161. Salem AR, Martínez Pulido P, Sanchez F, Sanchez Y, Español AJ, Sales ME. Effect of low dose metronomic therapy on MCF-7 tumor cells growth and angiogenesis. Role of muscarinic acetylcholine receptors. Int Immunopharmacol. 2020;84:106514
162. Español AJ, Salem A, Rojo D, Sales ME. Participation of non-neuronal muscarinic receptors in the effect of carbachol with paclitaxel on human breast adenocarcinoma cells. Roles of nitric oxide synthase and arginase. Int Immunopharmacol. 2015;29:87-92
163. Español AJ, Salem A, Di Bari M, Cristofaro I, Sanchez Y, Tata AM. et al. The metronomic combination of paclitaxel with cholinergic agonists inhibits triple negative breast tumor progression. Participation of M2 receptor subtype. PLoS One. 2020;15:e0226450
164. Reardon C, Duncan GS, Brüstle A, Brenner D, Tusche MW, Olofsson PS. et al. Lymphocyte-derived ACh regulates local innate but not adaptive immunity. Proc Natl Acad Sci U S A. 2013;110:1410-5
165. Petroff OA. GABA and glutamate in the human brain. Neuroscientist. 2002;8:562-73
166. McEntee WJ, Crook TH. Glutamate: its role in learning, memory, and the aging brain. Psychopharmacology (Berl). 1993;111:391-401
167. Nedergaard M, Takano T, Hansen AJ. Beyond the role of glutamate as a neurotransmitter. Nat Rev Neurosci. 2002;3:748-55
168. Foster AC, Kemp JA. Glutamate- and GABA-based CNS therapeutics. Curr Opin Pharmacol. 2006;6:7-17
169. Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1-105
170. Pál B. Involvement of extrasynaptic glutamate in physiological and pathophysiological changes of neuronal excitability. Cell Mol Life Sci. 2018;75:2917-49
171. Qian F, Tang FR. Metabotropic glutamate receptors and interacting proteins in epileptogenesis. Curr Neuropharmacol. 2016;14:551-62
172. Teh JL, Shah R, La Cava S, Dolfi SC, Mehta MS, Kongara S. et al. Metabotropic glutamate receptor 1 disrupts mammary acinar architecture and initiates malignant transformation of mammary epithelial cells. Breast Cancer Res Treat. 2015;151:57-73
173. Banda M, Speyer CL, Semma SN, Osuala KO, Kounalakis N, Torres Torres KE. et al. Metabotropic glutamate receptor-1 contributes to progression in triple negative breast cancer. PLoS One. 2014;9:e81126
174. Speyer CL, Bukhsh MA, Jafry WS, Sexton RE, Bandyopadhyay S, Gorski DH. Riluzole synergizes with paclitaxel to inhibit cell growth and induce apoptosis in triple-negative breast cancer. Breast Cancer Res Treat. 2017;166:407-19
175. Bastiaansen AEM, Timmermans AM, Smid M, van Deurzen CHM, Hulsenboom ESP, Prager-van der Smissen WJC. et al. Metabotropic glutamate receptor 1 is associated with unfavorable prognosis in ER-negative and triple-negative breast cancer. Sci Rep. 2020;10:22292
176. Mehta MS, Dolfi SC, Bronfenbrener R, Bilal E, Chen C, Moore D. et al. Metabotropic glutamate receptor 1 expression and its polymorphic variants associate with breast cancer phenotypes. PLoS One. 2013;8:e69851
177. Eelen G, Dubois C, Cantelmo AR, Goveia J, Brüning U, DeRan M. et al. Role of glutamine synthetase in angiogenesis beyond glutamine synthesis. Nature. 2018;561:63-9
178. Speyer CL, Hachem AH, Assi AA, Johnson JS, DeVries JA, Gorski DH. Metabotropic glutamate receptor-1 as a novel target for the antiangiogenic treatment of breast cancer. PLoS One. 2014;9:e88830
179. Sexton RE, Hachem AH, Assi AA, Bukhsh MA, Gorski DH, Speyer CL. Metabotropic glutamate receptor-1 regulates inflammation in triple negative breast cancer. Sci Rep. 2018;8:16008
180. Xiao B, Chen D, Zhou Q, Hang J, Zhang W, Kuang Z. et al. Glutamate metabotropic receptor 4 (GRM4) inhibits cell proliferation, migration and invasion in breast cancer and is regulated by miR-328-3p and miR-370-3p. BMC Cancer. 2019;19:891
181. Motiwala Z, Aduri NG, Shaye H, Han GW, Lam JH, Katritch V. et al. Structural basis of GABA reuptake inhibition. Nature. 2022;606:820-6
182. Sernagor E, Chabrol F, Bony G, Cancedda L. GABAergic control of neurite outgrowth and remodeling during development and adult neurogenesis: general rules and differences in diverse systems. Front Cell Neurosci. 2010;4:11
183. Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci. 2002;3:715-27
184. Oketch-Rabah HA, Madden EF, Roe AL, Betz JM. United States pharmacopeia (USP) safety review of gamma-aminobutyric acid (GABA). Nutrients. 2021;13:2742
185. Ben-Othman N, Vieira A, Courtney M, Record F, Gjernes E, Avolio F. et al. Long-term GABA administration induces alpha cell-mediated beta-like cell neogenesis. Cell. 2017;168:73-85.e11
186. Sizemore GM, Sizemore ST, Seachrist DD, Keri RA. GABA(A) receptor pi (GABRP) stimulates basal-like breast cancer cell migration through activation of extracellular-regulated kinase 1/2 (ERK1/2). J Biol Chem. 2014;289:24102-13
187. Gumireddy K, Li A, Kossenkov AV, Sakurai M, Yan J, Li Y. et al. The mRNA-edited form of GABRA3 suppresses GABRA3-mediated Akt activation and breast cancer metastasis. Nat Commun. 2016;7:10715
188. Li X, Wang H, Yang X, Wang X, Zhao L, Zou L. et al. GABRP sustains the stemness of triple-negative breast cancer cells through EGFR signaling. Cancer Lett. 2021;514:90-102
189. Zhang D, Li X, Yao Z, Wei C, Ning N, Li J. GABAergic signaling facilitates breast cancer metastasis by promoting ERK1/2-dependent phosphorylation. Cancer Lett. 2014;348:100-8
190. Wei B, Zhu Y, Yang P, Han Y, Wang S, Wang X. et al. GABAb1e promotes the malignancy of human cancer cells by targeting the tyrosine phosphatase PTPN12. iScience. 2021;24:103311
191. Benarroch EE. GABAB receptors: structure, functions, and clinical implications. Neurology. 2012;78:578-84
192. Young SZ, Bordey A. GABA's control of stem and cancer cell proliferation in adult neural and peripheral niches. Physiology (Bethesda). 2009;24:171-85
193. Bhat R, Axtell R, Mitra A, Miranda M, Lock C, Tsien RW. et al. Inhibitory role for GABA in autoimmune inflammation. Proc Natl Acad Sci U S A. 2010;107:2580-5
194. Opolski A, Mazurkiewicz M, Wietrzyk J, Kleinrok Z, Radzikowski C. The role of GABA-ergic system in human mammary gland pathology and in growth of transplantable murine mammary cancer. J Exp Clin Cancer Res. 2000;19:383-90
195. Klebig C, Seitz S, Arnold W, Deutschmann N, Pacyna-Gengelbach M, Scherneck S. et al. Characterization of {gamma}-aminobutyric acid type a receptor-associated protein, a novel tumor suppressor, showing reduced expression in breast cancer. Cancer Res. 2005;65:394-400
196. Chen X, Cao Q, Liao R, Wu X, Xun S, Huang J. et al. Loss of ABAT-mediated GABAergic system promotes basal-like breast cancer progression by activating Ca2+-NFAT1 axis. Theranostics. 2019;9:34-47
197. Neman J, Termini J, Wilczynski S, Vaidehi N, Choy C, Kowolik CM. et al. Human breast cancer metastases to the brain display GABAergic properties in the neural niche. Proc Natl Acad Sci U S A. 2014;111:984-9
198. Rao RD, Michalak JC, Sloan JA, Loprinzi CL, Soori GS, Nikcevich DA. et al. Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3). Cancer. 2007;110:2110-8
199. Mazurkiewicz M, Opolski A, Wietrzyk J, Radzikowski C, Kleinrok Z. GABA level and GAD activity in human and mouse normal and neoplastic mammary gland. J Exp Clin Cancer Res. 1999;18:247-53
200. Garcia-Recio S, Fuster G, Fernandez-Nogueira P, Pastor-Arroyo EM, Park SY, Mayordomo C. et al. Substance P autocrine signaling contributes to persistent HER2 activation that drives malignant progression and drug resistance in breast cancer. Cancer Res. 2013;73:6424-34
Author contact
![]() Corresponding authors: Jin Nan Gao or Xu Dong Zhang, LS3-49, Life Sciences Building, The University of Newcastle, Callaghan, NSW, Australia. Ph: 61 2 49218906. Fax: 61 2 49217311. Email: sxjinnancom or Xu.Zhangedu.au
Corresponding authors: Jin Nan Gao or Xu Dong Zhang, LS3-49, Life Sciences Building, The University of Newcastle, Callaghan, NSW, Australia. Ph: 61 2 49218906. Fax: 61 2 49217311. Email: sxjinnancom or Xu.Zhangedu.au
 Global reach, higher impact
Global reach, higher impact