13.3
Impact Factor
Theranostics 2023; 13(2):621-638. doi:10.7150/thno.72297 This issue Cite
Research Paper
FAM3C in circulating tumor-derived extracellular vesicles promotes non-small cell lung cancer growth in secondary sites
1. Cancer Science Institute of Singapore, National University of Singapore, Singapore 117599.
2. Department of Pharmacology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
3. Department of Pathology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
4. NUS Centre for Cancer Research (N2CR), Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
5. Division of Cellular and Molecular Research, National Cancer Centre, Singapore.
6. Department of Haematology-Oncology, National University Cancer Institute, National University Health System, Singapore.
7. Department of Pharmacy, Faculty of Science, National University of Singapore, Singapore.
8. INSERM Unit 1186, Comprehensive Cancer Center, Institut Gustave Roussy, Villejuif, France.
9. Nanyang Technological University, 60 Nanyang Drive, Singapore 637551, Singapore.
10. Zhejiang Provincial Laboratory of Life Sciences and Biomedicine, Key Laboratory of Structural Biology of Zhejiang Province, School of Life Sciences, Westlake University, China.
11. Institute of Basic Medical Sciences, Westlake Institute for Advanced Study, China.
12. Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
*Equal contributions
Abstract
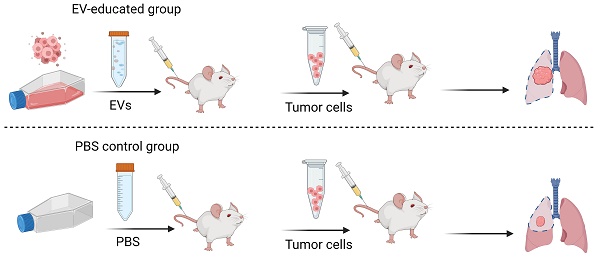
Rationale: Metastasis is a complex process with a molecular underpinning that remains unclear. We hypothesize that cargo proteins conducted by extracellular vesicles (EVs) released from tumors may confer growth and metastasis potential on recipient cells. Here, we report that a cytokine-like secreted protein, FAM3C, contributes to late-stage lung tumor progression.
Methods: EV protein profiling was conducted with an unbiased proteomic mass spectrometry analysis on non-small cell lung cancer (NSCLC) and normal lung fibroblast cell lines. Expression of FAM3C was confirmed in a panel of NSCLC cell lines, and correlated to the invasive and metastatic potentials. Functional phenotype of endogenous FAM3C and tumor-derived EVs (TDEs) were further investigated using various biological approaches in RNA and protein levels. Metastasis potential of TDEs secreted by FAM3C-overexpressing carcinoma cells was validated in mouse models.
Results: Transcriptomic meta-analysis of pan-cancer datasets confirmed the overexpression of FAM3C - a gene encoding for interleukin-like EMT inducer (ILEI) - in NSCLC tumors, with strong association with poor patient prognosis and cancer metastasis. Aberrant expression of FAM3C in lung carcinoma cells enhances cellular transformation and promotes distant lung tumor colonization. In addition, higher FAM3C concentrations were detected in EVs extracted from plasma samples of NSCLC patients compared to those of healthy subjects. More importantly, we defined a hitherto-unknown mode of microenvironmental crosstalk involving FAM3C in EVs, whereby the delivery and uptake of FAM3C via TDEs enhances oncogenic signaling - in recipient cells that phenocopies the cell-endogenous overexpression of FAM3C. The oncogenicity transduced by FAM3C is executed via a novel interaction with the Ras-related protein RalA, triggering the downstream activation of the Src/Stat3 signaling cascade.
Conclusions: Our study describes a novel mechanism for FAM3C-driven carcinogenesis and shed light on EV FAM3C as a driver for metastatic lung tumors that could be exploited for cancer therapeutics.
Keywords: FAM3C, non-small cell lung carcinoma, tumor-derived extracellular vesicles, predictive biomarker, tumor metastasis.
 Global reach, higher impact
Global reach, higher impact