13.3
Impact Factor
Theranostics 2023; 13(3):1091-1108. doi:10.7150/thno.78872 This issue Cite
Review
Saliva-based microfluidic point-of-care diagnostic
1. Department of Chemistry, Colorado State University, Fort Collins, Colorado 80523, USA.
2. Centre for Biomedical Technologies, School of Biomedical Sciences, Faculty of Health, QUT.
3. Griffith Institute for Drug Discover, Griffith University, Nathan, Australia.
4. Menzies Health Institute, Griffith University, Gold Coast, Australia.
5. Translational Research Institute, Woolloongabba, Australia.
6. Metallurgy and Materials Science Research Institute, Chulalongkorn University, Soi Chula 12, Phayathai Rd., Pathumwan, Bangkok 10330, Thailand.
* Equal contribution
Abstract
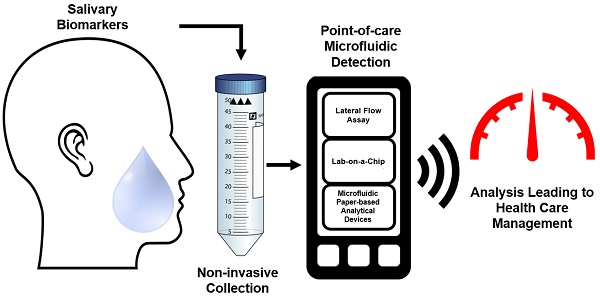
There has been a long-standing interest in point-of-care (POC) diagnostics as a tool to improve patient care because it can provide rapid, actionable results near the patient. Some of the successful examples of POC testing include lateral flow assays, urine dipsticks, and glucometers. Unfortunately, POC analysis is somewhat limited by the ability to manufacture simple devices to selectively measure disease specific biomarkers and the need for invasive biological sampling. Next generation POCs are being developed that make use of microfluidic devices to detect biomarkers in biological fluids in a non-invasive manner, addressing the above-mentioned limitations. Microfluidic devices are desirable because they can provide the ability to perform additional sample processing steps not available in existing commercial diagnostics. As a result, they can provide more sensitive and selective analysis. While most POC methods make use of blood or urine as a sample matrix, there has been a growing push to use saliva as a diagnostic medium. Saliva represents an ideal non-invasive biofluid for detecting biomarkers because it is readily available in large quantities and analyte levels reflect those in blood. However, using saliva in microfluidic devices for POC diagnostics is a relatively new and an emerging field. The overarching aim of this review is to provide an update on recent literature focused on the use of saliva as a biological sample matrix in microfluidic devices. We will first cover the characteristics of saliva as a sample medium and then review microfluidic devices that are developed for the analysis of salivary biomarkers.
Keywords: Saliva, Diagnostics, point-of-care, lab-on-chip, microfluidic.
 Global reach, higher impact
Global reach, higher impact