13.3
Impact Factor
Theranostics 2023; 13(3):931-954. doi:10.7150/thno.78639 This issue Cite
Review
Targeted and responsive biomaterials in osteoarthritis
1. Institute of Translational Medicine, Shanghai University, Shanghai, 200444, China.
2. Organoid Research Center, Shanghai University, Shanghai, 200444, China.
3. School of Medicine, Shanghai University, Shanghai 200444, China.
4. School of Life Sciences, Shanghai University, Shanghai 200444, China.
*These authors contributed equally to this study.
Received 2022-9-4; Accepted 2022-11-7; Published 2023-1-16
Abstract
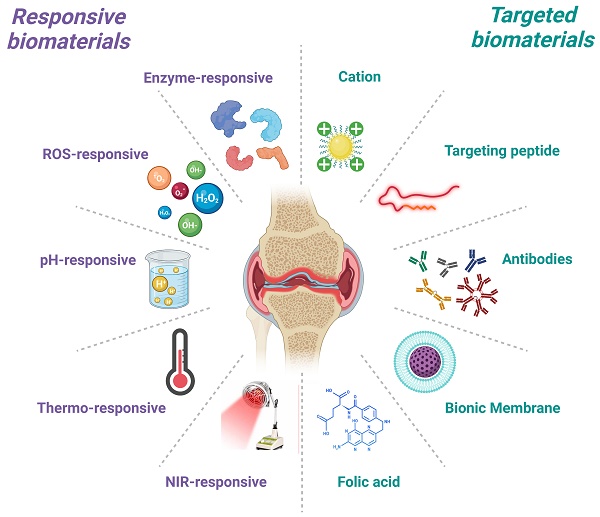
Osteoarthritis (OA) is a degenerative disease characterized by loss of articular cartilage and chronic inflammation, involving multiple cellular dysfunctions and tissue lesions. The non-vascular environment and dense cartilage matrix in the joints tend to block drug penetration, resulting in low drug bioavailability. There is a desire to develop safer and more effective OA therapies to meet the challenges of an aging world population in the future. Biomaterials have achieved satisfactory results in improving drug targeting, prolonging the duration of action, and achieving precision therapy. This article reviews the current basic understanding of the pathological mechanisms and clinical treatment dilemmas of OA, summarizes and discusses the advances for different kinds of targeted and responsive biomaterials in OA, seeking to provide new perspectives for the treatment of OA. Subsequently, limitations and challenges in clinical translation and biosafety are analyzed to guide the development of future therapeutic strategies for OA. As the need for precision medicine rises over time, emerging multifunctional biomaterials based on tissue targeting and controlled release will become an irreplaceable part of OA management.
Keywords: Osteoarthritis, articular cartilage, targeted biomaterial, responsive biomaterial, drug delivery
Introduction
Osteoarthritis (OA) is one of the most common chronic degenerative joint diseases worldwide, often occurring in middle-aged and older adults over the age of 45 [1]. About 10% of men and 18% of women over 60 years old worldwide suffer from OA, and there is a gradual trend toward younger patients with OA due to the effects of obesity and strenuous exercise [2]. The financial cost of an OA patient is estimated to be between $700 and $15,600 a year, placing a significant burden on both society and the individual [3]. The pathological changes of OA mainly include wear and tear degeneration of articular cartilage, formation of bone fragments, synovial inflammation and subchondral bone remodeling [4]. As the specific pathogenesis of OA is still unknown, the current clinical treatment of OA is mainly focused on relieving joint pain and delaying the development of OA [5-7]. Currently, drug therapy, physical therapy and surgery are the primary treatments [8, 9]. In the early stages of OA, pain relief and reduction of inflammation are achieved through appropriate exercise and weight loss and are supplemented with oral non-steroidal anti-inflammatory drugs (NSAIDs) [10]. For patients with advanced OA, accompanied by severe articular cartilage wear, short-term symptomatic relief can be provided by intra-articular injections of hyaluronic acid (H) or glucocorticoids [11, 12]. Due to the unique physiology of the joint cavity and the presence of synovial fluid, many drugs are characterized by poor water solubility, low cellular uptake, premature release or degradation. Targeted delivery and nonspecific release of drugs remain significant limitation to drug efficacy [13]. A well-designed drug delivery system should have the appropriate size, biological barrier permeability, and proper drug release capacity [14-16]. Therefore, many researchers hope to achieve OA treatment by developing novel and efficient drug carriers and delivery systems.
In recent times, many biomaterial carriers with cartilage or synovial targeting and responsiveness are emerging. Biomaterial carriers not only prolong the release of drug, but also exhibit higher tissue and cell specificity [17, 18]. Among the various types of biomaterials, targeted and responsive biomaterials are more suitable for OA applications due to their small size and localizable delivery. They are often designed and assembled into functional structures to ensure accurate drug delivery [19-21]. Currently, researchers developed various dual-functional biomaterials for OA treatment, such as cartilage-targeted combined enzyme/pH/ROS/NIR response [22-25], macrophage-targeted combined enzyme/ultrasound/pH response [26-28], and endothelial cell-targeted combined ultrasound response [29]. Compared to passive cellular endocytosis, the introduction of chondrocyte-targeting peptides allows more efficient entry of responsive biomaterials into chondrocytes [22]. Furthermore, Deng et al. designed a bifunctional biomaterial to target subchondral bone osteoclasts and synovial macrophages via RGD-αvβ3 integrin interactions [26]. Subsequently, these biomaterials induced apoptosis by releasing celastrol in response to local MMP-9 [26]. Pro-inflammatory cell infiltration was found to be reduced and bone erosion improved in rats with advanced arthritis [26]. Researchers have assembled engineered modified polymers into nanocarriers or hydrogels containing hydrophilic or hydrophobic regions loaded with drugs for targeted drug delivery or responsive drug release [13, 30]. Targeted drug delivery is mainly divided into two types: passive targeting and active targeting. The former increases the local drug concentration by physical methods such as local injection, particle size reduction, and surface charge change, while the latter enhances targeted drug delivery by chemical methods such as modification of specific antibodies or affinity peptides on the carrier surface [31-33]. In contrast, responsive biomaterials are usually stimulated by enzymes, reactive oxygen species (ROS), pH or external stimuli at the lesion site to release drugs, thus dramatically preventing the liberation and uptake of drugs in non-lesioned tissues [34].
In this review, we briefly overview the underlying physiopathology of OA and the currently used clinical treatments and their dilemmas. Then, we describe and summarize the characteristics of different types of targeted and responsive biomaterials and their research progress in OA. Finally, we discuss the potential limitations and challenges of biomaterials in the translation of clinical treatments for OA. We hope to provide further references and new perspectives for the future development of targeted and responsive biomaterials in OA therapy.
Physiopathology of osteoarthritis
Physiological properties of articular cartilage
The anatomy of articular cartilage is divided into hyaline cartilage, tidemark, and calcified cartilage [35]. Articular cartilage transmits mechanical stress and aids in joint motion by providing an elastic and lubricated surface overlying the articular bone [36]. It is a layer of avascular, nerve-free connective tissue composed of chondrocytes and extracellular matrix (ECM) [35, 37]. Chondrocytes regulate the composition of the ECM through their own anabolism and catabolism, which constitute the most basic cellular units of cartilage homeostasis [38]. ECM is composed of fibrin and proteoglycan, which has a certain mechanical strength and elasticity [36]. Among them, supramolecular arrays with diameters between 25-400 nm are assembled from collagen types I, II, III, V and XI, which constitute the classical fibrous structure [39]. Type II collagen, which accounts for 80% of the total collagen dry weight, is composed of three α1(II)-chains and has more glucosyl and galactosyl residues than other collagens so that they can interact with proteoglycans [39]. The pore size between the cross-longitudinal collagen network is approximately 50-60 nm, which would allow small molecules of nutrients or drugs to disperse in the ECM and penetrate and act on the chondrocytes or subchondral bone [40]. Proteoglycans consist of a number of core proteins attached to glycosaminoglycan (GAG) chains bonded non-covalently to long chains of hyaluronic acid, which give ECM its integral electronegativity, viscosity and resistance to compression [41]. The dynamic stabilization of the relative ratios of collagen fibers and proteoglycans provides the extracellular environment that supports normal metabolism, proliferation, and differentiation of chondrocytes [42]. Stimulated by mechanical stress or pro-inflammatory factors, anomalous higher expression of matrix metalloproteinases (MMPs) disrupts the dynamic balance in the ECM, which ultimately leads to chondrocyte apoptosis and articular cartilage degradation, thus further contributing to the progression of OA [43].
Pathogenesis and pathological changes of osteoarthritis
According to the causative factors, OA can be broadly divided into primary OA caused by gene, age and gender factors, and secondary OA caused by sports trauma, metabolic disorders, obesity, etc. [44, 45]. The latest data show that about 240 million people worldwide are afflicted by OA [2]. Among the aging population over 60 years old, women (18%) are more likely to suffer from OA than men (10%) [2]. In England, 53.23% of respondents over the age of 50 reported that they suffer from OA-related pain in one or more joints [2]. The pathological changes of OA involve the entire joint including the synovial membrane and subchondral bone (Figure 1). First, the articular cartilage becomes softened and inelastic, and the cartilage layer velvety erupts and exposes the subchondral bone plate [4, 46]. Secondly, abnormal ossification of the cartilage at the edge of the joint forms an osteoid with neurovascular invasion and causes joint pain [4]. Subsequently, vascularization at the edge of the cartilage or at the tendon attachment forms a bony mass via cartilage ossification, and this causes joint pain, stiffness, deformity and dysfunction [4]. Finally, collateral synovial inflammation and subchondral bone osteophytes will lead to macrophage infiltration and neurovascular invasion, further exacerbating the OA process [4].
The physiological structure of articular cartilage and pathological changes of OA. Created with BioRender.com.
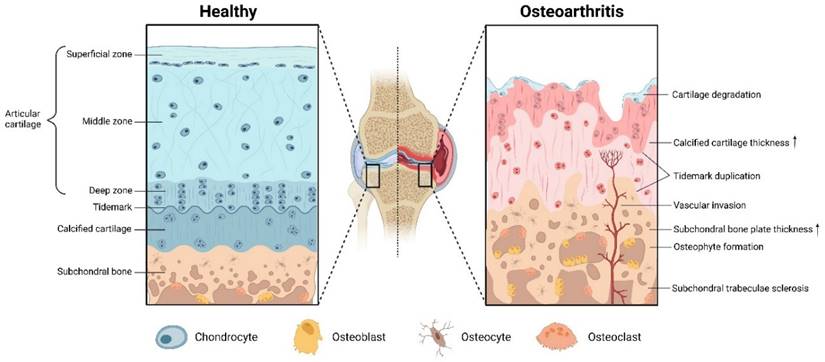
At the molecular level, Interleukin-1β (IL-1β), produced by chondrocytes, osteoblasts, synoviocytes and macrophages, is considered to be the most critical inflammatory factor in the pathogenesis of OA, which can act independently or synergistically with other cytokines to cause articular cartilage degradation and joint inflammatory responses [47, 48]. IL-1β increases nitric oxide (NO) and prostaglandin E2 (PGE2) expression by activating its downstream transcription factors, triggering synovial vasodilatation hyperplasia and joint cavity pain [49]. At the same time, the autocrine production of tumor necrosis factor-α (TNF-α) and Interleukin-6 (IL-6) by the above cells is stimulated, synergistically enhancing the pro-inflammatory effect of IL-1β and producing proteases such as the MMPs family and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) family to cleave and disassemble type II collagen and aggrecan in the ECM, thereby destroying cartilage structure [47, 48, 50]. Furthermore, because ADAMTS plays a critical role in overall tissue renewal, elevated ADAMTS expression is not only associated with joint degeneration and deterioration, but may also reflect the persistence of OA inflammation and injury [51]. Some scholars have also suggested that alterations in the subchondral bone microenvironment precede the occurrence of cartilage destruction, where abnormal mechanical loading and activation of TGF-β in bone-chondral crosstalk are essential factors leading to anomalous bone remodeling, angiogenesis, and sensory innervation [4]. Although numerous studies are available to explain the pathogenesis of OA at the cellular and molecular levels, a substantial body of research combined with clinical data is still needed to fully understand the underlying causative factors of OA.
Clinical treatment of osteoarthritis and its dilemma
The clinical treatment of OA is divided into two main categories: medication and surgery, while medication is split into two groups: symptom relief and condition improvement [7]. NSAIDs are the most widely used drugs that diminish the production of inflammatory factors and exert analgesic effects [52]. However, NSAIDs have gastrointestinal, neurological, hematologic and allergic side effects, as well as symptomatic treatment and relief of patient suffering, but not slowing down the progression of the disease [53, 54]. Another group of drugs can significantly relieve the clinical symptoms of OA is glucocorticoids, which have a potent anti-inflammatory effect and can suppress the proliferation of aberrant tissues and reduce joint effusion, thus achieving a significant effect of detumescence and analgesia [55]. However, long-term use of glucocorticoids leads to bone loss and accelerates articular cartilage and subchondral bone lesions, which limits their clinical application and eliminates them as a routine treatment option [56]. HA is an integral component of the joint fluid and cartilage matrix, commonly used in intra-articular injection for the treatment of OA [57]. HA could restore the viscoelasticity of joint fluid, enhance lubrication and shock absorption cushioning, reduce joint friction, relieve pain, prevent cartilage degradation, and lower the production of MMPs, which has significant efficacy in clinical practice [12, 58]. Nevertheless, joint cavity injection of HA has high demands on operation technique and operation environment, and may cause short-term complications such as joint edema and infection, so there are still plenty of questions and controversies concerning the use of HA [59]. Glucosamine and chondroitin sulfate (CS) suppress the activity of proteases and degradative enzymes, which also target articular cartilage, making them ideal drugs for improving OA [60].
In advanced OA, severe joint deformities will occur, rendering pharmacological treatment futile, and surgical treatment is the only option, most commonly with arthroscopic debridement and total joint replacement [61]. Arthroscopic debridement is indicated for mild to moderate OA and often provides a more satisfactory outcome [62]. Total joint replacement, on the other hand, is suitable for patients with severe OA and can not only significantly reduce pain, but also correct joint deformities and restore joint function, making it the last choice for all OA patients [63]. Although surgery can achieve effective treatment, it is expensive and may lead to serious postoperative complications and medical risks [64]. For example, patients undergoing total knee arthroplasty have twice or more the incidence and mortality of venous thromboembolism due to postoperative complications such as coagulation disorders, electrolyte disturbances, and pulmonary complications [64, 65]. In addition, the recruitment of bone marrow cells by microfracture to form a smooth and strong repair tissue can also replace the role of cartilage [66]. The recovery from microfracture surgery is directly related to the patient's own ability to repair [66]. Significant efficacy in young, low-weight patients, but poor efficacy in older, obese patients. After surgery, patients need to recover for 12 months or more with the assistance of a stent [67, 68]. With the maturation of in vitro culture techniques, autologous chondrocyte implantation (ACI) is better able to regenerate normal articular cartilage structures [68]. ACI allows for the implantation of mature and active hyaline cartilage in a single operation, while avoiding immune rejection [68]. But ACI technology is still in its infancy, and lab-cultured chondrocytes often mean higher treatment costs [69, 70]. Collectively, owing to the drawbacks and limitations of existing clinical treatment strategies, there is an imminent demand for the development of effective, versatile, targeted, and responsive OA therapies.
Responsive biomaterials in osteoarthritis
Currently available anti-inflammatory and analgesic drugs, either orally or by intra-articular injection, suffer from poor water solubility, low cellular uptake, poor drug concentration distribution or premature degradation [71]. By focusing on the responsiveness of biomaterials to different substrates or external stimuli, researchers are developing responsive biomaterials that can release encapsulated drugs efficiently and accurately [34, 72]. These responsive biomaterials can minimize early or abrupt release behavior to address the problems of low bioavailability and low efficacy of anti-OA drugs. In this section, the advances in research on enzymes, ROS, pH responsive and external stimulus responsive biomaterials in the treatment of OA are reviewed (Table 1).
Enzyme-responsive biomaterials
Since enzymes possess a high degree of selectivity and efficiency, drug release in a variety of ways such as surface ligand activation and chemical bond breaking can be achieved by introducing functional groups sensitive to enzymes aberrantly expressed in the OA microenvironment into biomaterials [99]. With a high secretion of multiple matrix-degrading enzymes and degradation-activating enzymes in OA, enzyme-responsive biomaterials are designed to control drug release by exploiting the differences in enzyme activity in tissues [100]. Namely, when administered early in OA or prophylactically, the drug is released in low or no volume to avoid side effects, while a large volume of drug can be unleashed swiftly in advanced disease. Enzyme-responsive wise biomaterials often have enzyme-sensitive backbones or biodegradable ester bonds as linkages, resulting in efficient controlled release behavior [71].
Responsive biomaterials for osteoarthritis treatment
| Materials | Responsive stimulus | Responsive components | Particle size | Drugs | Biological functions | References |
|---|---|---|---|---|---|---|
| MRC-PPL@PSO | MMP-13 | Specific peptide substrate of MMP-13 (H2N-GPLGVRGC-SH) | 121.5 ± 26.1 nm | Psoralidin | Resist chondrocyte inflammation and lower MMP-13 expression | [22] |
| CMFn@HCQ | MMP-13 | Specific peptide substrate of MMP-13 (H2N-GPLGVRGC-SH) | 22 nm | Hydroxychloroquine | Increase cartilage retention time and restore the complete structure of the ECM | [73] |
| TG-18 hydrogel | MMPs | Triglycerol monostearate (TG-18) | Not available | Triamcinolone acetonide | Release medication on demand and relieve paw swelling | [74] |
| Gelatin microspheres | Collagenase | Gelatin | 10~30 μm | IL-4, IL-10 and IL-13 | Decrease NO production in chondrocytes by 80% in vitro | [75] |
| PTKU@DEX | ROS | Polythioketal (PTK) | 460 nm | Dexamethasone | Reverse the expression of iNOS and Arg-1 in synovial macrophages and prevent their polarization to M1 type. | [76] |
| TKCP@DEX | ROS | Thioketal (TK) | 60 nm | Dexamethasone | Enrich Dex in articular cartilage to improve cartilage wear and reduce the expression of IL-6 and MMP-13 | [24] |
| Dex-pPADN | ROS | Phenylboronic acid | 89.6 ± 9.5 nm | Dexamethasone | Dramatically inhibit synovial neutrophil infiltration and restore aggrecan content in the cartilage layer | [77] |
| RRHMs | ROS | Fe2+ and sodium bicarbonate | 344.2 ± 10.3 μm | Dexamethasone sodium phosphate | Release anti-inflammatory drugs via Fenton response to scavenge ROS and rescue collagen type X content | [78] |
| DEX@PPNP | ROS | Tetrahydroxydiboron (THDB) | 190 nm | Dexamethasone | Suppress pain behavior and angiogenesis of subchondral bone in OA mice | [79] |
| AG@MSNs-PAA | pH | Polyacrylic acid (PAA) | 120 nm | Andrographolide | Restore IL-1β-induced chondrocyte apoptosis and Col2α1 gene expression. | [80] |
| MOF@HA@PCA | pH | MIL-100 (Fe) | 123.4 nm | Protocatechuic acid | Inhibit osteophyte formation, promote chondrocyte proliferation and articular cartilage regeneration in ACLT model | [81] |
| NP-BP | pH | Nano-apatite | 400 nm | Bisphosphonates | Impair subchondral bone TRAP+ osteoclast bone resorption activity and angiogenesis | [82] |
| Rh-PLGA-NPs@NH4 | pH | NH4HCO3 | 190.7 ± 1.2 nm | Rhein | Upregulate chondrocyte uptake and scavenge ROS in vitro | [83] |
| ACP | pH | Tertiary amine groups | 170 nm | Curcumin | Recover articular cartilage ECM integrity by targeting enrichment in OA joints by responding to acidic environments | [84] |
| CSL@HMSNs-Cs | pH | Chitosan | 260 nm | Celastrol | Restrain chondrocyte NF-κB signaling pathway activation and diminish the concentration of inflammatory factors and MMPs in the supernatant | [85] |
| F127/COS/KGNDCF | Thermo | Pluronic F-127 | 650 nm (4°C) and 305 nm (37°C) | Kartogenin and diclofenac | Minimize matrix loss of bone surface, sclerotic bone and microfracture on 14 weeks | [86] |
| pNIPAM+HA&DS | Thermo | Poly(N-isopropylacrylamide) | Not available | Hyaluronic acid and diclofenac sodium | Reverse OA synovial hyperplasia and promote GAG deposition to protect chondrocytes from degeneration | [87] |
| RB@MPMW | NIR | Polydopamine | 114.1 nm | Rapamycin and bilirubin | Significantly reduce autophagy-related p65 phosphorylation and restore mitochondrial homeostasis | [25] |
| MoS2@CS@Dex | NIR | MoS2 | 78 ± 18 nm | Dexamethasone | Invoke activated macrophage apoptosis pathway and reverse mitochondrial depolarization in chondrocytes | [88] |
| PEG-MNPs | Magnetic field | Metallic spherical cobalt | 30 nm | Not available | Accelerate transport in bovine articular cartilage by nearly 50-fold under the action of an alternating magnetic field | [89] |
| MC-MNCs | Magnetic field | Magnetic iron oxide | 258 nm | Meloxicam | Reduce arthritic pain and loss of collagen in articular cartilage of rats | [90] |
| MTX-Au/Fe/Au | Magnetic field | Iron-shell nanoparticle | 135 nm | Methotrexate | Accumulate at the site of arthritis and prolong the retention time to relieve synovial hyperplasia | [91] |
| AuSPION | Magnetic field | Magnetic iron oxide | 10 ± 3 nm | Methotrexate | Reduce immunostaining positive cells for TNF-α and IL-1β in synovium | [92] |
| SPIONs | Magnetic field | Magnetic iron oxide | 1 μm、10 μm | Dexamethasone | Uptake by synovial fibroblasts and increase residence time of Dex | [93] |
| PEI-SPIO/siRNA | Magnetic field | Magnetic iron oxide | 10 nm | siRNA | Suppress macrophage activation and cartilage destruction at joint edges | [94] |
| CAG-MSCs scaffolds | Magnetic field | Magnetic iron oxide | Not available | Not available | Promote BMSC chondrogenic differentiation and restore collagen content at the site of cartilage defects in rabbits | [95] |
| US-MB | Ultrasound | Microbubbles | Not available | Diclofenac sodium | Suppress inflammation and neovascularization in peri-ankle tissues | [96] |
| iELPs | Ultrasound | Microbubbles | 113.3 ± 4.6 nm | Methotrexate | Restore mean clinical indices and ankle disorders in arthritic mice | [29] |
| DexSP@LPs-PEG-FA | Ultrasound | Microbubbles | 158 ± 1.4 nm | Dexamethasone | Increase the uptake of liposomes into activated macrophages and decrease the level of inflammatory cytokines in the blood | [27] |
| PFP-Dex@NDs-PEG-FA | Ultrasound | Perfluorocarbon nanodroplets | 311.6 ± 3.8 nm | Dexamethasone | Down-regulate joint inflammatory cell infiltration and pannus formation | [97] |
| Rh/SPX-HSA | Ultrasound | Sparfloxacin | 10 nm | Rhodium nanozyme | Produce oxygen to resist angiogenesis and generate reactive oxygen species to induce apoptosis in synovial fibroblasts | [98] |
Enzyme-responsive biomaterials for OA treatment: (A) Schematic illustration of the synthesis and working mechanism of MMP-13 and pH responsive theranostic MRC-PPL@PSO nano-micelles for osteoarthritis [22]. High expression of MMP-13 in OA joints generates fluorescent signals by cleaving sensitive peptides to release Cy5.5. In addition, the low pH environment moderates the release of PSO in micelles to treat cartilage inflammation. Copyright 2020, BMC. (B) Schematic illustration of the CMFn@HCQ as a cartilage-targeting and MMP-13/pH dual-stimuli activatable theranostic nanoprobe for in vivo MMP-13 imaging and precision therapy of osteoarthritis [73]. Ferritin in the CMFn@HCQ nanocage dissociates in an acidic environment to release HCQ. Simultaneously, MMP-13 severs the short peptide that connects the burster and the free NIR dye can visualize the concentration of disease progressing MMP-13 in OA. Copyright 2019, Elsevier. (C) In vivo fluorescence imaging of the major organs and bilateral joints 21 days after MRC-PPL joint cavity injection [22]. Copyright 2020, BMC. (D) Bioresponsive microspheres for delivery of the anti-inflammatory cytokines in osteoarthritis [75]. Gelatin microspheres are degraded by the rate of catabolic factors produced by OA microenvironment cells to release IL-4, IL-10 and IL-13 to reduce chondrocyte inflammation. Copyright 2019, Wiley.
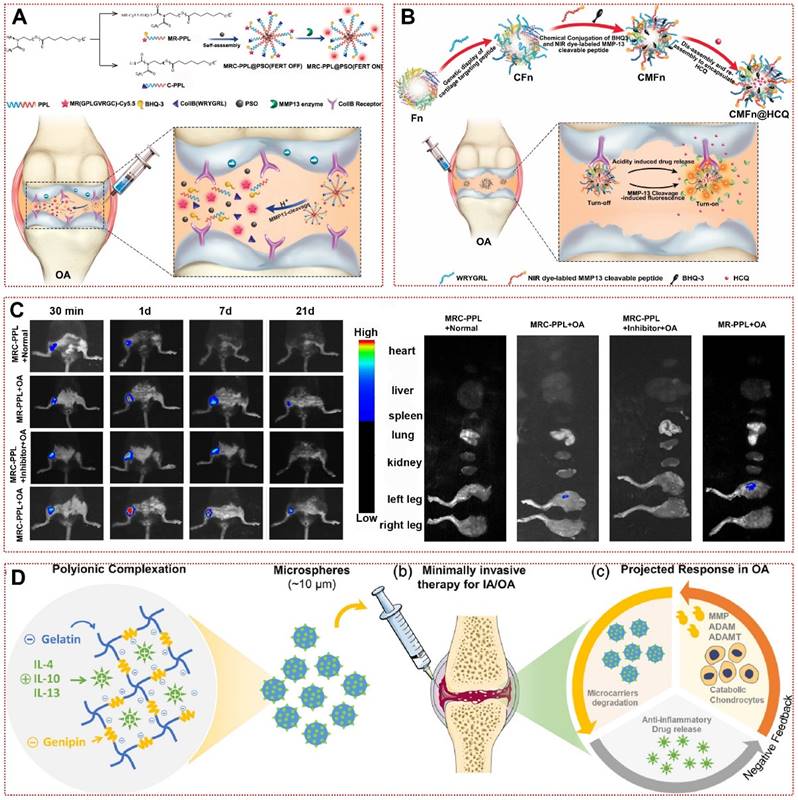
Although enzymes have received extensive attention in studies on their application as triggers in malignancies and cardiovascular diseases, their application in OA therapy is still in its infancy so far [101]. MMPs, one of the key enzymes in the deterioration of OA, are commonly manufactured by chondrocytes under inflammatory conditions and lead to cartilage erosion by breaking down type II collagen and aggrecan in the joint matrix [43]. Out of this inspiration, Lan et al. created an MMP-13 responsive therapeutic integrated nanoplatform by modifying specific peptide substrates of MMP-13 [22]. By respective coupling type II collagen-binding peptide and Cy5.5-labeled MMP-13-responsive peptide substrate to poly(2-ethyl-2-oxazoline)-poly(ε-caprolactone) (MRC-PPL), self-assembling and loading the Chinese herbal active ingredient psoralidin (PSO), the multifunctional MRC-PPL@PSO achieved the release of therapeutic drugs while being able to report OA via fluorescent signals (Figure 2A) [22]. The fluorescent signal of MRC-PPL micelles in healthy joints or MMP-13 inhibitor-treated OA joints was significantly lower than in the OA group, representing that MRC-PPL micelles were mediated by MMP-13 activity in response to the onset of OA joints (Figure 2C) [22]. Similarly, Chen et al. achieved responsiveness to altered MMP-13 expression in OA by loading hydroxychloroquine with MMP-13-responsive ferritin nanocages (CMFn@HCQ) in an almost identical manner to the former (Figure 2B) [73]. This shows the promise of multifunctional responsive biomaterials for image-guided OA precision therapy. In addition to MMP-13, synovial inflammation and subchondral bone lesions in OA are often accompanied by MMP-2/MMP-9-mediated matrix remodeling and angiogenesis [102]. Enzyme-responsive hydrogel loaded with triamcinolone acetonide (TA) have favorable biocompatibility. When it was incubated in PBS at 37°C or in synovial fluid without MMPs for 30 days, only 20% of the drug was released [74]. The addition of MMP-2/MMP-9 or inflammatory synovial fluid resulted in a sustained release of TA over 30 days, which was reversed by the addition of MMPs inhibitors [74]. Furthermore, responsive biomaterials using osteoblasts or vascular endothelial cells highly expressing MMP-2/MMP-9 as enzymatic response targets might be a new strategy to treat OA to restore subchondral bone structure and mechanical stress disorders [103, 104]. More interestingly, gelatin microspheres delivering anti-inflammatory cytokines in response to collagenase produced by inflammatory cells were able to effectively restrict IL-1β or LPS-induced NO production, which may further validate the promising application of responsive biomaterials in OA (Figure 2D) [75].
ROS-responsive biomaterials
ROS broadly refers to oxygen-derived radicals and non-radicals, and contains superoxide anions (O2-), hydrogen peroxide (H2O2), hydroxyl radicals (OH·), nitric oxide radicals (NO·) and singlet oxygen (1O2), which have high chemical reactivity because they contain unpaired electrons [38]. ROS under physiological conditions is primarily generated by the electron transport chain of mitochondria, which in physiological concentrations facilitate immune defense, apoptosis regulation, and intracellular signal transduction [105]. After the injury occurrence, the intracellular or microenvironmental antioxidants such as glutathione (GSH) and superoxide dismutase (SOD) are insufficient to eliminate the excessive production of ROS, which will trigger intracellular metabolic disorders and biomolecular damages, eventually leading to apoptosis [105, 106]. Recent studies have shown that ROS levels are significantly increased in the joints of OA patients, moreover, it has been shown that ROS clearance can be effective in relieving OA [105, 107, 108]. Oxidative stress generated by excessive ROS in the joint will lead to chondrocyte membrane disruption and synthesis of proteoglycan matrix [109]. ROS-induced activation of the NF-κB pathway stalled chondrocyte growth and secreted MMPs, exacerbating cartilage inflammation [105]. With the progression of aging, chondrocytes will become increasingly sensitive to ROS damage, inducing more cellular senescence and apoptosis [110]. Such evidence reveals that abnormally elevated ROS is inextricably linked to OA pathological progression, suggesting that utilizing high levels of ROS in the OA tissue microenvironment is a promising trigger for responsive drug release [110].
The mechanisms of ROS-responsive biomaterials are divided into two types: chemical bond breaking (e.g., polythioketal, polyproline, phenylboronic acid, and ester-containing polymers) and hydrophilic-hydrophobic conversion (e.g., polypropylene sulfide, thioether-containing polymers, monotellurium polymers, and monoselenium polymers) [111]. Zhang et al. used polythioketal (PTK) as a soft linkage, 1,4-butanediol as a chain extender, and Hexamethylene Diisocyanate (HDI) as a hard linkage to synthesize a ROS-responsive polyurethane (PTKU) with superior mechanical properties (Figure 3A) [76]. Compared to the control, PTKU NPs underwent a dramatic morphological transformation after incubation with OH· (1000 mM), and all thioketal bonds were broken, leading to nanoparticle depolymerization and structural relaxation [76]. In vivo experiments, PTKU NPs not only demonstrated favorable articular targeting, but also boosted the shift of synovial macrophages to M2 type and downregulate the expression of MMP-2 and various inflammatory factors in articular cartilage [76]. Similarly, TKCP@Dex NPs could also be obtained by encapsulating dexamethasone (Dex) with thioketal (TK) and cartilage-targeting peptide (CAPDWRVIIPPRPSA) functionalized PEG (Figure 3B) [24]. Under the in vitro catalysis of KO2, TKCP@Dex NPs progressively broke down the thioketone bond to release Cy5.5 and Dex to visualize the treatment, scavenging IL-6 and MMP-13 without causing cytotoxicity [24]. In the monosodium iodoacetate (MIA)-induced OA model, high concentrations of ROS in the joint facilitated aggregation of the nanoprobe and rapidly peaked within 24 h [24]. H&E and safranin O-fast green staining revealed a better recovery of the cartilage matrix than Dex injection, demonstrating the effectiveness of the vector in the organism [24]. Zhao et al. achieved high loading and controlled release of Dex by assembling L-dopamine modified with phenylboronic acid into nanoparticles (Dex-pPADN) in aqueous solution [77]. Through a cascade of redox reactions triggered by ROS at the site of inflammatory damage, Dex-pPADN formed melanin-like structures and exhibited extremely strong anti-inflammatory and antioxidant effects (Figure 3C) [77]. Due to the structural transformation after oxidation by ROS, Dex-pPADN was available as a contrast agent to detect the progression of OA noninvasively, with luminescence intensity proportional to the severity of OA [77].
ROS-responsive biomaterials for OA treatment: (A) Schematic illustration of the ROS scavenging/responsive mechanism of the thioketal repeating unit in PTKU@DEX NPs for OA treatment [76]. Copyright 2021, Elsevier. (B) Schematic illustration of the self-assembly of ROS-responsive nanoparticles for bioimaging and targeted therapy of osteoarthritis in vivo [24]. Copyright 2021, BMC. (C) Schematic illustration of the preparation of Dex-pPADN for the treatment of osteoarthritis [77]. Copyright 2021, Wiley. (D) Composition/structure of the ultrasensitive ROS-responsive gas-generating HM developed herein and its mechanism in the treatment of OA [78]. Copyright 2015, American Chemical Society.
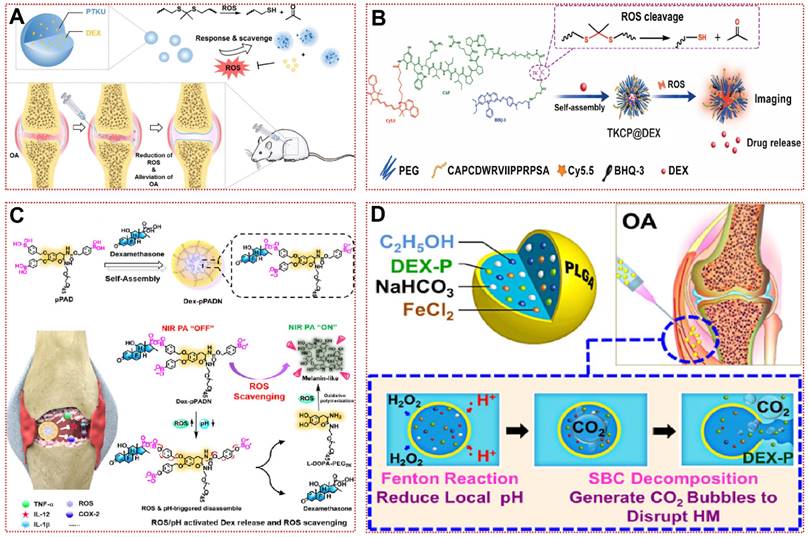
Compared with the previous study, the water-in-oil-in-water biphasic hollow microspheres (HM) composed of ethanol, ferrous chloride, Dex, and sodium bicarbonate achieved ultra-sensitive ROS responsiveness as shown in Figure 3D [78]. The low concentration of H2O2 passes through the PLAG shell and then oxidizes ethanol to acetic acid by the Fenton reaction with Fe2+ catalysis, subsequently sodium bicarbonate decomposes in an acidic environment to produce abundant carbon dioxide (CO2), which eventually leads to the collapse of HM [78]. As shown in the SEM images, HM is uniformly dispersed hollow spheres and form large pores at 50 μM H2O2/pH 6.8, making this unique structure ideal for the release of cargo [78]. Nevertheless, researchers should be mindful of the potential toxicity of these responsive biomaterials with excessive local Dex concentrations. In addition, tannic acid and tetrahydroxydiboron have been reported to form borates with ROS response and NO scavenging ability [79]. In conclusion, ROS-responsive biomaterials hold exciting promise for the treatment of inflammatory diseases not restricted to OA.
pH-responsive biomaterials
In OA lesioned joints, synovial fluid pH decreases from 7.4 to 6.0 owing to local tissue hypoxia metabolism and acidosis [112, 113]. Even in different areas of the lesion, the pH of the joint tissue reflects some variability [114]. Articular cartilage (6.3) had a slightly lower pH than the meniscus (6.5) [114]. This significant pH shift in the pathological microenvironment is considered as an ideal response condition to modulate drug delivery [115, 116]. Most pH-responsive nanomaterials are based on chemical bond breakage of cleavable groups or protonated dissociation of ionizable groups, leading to carrier acidolysis, representative of which are esters, Schiff bases, amides, vinyl ethers, and imines [34]. To overcome the high lipophilicity limitation of the anti-inflammatory drug andrographolide (AG), He et al. formulated a ph-responsive biomaterial (AG@MSNs-PAA) based on polyacrylic acid (PAA) [80]. Since PAA degrades in an acidic environment, the progressive release of AG over 72 h reversed IL-1β-induced chondrocyte apoptosis, showing recovery of Col2α1 and Aggrecan [80]. Beyond polymers, metal-organic frameworks (MOF) and inorganic materials also hold promising promise for the development of pH-responsive biomaterials [81, 82]. Xiong et al. synthesized an acid-dissolvable biomaterial MOF@HA@PCA by HA modification and loading with protocatechuic acid (PCA) [81]. The release of cargoes in MOF@HA@PCA showed “fast followed by slow” pattern, with a 1.6-fold increase in PCA release rate in PBS at pH=5.6 relative to pH=7.4 [81]. MOF@HA@PCA demonstrated a favorable safety and potent anti-inflammatory effect, substantially downregulating the expression of inflammatory markers including MMP-3, MMP-13, COX2, and iNos [81]. More interestingly, the bisphosphonate-conjugated nano-apatite (NP-BP) exhibited distinct release behaviors at different pH [82]. NP-BP targets overactive osteoclasts in subchondral bone to reverse cartilage fibrosis and angiogenesis, reflecting a novel therapeutic strategy for OA [82].
pH-responsive biomaterials for OA treatment: (A) Schematic assembly procedure of CSL@HMSNs-Cs and mechanism of intra-articular injection of CSL@HMSNs-Cs serving as a pH-responsive medicine for anti-inflammatory therapy of osteoarthritis [85]. Copyright 2020, BMC. (B) TEM images of HMSN, CSL@HMSNs-CS and cumulative release curves of CSL@HMSNs-CS in different pH PBS [85]. Copyright 2020, BMC. (C) Schematic showing pH-responsive polymeric prodrug of curcumin as a therapeutic system for osteoarthritis [84]. Copyright 2019, Elsevier. (D) Fluorescence images and quantitative analysis of MIA joints injected with ACP micelles [84]. Copyright 2019, Elsevier.
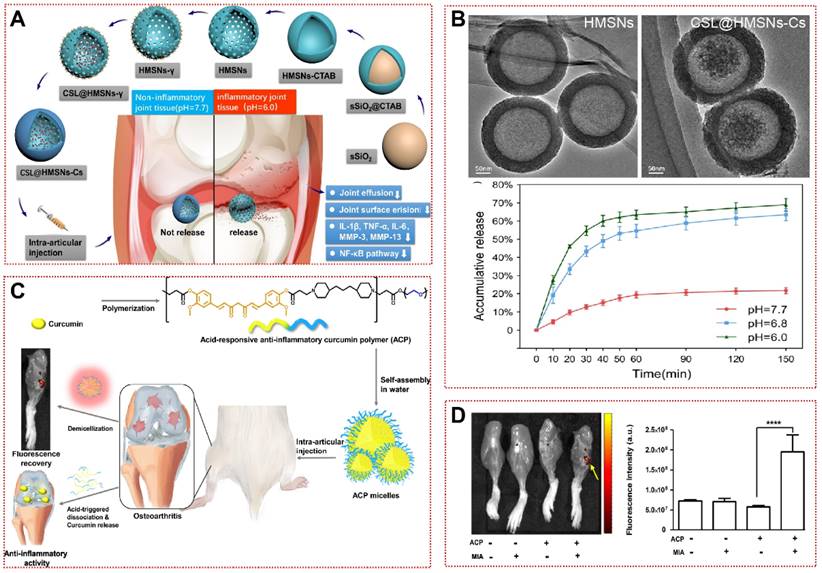
Additionally, some strategies are based on pH-dependent hydrophobic alterations or internal gas generation, allowing for overall morphological structural changes to achieve on-demand drug release [83-85]. Jin et al. constructed chitosan-coated hollow mesoporous silica nanoparticles (CSL@HMSNs-CS) loaded with poorly hydrosoluble Celastrol (CSL, a pentacyclic triterpene compound) [85]. The hollow structure (HMSN) was first prepared by hydrothermal etching, followed by anchoring the chitosan to the HMSN surface by cross-linking agent to enclose the CSL in the cavity inside the sphere (Figure 4A and 4B) [85]. When H+ is present at high levels, the protonation of free NH2 in Cs leads to increased water solubility and rupture of the protective layer, triggering pH-responsive cargo release [117]. CSL@HMSNs-CS significantly reduced IL-1β and TNF-α in chondrocyte culture supernatants by inhibiting the NF-κB signaling pathway, as well as alleviating pain behavior in OA [85]. In the MIA-induced OA model, poly(β-amino ester) (PAE) amphiphilic polymers released curcumin in a pH-sensitive manner, demonstrating superior cartilage matrix reconstitution than the equivalent curcumin (Figure 4C) [84]. ACP micelles will aggregate more significantly in OA joints (Figure 4D). Hu et al. synthesized a responsive biomaterial with carbonates for the treatment of OA [83]. Protons in the acidic microenvironment could penetrate the PLGA shell to produce CO2 with NH4HCO3 to lead to lactate disintegration, then Rh-PLGA-NPs@NH4 released three times as much rhein (Rh) as the control in the OA synovial fluid mimic [83]. In future applications, we must consider the possible safety hazards associated with these degradation products and improve the sensitivity of biomaterials to environmental pH changes to adapt to the needs of different environments in OA therapy.
Stimulus-responsive biomaterials
In contrast to endogenous factors like enzymes, ROS, and pH, simple and manipulable exogenous stimuli have also been used to initiate the release of loaded drugs [118, 119]. Operators can modulate the release behavior of responsive biomaterials by applying light, thermal, magnetic or ultrasound to the lesion site [120]. Moreover, as a high-precision drug delivery system, stimulus-responsive biomaterials can be used to add or remove external stimuli at will, or to achieve simultaneous release of multiple sites, as required, ultimately enabling individualized OA treatment modalities (continuous or intermittent) [121]. Thus, all these strengths offer great possibilities for the application of stimulus-responsive biomaterials in OA therapeutics.
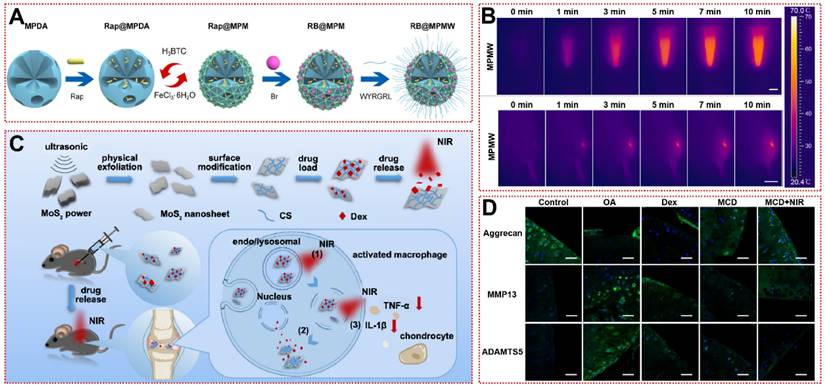
After the onset of inflammation, clinical practice reveals swelling of the knee joint accompanied by an increase in temperature of 2-3°C [34]. Kang et al. reported a thermo-responsive nanosphere that can independently release kartogenin (KGN) and diclofenac (DCF) at different rates in response to variations in temperature [86]. The nanospheres consist of a cross-linked network of amphiphilic tri-block polyoxyethylene, pluronic F127 and chitosan loaded with KGN on the outside, wrapped with polyoxypropylene and a pluronic F127 core loaded with DCF [86]. Nanospheres not only promote chondrogenesis of hBMSC in vitro, but also rescue cartilage matrix deficiency and abnormal ossification (e.g. osteophyte, bone spurs) in vivo [86]. The inverse opal hydrogel scaffold was modified with poly(N-isopropylacrylamide) to acquire temperature responsiveness, releasing diclofenac sodium only in response to inflammation or exercise fever, which greatly enhances drug utilization [87]. But endogenous heat is not sufficient for high-precision and spatiotemporally controllable drug release, limiting the clinical translation of thermo-responsive biomaterials. Considering that near-infrared (NIR, λ=780~1100 nm) light has superior tissue penetration (5~10 mm), researchers have developed photothermal therapy (PTT) with specific wavelengths of laser as the arouser [122-124]. Xue et al. constructed a MOF-based mesoporous polydopamine (PDA) multifunctional targeting nanocarrier (RB@MPMW) loaded with rapamycin (Rap) and bilirubin (Br) for the first time (Figure 5A) [25]. Whether in articular cavity or aqueous solution, RB@MPMW exhibited outstanding photothermal properties, reaching over 40°C within 3 min (Figure 5B) [25]. Meanwhile, under NIR irradiation, Rap and Br released by RB@MPMW intensely suppressed P65 phosphorylation and mitochondrial dysfunction, suggesting the restoration of chondrogenic anabolism [25]. In an anterior cruciate ligament transection (ACLT) rat model, the RB@MPMW group had the lowest OARSI score and LC3B autophagy-positive cell count, confirming the efficacy of NIR-responsive biomaterials in OA [25]. Similarly, Dex-loaded molybdenum disulfide nanosheets diminished macrophage-derived TNF-α and IL-1β by NIR exposure that rescued joint surface erosion and bradykinesia in OA mice (Figure 5C) [88]. Immunofluorescence showed that cartilage lesion caused by OA was reversed (Figure 5D) [88].
Magnetic manipulation has attracted interest due to its biocompatibility and non-invasive operation [125]. It is a magnetically driven manipulation achieved by introducing magnetic nanoparticles or magnetic fields. In the presence of an alternating magnetic field, the magnetic nanoparticle transit rate in bovine articular cartilage was increased by nearly 50-fold [89]. Magnetic nanoparticles are often made of trivalent iron complexes. After chitosan modification, meloxicam achieves an encapsulation rate of 82% [90]. In addition, nanoparticles with a gold-iron-gold shell structure coupled PPT and in vivo MRI, enabling the integration of diagnosis and treatment [91]. The methotrexate loaded in the core is precisely released in the inflamed joint by an external magnetic field [91, 92]. Superparamagnetic iron oxide particles integrated diagnosis and therapy under an applied magnetic field. It carries anti-inflammatory drugs to combat local inflammation while enhancing magnetic resonance imaging sensitivity [93, 125]. Magnetic nanoparticles with a superparamagnetic iron oxide nanoparticle core were used for responsive delivery of active siRNA to rat joints [94]. Magnetically responsive nanoparticles improved siRNA stabilization and macrophage uptake [94]. The subsequently released siRNA inhibited IL-2/IL-15Rβ expression to alleviate joint inflammation [94]. In addition, the addition of dynamic magnetic field is more favorable for the chondrogenic differentiation of BMSC on the surface of biomaterials [95]. All of these results demonstrate the feasibility and efficiency of magnetically responsive biomaterials for OA therapy.
Ultrasound-responsive biomaterials for arthritis treatment are being developed in recent years. Liao et al. successfully delivered diclofenac sodium to rat joints by ultrasound responsiveness [96]. This transdermal delivery reduced angiogenesis and joint swelling in the inflamed area [96]. Under safe low frequency ultrasound stimulation, ultrasound-responsive liposomes are enriched in the joint and release methotrexate to inhibit arthritis progression [29]. Ultrasound turns drug-loaded liposomes into “nanobombs” by locally triggering the rupture of the liposomes [27, 97]. Under folic acid-mediated targeted delivery, the uptake of nanobombs by activated macrophages is increased [27, 97]. Subsequently, localized and precise release of Dex alleviated inflammatory cell infiltration and cartilage destruction in the joint cavity under ultrasound stimulation [27, 97]. Alternatively, ultrasound responsiveness of biomaterials can be conferred by introducing sonosensitizer sparfloxacin into the structure [98]. A recent study demonstrated that ultrasound-activated H2O2 nanoenzymes produce O2 to alleviate hypoxia in the joint cavity, thereby downregulating hypoxia-inducible factors to prevent angiogenesis [98]. Unfortunately, the biggest disadvantage of ultrasound is that it is strongly attenuated by the dense bone. The lack of sufficient penetration may also be an important reason limiting the use of ultrasound-responsive biomaterials in bone-related fields. Overall, the application of stimulus-responsive biomaterials for OA therapy is still in the infancy of simple assembly of material precursors and drugs. More systematic and comprehensive preclinical studies are needed in the future to confirm their reliability and stability.
Targeted biomaterials in osteoarthritis
In the treatment of OA, whether by intra-articular injection or systemic injection, the disorganized diffusion of the drug agent in the organism is fundamental to the efficacy of the drug [126]. The diminished efficacy and low utilization of the drug brought about by this disorganized proliferation forced physicians to raise the dose, creating a vicious cycle that ultimately leads to side effects [126]. Therefore, the targeted delivery of drugs by designing reliable OA targeting strategies has become a hot topic of recent research. Targeted biomaterials not only expand the therapeutic window of the drug, but also enhance penetration and retention time [127, 128]. Next, we introduce various passive and active targeting strategies in the hope of achieving the healing of OA through targeted drug delivery to cartilage or synovium (Table 2).
Stimulus-responsive biomaterials for OA treatment: (A) The schematic diagram of fabrication of RB@MPMW [25]. Copyright 2021, Elsevier. (B) In vitro and in vivo photothermal performance of MPMW under 808 nm NIR laser irradiation at different times [25]. Copyright 2021, Elsevier. (C) Synthesis process of the drug-loaded nanosystem and its drug release in response to NIR light in vitro [88]. Copyright 2019, American Chemical Society. (D) Immunohistochemical fluorescence staining of articular cartilage matrix markers (Aggrecan) and cartilage inflammation markers (MMP13, ADAMTS5) in mice after 28 days of treatment with Dex-loaded molybdenum disulfide nanosheets [88]. Copyright 2019, American Chemical Society.
Targeted biomaterials for osteoarthritis treatment
| Materials | Targeted strategies | Targeted cells | Particle size | Drugs | Biological functions | References |
|---|---|---|---|---|---|---|
| AuNPs | Small size | Synoviocytes | 5 nm | Not available | Reduced MMPs and LDH activity as well as HA concentration, but did not affect PGE2 levels | [129] |
| PEG-MnO2 NPs | Small size and cation | Chondrocytes | 10.92 nm | Not available | Down-regulate the transcription of various antioxidant genes such as superoxide dismutase to baseline levels to restore normal chondrocyte function | [130] |
| CPC | Cationic peptide | Chondrocytes | Not available | Not available | Provide sufficient electrostatic driving force to deliver the carrier into the articular cartilage space without affecting chondrocyte viability | [131] |
| mAv-Dex | Cationic Avidin | Chondrocytes | 7.6 nm | Dexamethasone | Lower IL-1α-induced GAG loss, chondrocyte death and ROS inflammatory response | [132] |
| Dendrimer-IGF-1 | Cationic polyamidoamine | Chondrocytes | Not available | Insulin-like growth factor 1 | Penetrate the entire layer of cartilage and prolong the residence time of IGF-1 to save cartilage from degeneration | [133] |
| HGdPDW | Cartilage affinity peptide (DWRVIIPPRPSA) | Chondrocytes | 110 nm | Hesperetin | Down-regulate TLR-2/NF-κB/Akt phosphorylation activation to resist chondrocyte apoptosis and ECM loss | [134] |
| PLGA NPs | Type II collagen-targeting peptide (WYRGRL) | Chondrocytes | 256 nm | Not available | Guide nanoparticles to bind tightly to chondrocytes for in vitro fluorescence imaging | [135] |
| CT-Fn/Met | Type II collagen-targeting peptide (WYRGRL) | Chondrocytes | 20 nm | Metformin | Significantly reduce the number of MMP-13 positive cells, bone flab formation and cartilage surface lesions | [23] |
| ctLP-NPs (MK) | Type II collagen-targeting peptide (WYRGRL) | Chondrocytes | 25 nm | MK-8722 | Repair cartilage structural damage and depress TNF-α and IL-6 concentrations in OA femurs | [136] |
| WPV-CuO NPs | Type II collagen-targeting peptide (WYRGRL), MSC-targeting peptide (VTAMEPGQ), MMP-2 sensitive sequence (GALGLP) | Chondrocytes, MSCs | 5 nm | Not available | Recruit MSCs to differentiate into chondrocytes and repress PI3K/AKT/mTOR signaling pathway to improve tissue damage | [137] |
| MAbCII liposome | Type II collagen antibodies | Chondrocytes | 200 nm | Not available | Guide the tail vein injection of liposomes to selectively accumulate in the articular cartilage at the OA lesion, rather than in healthy cartilage | [138] |
| MAbCII liposome | Type II collagen antibodies | Chondrocytes | 100 nm | Not available | Enhance joint aggregation with OA severity for in vivo real-time fluorescence imaging | [139] |
| MAbCII-siNPs | Type II collagen antibodies | Chondrocytes | 124 nm | MMP-13 siRNA | Targeted silence MMP-13 expression in mice to protect against cartilage damage and prevent ectopic calcification of synovium | [140] |
| HA-NPs | Bindng to CD44 | Chondrocytes | 221 ± 1 nm | Not available | Reduce NK-κB promoter activity and block CD44-induced expression of MMP3, MMP13 and COX2 | [141] |
| Nanoghosts | MSC membrane | Chondrocytes | 200 nm | Not available | Alleviate cartilage tissue damage and reduce production of multiple pro-inflammatory cytokines | [142] |
| AM2M | Macrophage membrane | Chondrocytes | 608.57 ± 11.37 nm | Chondroitin sulfate | Mimic physiological environment cytokine release to promote chondrocyte proliferation and restore articular cartilage GAG content | [143] |
| RGD-PEG-L | RGD peptide | Vascular endothelial cells | Not available | Dexamethasone sodium phosphate | Targeted deliver to blood vessels in areas of inflammation to enhance the efficacy of Dex-p | [144] |
| tBNPs-MTX | Synovial targeting peptide (CKSTHDRLC) | Synovial vascular endothelial cells | 160 nm | Methotrexate | Disrupt synovial neovascularization by promoting CD34+ endothelial cell death | [145] |
| SOD-NPs | Permeable porous polymersomes | Synovial fibroblasts | 120 nm | Superoxide dismutase | Reduce MMP-13 and Adamts-5 secretion after endocytosis by synovial fibroblasts to reduce synovitis | [146] |
| IL-1Ra NPs | IL-1 receptor antagonist | Synoviocytes | 270 ± 5 nm | Not available | Extend the joint retention of IL-1Ra and specifically block IL-1β-induced NK-κB activation | [147] |
| ZIF-8 NPs | Anti-CD16/32 antibody | Macrophages | 160 nm | S-methylisothiourea hemisulfate salt and catalase | Eliminate H2O2 and NO production in M1 macrophages and reprogram mitochondrial metabolism to facilitate M2 conversion | [148] |
| Rap-FA@MgDHIA | Folic acid | Macrophages | 3 nm | Rapamycin | Promote the conversion of M1 macrophages to M2 macrophages to reduce the inflammatory cell infiltration of synovium and synovitis score | [149] |
| CPHs | Folic acid | Macrophages | 270 ± 1 nm | CORM-401 | Release CO to deplete intracellular H2O2 and downregulate LPS-induced cellular inflammation to protect cartilage ECM from degradation | [150] |
| D-CuS@NR NPs | Neutrophil-erythrocyte membrane | Macrophages | 178 nm | Dexamethasone sodium phosphate | Extend the circulation time in vivo to adsorb plasma inflammatory factors and accumulate to the site of inflammation | [151] |
| DS-TA NPs | Binding to the scavenger receptor class A | Macrophages | 70 nm | Triamcinolone acetonide | Reduce expression of IL-1β and IL-6 in articular cartilage and restore bone remodeling in subchondral bone | [151] |
Passive targeting strategy
As an avascular dense connective tissue, the cross-linked network formed by type II collagen with a pore size of about 50-60 nm and aggrecan with a chain spacing of about 20 nm hinders the uptake of most drugs [152-154]. Many promising experimental drugs have failed in clinical practice due to the fact that they remain on the surface of the cartilage and do not penetrate into the interior and subchondral bone [155-157]. The pore size and porosity of the cartilage matrix also vary with the progression of OA, and either too large or too small a particle size will result in poor penetration [158, 159]. Thus, suitable and homogeneous particle size will be a shortcut to increasing the effective dose and penetration of the drug. Several studies have shown that morphologically intact healthy cartilage only allows particles below 10 nm to enter the deeper layers, while 15 nm particles remain in the cartilage surface after 24 h [160, 161]. However, during the progression of OA, ECM will allow the entry of nanoparticles up to 300 nm, which may be due to the disruption of the cartilage matrix [135, 147]. Endocytosis of 15 nm MnO2 nanoparticles by chondrocytes was observed at 30 μm after 24 h incubation with bovine cartilage explants [130]. Furthermore, the penetration of nanoparticles in the synovium is similarly size-dependent. Only 5-nm gold nanoparticles successfully penetrated and would reduce synovial inflammatory biomarker concentrations, corroborating the importance of particle size in OA treatment [129]. Overall, smaller particle size tends to exhibit higher cartilage penetration and cellular uptake, which is one of the important bases for achieving OA targeting.
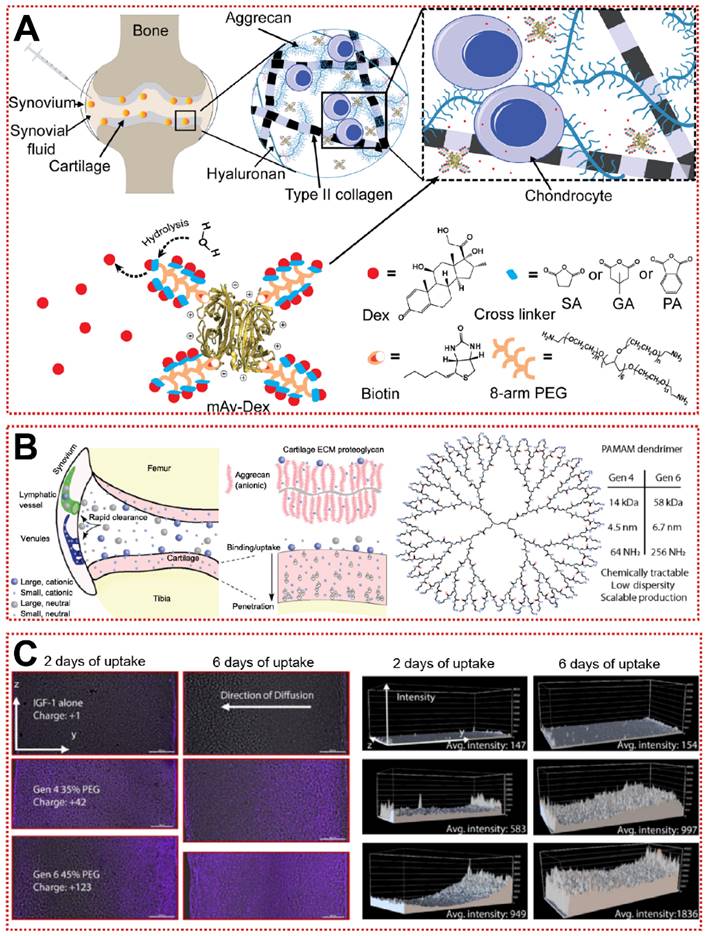
In addition, the constituent monomers of Aggrecan contain a large number of negatively charged carboxyl residues and sulfate residues, allowing cationic biomaterials to be attracted to the joints by electrostatic forces [162]. Utilizing this unique property, nanoparticles with a positive charge tend to target the ECM and increase the uptake of chondrocytes through electrostatic attraction [162]. A well-designed green fluorescent protein (GFP) was used to probe the optimal range of positive charge [163]. As the GFP surface charge increased from +9 to +36, the permeability of GFP in the cartilage layer gradually diminished and was replaced by a stronger cellular uptake rate [163]. The +9 GFP can be efficiently taken up by chondrocytes and human knee joints, while the electrically neutral GFP is completely inaccessible to cartilage explants [163]. Similarly, when using cationic peptides as carriers, the net charge of +14 exhibited the best cartilage diffusion ability, suggesting that cations need to be applied with attention to the optimal charge range [131]. Bajpayee et al. assembled a multi-armed anti-biotin protein, succinic acid (SA), glutaric acid (GA) and phthalic anhydride (PA) as a +25 mV cationic nanocarrier (mAv-Dex) to deliver Dex (Figure 6A) [132]. mAv-Dex effectively inhibited IL-1α-induced ECM loss and nitrite release within two weeks [132]. Polyethyleneglycol-functionalized polyamidoamines (PAMAM) with hundreds of primary amine residues are ideal cationic carriers for the treatment of OA (Figure 6B) [133]. PAMAM loaded with insulin-like growth factor 1 (IGF-1) penetrated into cartilage to an unprecedented depth of 1 mm and extended the retention time of IGF-1 to 30 days (Figure 6C) [133]. Relative to the poor efficacy of free IGF-1, PAMAM-IGF-1 significantly alleviated osteophyte formation and aggrecan degradation in rats, significantly delaying the pathological changes of OA [133]. Notably, since the interaction of synovial fluid with biomaterials in the articular cavity may lead to “off-targeting”, more efficient targeting strategies must be sought, rather than just staying with physicochemical properties [164].
Proactive targeting strategy
Targeting cartilage and extracellular matrix
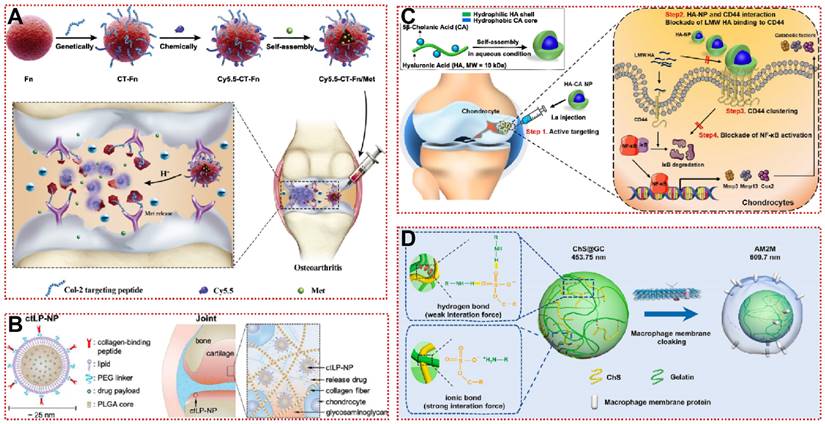
The identification of highly specific targeting ligands is often the paving stone for the study of active targeting strategies. In 2011 cartilage-targeting peptides (CAP, DWRVIIPPRPSA) screened by phage display technology have been shown to specifically induce the binding of nanoparticles to chondrocytes [165]. They are not species-specific for in vivo studies in different model animals [165]. Due to the unique three-dimensional structure and binding sites of short peptides, peptide-functionalized biomaterials have lower immunogenicity and higher operability [166]. Ouyang et al. reported a cartilage-targeted gadolinium carbonate-PDA nanoparticle loaded with hesperidin (HGdPDW) that can be used for magnetic resonance imaging and OA therapy [134]. CAP-modified HGdPDW has a homogeneous morphological structure and elemental distribution, accumulating abundantly in the cartilage of the lower femur and upper tibia in vivo [134]. HGdPDW decreased Toll-like receptor 2 (TLR-2) expression on the plasma membrane surface, thereby downregulating NF-κB/Akt activation and reversing IL-1β-induced chondrocyte apoptosis [134]. In the ACLT model, HGdPDW alleviated chondrogenic malformation and apoptosis marker caspase-3 protein production, revealing a potential mechanism for chondrocyte protection by HGdPDW [134]. In addition to CAP, Rothenfluh et al. identified a type II collagen-targeting peptide (WYRGRL) that is now widely used in biomaterial modification [152]. WYRGRL directs fluorescent probes into the cartilage matrix, so researchers can track articular cartilage lesion progression through in vitro live imaging [167]. As shown in Figure 7A, Ho et al. attached WYRGRL to the surface of ferritin nanocages (CT-Fn) to overcome the drawbacks of conventional oral administration of metformin (Met) [23]. The modified CT-Fn targeted type II collagen in articular cartilage, reducing cartilage macroscopic scores by 47% and 68% at weeks 2 and 6 [23]. Type II collagen-targeting peptide-modified liposomes also exhibited smooth penetration and prolonged retention in the mouse cartilage matrix (Figure 7B) [136]. Based on the previous work, Lu et al. designed a functionalized nanoparticle (WPV-CuO) with MMP-2 cleavage peptide as a linker for dual-targeting peptides of type II collagen and MSC [137]. WPV-CuO actively homed to cartilage and recruited MSC to cartilage differentiation in vivo [137]. Transcriptome analysis revealed that phosphorylation activation of AKT was significantly downregulated in the WPV-CuO group, implying that the potential therapeutic mechanism of WPV-CuO is through inhibition of the PI3K/AKT/mTOR signaling pathway [137].
Passive targeting strategy for OA treatment: (A) Schematic of charge-based intra-cartilage drug delivery of the nano-construct multi-arm Avidin conjugated with a small molecule drug [132]. Copyright 2020, Elsevier. (B) Schematic of drug and carrier fates within joints following intra-articular injection based on size and charge [133]. Copyright 2018, AAAS. (C) Confocal microscopy images of IGF-1 (purple) diffusion in articular cartilage and quantitative analysis of fluorescence [133]. Copyright 2018, AAAS.
Cartilage targeting strategy for OA treatment: (A) Schematic illustration of synthesis, structure and anti-inflammatory mechanism of CT-Fn/Met drug delivery system in OA [23]. Copyright 2020, Elsevier. (B) The design of collagen‐targeting ultrasmall lipid‐polymer hybrid nanoparticles (ctLP‐NPs) for targeted drug delivery to the joints [136]. Copyright 2020, Wiley. (C) Schematic illustration of HA-NPs for treatment of OA [141]. Copyright 2021, Elsevier. (D) Preparation procedure of macrophage membrane-cloaked ChS@GC (artificial M2 macrophage, AM2M) that loaded chondroitin sulfate (ChS) through multi-component ionic cross-linking and simple physical adsorption via ionic bond and hydrogen bond [143]. Copyright 2021, Elsevier.
To further improve the active targeting of vectors, specific antibodies against articular cartilage have been incorporated into biomaterial fields of application. Compared with other methods, the antibody only binds and tightly attaches to the antigenic epitope on the surface of the corresponding antigen with high affinity and specificity, greatly improving the targeting of the vector [168]. Type II collagen monoclonal antibody (MabCⅡ)-modified liposomes can be used for in vivo imaging by accumulating at the injured joint after intravenous injection [138]. The amount of MabCⅡ binding correlated positively with the severity of OA, while no fluorescent signal was detected in healthy joints [139]. Compared to the negative control, 124nm polymers coupled to MabCII (mAbCII-siNPs) achieved efficient siRNA chondrogenic delivery [140]. mAbCII-siNPs not only silenced the expression of MMP13 and downstream related genes in chondrocytes, but also showed in vivo protection against ectopic calcification of synovial membrane, which was superior to steroid treatment [140]. This suggests that specific antibody-based cartilage targeting strategies are worthy of further long-term study and promising for clinical trials.
For biocompatibility and adaptability considerations, some bionic biomaterials have also been applied for active targeting of OA [169, 170]. The use of HA nanoparticles (HA-NP), rather than direct HA injection, allows targeted binding of highly expressed CD44 in damaged cartilage to competitively block activation of the NF-κB signaling pathway (Figure 7C) [141]. HA-NP not only exhibited hyaluronidase resistance and cartilage penetration, but also dramatically alleviated destabilization of the medial meniscus (DMM)-induced cartilage destruction and subchondral bone plate thickening [141]. The efficacy remained significant at intervals of up to 4 weeks of administration, suggesting that joint cavity injection of HA-NP may be a potential targeted therapeutic strategy for OA [141]. Nanoghosts (NGs) made from mesenchymal stem cell membranes were reported to be a cell-free cartilage targeting platform, and the intracellular transport of NGs was analyzed by fluorescence co-localization techniques [142]. Due to their inherent immunomodulatory and inflammatory homing ability, NGs were first endocytosed by chondrocytes and then entered the lysosome, subsequently downregulating the expression of COX2 and PEG2 at the mRNA and protein levels compared to the untreated group [142]. Moreover, when using M2 macrophage membranes as a shell (Figure 7D), artificial macrophages (AM2M) block interleukin-induced acute inflammatory injury while avoiding chondroitin sulfate (ChS)-induced immune stimulation [143]. AM2M has the greatest articular retention time and stable ChS release rate, successfully restoring the expression of MMP13 to normal levels [143]. It is demonstrated that it is indeed feasible to mimic the secretion of anti-inflammatory cytokines by M2 macrophages in OA. For bionic biomaterials used in OA, there is a lack of sufficient systematic in vivo and in vitro studies to clarify the action mechanisms and long-lasting therapeutic effects, restricting their clinical translation. Overall, active targeting strategies based on cartilage and extracellular matrix are still in their infancy, further development of more desirable OA-targeting biomaterials can be achieved in the future by drawing on experience from other diseases such as cancer or immunomodulation.
Targeting synovium
Synovium is composed of macrophages, fibroblasts and vascular endothelial cells, which perform the functions of material exchange and secretion of synovial fluid [171]. Apart from cartilage damage, synovial inflammation is also a major pathological characteristic of OA lesions [172, 173]. Topical or systemic administration of corticosteroid is often used for synovial inflammation in OA, but their potential toxicity and excessive metabolism both make long-term use impossible and increase the risk of osteoporosis [174]. For this reason, biomaterial therapeutic strategies targeting synovium have attracted interest. Initially, the researchers hoped to achieve synovial-targeted delivery by using RGD peptide binding to integrin αvβ3 on synovial vascular endothelial cells [144]. However, the expression of integrin αvβ3 is not synovial specific and may lead to a non-specific accumulation of biomaterials extra-synovial [175, 176]. Colombo et al. achieved targeted delivery of degradable nanoparticles (tBNPs-MTX) by coupling cyclic synovial homing peptides [145]. This cyclic peptide showed strong binding capacity to von Willebrand Factor+ (VWF+) vascular endothelial cells and CD34+ hematopoietic stem cells in synovial tissue [145]. tBNPs-MTX significantly reduced neovascularization density and leukocyte counts in rat synovium, further demonstrating the great potential of synovium as a therapeutic target for OA [145]. In addition, a block polymer-based superoxide dismutase nanoparticle (SOD-NP) was reported to have a unique targeting ability to synovial fibroblasts, overcoming the excessive degradation of SOD [146].
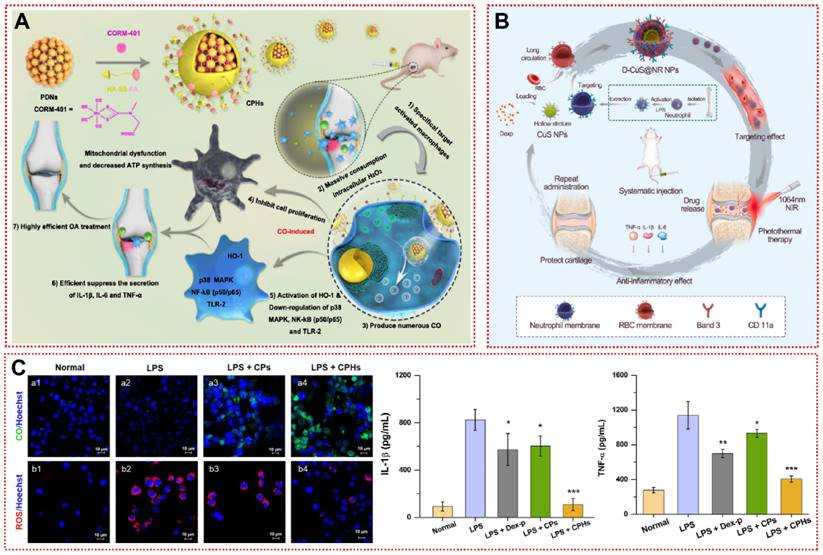
In the inflammatory microenvironment, interferon-γ (IFN-γ) and TNF-α induce synovial macrophages to polarize toward the pro-inflammatory M1 type, leading to the release of downstream inflammatory factors IL-1β and IL-6 [177]. Modulation of the M1/M2 ratio of macrophages to suppress the progression of inflammation may be a promising intervention for OA [178]. Zhou et al. grafted an anti-CD16/32 antibody onto a modified zeolitic imidazolate framework-8 (ZIF-8) to restrain hypoxia-inducible factor-1α (HIF-1α) and recover macrophage mitochondrial dysfunction [148]. After macrophages were reprogrammed to M2 type, angiogenesis and inflammatory cell infiltration in the synovium were dramatically reduced, and ACLT-induced cartilage damage was reversed [148]. More interestingly, Chen et al. designed and synthesized novel magnesium-based ligand containers (Rap-FA@MgDHIA) for the stepwise loading and release of Rap utilizing folic acid (FA) as a surface ligand [149]. Folate receptor β (FRβ) is highly expressed on the membrane surface of M1 macrophages [149]. Rap-FA@MgDHIA achieves targeting M1 type macrophages by binding to FRβ [149]. Rap-FA@MgDHIA subsequently releases Rap to convert macrophages from a pro-inflammatory phenotype (M1) to an anti-inflammatory phenotype (M2) [149]. After 14 days of Rap-FA@MgDHIA local injection, iNOS-positive M1-type macrophages in synovial tissue were remarkably minimized [149]. Rap-FA@MgDHIA alleviated synovial inflammatory cell infiltration while articular cartilage and subchondral bone were successfully conserved [149]. In another study targeting synovial macrophages, FA-modified nanoparticles (CPHs) acted as CO donors to inhibit MIA-induced articular damage in rats (Figure 8A) [150]. CPHs induced apoptosis by depleting H2O2 in activated macrophages while decreasing mitochondrial membrane potential and ATP synthesis, yet showed a satisfactory safety profile for normal macrophages [150]. Lower ROS levels and higher CO levels reduced the macrophage-mediated inflammatory response (Figure 8B) [150]. Considering the potential uptake by macrophages and lack of targeting in the circulation by conventional drug delivery, Xue et al. fused erythrocyte membranes and neutrophil membranes on a hollow copper sulfide surface to provide a promising platform (D-CuS@NR NPs) for inflammation-associated diseases (Figure 8C) [151]. The modification of the composite membrane not only non-specifically adsorbs a variety of serum inflammatory factors, but also rapidly accumulates in the articular cavity at 60 min to inhibit cartilage wear [151]. D-CuS@NR NPs can release Dex while also responding to NIR to produce photothermal effects for adjunctive therapy, demonstrating a new mode of OA treatment [151]. Additionally, self-assembled micellar nanoparticles (DS-TA NPs) of dextran sulfate-triamcinolone acetonide conjugates specifically target the scavenger receptor class A (SR-A) on the surface of activated macrophages to reduce the expression of pro-inflammatory cytokines for promising targeted therapy of OA [179]. In summary, the engineering of targeted biomaterials for synovial cells optimizes the biodistribution and potency of drugs in vivo as a promising therapeutic strategy for OA.
Synovial targeting strategy for OA treatment: (A) Schematic illustration for the preparation of CPHs as well as its related mechanisms for the effective treatment of osteoarthritis [150]. Copyright 2020, Elsevier. (B) Changes in ROS/CO levels in activated macrophages and inhibition of inflammatory factor secretion after treatment with CPHs [150]. Copyright 2020, Elsevier. (C) Schematic diagram of the preparation and therapy of the D-CuS@NR NPs [151]. Copyright 2022, Elsevier.
Targeting subchondral bone
Although cartilage degeneration and ECM loss have long been considered the primary causative factors of OA, there is growing evidence that subchondral bone lesions precede the onset of cartilage loss [4]. Bone trabeculae and platelets in the subchondral bone are arranged in an appropriate proportional distribution to disperse mechanical stress, and osteoclast-mediated bone resorption and osteoblast-mediated osteogenesis are counterbalanced [4]. After the onset of the lesion, the over-activated osteoclasts of the subchondral bone induce the invasion of blood vessels and nerves into the cartilage layer, triggering inflammation and pain [180]. Several preclinical studies have been conducted to show the effectiveness of OA treatment options targeting abnormal subchondral bone remodeling and angiogenesis [181-183]. With only the simplest drug injections to discuss efficacy on subchondral bone, rather than specific delivery, there remains a potential risk of nonspecific distribution of the drug in the articular cavity or systemically. Bisphosphonates (BPs) are used as highly effective bone targeting agents to inhibit osteoclast activity, and biomaterials coupled to BPs are also used to carry other active molecules for basic bone-related therapies [82]. BPs-modified nano-apatite (NP-BP) achieves targeting of subchondral bone by responding to lower pH in the microenvironment [82]. Since the bone resorption of subchondral bone in the early stage of OA was inhibited by NP-BP, the pain behavior of rats was relieved and the three-dimensional structure of the cartilage layer was preserved intact within 90 days, demonstrating that targeting subchondral bone has favorable feasibility and prospect for the management of OA [82]. In addition, targeting crystalline mineral loss and reduction in collagen mineralization in subchondral bone may also be a potential target for OA [184]. Yet, treatment options that target subchondral bone present enormous difficulties due to the natural barrier of articular cartilage and the low blood circulation to the subchondral bone. Although there are limited studies on targeting subchondral bone, the future development of subchondral bone-targeting biomaterials based on surface modification and innovative structures will be a new direction for OA treatment.
Limitations and challenges of clinical translation
Although biomaterials hold exciting promise for improving drug solubility and precision release, the potential risks and unknown chronic toxicity of these materials in living organisms limit their clinical translation. The size of most biomaterials reaches the nanometer dimension, which allows them to easily cross multiple biological barriers and be non-specifically endocytosed by immune cells [185]. Although no visceral toxicity or other complications have been reported in any of the biomaterials discussed above, the risk of temporary organ accumulation, sudden release or accidental off-target remains is still a concern. In addition to focusing on recovery from OA lesions, researchers should have a sufficient long-term understanding of the normal physiological functions of joint tissue (e.g., mechanical strength, synovial fluid composition, and nutrient supply) to ensure the safety of biomaterials. The polymers synthesized in the experiments usually do not reach 100% polymerization, although the polymers themselves are not toxic, the residual polymer monomers may promote apoptosis or genotoxicity [186]. Around 100 nm chitosan, the most common natural biopolymer in the biomedical field, would cause significant neurotoxicity, liver damage and reduced hatchability in zebrafish [187, 188]. In addition, joints are one of the most important motor tissues in the body, implant particles from sportswear will activate the NF-κB signaling pathway in macrophages and induce bone mineral loss [189]. Chronic inflammation due to prosthetic wear particles may present an unpleasant challenge for patients with advanced OA undergoing total joint arthroplasty [189]. Also, in vivo evaluation of simulated biomaterials using in vitro stem cell models or bone like organs has recently received much attention, which would be more convincing than toxicity experiments with single cell lines [190, 191].
The biodistribution and toxicity of the delivery platforms within the joint will also be influenced by the strength of the surface charge. Positively charged cationic materials tend to have easier uptake by cells and are therefore often used for therapeutic RNA delivery [192]. However, when the charge intensity or concentration is too high, either cation or anion, it will trigger mitochondria-mediated cellular autophagy and plasma membrane damage [193]. In previous studies, cationic nanoparticles were considered as ideal carriers for targeting cartilage using electrostatic attraction. However, as the surface charge continues to rise, excessive electrostatic interactions will prevent the penetration of nanoparticles larger than +14 mV into deeper layers, while other non-covalent bonds (e.g. hydrogen bonds, hydrophobic bonds) formed between GAG and cationic peptides will also affect their permeability [131]. On the other hand, for carriers applied for systemic injection, liposomes with high surface charge have increased hepatic accumulation and complement protein-mediated immune clearance relative to electroneutral liposomes [194]. Researchers should be more cautious to examine whether the loss of GAG in the late stages of OA may lead to off-target effects of cation-targeting strategies and subsequently increase the cumulative toxicity in non-targeted tissues.
Due to the presence of synovial fluid, adsorption of synovial proteins on the surface of biomaterials will probably interfere responsive group breakage and exposure of targeting ligands [195]. Although PEG modification can suppress protein adsorption and protein corona formation, it does not completely avoid albumin or complement protein binding [196]. Brown et al. indicated that incubation in the synovial fluid would lead to an increase in cationic nanoparticle size and charge reversal [195]. It is speculated that the protein corona formed by adsorbed synovial proteins may mask the functional ligands on the nanoparticle surface and subsequently affect their tissue transportation and responsive release within the joint. Hence, how to overcome the interference of synovial fluid with biomaterials to achieve efficient OA treatment will be a great challenge for clinical translation. They should be further tested in a diversity of joint tissues or in large animals to understand and confirm the efficacy of OA.
Finally, researchers should be cautious in their choice of model animals and means of inducing OA. Due to differences in species, age, and sex, none of the animal models can fully replicate all pathological processes in human OA [197]. Researchers need to consider a combination of study objectives and concerns to determine the modeling approach. For example, spontaneous OA allows researchers to analyze the disease process from early to late stages in different time sequences. However, such OA models are time-consuming and expensive, while the variation between animals can be significant. Invasive surgery allows experimenters to achieve a less variable OA development and is currently the most commonly used and stable animal model of OA [198]. Nevertheless, it is not suitable for studying the changes in OA during long-term wear and aging [198]. Induction by chemical adjuvants such as collagenase can produce significant cartilage wear and pain perception in a short period of time [198]. Yet, this is far from the pathogenesis of human OA. These different mechanisms of pathogenesis will perhaps influence the efficiency of drug targeting. Some models that produce large amounts of ROS or pH reduction rapidly may exhibit more pronounced responsive drug release relative to naturally occurring OA [199]. Thus, a single model of OA treatment strategy cannot be fully equated with clinical efficacy. Future studies should include more convincing evidence to compare the efficiency of targeted release of drug-loaded biomaterials in different OA models.
Conclusion
OA is a degenerative disease characterized by loss of articular cartilage and local inflammation that affects tens of millions of people worldwide. The unknown pathogenesis of OA leads to clinical treatment strategies that do not completely eradicate cartilage degradation. Conventional intra-articular injections or oral administration of drugs are often rapidly cleared by phagocytes or metabolic organs resulting in low drug utilization and a narrow therapeutic window. The application of targeted and responsive biomaterials in the biomedical field is bringing new light to the treatment of OA. Efficient drug delivery systems based on the OA microenvironment (enzymes, ROS, pH, stimulation) and lesion sites (cartilage, synovium, subchondral bone) have shown promising applications. Not only do they maximize the therapeutic potential of drug molecules, but more importantly they improve the biodistribution and delivery of drugs. The "bio-missile" based on biomaterials penetrates nanoscale pores and has long-lasting controlled release, avoiding the pain and economic burden of multiple high-dose administrations to patients. By modifying and optimizing the structure and physicochemical properties, biomaterials overcome the therapeutic bottleneck of drug monomers and enable the integration of treatment. The controlled release of drugs in combination with microenvironmental changes assists in better mastering the pathological stages of OA, while the coupling of targeted ligands opens new horizons for precision therapy. Moreover, these targeted and responsive biomaterials may be used for other OA-affected joint tissues in the future, including fat pads and joint ligaments.
Nevertheless, biomaterial-based clinical therapies for OA are still in their infancy, and there is a lack of sufficient clinical studies to demonstrate the efficacy and safety of these strategies. Previous studies have also shown that the selection of natural cartilage components based on hyaluronic acid and collagen as carriers may yield better therapeutic benefits and safety. Also, the cartilage layer is thicker in primates, which would greatly prolong the diffusion of biomaterials. These findings should be further validated in a more closely related human model (e.g., rhesus monkey). In addition, further validation is needed to determine if the effectiveness of these targeted ECM strategies is compromised in the late stages of OA when the collagen network of chondrocytes is depleted. On the other hand, new multifunctional biomaterials involve more complex and sophisticated technologies, often leading to expensive economic costs in clinical applications, which should be taken into account. Since the criteria for toxicity evaluation of biomaterials at the development stage are not yet standardized, the absence of a quality evaluation system will lengthen the cycle of the approval process, which is not conducive to the clinical translation of biomaterials. To break the existing barriers and accelerate the clinical translation of targeted and responsive biomaterials, we need to deepen our understanding of OA not only at the molecular and cellular levels, but also to speed up the research process of novel active drug molecules and the search for more specific drug carriers to achieve stronger delivery effects. To meet these challenges, broad and close collaboration and cross-disciplinary research among materials scientists, biologists and clinicians are required. Looking ahead, with the continuous improvement of regulatory mechanisms and experimental techniques, the targeted and responsive biomaterials are expected to become a panacea for OA patients.
Abbreviations
OA: osteoarthritis; NSAIDs: non-steroidal anti-inflammatory drugs; HA: hyaluronic acid; ROS: reactive oxygen species; ECM: extracellular matrix; GAG: glycosaminoglycan; MMPs: matrix metalloproteinases; IL-1β: interleukin-1β; NO: nitric oxide; PGE2: prostaglandin E2; TNF-α: tumor necrosis factor-α; IL-6: interleukin-6; ADAMTS: a disintegrin and metalloproteinase with thrombospondin motifs; CS: chondroitin sulfate; ACI: autologous chondrocyte implantation; MRC-PPL: poly(2-ethyl-2-oxazoline)-poly(ε-caprolactone); PSO: psoralidin; TA: triamcinolone acetonide; O2-: superoxide anions; H2O2: hydrogen peroxide; OH·: hydroxyl radicals; NO·: nitric oxide radicals; 1O2: singlet oxygen; GSH: glutathione; SOD: superoxide dismutase; PTK: polythioketal; HDI: hexamethylene diisocyanate; PTKU: polyurethane; Dex: dexamethasone; TK: thioketal; MIA: monosodium iodoacetate; HM: hollow microspheres; CO2: carbon dioxide; AG: andrographolide; PAA: polyacrylic acid; MOF: metal-organic frameworks; PCA: protocatechuic acid; NP-BP: bisphosphonate-conjugated nano-apatite; CSL: celastrol; PAE: poly(β-amino ester); Rh: rhein; KGN: kartogenin; DCF: diclofenac; NIR: near-infrared; PTT: photothermal therapy; PDA: polydopamine; Rap: rapamycin; Br: bilirubin; ACLT: anterior cruciate ligament transection; GFP: green fluorescent protein; SA: succinic acid; GA: glutaric acid; PA: phthalic anhydride; PAMAM: polyamidoamines; IGF-1: insulin-like growth factor 1; CAP: cartilage-targeting peptides; TLR-2: toll-like receptor 2; Met: metformin; MabCⅡ: Type II collagen monoclonal antibody; DMM: destabilization of the medial meniscus; NGs: nanoghosts; ChS: chondroitin sulfate; VWF: von willebrand factor; SOD: superoxide dismutase; IFN-γ: interferon-γ; ZIF-8: zeolitic imidazolate framework-8; HIF-1α: hypoxia-inducible factor-1α; FA: folic acid; FRβ: folate receptor β; BPs: bisphosphonates.
Acknowledgements
This work was supported by the National Key R&D Program of China (2018YFC2001500), Key Project of the National Natural Science Foundation of China (82230071) and National Natural Science Foundation of China (82172098, 32101084).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Martel-Pelletier J, Barr AJ, Cicuttini FM, Conaghan PG, Cooper C, Goldring MB. et al. Osteoarthritis. Nat Rev Dis Primers. 2016;2:16072
2. Allen KD, Thoma LM, Golightly YM. Epidemiology of osteoarthritis. Osteoarthr Cartilage. 2022;30:184-95
3. Leifer VP, Katz JN, Losina E. The burden of OA-health services and economics. Osteoarthritis Cartilage. 2022;30:10-6
4. Hu Y, Chen X, Wang S, Jing Y, Su J. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res. 2021;9:20
5. Zhou Y, Ni J, Wen C, Lai P. Light on osteoarthritic joint: from bench to bed. Theranostics. 2022;12:542-57
6. Gao J, Xia Z, Mary HB, Joseph J, Luo JN, Joshi N. Overcoming barriers for intra-articular delivery of disease-modifying osteoarthritis drugs. Trends Pharmacol Sci. 2022;43:171-87
7. Katz JN, Arant KR, Loeser RF. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA. 2021;325:568-78
8. Latourte A, Kloppenburg M, Richette P. Emerging pharmaceutical therapies for osteoarthritis. Nat Rev Rheumatol. 2020;16:673-88
9. Jiang YY, Tao YL, Chen YT, Xue X, Ding GY, Wang SC. et al. Role of Phosphorus-Containing Molecules on the Formation of Nano-Sized Calcium Phosphate for Bone Therapy. Front Bioeng Biotech. 2022 10
10. Mahmoudian A, Lohmander LS, Mobasheri A, Englund M, Luyten FP. Early-stage symptomatic osteoarthritis of the knee - time for action. Nat Rev Rheumatol. 2021;17:621-32
11. Conaghan PG, Cook AD, Hamilton JA, Tak PP. Therapeutic options for targeting inflammatory osteoarthritis pain. Nat Rev Rheumatol. 2019;15:355-63
12. Jones IA, Togashi R, Wilson ML, Heckmann N, Vangsness CT Jr. Intra-articular treatment options for knee osteoarthritis. Nat Rev Rheumatol. 2019;15:77-90
13. Saeedi T, Alotaibi HF, Prokopovich P. Polymer colloids as drug delivery systems for the treatment of arthritis. Adv Colloid Interface Sci. 2020;285:102273
14. Xue X, Hu Y, Deng YH, Su JC. Recent Advances in Design of Functional Biocompatible Hydrogels for Bone Tissue Engineering. Adv Funct Mater. 2021 31
15. Xue X, Zhang H, Liu H, Wang SC, Li JD, Zhou QR. et al. Rational Design of Multifunctional CuS Nanoparticle-PEG Composite Soft Hydrogel-Coated 3D Hard Polycaprolactone Scaffolds for Efficient Bone Regeneration. Adv Funct Mater. 2022 32
16. Liu H, Zhang H, Han Y, Hu Y, Geng Z, Su J. Bacterial extracellular vesicles-based therapeutic strategies for bone and soft tissue tumors therapy. Theranostics. 2022;12:6576-94
17. Kou L, Xiao S, Sun R, Bao S, Yao Q, Chen R. Biomaterial-engineered intra-articular drug delivery systems for osteoarthritis therapy. Drug Deliv. 2019;26:870-85
18. Xue X, Hu Y, Wang SC, Chen X, Jiang YY, Su JC. Fabrication of physical and chemical crosslinked hydrogels for bone tissue engineering. Bioactive Materials. 2022;12:327-39
19. Bottini M, Bhattacharya K, Fadeel B, Magrini A, Bottini N, Rosato N. Nanodrugs to target articular cartilage: An emerging platform for osteoarthritis therapy. Nanomedicine. 2016;12:255-68
20. Ren XX, Chen X, Geng Z, Su JC. Bone-targeted biomaterials: Strategies and applications. Chem Eng J. 2022 446
21. Gao F, Yin JH, Chen Y, Guo CY, Hu HG, Su JC. Recent advances in aptamer-based targeted drug delivery systems for cancer therapy. Front Bioeng Biotech. 2022 10
22. Lan Q, Lu R, Chen H, Pang Y, Xiong F, Shen C. et al. MMP-13 enzyme and pH responsive theranostic nanoplatform for osteoarthritis. J Nanobiotechnology. 2020;18:117
23. He Y, Ren E, Lu Z, Chen H, Qin Z, Wang J. et al. Rational engineering of ferritin nanocages for targeted therapy of osteoarthritis. Nanomedicine. 2020;28:102210
24. Shen C, Gao M, Chen H, Zhan Y, Lan Q, Li Z. et al. Reactive oxygen species (ROS)-responsive nanoprobe for bioimaging and targeting therapy of osteoarthritis. J Nanobiotechnology. 2021;19:395
25. Xue S, Zhou X, Sang W, Wang C, Lu H, Xu Y. et al. Cartilage-targeting peptide-modified dual-drug delivery nanoplatform with NIR laser response for osteoarthritis therapy. Bioact Mater. 2021;6:2372-89
26. Deng CF, Zhang Q, He PH, Zhou B, He K, Sun X. et al. Targeted apoptosis of macrophages and osteoclasts in arthritic joints is effective against advanced inflammatory arthritis. Nat Commun. 2021 12
27. Wang LY, Zhu BH, Huang JB, Xiang X, Tang YJ, Ma L. et al. Ultrasound-targeted microbubble destruction augmented synergistic therapy of rheumatoid arthritis via targeted liposomes. Journal of Materials Chemistry B. 2020;8:5245-56
28. Li XY, Yu CH, Meng XX, Hou YF, Cui YX, Zhu TY. et al. Study of double-targeting nanoparticles loaded with MCL-1 siRNA and dexamethasone for adjuvant-induced arthritis therapy. Eur J Pharm Biopharm. 2020;154:136-43
29. Wu HH, He YN, Wu H, Zhou MJ, Xu ZL, Xiong R. et al. Near-infrared fluorescence imaging-guided focused ultrasound-mediated therapy against Rheumatoid Arthritis by MTX-ICG-loaded iRGD-modified. Theranostics. 2020;10:10092-105
30. Meng FY, Yin ZF, Ren XX, Geng Z, Su JC. Construction of Local Drug Delivery System on Titanium-Based Implants to Improve Osseointegration. Pharmaceutics. 2022 14
31. Brown S, Kumar S, Sharma B. Intra-articular targeting of nanomaterials for the treatment of osteoarthritis. Acta Biomater. 2019;93:239-57
32. Chen YL, Wu XM, Li JD, Jiang YY, Xu K, Su JC. Bone-Targeted Nanoparticle Drug Delivery System: An Emerging Strategy for Bone-Related Disease. Front Pharmacol. 2022 13
33. Zhou ZY, Cui J, Wu SL, Geng Z, Su JC. Silk fibroin-based biomaterials for cartilage/ osteochondral repair. Theranostics. 2022;12:5103-24
34. Wang Z, Wang S, Wang K, Wu X, Tu C, Gao C. Stimuli-Sensitive Nanotherapies for the Treatment of Osteoarthritis. Macromol Biosci. 2021;21:e2100280
35. Carballo CB, Nakagawa Y, Sekiya I, Rodeo SA. Basic Science of Articular Cartilage. Clin Sports Med. 2017;36:413-25
36. Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1:461-8
37. Wang FX, Guo JW, Wang SC, Wang YL, Chen J, Hu Y. et al. B-cell lymphoma-3 controls mesenchymal stem cell commitment and senescence during skeletal aging. Clinical and Translational Medicine. 2022 12
38. Bolduc JA, Collins JA, Loeser RF. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic Biol Med. 2019;132:73-82
39. Gelse K, Poschl E, Aigner T. Collagens-structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55:1531-46
40. Luo Y, Sinkeviciute D, He Y, Karsdal M, Henrotin Y, Mobasheri A. et al. The minor collagens in articular cartilage. Protein Cell. 2017;8:560-72
41. Gao Y, Liu S, Huang J, Guo W, Chen J, Zhang L. et al. The ECM-cell interaction of cartilage extracellular matrix on chondrocytes. Biomed Res Int. 2014;2014:648459
42. Guilak F, Nims RJ, Dicks A, Wu CL, Meulenbelt I. Osteoarthritis as a disease of the cartilage pericellular matrix. Matrix Biol. 2018;71-72:40-50
43. Peng Z, Sun H, Bunpetch V, Koh Y, Wen Y, Wu D. et al. The regulation of cartilage extracellular matrix homeostasis in joint cartilage degeneration and regeneration. Biomaterials. 2021;268:120555
44. Blagojevic M, Jinks C, Jeffery A, Jordan KP. Risk factors for onset of osteoarthritis of the knee in older adults: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2010;18:24-33
45. Sun AR, Udduttula A, Li J, Liu Y, Ren PG, Zhang P. Cartilage tissue engineering for obesity-induced osteoarthritis: Physiology, challenges, and future prospects. J Orthop Translat. 2021;26:3-15
46. Hu Y, Cui J, Liu H, Wang SC, Zhou QR, Zhang H. et al. Single-cell RNA-sequencing analysis reveals the molecular mechanism of subchondral bone cell heterogeneity in the development of osteoarthritis. Rmd Open. 2022 8
47. Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33-42
48. Wang T, He C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018;44:38-50
49. Wojdasiewicz P, Poniatowski LA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459
50. Li J, Yin Z, Huang B, Xu K, Su J. Stat3 Signaling Pathway: A Future Therapeutic Target for Bone-Related Diseases. Front Pharmacol. 2022;13:897539
51. Bay-Jensen AC, Kjelgaard-Petersen CF, Petersen KK, Arendt-Nielsen L, Quasnichka HL, Mobasheri A. et al. Aggrecanase degradation of type III collagen is associated with clinical knee pain. Clin Biochem. 2018;58:37-43
52. D'Arcy Y, Mantyh P, Yaksh T, Donevan S, Hall J, Sadrarhami M. et al. Treating osteoarthritis pain: mechanisms of action of acetaminophen, nonsteroidal anti-inflammatory drugs, opioids, and nerve growth factor antibodies. Postgrad Med. 2021;133:879-94
53. Rannou F, Pelletier JP, Martel-Pelletier J. Efficacy and safety of topical NSAIDs in the management of osteoarthritis: Evidence from real-life setting trials and surveys. Semin Arthritis Rheum. 2016;45:S18-21
54. Zhou D, Zhang H, Xue X, Tao Y, Wang S, Ren X. et al. Safety Evaluation of Natural Drugs in Chronic Skeletal Disorders: A Literature Review of Clinical Trials in the Past 20 years. Front Pharmacol. 2021;12:801287
55. Tenti S, Cheleschi S, Mondanelli N, Giannotti S, Fioravanti A. New Trends in Injection-Based Therapy for Thumb-Base Osteoarthritis: Where Are We and where Are We Going? Front Pharmacol. 2021;12:637904
56. Delsmann MM, Schmidt C, Muhlenfeld M, Jandl NM, Boese CK, Beil FT. et al. Prevalence of osteoporosis and osteopenia in elderly patients scheduled for total knee arthroplasty. Arch Orthop Trauma Surg. 2021
57. Gupta RC, Lall R, Srivastava A, Sinha A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front Vet Sci. 2019;6:192
58. Maheu E, Bannuru RR, Herrero-Beaumont G, Allali F, Bard H, Migliore A. Why we should definitely include intra-articular hyaluronic acid as a therapeutic option in the management of knee osteoarthritis: Results of an extensive critical literature review. Semin Arthritis Rheum. 2019;48:563-72
59. Henrotin Y, Raman R, Richette P, Bard H, Jerosch J, Conrozier T. et al. Consensus statement on viscosupplementation with hyaluronic acid for the management of osteoarthritis. Semin Arthritis Rheum. 2015;45:140-9
60. Bruyere O, Honvo G, Veronese N, Arden NK, Branco J, Curtis EM. et al. An updated algorithm recommendation for the management of knee osteoarthritis from the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Semin Arthritis Rheum. 2019;49:337-50
61. Lutzner J, Kasten P, Gunther KP, Kirschner S. Surgical options for patients with osteoarthritis of the knee. Nat Rev Rheumatol. 2009;5:309-16
62. de Klerk HH, Welsink CL, Spaans AJ, Verweij LPE, van den Bekerom MPJ. Arthroscopic and open debridement in primary elbow osteoarthritis: a systematic review and meta-analysis. EFORT Open Rev. 2020;5:874-82
63. Neuprez A, Neuprez AH, Kaux JF, Kurth W, Daniel C, Thirion T. et al. Total joint replacement improves pain, functional quality of life, and health utilities in patients with late-stage knee and hip osteoarthritis for up to 5 years. Clin Rheumatol. 2020;39:861-71
64. Dai WL, Lin ZM, Shi ZJ, Wang J. Venous Thromboembolic Events after Total Knee Arthroplasty: Which Patients Are at a High Risk? J Knee Surg. 2020;33:947-57
65. Malcolm TL, Knezevic NN, Zouki CC, Tharian AR. Pulmonary Complications After Hip and Knee Arthroplasty in the United States, 2004-2014. Anesth Analg. 2020;130:917-24
66. Albright JC, Daoud AK. Microfracture and Microfracture Plus. Clin Sports Med. 2017;36:501-7
67. Krych AJ, Saris DBF, Stuart MJ, Hacken B. Cartilage Injury in the Knee: Assessment and Treatment Options. J Am Acad Orthop Surg. 2020;28:914-22
68. Redondo ML, Beer AJ, Yanke AB. Cartilage Restoration: Microfracture and Osteochondral Autograft Transplantation. J Knee Surg. 2018;31:231-8
69. Hinckel BB, Gomoll AH. Autologous Chondrocytes and Next-Generation Matrix-Based Autologous Chondrocyte Implantation. Clin Sports Med. 2017;36:525-48
70. Mistry H, Connock M, Pink J, Shyangdan D, Clar C, Royle P. et al. Autologous chondrocyte implantation in the knee: systematic review and economic evaluation. Health Technol Assess. 2017;21:1-294
71. Majumder J, Minko T. Multifunctional and stimuli-responsive nanocarriers for targeted therapeutic delivery. Expert Opin Drug Deliv. 2021;18:205-27
72. Chen W, Zhou Z, Chen D, Li Y, Zhang Q, Su J. Bone Regeneration Using MMP-Cleavable Peptides-Based Hydrogels. Gels. 2021 7
73. Chen H, Qin Z, Zhao J, He Y, Ren E, Zhu Y. et al. Cartilage-targeting and dual MMP-13/pH responsive theranostic nanoprobes for osteoarthritis imaging and precision therapy. Biomaterials. 2019;225:119520
74. Joshi N, Yan J, Levy S, Bhagchandani S, Slaughter KV, Sherman NE. et al. Towards an arthritis flare-responsive drug delivery system. Nat Commun. 2018;9:1275
75. Park E, Hart ML, Rolauffs B, Stegemann JP, R TA. Bioresponsive microspheres for on-demand delivery of anti-inflammatory cytokines for articular cartilage repair. J Biomed Mater Res A. 2020;108:722-33
76. Zhang HL, Xiong H, Ahmed W, Yao YJ, Wang SQ, Fan CY. et al. Reactive oxygen species-responsive and scavenging polyurethane nanoparticles for treatment of osteoarthritis in vivo. Chem Eng J. 2021 409
77. Zhao C, Chen J, Ye J, Li Z, Su L, Wang J. et al. Structural Transformative Antioxidants for Dual-Responsive Anti-Inflammatory Delivery and Photoacoustic Inflammation Imaging. Angew Chem Int Ed Engl. 2021;60:14458-66
78. Chung MF, Chia WT, Wan WL, Lin YJ, Sung HW. Controlled Release of an Anti-inflammatory Drug Using an Ultrasensitive ROS-Responsive Gas-Generating Carrier for Localized Inflammation Inhibition. Journal of the American Chemical Society. 2015;137:12462-5
79. Li X, Wang X, Liu Q, Yan J, Pan D, Wang L. et al. ROS-Responsive Boronate-Stabilized Polyphenol-Poloxamer 188 Assembled Dexamethasone Nanodrug for Macrophage Repolarization in Osteoarthritis Treatment. Adv Healthc Mater. 2021;10:e2100883
80. He M, Qin Z, Liang X, He X, Zhu B, Lu Z. et al. A pH-responsive mesoporous silica nanoparticles-based drug delivery system with controlled release of andrographolide for OA treatment. Regen Biomater. 2021;8:rbab020
81. Xiong F, Qin ZN, Chen HM, Lan QM, Wang ZT, Lan NH. et al. pH-responsive and hyaluronic acid-functionalized metal-organic frameworks for therapy of osteoarthritis. J Nanobiotechnol. 2020 18
82. Geng Z, Wang XG, Yu YM, Ji LL, Wang J, Liu CS. Attenuating osteoarthritis by a high efficient anti-bone resorption injectable pH-responsive bisphosphonate-conjugated nano-apatite system. Chem Eng J. 2021 420
83. Hu B, Gao F, Li C, Zhang B, An M, Lu M. et al. Rhein laden pH-responsive polymeric nanoparticles for treatment of osteoarthritis. AMB Express. 2020;10:158
84. Kang C, Jung E, Hyeon H, Seon S, Lee D. Acid-activatable polymeric curcumin nanoparticles as therapeutic agents for osteoarthritis. Nanomed-Nanotechnol. 2020 23
85. Jin T, Wu D, Liu XM, Xu JT, Ma BJ, Ji Y. et al. Intra-articular delivery of celastrol by hollow mesoporous silica nanoparticles for pH-sensitive anti-inflammatory therapy against knee osteoarthritis. J Nanobiotechnology. 2020;18:94
86. Kang ML, Kim JE, Im GI. Thermoresponsive nanospheres with independent dual drug release profiles for the treatment of osteoarthritis. Acta Biomater. 2016;39:65-78
87. Yang L, Liu Y, Shou X, Ni D, Kong T, Zhao Y. Bio-inspired lubricant drug delivery particles for the treatment of osteoarthritis. Nanoscale. 2020;12:17093-102
88. Zhao Y, Wei C, Chen X, Liu J, Yu Q, Liu Y. et al. Drug Delivery System Based on Near-Infrared Light-Responsive Molybdenum Disulfide Nanosheets Controls the High-Efficiency Release of Dexamethasone To Inhibit Inflammation and Treat Osteoarthritis. ACS Appl Mater Interfaces. 2019;11:11587-601
89. Jafari S, Mair LO, Chowdhury S, Nacev A, Hilaman R, Stepanov P. et al. Magnetically targeted delivery through cartilage. Aip Adv. 2018 8
90. Subbiah L, Palanisamy S, Thamizhmurasu S, Joseph ABM, Thangavelu P, Ganeshan M. et al. Development of Meloxicam-chitosan magnetic nanoconjugates for targeting rheumatoid arthritis joints: Pharmaceutical characterization and preclinical assessment on murine models. J Magn Magn Mater. 2021 523
91. Kim HJ, Lee SM, Park KH, Mun CH, Park YB, Yoo KH. Drug-loaded gold/iron/gold plasmonic nanoparticles for magnetic targeted chemo-photothermal treatment of rheumatoid arthritis. Biomaterials. 2015;61:95-102
92. Carneiro MFH, Machado ART, Antunes LMG, Souza TE, Freitas VA, Oliveira LCA. et al. Gold-Coated Superparamagnetic Iron Oxide Nanoparticles Attenuate Collagen-Induced Arthritis after Magnetic Targeting. Biol Trace Elem Res. 2020;194:502-13
93. Butoescu N, Seemayer CA, Foti M, Jordan O, Doelker E. Dexamethasone-containing PLGA superparamagnetic microparticles as carriers for the local treatment of arthritis. Biomaterials. 2009;30:1772-80
94. Duan JL, Dong JL, Zhang TT, Su ZY, Ding J, Zhang Y. et al. Polyethyleneimine-functionalized iron oxide nanoparticles for systemic siRNA delivery in experimental arthritis. Nanomedicine. 2014;9:789-801
95. Zhang JB, Zhang M, Lin RC, Du YG, Wang LM, Yao QQ. et al. Chondrogenic preconditioning of mesenchymal stem/stromal cells within a magnetic scaffold for osteochondral repair. Biofabrication. 2022 14
96. Liao AH, Chung HY, Chen WS, Yeh MK. Efficacy of Combined Ultrasound-and-Microbubbles-Mediated Diclofenac Gel Delivery To Enhance Transdermal Permeation in Adjuvant-Induced Rheumatoid Arthritis in the Rat. Ultrasound Med Biol. 2016;42:1976-85
97. Zhu BH, Wang LY, Huang JB, Xiang X, Tang YJ, Cheng C. et al. Ultrasound-triggered perfluorocarbon-derived nanobombs for targeted therapies of rheumatoid arthritis. Journal of Materials Chemistry B. 2019;7:4581-91
98. Li W, Song YL, Liang XY, Zhou Y, Xu M, Lu Q. et al. Mutual-reinforcing sonodynamic therapy against Rheumatoid Arthritis based on sparfloxacin sonosensitizer doped concave-cubic rhodium nanozyme. Biomaterials. 2021 276
99. He Q, Chen J, Yan J, Cai S, Xiong H, Liu Y. et al. Tumor microenvironment responsive drug delivery systems. Asian J Pharm Sci. 2020;15:416-48
100. Karimi M, Ghasemi A, Sahandi Zangabad P, Rahighi R, Moosavi Basri SM, Mirshekari H. et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem Soc Rev. 2016;45:1457-501
101. Wang M, Gao B, Wang X, Li W, Feng Y. Enzyme-responsive strategy as a prospective cue to construct intelligent biomaterials for disease diagnosis and therapy. Biomater Sci. 2022;10:1883-903
102. Lavrador P, Gaspar VM, Mano JF. Stimuli-responsive nanocarriers for delivery of bone therapeutics - Barriers and progresses. J Control Release. 2018;273:51-67
103. Qi H, Yang L, Li X, Sun X, Zhao J, Hou X. et al. Systemic administration of enzyme-responsive growth factor nanocapsules for promoting bone repair. Biomater Sci. 2019;7:1675-85
104. Li N, Qiao Y, Xue L, Xu S, Zhang N. Targeted and MMP-2/9 responsive peptides for the treatment of rheumatoid arthritis. Int J Pharm. 2019;569:118625
105. Tudorachi NB, Totu EE, Fifere A, Ardeleanu V, Mocanu V, Mircea C. et al. The Implication of Reactive Oxygen Species and Antioxidants in Knee Osteoarthritis. Antioxidants (Basel). 2021 10
106. Zhang WQ, Chen L, Xiong Y, Panayi AC, Abududilibaier A, Hu YQ. et al. Antioxidant Therapy and Antioxidant-Related Bionanomaterials in Diabetic Wound Healing. Front Bioeng Biotech. 2021 9
107. Hou W, Ye C, Chen M, Gao W, Xie X, Wu J. et al. Excavating bioactivities of nanozyme to remodel microenvironment for protecting chondrocytes and delaying osteoarthritis. Bioact Mater. 2021;6:2439-51
108. Li Y, Chen M, Yan J, Zhou W, Gao S, Liu S. et al. Tannic acid/Sr(2+)-coated silk/graphene oxide-based meniscus scaffold with anti-inflammatory and anti-ROS functions for cartilage protection and delaying osteoarthritis. Acta Biomater. 2021;126:119-31
109. Wang XY, Zhao HJ, Liu ZC, Wang YT, Lin D, Chen L. et al. Polydopamine nanoparticles as dual-task platform for osteoarthritis therapy: A scavenger for reactive oxygen species and regulator for cellular powerhouses. Chem Eng J. 2021 417
110. Portal-Núñez S, Esbrit P, Alcaraz MJ, Largo R. Oxidative stress, autophagy, epigenetic changes and regulation by miRNAs as potential therapeutic targets in osteoarthritis. Biochem Pharmacol. 2016;108:1-10
111. Ren X, Liu H, Wu X, Weng W, Wang X, Su J. Reactive Oxygen Species (ROS)-Responsive Biomaterials for the Treatment of Bone-Related Diseases. Front Bioeng Biotechnol. 2021;9:820468
112. Zhou M, Wen K, Bi Y, Lu H, Chen J, Hu Y. et al. The Application of Stimuli-responsive Nanocarriers for Targeted Drug Delivery. Curr Top Med Chem. 2017;17:2319-34
113. Li C, Li H, Wang Q, Zhou M, Li M, Gong T. et al. pH-sensitive polymeric micelles for targeted delivery to inflamed joints. J Control Release. 2017;246:133-41
114. Lombardi AF, Ma YJ, Jang H, Jerban S, Tang QB, Searleman AC. et al. AcidoCEST-UTE MRI Reveals an Acidic Microenvironment in Knee Osteoarthritis. Int J Mol Sci. 2022 23
115. Dou C, Li J, He J, Luo F, Yu T, Dai Q. et al. Bone-targeted pH-responsive cerium nanoparticles for anabolic therapy in osteoporosis. Bioact Mater. 2021;6:4697-706
116. Yang M, Li J, Gu P, Fan X. The application of nanoparticles in cancer immunotherapy: Targeting tumor microenvironment. Bioact Mater. 2021;6:1973-87
117. Yan T, He J, Liu R, Liu Z, Cheng J. Chitosan capped pH-responsive hollow mesoporous silica nanoparticles for targeted chemo-photo combination therapy. Carbohydr Polym. 2020;231:115706
118. Montoya C, Du Y, Gianforcaro AL, Orrego S, Yang M, Lelkes PI. On the road to smart biomaterials for bone research: definitions, concepts, advances, and outlook. Bone Res. 2021;9:12
119. Huang B, Tan L, Liu XM, Li J, Wu SL. A facile fabrication of novel stuff with antibacterial property and osteogenic promotion utilizing red phosphorus and near-infrared light. Bioactive Materials. 2019;4:17-21
120. Wei H, Cui J, Lin K, Xie J, Wang X. Recent advances in smart stimuli-responsive biomaterials for bone therapeutics and regeneration. Bone Res. 2022;10:17
121. Zhang M, Hu W, Cai C, Wu Y, Li J, Dong S. Advanced application of stimuli-responsive drug delivery system for inflammatory arthritis treatment. Mater Today Bio. 2022;14:100223
122. Xiang YM, Zhou QL, Li ZY, Cui ZD, Liu XM, Liang YQ. et al. A Z-scheme heterojunction of ZnO/CDots/C3N4 for strengthened photoresponsive bacteria-killing and acceleration of wound healing. J Mater Sci Technol. 2020;57:1-11
123. Mao CY, Xiang YM, Liu XM, Cui ZD, Yang XJ, Li ZY. et al. Repeatable Photodynamic Therapy with Triggered Signaling Pathways of Fibroblast Cell Proliferation and Differentiation To Promote Bacteria-Accompanied Wound Healing. Acs Nano. 2018;12:1747-59
124. Mao CY, Xiang YM, Liu XM, Cui ZD, Yang XJ, Yeung KWK. et al. Photo-Inspired Antibacterial Activity and Wound Healing Acceleration by Hydrogel Embedded with Ag/Ag@AgCl/ZnO Nanostructures. Acs Nano. 2017;11:9010-21
125. Liu QT, Wang L, Jiang H, Chen T, Zhang WG, Jiang T. Application of Magnetic Nano-MR Molecular Imaging Technology in the Relationship Between Meniscus Injury and Knee Joint Movement. Nanosci Nanotech Let. 2020;12:1200-6
126. Mao L, Wu W, Wang M, Guo J, Li H, Zhang S. et al. Targeted treatment for osteoarthritis: drugs and delivery system. Drug Deliv. 2021;28:1861-76
127. Huang H, Lou Z, Zheng S, Wu J, Yao Q, Chen R. et al. Intra-articular drug delivery systems for osteoarthritis therapy: shifting from sustained release to enhancing penetration into cartilage. Drug Deliv. 2022;29:767-91
128. Hu Y, Li XQ, Zhang Q, Gu ZR, Luo Y, Guo JW. et al. Exosome-guided bone targeted delivery of Antagomir-188 as an anabolic therapy for bone loss. Bioactive Materials. 2021;6:2905-13
129. Labens R, Lascelles BD, Charlton AN, Ferrero NR, Van Wettere AJ, Xia XR. et al. Ex vivo effect of gold nanoparticles on porcine synovial membrane. Tissue Barriers. 2013;1:e24314
130. Kumar S, Adjei IM, Brown SB, Liseth O, Sharma B. Manganese dioxide nanoparticles protect cartilage from inflammation-induced oxidative stress. Biomaterials. 2019;224:119467
131. Vedadghavami A, Wagner EK, Mehta S, He T, Zhang C, Bajpayee AG. Cartilage penetrating cationic peptide carriers for applications in drug delivery to avascular negatively charged tissues. Acta Biomater. 2019;93:258-69
132. He T, Zhang C, Vedadghavami A, Mehta S, Clark HA, Porter RM. et al. Multi-arm Avidin nano-construct for intra-cartilage delivery of small molecule drugs. J Control Release. 2020;318:109-23
133. Geiger BC, Wang S, Padera RF Jr, Grodzinsky AJ, Hammond PT. Cartilage-penetrating nanocarriers improve delivery and efficacy of growth factor treatment of osteoarthritis. Sci Transl Med. 2018 10
134. Ouyang Z, Tan T, Liu C, Duan J, Wang W, Guo X. et al. Targeted delivery of hesperetin to cartilage attenuates osteoarthritis by bimodal imaging with Gd2(CO3)3@PDA nanoparticles via TLR-2/NF-kappaB/Akt signaling. Biomaterials. 2019;205:50-63
135. Jiang T, Kan HM, Rajpura K, Carbone EJ, Li Y, Lo KW. Development of Targeted Nanoscale Drug Delivery System for Osteoarthritic Cartilage Tissue. J Nanosci Nanotechnol. 2018;18:2310-7
136. Ai X, Duan Y, Zhang Q, Sun D, Fang RH, Liu-Bryan R. et al. Cartilage-targeting ultrasmall lipid-polymer hybrid nanoparticles for the prevention of cartilage degradation. Bioeng Transl Med. 2021;6:e10187
137. Lu Y, Chen J, Li L, Cao Y, Zhao Y, Nie X. et al. Hierarchical functional nanoparticles boost osteoarthritis therapy by utilizing joint-resident mesenchymal stem cells. J Nanobiotechnology. 2022;20:89
138. Cho HS, Stuart JM, Magid R, Danila DC, Hunsaker T, Pinkhassik E. et al. Theranostic immunoliposomes for osteoarthritis. Nanomed-Nanotechnol. 2014;10:619-27
139. Cho H, Kim BJ, Park SH, Hasty KA, Min BH. Noninvasive visualization of early osteoarthritic cartilage using targeted nanosomes in a destabilization of the medial meniscus mouse model. Int J Nanomed. 2018;13:1215-24
140. Bedingfield SK, Yu F, Liu DD, Jackson MA, Himmel LE, Cho H. et al. Matrix-targeted Nanoparticles for MMP13 RNA Interference Blocks Post-Traumatic Osteoarthritis. bioRxiv. 2020. 2020 01.30.925321
141. Kang LJ, Yoon J, Rho JG, Han HS, Lee S, Oh YS. et al. Self-assembled hyaluronic acid nanoparticles for osteoarthritis treatment. Biomaterials. 2021;275:120967
142. D'Atri D, Zerrillo L, Garcia J, Oieni J, Lupu-Haber Y, Schomann T. et al. Nanoghosts: Mesenchymal Stem cells derived nanoparticles as a unique approach for cartilage regeneration. Journal of Controlled Release. 2021;337:472-81
143. Ma Y, Yang H, Zong X, Wu J, Ji X, Liu W. et al. Artificial M2 macrophages for disease-modifying osteoarthritis therapeutics. Biomaterials. 2021;274:120865
144. Koning GA, Schiffelers RM, Wauben MH, Kok RJ, Mastrobattista E, Molema G. et al. Targeting of angiogenic endothelial cells at sites of inflammation by dexamethasone phosphate-containing RGD peptide liposomes inhibits experimental arthritis. Arthritis Rheum. 2006;54:1198-208
145. Colombo F, Durigutto P, De Maso L, Biffi S, Belmonte B, Tripodo C. et al. Targeting CD34(+) cells of the inflamed synovial endothelium by guided nanoparticles for the treatment of rheumatoid arthritis. J Autoimmun. 2019;103:102288
146. Gui T, Luo L, Chhay B, Zhong L, Wei Y, Yao L. et al. Superoxide dismutase-loaded porous polymersomes as highly efficient antioxidant nanoparticles targeting synovium for osteoarthritis therapy. Biomaterials. 2022;283:121437
147. Whitmire RE, Wilson DS, Singh A, Levenston ME, Murthy N, Garcia AJ. Self-assembling nanoparticles for intra-articular delivery of anti-inflammatory proteins. Biomaterials. 2012;33:7665-75
148. Zhou F, Mei JT, Yang SB, Han XG, Li HJ, Yu ZF. et al. Modified ZIF-8 Nanoparticles Attenuate Osteoarthritis by Reprogramming the Metabolic Pathway of Synovial Macrophages. Acs Appl Mater Inter. 2020;12:2009-22
149. Chen XZ, He C, Sheng TP, Wang ZQ, Xu WF, Dai FR. et al. A magnesium-based coordination container as a multi-drugs co-loaded system for boosting anti-inflammatory therapy in joints. Chem Eng J. 2021 415
150. Yang GZ, Fan MN, Zhu JW, Ling C, Wu LH, Zhang X. et al. A multifunctional anti-inflammatory drug that can specifically target activated macrophages, massively deplete intracellular H2O2, and produce large amounts CO for a highly efficient treatment of osteoarthritis. Biomaterials. 2020 255
151. Xue X, Liu H, Wang S, Hu Y, Huang B, Li M. et al. Neutrophil-erythrocyte hybrid membrane-coated hollow copper sulfide nanoparticles for targeted and photothermal/ anti-inflammatory therapy of osteoarthritis. Composites Part B: Engineering. 2022;237:109855
152. Rothenfluh DA, Bermudez H, O'Neil CP, Hubbell JA. Biofunctional polymer nanoparticles for intra-articular targeting and retention in cartilage. Nat Mater. 2008;7:248-54
153. Ng L, Grodzinsky AJ, Patwari P, Sandy J, Plaas A, Ortiz C. Individual cartilage aggrecan macromolecules and their constituent glycosaminoglycans visualized via atomic force microscopy. J Struct Biol. 2003;143:242-57
154. Martel-Pelletier J, Boileau C, Pelletier JP, Roughley PJ. Cartilage in normal and osteoarthritis conditions. Best Pract Res Clin Rheumatol. 2008;22:351-84
155. Karsdal MA, Michaelis M, Ladel C, Siebuhr AS, Bihlet AR, Andersen JR. et al. Disease-modifying treatments for osteoarthritis (DMOADs) of the knee and hip: lessons learned from failures and opportunities for the future. Osteoarthritis Cartilage. 2016;24:2013-21
156. Wang FX, Guo JW, Wang YL, Hu Y, Zhang H, Chen J. et al. Loss of Bcl-3 delays bone fracture healing through activating NF-?B signaling in mesenchymal stem cells. J Orthop Transl. 2022;35:72-80
157. Pan PP, Chen X, Xing HR, Deng YH, Chen JD, Alharthi FA. et al. A fast on-demand preparation of injectable self-healing nanocomposite hydrogels for efficient osteoinduction. Chinese Chem Lett. 2021;32:2159-63
158. Majda D, Bhattarai A, Riikonen J, Napruszewska BD, Zimowska M, Michalik-Zym A. et al. New approach for determining cartilage pore size distribution: NaCl-thermoporometry. MICROPOROUS AND MESOPOROUS MATERIALS. 2017;241:238-45
159. Wu SL, Wu XM, Wang XH, Su JC. Hydrogels for bone organoid construction: From a materiobiological perspective. J Mater Sci Technol. 2023;136:21-31
160. DiDomenico CD, Goodearl A, Yarilina A, Sun V, Mitra S, Sterman AS. et al. The Effect of Antibody Size and Mechanical Loading on Solute Diffusion Through the Articular Surface of Cartilage. J Biomech Eng. 2017 139
161. Bajpayee AG, Scheu M, Grodzinsky AJ, Porter RM. Electrostatic interactions enable rapid penetration, enhanced uptake and retention of intra-articular injected avidin in rat knee joints. J Orthop Res. 2014;32:1044-51
162. Kumar S, Sharma B. Leveraging Electrostatic Interactions for Drug Delivery to the Joint. Bioelectricity. 2020;2:82-100
163. Krishnan Y, Rees HA, Rossitto CP, Kim SE, Hung HK, Frank EH. et al. Green fluorescent proteins engineered for cartilage-targeted drug delivery: Insights for transport into highly charged avascular tissues. Biomaterials. 2018;183:218-33
164. Brown S, Pistiner J, Adjei IM, Sharma B. Nanoparticle Properties for Delivery to Cartilage: The Implications of Disease State, Synovial Fluid, and Off-Target Uptake. Mol Pharm. 2019;16:469-79
165. Pi Y, Zhang X, Shi J, Zhu J, Chen W, Zhang C. et al. Targeted delivery of non-viral vectors to cartilage in vivo using a chondrocyte-homing peptide identified by phage display. Biomaterials. 2011;32:6324-32
166. Zhu M, Zhong W, Cao W, Zhang Q, Wu G. Chondroinductive/chondroconductive peptides and their-functionalized biomaterials for cartilage tissue engineering. Bioact Mater. 2022;9:221-38
167. Yi W, Zhou H, Li A, Yuan Y, Guo Y, Li P. et al. A NIR-II fluorescent probe for articular cartilage degeneration imaging and osteoarthritis detection. Biomater Sci. 2019;7:1043-51
168. Ye QN, Wang Y, Shen S, Xu CF, Wang J. Biomaterials-Based Delivery of Therapeutic Antibodies for Cancer Therapy. Advanced Healthcare Materials. 2021 10
169. Jiang Y, Li J, Xue X, Yin Z, Xu K, Su J. Engineered extracellular vesicles for bone therapy. Nano Today. 2022;44:101487
170. Liu H, Zhang Q, Wang SC, Weng WZ, Jing YY, Su JC. Bacterial extracellular vesicles as bioactive nanocarriers for drug delivery: Advances and perspectives. Bioactive Materials. 2022;14:169-81
171. Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6:625-35
172. Cao X, Cui Z, Ding Z, Chen Y, Wu S, Wang X. et al. An osteoarthritis subtype characterized by synovial lipid metabolism disorder and fibroblast-like synoviocyte dysfunction. J Orthop Translat. 2022;33:142-52
173. Mathiessen A, Conaghan PG. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther. 2017;19:18
174. Chen L, Ni Z, Huang J, Zhang R, Zhang J, Zhang B. et al. Long term usage of dexamethasone accelerating accelerates the initiation of osteoarthritis via enhancing chondrocyte apoptosis and the extracellular matrix calcification and apoptosis of chondrocytes. Int J Biol Sci. 2021;17:4140-53
175. Scheinman RI, Trivedi R, Vermillion S, Kompella UB. Functionalized STAT1 siRNA nanoparticles regress rheumatoid arthritis in a mouse model. Nanomedicine (Lond). 2011;6:1669-82
176. Wilder RL. Integrin alpha V beta 3 as a target for treatment of rheumatoid arthritis and related rheumatic diseases. Ann Rheum Dis. 2002;61(Suppl 2):ii96-9
177. Utomo L, van Osch GJ, Bayon Y, Verhaar JA, Bastiaansen-Jenniskens YM. Guiding synovial inflammation by macrophage phenotype modulation: an in vitro study towards a therapy for osteoarthritis. Osteoarthritis Cartilage. 2016;24:1629-38
178. Kou L, Huang H, Tang Y, Sun M, Li Y, Wu J. et al. Opsonized nanoparticles target and regulate macrophage polarization for osteoarthritis therapy: A trapping strategy. J Control Release. 2022;347:237-55
179. She P, Bian S, Cheng YQ, Dong SJ, Liu JG, Liu WG. et al. Dextran sulfate-triamcinolone acetonide conjugate nanoparticles for targeted treatment of osteoarthritis. International Journal of Biological Macromolecules. 2020;158:1082-9
180. Zhou F, Han X, Wang L, Zhang W, Cui J, He Z. et al. Associations of osteoclastogenesis and nerve growth in subchondral bone marrow lesions with clinical symptoms in knee osteoarthritis. J Orthop Translat. 2022;32:69-76
181. Fang C, Guo JW, Wang YJ, Li XQ, Zhang H, Cui J. et al. Diterbutyl phthalate attenuates osteoarthritis in ACLT mice via suppressing ERK/c-fos/NFATc1 pathway, and subsequently inhibiting subchondral osteoclast fusion. Acta Pharmacologica Sinica. 2022;43:1299-310
182. Ma L, Zhao X, Liu YB, Wu J, Yang XC, Jin QH. Dihydroartemisinin attenuates osteoarthritis by inhibiting abnormal bone remodeling and angiogenesis in subchondral bone. Int J Mol Med. 2021 47
183. Yajun W, Jin C, Zhengrong G, Chao F, Yan H, Weizong W. et al. Betaine Attenuates Osteoarthritis by Inhibiting Osteoclastogenesis and Angiogenesis in Subchondral Bone. Front Pharmacol. 2021;12:723988
184. Zhai MY, Lu YF, Fu JJ, Zhu YK, Zhao Y, Shang LW. et al. Fourier transform infrared spectroscopy research on subchondral bone in osteoarthritis. Spectrochim Acta A. 2019;218:243-7
185. Foroozandeh P, Aziz AA. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res Lett. 2018 13
186. Juranova J. Illuminating the cellular and molecular mechanism of the potential toxicity of methacrylate monomers used in biomaterials. Drug Chem Toxicol. 2020;43:266-78
187. Abou-Saleh H, Younes N, Rasool K, Younis MH, Prieto RM, Yassine HM. et al. Impaired Liver Size and Compromised Neurobehavioral Activity are Elicited by Chitosan Nanoparticles in the Zebrafish Embryo Model. Nanomaterials-Basel. 2019 9
188. Wang YB, Zhou JR, Liu L, Huang CJ, Zhou DQ, Fu LL. Characterization and toxicology evaluation of chitosan nanoparticles on the embryonic development of zebrafish, Danio rerio. Carbohyd Polym. 2016;141:204-10
189. Lin TH, Pajarinen J, Sato T, Loi F, Fan CC, Cordova LA. et al. NF-kappa B decoy oligodeoxynucleotide mitigates wear particle-associated bone loss in the murine continuous infusion model. Acta Biomater. 2016;41:273-81
190. Ahmed U, Ahmed R, Masoud MS, Tariq M, Ashfaq UA, Augustine R. et al. Stem cells based in vitro models: trends and prospects in biomaterials cytotoxicity studies. Biomed Mater. 2021 16
191. Chen SS, Chen X, Geng Z, Su JC. The horizon of bone organoid: A perspective on construction and application. Bioactive Materials. 2022;18:15-25
192. Li MY, Li Y, Li SQ, Jia L, Wang HM, Li M. et al. The nano delivery systems and applications of mRNA. Eur J Med Chem. 2022 227
193. Ruenraroengsak P, Tetley TD. Differential bioreactivity of neutral, cationic and anionic polystyrene nanoparticles with cells from the human alveolar compartment: robust response of alveolar type 1 epithelial cells. Part Fibre Toxicol. 2015 12
194. Ren HW, He YW, Liang JM, Cheng ZK, Zhang M, Zhu Y. et al. Role of Liposome Size, Surface Charge, and PEGylation on Rheumatoid Arthritis Targeting Therapy. Acs Appl Mater Inter. 2019;11:20304-15
195. Brown S, Pistiner J, Adjei IM, Sharma B. Nanoparticle Properties for Delivery to Cartilage: The Implications of Disease State, Synovial Fluid, and Off-Target Uptake. Mol Pharmaceut. 2019;16:469-79
196. Suk JS, Xu QG, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliver Rev. 2016;99:28-51
197. Makarczyk MJ, Gao Q, He Y, Li Z, Gold MS, Hochberg MC. et al. Current Models for Development of Disease-Modifying Osteoarthritis Drugs. Tissue Eng Part C Methods. 2021;27:124-38
198. Lampropoulou-Adamidou K, Lelovas P, Karadimas EV, Liakou C, Triantafillopoulos IK, Dontas I. et al. Useful animal models for the research of osteoarthritis. Eur J Orthop Surg Traumatol. 2014;24:263-71
199. Yamada EF, Salgueiro AF, Goulart ADS, Mendes VP, Anjos BL, Folmer V. et al. Evaluation of monosodium iodoacetate dosage to induce knee osteoarthritis: Relation with oxidative stress and pain. Int J Rheum Dis. 2019;22:399-410
Author contact
![]() Corresponding authors: Yan Hu, E-mail: xjhuyanedu.cn; Zhen Geng, E-mail: nanboshan1987com; Jiacan Su, E-mail: drsujiacancom.
Corresponding authors: Yan Hu, E-mail: xjhuyanedu.cn; Zhen Geng, E-mail: nanboshan1987com; Jiacan Su, E-mail: drsujiacancom.
 Global reach, higher impact
Global reach, higher impact