13.3
Impact Factor
Theranostics 2023; 13(1):161-196. doi:10.7150/thno.79639 This issue Cite
Review
Recent advances in the medical applications of hemostatic materials
1. Department of the General Surgery, Jilin University Second Hospital, Changchun, China.
2. Department of Operating Theater and Anesthesiology, Jilin University Second Hospital, Changchun, China.
3. Department of the Dermatology, Jilin University Second Hospital, Changchun, China.
* Equally contributed to this work.
Received 2022-10-7; Accepted 2022-11-14; Published 2023-1-1
Abstract
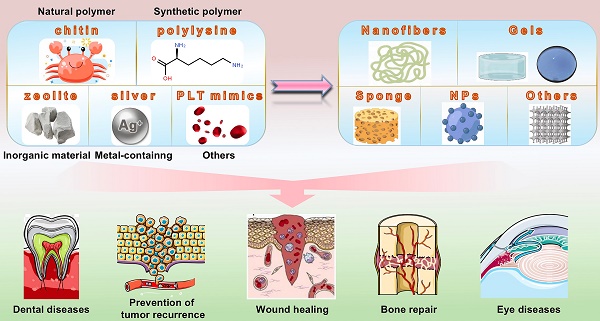
Bleeding caused by trauma or surgery is a serious health problem, and uncontrollable bleeding can result in death. Therefore, developing safe, effective, and convenient hemostatic materials is important. Active hemostatic agents currently used to investigate the field of hemostasis are divided into four broad categories: natural polymers, synthetic polymers, inorganic materials, and metal-containing materials. Hemostatic materials are prepared in various forms for wound care applications based on the active ingredients used. These materials include nanofibers, gels, sponges, and nanoparticles. Hemostatic materials find their applications in the field of wound care, and they are also used for hemostasis during malignant tumor surgery. Prompt and effective hemostasis can reduce the possibility of the spread of tumor cells with blood. This review discusses the outcomes of current research conducted in the field and the problems persisting in the field of developing hemostatic materials. The review also presents a platform for the further development of hemostatic materials. Bleeding caused by trauma or surgery is a serious health problem, and uncontrollable bleeding can result in death. Therefore, developing safe, effective, and convenient hemostatic materials is important. Active hemostatic agents currently used to investigate the field of hemostasis are divided into four broad categories: natural polymers, synthetic polymers, inorganic materials, and metal-containing materials. Hemostatic materials are prepared in various forms for wound care applications based on the active ingredients used. These materials include nanofibers, gels, sponges, and nanoparticles. Hemostatic materials find their applications in the field of wound care, and they are also used for hemostasis during malignant tumor surgery. Prompt and effective hemostasis can reduce the possibility of the spread of tumor cells with blood. This review discusses the outcomes of current research conducted in the field and the problems persisting in the field of developing hemostatic materials. The review also presents a platform for the further development of hemostatic materials.
Keywords: hemostatic materials, polymer, nanofibers, hydrogels, medical use.
Introduction
Post-traumatic hemorrhage is the second leading cause of human trauma-related deaths, accounting for 15% of all trauma deaths [1, 2]. The amount of blood in a normal person remains relatively constant during the life of the person, and the blood volume is proportional to the person's body weight. The amount of blood in a normal human has been reported to be approximately 7-8% of the total body weight [3]. When blood loss reaches 20% of total blood volume, it becomes difficult to maintain normal blood volume and blood pressure. Symptoms such as dizziness and reduced urine output are observed under these conditions. When the extent of blood loss exceeds 40% in a short time, life-threatening conditions develop if blood is not transfused in time [4]. Therefore, it is important to control the extent of bleeding. Cessation of bleeding from small wounds is realized through a series of events such as vasoconstriction, platelet thrombosis, and blood clotting [5].
The rupture of blood vessels can result in vasoconstriction, reducing blood flow to the injured site. At the same time, some hemostatic agents, such as thromboxane, are released into the bloodstream. These promote the local constriction of small arteries [6]. Injury exposes subendothelial collagen and a small number of platelets adhering to the damaged endothelium. The release of adenosine diphosphate (ADP) from erythrocytes and thrombin generated during coagulation can trigger the induction of the functions of platelets. The activated platelets promote the release of endogenous ADP and thromboxane 2 (TXA2), resulting in the activation and recruitment of a large number of platelets. This results in the formation of platelet thrombi that plug wounds, and the process is known as primary hemostasis [7]. The process of coagulation cascade can activate multiple coagulation factors in a certain sequence following two pathways (endogenous and exogenous). Eventually, soluble fibrinogen in plasma is converted into insoluble fibrin, which interweaves to reinforce the hemostatic plug. The endogenous coagulation pathway is initiated by the activation of a surface factor (Factor XII) by damaged endothelial cells, whereas the exogenous coagulation pathway is activated following the exposure of a tissue factor (Factor III) to blood. The two pathways converge by activating the respective downstream coagulation factors. This results in the activation of the Stuart Prower factor (Factor X) pathway, which activates thrombinogen. Thrombin could activate the fibrin stabilizing factor (Factor XIII), which, in the presence of Ca2+, could cause the fibrin monomers to polymerize with each other, resulting in the formation of water-insoluble multimeric clots. The platelet emboli formed during the primary hemostasis phase are reinforced under these conditions [8, 9]. Under normal circumstances, rapid hemostasis can be achieved in the presence of a large number of local adherent platelets and clotting factors. Thrombotic disorders are observed when the number of platelets increases beyond a certain range [10]. However, severe post-traumatic bleeding requires aggressive human intervention to promote coagulation. Uncontrolled post-traumatic hemorrhage is one of the leading causes of preventable death. Massive blood loss can sometimes lead to unavoidable serious complications requiring amputation. Severe infection may also occur [11].
It is important to develop safe, efficient, and stable hemostatic materials urgently. Hemostatic materials can be used to stop bleeding, and the process is realized following various ways. For example, gauze, a traditional hemostatic material, can be used to stop bleeding under conditions of physical compression (realized using pressure wraps) [12]. Hemostatic sponges function as efficient absorbents. These swell when local blood gets absorbed, resulting in local compression. Polyurethane (PU) sponges, which are used for local compression, are used as nasal tamponades [13]. Several hemostatic materials can activate the hemostatic pathways in the human body in various ways. For example, chitosan sponges can promote hemostasis by activating and aggregating platelets exploiting the negative charges on the chitosan surface [14]. At the same time, the absorbent property of the sponge helps increase the local concentration of platelets and coagulation factors in the wound. This results in the promotion of the coagulation cascade. Several hemostatic materials can also be loaded with hemostatic drugs. It has been observed that the thrombin-loaded gelatin sponges that have been used to study a porcine liver hemorrhage model can be used to achieve excellent hemostasis [15]. Nanofibers have been found to be excellent drug carriers, and these can be used to stop bleeding and promote the process of wound healing [16, 17]. There are many commercially available hemostatic materials, such as Hemcon® (chitosan-based), TraumaDEX® (Potato starch-based microparticles), and QuickClot® (zeolite-based), which can efficiently reduce the bleeding time to a certain extent. These agents exhibit antimicrobial activity but also suffer from poor biodegradability. These cannot be used to treat complex wounds and are difficult to remove.
Natural polymers, synthetic polymers, inorganic materials, and metal-containing materials are primarily used to realize hemostasis (Table 1). Natural polymers used in the field of medicine for hemostasis include chitosan, cellulose, hyaluronic acid (HA), alginate, collagen, and fibrin. Peptides, polyethylene glycol (PEG), polyurethane (PU), polyvinyl alcohol (PVA), polycaprolactone (PCL), and poly (ethylene oxide) (PEO) are used to prepare the synthetic hemostatic materials. Most studies on inorganic hemostatic materials have focused on zeolites, mesoporous silica, and graphene. Calcium, zinc, and silver are primarily used to prepare metal-containing hemostatic materials. It is challenging to process these materials containing different types of active ingredients and develop different chemically modified hemostatic materials that exhibit powerful wound healing properties in addition to the basic hemostatic properties. The type of hemostatic material is strongly related to its function. Nanocellulose, hydrogels, nanoparticles, etc., are widely used for the development of hemostatic materials. Chitosan hydrogels, chitosan nanoparticles, and chitosan hemostatic sponges, which can be used efficiently to treat a wide range of wounds, are different types of chitosan-based hemostatic materials. Hemostatic materials in the form of hydrogels tend to promote clotting by thickening the blood and increasing the concentration of platelets and clotting factors. These materials exert a local compression effect by expanding in size after absorbing water. However, hydrogels are notoriously difficult to remove and cannot be efficiently used to treat complex, deep wounds. Nanoparticles can be used effectively to realize the hemostasis of deep and irregular wounds. However, these are difficult to remove. Different forms of hemostatic materials prepared using the same active ingredient can be used to treat different types of wounds. For example, Hemcon® and Chitoflex® are lyophilized chitosan-based materials used to treat post-traumatic or surgical bleeding. It should be noted that Chitoflex® is more flexible than Hemcon®, and the former is more suitable than the latter to stop bleeding occurring near joints. The composite material is strong and suitable for treating complex and diverse wounds.
This review presents the mechanisms through which different substances promote coagulation. We also present the results obtained when various nanomaterial-based hemostatic materials were studied. Finally, we have focused on the pros and cons of the diverse forms of hemostatic materials and have reported the application prospects and importance of hemostatic materials in the field of healthcare. The data presented herein provide an outlook for future research directions.
Chemical composition of hemostatic materials
Natural polymers, synthetic polymers, inorganic materials, and metal-containing materials are currently used to prepare hemostatic materials. In this section, we present the mechanisms following which these substances accelerate wound hemostasis.
Natural polymers
The widely available natural polymeric hemostatic materials currently in use are polysaccharides and collagens. Polysaccharides exhibit excellent properties. These are non-toxic to human tissues. These are non-irritants, non-immunogenic, non-hemolytic, histocompatible, and naturally degradable. These materials are widely used to manufacture artificial skin [45, 46], absorbable sutures [47, 48], and drug carriers [49-51]. Well-studied polysaccharide-based hemostatic materials include chitosan, cellulose, HA, and alginate.
Chitosan is a natural polysaccharide extracted from the shells of crustaceans. Chitosan can increase the intracellular calcium ion concentration of platelets, facilitating platelet aggregation and mutual adhesion [52]. Activation of platelets results in the production of a large number of negatively charged phosphatidylserine units on the surface, which can interact electrostatically with positively charged chitosan, increasing the viscosity of platelets. This results in the rapid formation of thrombi. The positive charge on the surface of chitosan polymers interacts with receptors containing neuraminic acid residues (negative charge) on the surface of red blood cells to promote aggregation [53]. Chitosan is abundant in nature and can be modified to improve hemostatic effects and increase the application prospects of chitosan-based materials. The cationic properties of chitosan significantly influence the function of the material as a coagulant of red blood cells and platelets. Tan et al. reacted N,N,N-trimethyl chitosan with chloroacetyl chloride to obtain chitosan derivatives. Subsequently, they reacted tricyclohexylphosphine with triphenylphosphine to obtain highly cationic chitosan derivatives [54]. The introduction of quaternary ammonium groups into chitosan improved the blood cell adhesion and antibacterial properties of the material [55]. Wibel et al. formulated thiol chitosan by incorporating L-cysteine with amide bonds. This helped improve the mucosal adhesion and penetration ability of the materials [56]. Jayakumar et al. synthesized phosphorylated chitosan by grafting mono(2-methacryloyloxyethyl) ester of phosphoric acid into chitosan to improve the stability and antimicrobial properties of the materials [57]. In addition to direct modification, Schnurch et al. grafted ethylenediaminetetraacetic acid (EDTA) onto chitosan polymers. The chelation of magnesium ions resulted in the generation of antimicrobial effects [58]. Alkylated chitosan units are hydrophobic, and these can be inserted into blood cell membranes to promote blood clotting [59]. The hydrophobically modified surface hinders the process of local fluid exchange between wounds and the external environment. This helps prevent bacterial and other contamination of the wounds. Chitosan-based hemostatic materials have been extensively studied in rat wound models, rat femoral artery trauma models, and rat liver injury models.
Various components and forms of hemostatic materials used to realize hemostasis.
| Material | Form | Testing model | Efficacy | Ref. |
|---|---|---|---|---|
| Chitosan | Chitosan/PVA/ZnO nanofiber mat | Diabetic rabbit subcutaneous wound model | Excellent antibacterial properties observed; promotes the production of granulation tissues and wound healing at the injured tissue. | [18] |
| Chitosan/silk fibroin/ Mg (OH)2 nanoparticles hydrogel | In vitro | The composite material exhibits favorable biocompatibility and can effectively inhibit the growth of Pseudomonas aeruginosa. | [19] | |
| O-nitrobenzyl alcohol/ carboxymethyl chitosan hydrogel | Rat liver injury model | Exhibits great adhesion and sealing properties; hemostasis is achieved within a short time period; decreases bleeding volume. | [20] | |
| Chitosan/Diatom-Biosilica Aerogel | Rat hemorrhage model. | Exhibits excellent water absorption properties; promotes the aggregation of red blood cells and adhesion and activation of platelets; accelerates hemostasis. | [21] | |
| Alkylated chitosan/ diatom-silica sponge | Rat tail transect model | Exhibits excellent biocompatibility; induces the activation, deformation, and aggregation of red blood cells and platelets, thus activating the intrinsic coagulation pathway and rapidly stopping bleeding. | [14] | |
| Quaternized chitosan/CNT cryogel | Mouse-liver injury model and mouse-tail amputation model | Demonstrates strong mechanical strength, superior blood absorption rate, and remarkably high blood cell and platelet adhesion and activation capacity. | [22] | |
| Cellulose/chitosan sponge | Mouse tail amputation model | Exhibits excellent absorbent and rapid shape recovery properties; exhibits effective antibacterial properties; cellulose/chitosan sponges have been studied in vitro and in vivo to achieve rapid hemostasis. | [23] | |
| HA | GelMA/HA-NB hydrogel | Porcine carotid artery hemorrhage and heart injury model | Gels quickly and adheres tightly to seal bleeding arteries and heart walls; withstands blood pressure of up to 290 mmHg, making it a superior wound sealant. | [24] |
| Gelatin | Gelatin nanofiber sponge | Rabbit ear artery injury model and liver trauma model | The composite sponge is characterized by low density, high surface area, and excellent fluid absorption capacity; aggregates and activates platelets in large quantities; accelerates the formation of platelet emboli; activates the extrinsic and intrinsic coagulation pathways. | [25] |
| Gelatin sponge with thrombin | Pig liver bleeding model | Easy to apply and can stop bleeding quickly to reduce the amount of blood loss realized. | [15] | |
| Peptide | Self-assembling peptide RADA16 nanofiber | Mouse diabetic wound model | Accelerates wound closure, collagen deposition, and tissue remodeling in healthy and diabetic mice. | [26] |
| Polylysine/thiolated chitosan hydrogel | Rat liver injury model | Gels quickly and exhibits excellent tissue adhesion properties. | [27] | |
| PEG | Tetra-PEG hydrogel | Rabbit liver hemorrhage model | Exhibits rapid gelation properties, strong tissue adhesion ability, high mechanical strength, rapid degradation properties, and controlled dissolution properties. | [28] |
| TRAP-loaded starch/PEG sponge | Rat femoral artery and liver hemorrhage models | The superior water absorption property of the sponge helps absorb plasma, concentrate blood cells, and enhance blood clotting. Post water absorption, the shape-fixed sponge exhibits sufficient mechanical strength to apply pressure to the wounds to promote hemostasis. | [29] | |
| PU | PU nanoparticle | Rat neurosurgical model | Hemostatic agents containing PU NPs exhibit anti-inflammatory and neuroprotective property in vivo; promotes procedures involving fragile tissues or organs. | [30] |
| PU based adhesive | Porcine vascular suture model | Applied to porcine vascular sutures to effectively prevent perioperative suture line bleeding; does not produce an inflammatory response that affects healing. | [31] | |
| PVA | PVA/oxidized betaine polysaccharide Schiff base aerogel | Whole skin defect model | Exhibits promising antimicrobial and hemostatic properties that help reduce inflammation; promotes angiogenesis; accelerates epithelialization to accelerate wound healing. | [32] |
| PVF sponge with pore-filled chitosan hydrogel, CaCO3 particle, and silica particle | Wistar rat liver hemorrhage model | The application of PVF-based composites significantly reduced the bleeding time (78.3-90.4%) compared to the commercial hemostatic product Celox™. | [33] | |
| PCL | PCL/gelatin nanofibers loaded with 1% lawsone | Rat wound model | PCL/Gel nanofibers loaded with 1% lawsone significantly improves the cell attachment and proliferation properties; promotes wound healing. | [34] |
| CNT-coated PCL nanofiber | Rat calvarium bone defect animal model | Inhibits inflammatory response; promotes angiogenesis; drives MSC adhesion; promotes bone regeneration. | [35] | |
| PLGA | PLGA/PEG/ SiO2 nanofiber | Porcine liver laceration model | The composite nanofibers can be deposited directly onto the surgical site in the form of a solid fiber pad for ease of application. It exhibits excellent biocompatibility and sealing properties. | [36] |
| PEO | PEO/chitosan nanofiber mat | Porcine liver laceration model | Model experimental studies have confirmed the superior biocompatibility and hemostatic properties of PEO/chitosan nanofiber mats, which can be used to repair liver damage and achieve a high biodegradation rate. | [37] |
| Silica | Silica/chitosan sponges | Mouse tail amputation model | The silica/chitosan sponges are characterized by promising water absorption properties and mechanical strength; these help achieve excellent hemostasis in the mouse tail amputation model. | [14] |
| Silica nanoparticles for loading tannic acid | Mouse tail amputation model | Superior antibacterial properties and hemostatic properties. | [38] | |
| Chitosan/hydrocaffeic acid/SNP | Rat femoral artery trauma model | It can form a network structure with fibrin in the blood to improve the mechanical strength of blood clots and promote hemostasis and wound healing ability. | [39] | |
| Graphene | GO/gelatin aerogel | In vitro | Exhibits good water absorption properties; negative charge on its surface can promote hemostasis; red blood cells adhere to its surface, facilitating the formation of a stable fibrin network that promotes hemostasis. | [40] |
| Graphene/MMT composite sponge | Rabbit artery injury model | The composite sponge exhibits good water absorption capacity and can be used to rapidly stop bleeding in a rabbit arterial injury model without remaining in the blood vessels and causing thrombosis. | [41] | |
| CNF/copper-containing glass aerogel | In vitro | CNF/copper-containing glass aerogel can promote angiogenesis effectively; exerts excellent bactericidal effects, which promote hemostasis and wound healing. | [42] | |
| Platelet-derived nanovesicle | Natural platelet-derived nanoparticles prepared by hypotonic ultrasonication | Mouse tail transection bleeding model | The natural, biocompatible platelet-derived nanoparticles can be used to achieve hemostasis when injected; inflammatory reactions are not caused. | [43] |
| Platelet mimic | Liposomes with surface-decorated collagen and vWF-binding peptide | Mouse tail transection bleeding model | The surface of the liposomes is modified using a variety of mimetic peptides, which can effectively mimic the processes of platelet adhesion and aggregation to form platelet thrombi and achieve hemostasis. | [44] |
(PVA: polyvinyl alcohol; CNT: carbon nanotube; HA: hyaluronic acid; GelMA: gelatin methacrylate; PEG: polyethylene glycol; TRAP: thrombin receptor agonist peptides; PU: polyurethane; PVA: polyvinyl alcohol; PVF: poly(vinyl formal); PCL: polycaprolactone; PLGA: poly(lactic-co-glycolic acid); PEO: poly (ethylene oxide); SNP: silicon dioxide-based nanoparticle; GO: graphene oxide; CNF: carbon nanofiber)
Commercially available chitosan-based hemostatic materials (e.g., Hemcon®, Chitoflex®, and CELOX®) have exhibited excellent efficacy in trauma and surgical hemostatic applications. Chitosan-based hemostatic materials exhibit limited hemostatic effects and cannot be used to effectively control extensive bleeding [60]. These limit the application prospects of these materials.
Cellulose is a macromolecular polysaccharide that is present in abundance in plants and bacteria. It is used for hemostasis and can be used to absorb water quickly. Cellulose fills the site of bleeding, providing physical compression to stop bleeding [61]. Cellulose contains acidic carboxyl functional groups, which can bind with Fe3+ ions in hemoglobin. This results in the formation of brown gels that help close capillary ends and stop bleeding [62]. It can potentially activate platelets to accelerate hemostasis [63]. Oxidized cellulose (OC) is a cellulose derivative that is synthesized following the oxidation of TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxy). The method helps increase the anionic carboxyl group content on the cellulose surface. A large number of hydroxyl groups are oxidized to carboxyl groups to reduce surface water repellency [64, 65]. OC introduces a large number of carboxyl groups on the surface, lowering the local pH and causing non-specific platelet aggregation. This can potentially result in thrombosis and the promotion of hemostasis. The process can help in wound healing. The relatively low pH of OC helps inhibit the growth of a wide range of gram-positive and gram-negative microorganisms [66]. Cellulose is commonly prepared as hemostatic material together with other active coagulation ingredients. For example, OC-based nanofibers loaded with thrombin exhibit excellent biocompatibility and rapid water absorption properties. These can be used to study models of rat liver injury to significantly shorten clotting time and reduce bleeding volume [67]. The OC-based commercial hemostatic material Surgicel® is particularly effective in stopping bleeding during surgery. It can also be used to stop small arterial bleeding. The material is used during neurosurgery to reduce intracranial hematomas in bleeding patients [33]. However, the acidic environment can potentially generate an inflammatory response in tissues, prolonging the wound-healing process. This may also damage peripheral nerves. The slow degradation of cellulose-based hemostatic materials significantly hinders the application prospects of the materials. These may cause foreign body reactions and adhere to the wound area, interfering with epithelial growth and resulting in delayed healing [68, 69].
HA, an acidic mucopolysaccharide, is a natural component of the body with excellent biocompatibility [70]. HA contains a large number of hydroxyl groups and can form hydrogen bonds with water molecules to absorb water and increase the concentration of platelets and clotting factors [71]. It is also involved with the process of releasing inflammatory cytokines (such as TNF-α, IL-1β, and IL-8) [72]. It directs the processes of fibroblast invasion and proliferation to promote wound healing [71, 73]. It should be noted that HA is easily soluble in water and has a short residence time in tissues. The mechanical properties of HA were improved, and its degradation rate was controlled following the processes of cross-linking [74, 75], esterification [76, 77], molecular modifications [78, 79], and compounding [80, 81]. The addition of a certain amount of hydrazinolyzed 3,3'-dithiopropionic acid (DTP) to an aqueous solution of HA to obtain a mercapto-hyaluronic acid derivative (HA-DTPH) resulted in a decrease in the rate of degradation of HA and an improvement in the wet tissue [82, 83]. Some researchers used tyramine to modify HA by introducing phenol groups to the backbone of HA. This resulted in the production of hydrogels with controlled mechanical strength [84]. Hydrogels based on HA-tyrosine (HA-Tyr) prepared following the process of enzymatic cross-linking could improve the extent of hemostasis achieved and effectively increase the cell adhesion property of the material [74, 85-87]. Bagheri et al. studied the efficacy of phenolic hydroxy HA (HA-Ph)-based hydrogels (characterized by different degrees of cross-linking) on the cell adhesion and proliferation properties of the materials [75]. The drawbacks of using modified HA as a hemostatic material lie in the difficulty of removing the material when necessary. HA-based hemostatic materials have been examined in the mouse model of total skin injury, the arterial hemorrhage model, the liver injury model, and the porcine left ventricular penetration model [24].
Alginate, similar to HA, is highly absorbent and can increase the concentration of platelets and coagulation factors. Alginate can increase the local Ca2+ concentration and accelerate the coagulation process [88, 89]. Furthermore, alginate exhibits cellular chemotactic activity, which promotes wound repair. Alginate oligomers (AOS), which retain the physical and chemical properties of the parent polymer and can be modified, are the focus of current research [90, 91]. The hydroxyl group in the glyoxylate units can be transformed into an aldehyde group in the presence of strong oxidizing agents. This transformation helps to improve the degradation properties of the material. AOS can cross-link with various metal ions, such as Ca2+, to form gels that improve hemostasis and viscosity [92-95]. Alginate is not designed to treat dry wounds with hard crusts. Numerous studies have been conducted on the diabetic foot ulcer model, and the alginate-based commercial product Algosteril® has been approved clinically for wound care dressing.
Collagen, a porous material, expands following blood absorption. It forms a mesh for blood clotting and sealing cracks in blood vessels or wounds. It can activate platelets and induce the release of physiological aggregating agents such as ADP and TXA2, resulting in the irreversible aggregation of platelets. It also functions as a platelet adhesion matrix that helps form blood clots for hemostasis [96-98]. Collagen promotes the process of proliferation during the wound repair process [99]. Collagen tends to cause allergic reactions, exhibits poor adhesion properties, and cannot be easily degraded, and these properties hinder the practical applications of the material. Partial hydrolysis of collagen results in the production of gelatin, and it can be used to address (to a certain extent) the safety issues posed by collagen [100]. Gelatin has been processed in various forms for clinical applications, and the most commonly used gelatin-based material for hemostasis is the gelatin sponge [101, 102]. Slezak et al. compared the hemostatic effects exerted by the collagen and thrombin-containing powder agent Hemoblast® and the flowable gelatin-thrombin matrix Floseal® in a porcine liver hemorrhage model. The results revealed that the gelatin-the thrombin-based system was more efficient than the other model in reducing bleeding. The ease of applicability of the former was better than that of the latter [15]. Wang et al. used gold/silver clusters to functionalize gelatin sponges that exhibited good antibacterial properties [103]. Additionally, gelatin and collagen are popular materials for wound healing because they are biocompatible and biodegradable. Commercial gelatin-based hemostatic products, such as Floseal® and Endo Avitene®, exhibit excellent hemostatic efficacy in clinical settings. FloSeal® is a commercial hemostatic material consisting of animal collagen, glutaraldehyde, and human coagulation enzymes in fibrin glue. It is currently used to treat obstetric bleeding [104], nasal bleeding [105, 106], and joint bleeding [107]. Moreover, it is also used for intracranial tumor removal [108]. Collagen and gelatin are prone to allergic reactions and are liable to degradation.
Fibrin significantly affects the coagulation process and can form insoluble fibrin multimers under the effect of thrombin and Ca2+. Multimers trap platelets and blood cells to form blood clots [109, 110]. It can also stimulate the growth of capillary endothelial cells and fibroblasts, and the process promotes wound healing [111]. Tissuccol®, a commercial fibrin glue, produced satisfactory hemostatic results when used to treat upper gastrointestinal bleeding and splenic rupture. It could also be used to treat hepatectomy [81] efficiently. Fibrin-based materials cannot be used to stop bleeding from large arteries and veins, and this can be attributed to the long clotting time. The use of fibrin-based materials may result in animal or human blood-borne infections [112].
Natural polymers can facilitate platelet aggregation and activation through various mechanisms to achieve rapid hemostasis and promote wound healing. The aldehyde group in oxidized dextran can serve as a cross-linking agent for the preparation of hydrogels and sponges, and it can also cross-link with amino groups on the tissue surface to promote the local adhesion of hemostatic materials [113]. The high price, poor mechanical properties, and tendency of natural polymers to cause allergic reactions limit the development of natural polymer-based hemostatic materials.
Synthetic polymers
It is easier to functionalize synthetic polymers synthesized following physical or chemical methods than natural polymers. Hence, these materials have attracted immense attention in the field of research of hemostatic material research.
Solutions made of self-assembling peptides can gelate rapidly with blood, and the formed hydrogel can seal wounds. Thus, such hydrogels can be used as hemostatic agents. The molecules in the peptide solution can trigger the processes of self-assembly and gelation when they encounter charged amino acid molecules in the blood. When applied to a wound as hemostatic material, the peptide can rapidly absorb the surrounding fluid, increase the local concentration of platelets and clotting factors in the wound, initiate the peptide self-assembly process, and form a nanofibrous scaffold to promote the exudation of red blood cells and blood from wounds [114]. Polylysine contains abundant primary amine groups that are cationic in neutral aqueous solutions. The ionic attraction between these amino groups and the residues exposed under conditions of tissue damage demonstrate the potential tissue adhesion property of polylysine [115]. Nie et al. cross-linked thiolated chitosan and ε-polylysine through maleimide groups to form (in situ) a fast-curing hydrogel that could effectively adhere to rat liver wounds and help achieve rapid hemostasis [27]. Zhang et al. realized the in situ preparation of hydrogels characterized by high mechanical strength and short gelling time by cross-linking polyacrylamide units containing a large number of amide groups with ε-polylysine. The prepared systems were used to study a model of rat liver injury, and excellent tissue adhesion and hemostatic properties were observed. It was observed that the extent of bleeding from the synthetic hydrogel-treated wounds was 1/7th of the extent of bleeding observed for the untreated groups [116]. Peptide sequences other than polylysine also perform well in the field of hemostatic materials. Charbonneau et al. prepared a peptide sequence consisting of lysine and leucine residues bound to the surface of a biocompatible polymeric hydrogel matrix to form a hemostatic material. This material was used to test a rabbit ear bleeding model, and it was observed that the bleeding time could be reduced by 40% using this material. When a rabbit liver injury model was tested using the prepared peptide sequence, the extent of surface damage realized was less than the extent of surface damage realized using the commercial zeolite powder agent QuikClot® [117]. RADA-16 (RADARADARADARADA) is a short peptide sequence consisting of arginine, aspartic acid, and alanine. This system exhibits self-assembling properties and can spontaneously assemble to form b-folded and nanofiber lattice structures under certain conditions. The composition of the structure is stable. The structure is adjustable and is characterized by high biocompatibility and non-immunogenicity. These materials mimic the porosity and structure of the extracellular matrix in vivo, can provide a suitable microenvironment for cell growth, promote the repair and reconstruction of damaged tissues, and promote the drug delivery process [118]. Wang et al. used a RADA-16 self-assembled peptide system to rat spinal cord transection and liver injury models. They demonstrated that the peptide significantly reduced bleeding time and reduced bleeding volume [119]. Yang et al. prepared a copper peptide-functionalized RADA16 system that was used to study a diabetic mouse model to effectively accelerate the processes of wound closure and tissue repair [26]. Self-assembled peptide sequences can be potentially used to realize hemostasis, although some persistent disadvantages hinder their clinical applications. The peptides are not readily accessible, and some peptides need to be preheated and sonicated prior to use. The cost-inefficiency of the preparation process hinders the practical applications of the material.
PEG is widely applied in the medical field as they are characterized by excellent properties. Amphiphilic copolymers containing PEG are adsorbed on the surface of medical polymer materials to improve the biocompatibility of medical polymer materials in contact with blood [120, 121]. PEG adheres well to tissues and is commonly used as a drug carrier that promotes slow drug release [122, 123]. It is also used as a carrier of immobilized enzymes [124]. Furthermore, PEG can crosslink with tissues and form physical barriers to inhibit bleeding. PEG-based hydrogels exhibit good water absorption properties, and these can increase the concentration of platelets and coagulation factors around wounds. These materials are suitable for the hemostatic closure of traumatic organ injuries [125]. A high relative molecular weight characterizes Dobby PEG derivatives, and these can form hydrogels characterized by excellent water barrier and tissue activity. It can gradually be degraded in the body and completely excreted under conditions of in vivo hemostasis. The material degrades slowly and may cause foreign body reactions, delaying the process of wound healing. Wu et al. introduced cyclized succinate groups into the hydrogel matrix to enable the rapid degradation of the sealant to promote the degradation of PEG and reduce possible damage to tissues [28]. PEG reacts with other polymers to form highly functional copolymers. PEG and acrylic acid can be formed following esterification reactions, and rapid gelation to form the hydrogel from the resulting poly (ethylene glycol) diacrylate (PEGDA) could be triggered using a photoinitiator in the presence of ultraviolet (UV) light at room temperature [126]. This property makes the materials suitable for rapid wound closure [127]. The property of tissue adhesion significantly affects the applicability of hemostatic materials. This property can be improved by functionalizing PEG with aldehyde groups or dopamine. Hydrogen or Schiff base bonds are formed, and amino groups are exposed in the tissue. Each coin has two sides, and the preparation of functionalized hemostatic materials poses difficulties.
PU, a synthetic polymer, exhibits good biocompatibility and degradability. It is easy to process and is used in a wide range of fields, such as trauma dressing and tissue engineering. PU foams can induce the processes of coagulation cascades and platelet aggregation [128]. PU-based sponges can preserve wound moisture, promoting the process of repair of tissues based on the theory of “moist healing”. The mesh-like structure helps control the rate of water vapor transmission and resists bacterial invasion. The self-expanding PU foam provides rapid response and is characterized by rapid volume expansion. Close-fitting of the trauma surface can be realized using the material, and this can be used to study animal models of hypothermia in traumatic hemorrhagic shock (such as the porcine lethal liver hemorrhage model). It has been found that the materials can be used to rapidly and effectively reduce the amount of bleeding in cases of lethal trauma, significantly improve vital signs, ensure the perfusion of vital organs, and generate conditions for subsequent definitive hemostatic treatment [129]. The commercial formulation NasoPore® (a PU-based foam) demonstrated improved hemostasis and provided good patient satisfaction in a clinical trial, as reported by Pawel et al. The researchers used the model for nasal caulking following endoscopic sinus surgery [13]. The excellent tissue adhesion properties of PU make it suitable for application as a sealant in vascular procedures. Lisanne et al. used a PU-based adhesive to study a porcine model of vascular injury repair. The results revealed that the material could effectively improve the outcome of cardiovascular surgery, reduce the incidence of vascular anastomotic fistulae, and efficiently fix sutured vascular anastomoses rapidly and safely without interfering with the process of vascular wound healing [31]. Huang et al. exploited the anti-inflammatory properties of PU nanoparticles (NPs) by combining them with gelatin. The material was implanted in the cortex of injured rats in a rat neurosurgical model, and anti-inflammatory PU NPs were released. The process significantly shortened the hemostasis time and reduced the extent of brain edema. The degree of the inflammatory response generated in the brain was also reduced, facilitating the process of tissue repair [30]. PU materials degrade relatively slowly in vivo compared to other materials. The prolonged in vivo polymerization time of Tissue Glue®, a commercial PU-based formulation, hinders its application prospects.
The PVA-based hemostatic sponges, prepared following a series of processes such as cross-linking, foaming, curing, and sterilization, are characterized by non-fibrous porous structures [130]. These perform well and exhibit excellent hydrophilic and liquid-absorbing properties. These also exhibit good biocompatibility and stable chemical properties. PVA swells following water absorption and can be used to realize localized compression and physical hemostasis. Therefore, these materials are commonly used for compression hemostasis of narrow cavities in humans (such as nasal [131, 132] and anal cavities). Kim et al. used PVA-based nanocellulose sponges to study a rabbit nasal mucosal defect model, and experiments demonstrated that PVA sponges significantly promoted the mucosal regeneration during the early stages of mucosal wound healing of mucosal wounds in noses [132]. PVA does not contain pro-coagulants and relies primarily on the water-absorbing and swelling properties of the sponge material to stop bleeding. Thus, the material is characterized by a narrow application range and cannot be used to treat patients suffering from coagulation disorders.
PCL has been approved by the U.S. Food and Drug Administration (FDA) for a wide range of biomedical applications as it exhibits excellent biocompatibility and good biodegradability. PCL is widely used for producing and processing drug carriers, nanofiber spinning agents, and implantable plastic materials, as it is characterized by excellent shape memory and temperature control properties. PCL, used to develop hemostatic materials, can form compounds with chitosan [100, 133, 134], gelatin [100, 135], collagen fibers [136], and starch [100] to improve the biocompatibility and degradability of the materials without changing or improving the hemostatic properties. Cheng et al. used the process of phage incorporation to develop PCL/collagen fibers following the electrostatic spinning technique. They studied rabbit back injury and deep muscle injury models, and the results indicated that the compounds exhibited excellent antimicrobial effects and could be used to achieve rapid hemostasis [136]. The PCL material used for the preparation of nanofibers could efficiently cover the maximum contact surface area of wounds. Hu et al. fabricated alginate/PCL composite fibers that could be used to dress a mouse back wound model. The material could effectively promote the processes of wound healing and tissue repair. Alginate could provide a moist environment for the wound and promote PCL adhesion [137]. The hydrophobic nature of PCL does not promote adhesion. Hence, surface modification with hydrophilic or mucoadhesive materials is required to prepare hemostatic materials. The degradation rate of PCL is slow, and its degradation rate and drug release profile must be considered when it is used as a carrier of hemostatic drugs.
Acrylate polymers exhibit excellent biocompatibility, broad adhesive properties, water resistance, and durability, which dictate the development and application prospects of the materials in the field of medical adhesives [138]. Moreover, these materials are easy to formulate. Medical bioadhesives can adhere tightly to tissue surfaces and close wounds. There are two major acrylate-based tissue adhesives: polyacrylate adhesives and α-cyanoacrylate adhesives. Polyacrylate adhesives are extensively used as tissue adhesives and implant-filling materials. These are widely used as filling materials for orthodontics. These also find their applications in the fields of dental repair, artificial joint replacement, and bone defect filling [139]. Polymethylmethacrylate (PMMA)-based bone cement is used to treat unstable fractures and in the field of artificial joint replacements. This material significantly improves the firmness of a prosthesis and reduces the degree of long-term loosening of the prosthesis [140]. However, PMMA-based bone cement does not exert osteogenic effects to promote bone healing and does not match the mechanical properties of natural bone. These properties hinder their applications in clinical settings. Li et al. prepared PMMA-based bone cement-containing active nano-MgO particles (nano-MgO/PMMA), which significantly improved the biocompatibility of the material and significantly increased the expression of osteogenic genes. The use of the material also promoted the production of calcium nodules in bone tissues when applied to a model of critical cranial bone defect model [141]. Since 1950, α-cyanoacrylate-based adhesives have been introduced into the medical field, and these are the first and most widely available tissue adhesives. These adhesives can be used to realize rapid and intense bonding. They exhibit excellent biocompatibility and relatively low toxicity. The degree of tissue rejection is also low [142]. The α-cyanoacrylate unit contains negatively charged nitrile and ester groups. This makes the double bond in the monomer extremely reactive, and the process of anionic polymerization can be rapidly realized under these conditions under the influence of weak bases. Instantaneous bonding is realized under these conditions while encountering bleeding wounds. Several tissue adhesives, primarily α-cyanoacrylates, are widely used in a variety of surgical procedures. Alfredo Moreno-Egea conducted a randomized controlled trial to explore the effectiveness of n-hexyl-α-cyanoacrylate as a suture substitute in inguinal hernia surgery. The study included 208 patients, 102 of whom were treated with tissue adhesives. The results demonstrated that the use of n-hexyl-α-cyanoacrylate significantly reduced the mean operative time and decreased postoperative pain [143]. Cyanoacrylate was effectively used during the endoscopic ultrasound-guided treatment of fundic varices formed under conditions of portal hypertension [144]. The α-cyanoacrylate-based adhesive, applied to a dog model with severe liver injury, can close wounds and reduce bleeding [145]. Some problems still need to be addressed to increase the application prospects of α-cyanoacrylate-based adhesives. These adhesives may cause foreign body reactions in living tissues and hinder the process of tissue healing. The poor elasticity of the adhesion site and the poor flexibility with the surface tension of the organ limit the practical applications of the adhesive.
PEO is a water-soluble polymer that exhibits excellent biocompatibility, non-immunogenicity, and non-toxicity. It resists the non-specific adsorption of proteins and is frequently used to develop various drug carriers and anti-adsorption coatings [146]. In the field of hemostatic material development, PEO is primarily used as an additive to improve the performance of other active ingredients. Chitosan, as a positively charged hemostatic active substance, can promote wound hemostasis by aggregating red blood cells, activating platelets, and activating coagulation cascade reactions [53]. The chitosan-based nanofibers fabricated following the electrostatic spinning method adhere tightly to tissues and exhibit excellent biocompatibility and degradability. The application of PEO to the chitosan electrospinning process can effectively reduce the surface tension and viscosity of the chitosan solution, promoting the formation of nanofibers [147]. Deineka et al. used nanofiber mats formed using PEO and chitosan to study a rat liver injury model, and the composite nanofibers significantly reduced the bleeding time. The material exhibited improved degradability and tissue repair compared to the conventional chitosan sponge [37]. The composite nanofibers formed from PEO and chitosan exhibited reduced adhesion on the EpiSkin model. This helped to safely remove the hemostatic material from the wound surface without causing secondary bleeding [148]. Poly (propylene oxide) (PPO) is insoluble in water, biocompatible, and stable. It is usually combined with PEO to prepare high-performance materials. Pluronics® is a PEO-PPO-PEO copolymer that the FDA has approved for usage as an injectable pharmaceutical excipient [149]. PEO and PPO do not promote tissue hemostasis or blood coagulation by themselves, and these are mostly explored together with other hemostatic active ingredients for the development of hemostatic materials.
Most synthetic polymers lack the biological activity required to promote blood clotting. These function as substrates or cross-linking agents for hemostatic materials. It is difficult for simple polymers to meet the special performance requirements of biomedical materials. Researchers are widely compounding synthetic polymers with different biologically active natural polymers to integrate their strengths and obtain composite materials that can be effectively used in clinical settings. PU sponge is a porous foam obtained by foaming PU. The interior of the material appears porous, and the material exhibits excellent physical and mechanical properties. Aqueous PU and sulfated chitosan are blended using glutaraldehyde as the cross-linking agent to form a cross-linked semi-interpenetrating network characterized by excellent hemocompatibility [150].
Inorganic materials
Inorganic materials can be used effectively for hemostasis. Zeolite has long been noted for its hemostatic properties, and in 2002, QuikClot®, the first generation of zeolite hemostatic products, was introduced as first aid equipment for the U.S. military [151, 152]. Zeolite is a porous crystalline aluminosilicate, and its porous structure allows for positive water absorption. This helps increase the concentration of local platelets and clotting factors. The negative surface charge can activate positively charged Factor XII, triggering a cascade reaction of endogenous clotting [153, 154]. Zeolites not only tend to enter capillaries (resulting in thrombosis) but also tend to absorb water and release excessive heat. This can potentially damage the skin [155]. Fan et al. prepared calcium-based zeolites that effectively shorten clotting time and help avoid adverse effects. The FDA has approved the patented zeolite hemostatic gauze for use in clinical settings [156].
Mesoporous silica is characterized by a high density of silanol anions on its surface. These anions can interact with the positively charged amino acids on the surface of the coagulation factor XII to accelerate the coagulation cascade reaction and promote hemostasis. Mesoporous silica exhibits good biocompatibility and exerts low degrees of side effects when used in the human body [21]. Characterized by the presence of large pores, it can attach to thrombin [157], antimicrobial agents [158, 159], and analgesic drugs [160] to achieve effective wound treatment. Wang et al. used silica nanoparticles for loading tannic acid. These materials could effectively promote the activation of the coagulation cascade reaction and reduce the hemostasis time by 65%. These also exhibited excellent antibacterial properties [38]. Silica- and chitosan-based composite sponges were used to study a rat tail break model, and these materials exhibited excellent hemostatic properties [14]. The positive charge on the chitosan surface and the negative charge of the diatom attracts each other, reducing the amount of powder residue on the trauma surface.
Graphene is readily functionalized and widely used in various biomedical fields, such as drug delivery [161, 162], bioimaging [163, 164], and tumor therapy [162, 165]. Graphene oxide (GO) has a 2D sheet structure and contains numerous oxygen-containing functional groups on its surface. These groups can activate platelets to trigger aggregation, and the material exhibits excellent biocompatibility and hydrophilicity. Thus, GO can potentially be used for trauma treatment [40, 166]. Graphene-based sponges are characterized by good water absorption abilities, and these are functionalized on the surface. Thus, multiple functional composites can be prepared from GO, laying the foundation for their development as hemostatic materials. Li et al. prepared graphene/montmorillonite composite sponges (GMCS) using graphene-immobilized montmorillonite (MMT) particles. GMCS-activated Factor XII with a negative charge cascade on the surface of MMT could be used to accelerate hemostasis, and the entry of MMT particles into blood vessels could be prevented [41].
Metal-containing materials
Metal ions such as Ag, Zn, and Cu exhibit broad-spectrum antimicrobial properties, superior heat resistance, good dispersibility, and low drug resistance. These materials are widely used to develop various biological materials and medical devices. Various materials containing metal ions have been effectively used in the field of hemostasis. Ca2+ significantly affects the physiological coagulation pathway and can activate Factor X. It participates in the formation of prothrombin complexes and activates Factor XIII to form insoluble fibrin multimers that exert hemostatic effects [167-169]. The precipitation of calcium ions on the surface of chitosan nanofibers can effectively accelerate the platelet activation process and shorten the hemostasis time. The composite material prepared by combining hydroxyapatite with bacterial cellulose promotes cell proliferation [170]. The calcium-based zeolite can be used effectively to realize hemostasis [156].
Silver ions are antimicrobial agents, and their incorporation into mesoporous silica-chitosan composites can improve the antimicrobial properties of the composites without increasing cytotoxicity [171]. Microporous chitosan-polyethylene glycol hydrogels containing copper ions exhibit good stability. These compounds exhibit excellent antimicrobial activity and generate an excellent keratin-forming cell response [172]. Zinc is considered a platelet modulator that can promote the process of platelet activation [173]. Cellulose oxide nanoparticles loaded with Zn ions can significantly improve the mechanical and antimicrobial properties of bionic calcium alginate hydrogels [174]. Carboxymethyl chitosan zinc (CMCS-Zn) is a degradable biomaterial that can be used to treat bone defects effectively, and this composite inhibits the occurrence of bone infections [175]. Cerium oxide exerts a significant neuroprotective effect under conditions of oxidative stress [176]. Liu J et al. synthesized cerium-containing spherical mesoporous bioglass particles, effectively reducing the clotting time and promoting cell proliferation. The results were arrived at by conducting in vitro tests [177]. Magnesium ions significantly affect the processes of cell adhesion, proliferation, and mineralization [178]. Bian et al. prepared bioactive hydrogels based on HA and bisphosphonate-magnesium nanoparticles, which could significantly upregulate the production of alkaline phosphatase (ALP) and effectively promote the repair of bone tissues [179]. Lu et al. loaded Fe3+ onto the surface of sodium alginate grafted dopamine-modified poly (lactic acid) (PLLA) complexes. The resulting material could effectively concentrate local blood components and shorten coagulation time. The material exhibited excellent antibacterial properties against E. coli and Staphylococcus aureus [180]. Zinc ions also exhibit significantly high antibacterial activity, and these are used to develop hemostatic materials [18].
Others
Platelet-derived nano-vesicles and synthetic platelet mimics can be used to develop hemostatic materials. Platelets are small pieces of cytoplasm that are shed from the cytoplasm of mature megakaryocytes. These participate in hemostasis and coagulation. The von-Willebrand Factor (vWF) receptors on the surface of collagen fibers are exposed when vascular endothelial cells are damaged. Glycoprotein Ib (GPIb) on the platelet surface recognizes the bound vWF receptors and rapidly adheres to the site of injury. Activated platelets release epinephrine, ADP, and thrombin, which promote the process of platelet aggregation, resulting in the formation of thrombi. Platelets present a phospholipid surface that adsorbs large amounts of coagulation factors, increasing the speed of the clotting response. Platelet-derived particles can promote the processes of megakaryocyte differentiation and platelet production. Jung et al. developed platelet-derived nanovesicles following a hypotonic ultrasound method. The nanovesicles can adhere and aggregate to stop bleeding in the presence of calcium ions and thrombin. Reduced pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-1β, which can potentially hinder the processes of macrophage activation and phagocytosis, are produced under these conditions [43]. Platelet mimics can mimic and enhance the hemostatic capacity of platelets. The shelf life is increased, and the degree of contamination is low. These materials can be used to develop hemostatic materials in the future. Christa et al. used liposomes as model particles with collagen, vWF binding peptides, and fibrinogen mimetic peptide (FMP) decorated on the liposome surface. The functionally integrated platelet mimetic liposome structure exhibited significantly high hemostatic efficacy when used to study the mouse tail transection model [44]. James et al. developed Arg-Gly-Asp (RGD)-based functionalized nanoparticle platelet mimics. Poly (lactic-co-glycolic acid)-poly-L-lysine (PLGA-PLL) was used as the core, and RGD sequences were combined with nanospheres via PEG to form the synthetic systems. The material could be used to effectively reduce the bleeding time in a severely traumatized rat model [181]. Several biomaterials are also used to develop hemostatic materials. Extracts of lactic acid bacteria combined with nitric oxide donors can be used to form biomimetic phage microparticles exhibiting antimicrobial properties. These microparticles are further encapsulated using graphene oxide, and the resulting antimicrobial hydrogels are prepared to promote the process of wound healing [182]. Herbal components such as curcumin have also been investigated for the development of hemostatic materials, and the materials exhibit good antibacterial activity [183].
Forms of hemostatic materials
The effectiveness of hemostatic materials depends not only on the active chemical composition but also on the selection of the appropriate form of the material to be processed. Nanofibers, hydrogels, nanoparticles, and sponges are common forms of hemostatic materials. Different forms of hemostatic materials are used under different conditions, and the pros and cons of using these materials differ based on the type and form of the material used.
Nanofibers
Any fiber with a diameter of less than 1 μm is a nanofiber. These ultrafine fibers have a specific surface area that generates excellent adsorption properties and surface activity. The pore sizes of the lattices and films composed of these extremely fine interwoven nanofibers are significantly small. The materials are characterized by high porosity and good surface adsorption properties. The high porosity of the nanofiber surface helps achieves moderate levels of wound drying, which promotes wound healing. Nanofibers can be classified into two types based on the preparation methods (spinning and molecular technology) followed. In this section, we introduce the application of nanofibers, prepared following the two methods, in the field of hemostatic materials.
The electrostatic spinning technique was applied to prepare PCL-gelatin nanofibers loaded with different concentrations of Lawsone, and they were experimentally evaluated for wound healing effects in a rat wound model. Reproduced with permission from [34]. © 2018 Elsevier B.V. All rights reserved.
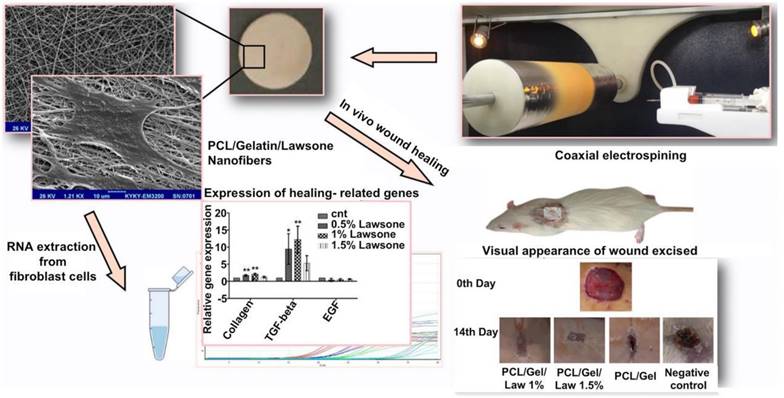
Spinning methods include electrostatic spinning [184], solution blowing, and centrifugal spinning [185] methods. The electrostatic spinning method is the most commonly used method for nanofiber preparation as the method is easy to execute. Moreover, the method is widely used in various fields and is characterized by high production efficiency. The electrostatic spinning technology involves the use of the electrostatic force generated under conditions of a high-voltage electric field to prepare the polymer solution extruded from a syringe at a certain flow rate [186]. The surface tension is overcome under these conditions, and a fine stream of charged polymer is ejected. The process is influenced by the electrostatic force, and the charged jet further accelerates to stretch and form a continuous ultra-fine fiber that is collected at the receiving end. Sardou et al. loaded the nanofibers prepared following the electrostatic spinning method with the PCL/gelatin mixture containing different concentrations of lawsone. The results of in vitro cell adhesion and proliferation experiments and those obtained by studying rat wound models revealed that PCL/gelatin nanofibers loaded with 1% lawsone can effectively promote the processes of fibroblast adhesion and proliferation. These can also promote wound healing and the formation of granulation tissues [34] (Figure 1). Ahmed et al. synthesized nanofiber mats by encapsulating ZnO particles with chitosan/PVA nanofibers prepared following the electrospinning process. Results of in vitro experiments revealed the significantly high inhibitory effects exerted by chitosan/PVA/ZnO nanofibers on a variety of bacteria, including Staphylococcus aureus, E. coli, and Pseudomonas aeruginosa. The chitosan/PVA/ZnO nanofibers exhibited strong antioxidant capacity in a diabetic rabbit wound model, inhibiting the development of local inflammation and promoting wound healing [18]. The polymer solution should be characterized by a certain level of conductivity for the efficient preparation of nanofibers following the electrostatic spinning method. This limits the choice of conductive polymers. Moreover, the electrostatic spinning method cannot be used for industrial production, as the products are obtained in low yields when this method is used. This resulted in the development of the solution spinning technology. Unlike the electrostatic spinning method, the solution-blowing spinning method involves the exploitation of the jet effect generated by high-pressure airflow when droplets overcome surface tension to form nanofibers [185]. The solution blow spinning technique is a highly efficient method that can be used to prepare materials within a short time. Electrostatic interactions and dielectric constants do not limit the process. Daristotle et al. prepared a mixture of solutions containing PLGA, PEG, and SiO2 nanoparticles with acetone as the solvent and deposited polymer nanofibers on a porcine liver wound model using a solution blow-spinning device. The results demonstrated the good hemostatic effect exerted by the nanofibers [36]. It should be noted that the fibers are formed randomly, and the expected morphology is not achieved when the solution spinning technology is used. Xie et al. manufactured nanofiber capsules following the processes of electrospinning, gas foaming, coating, and cross-linking. The capsules can be swallowed. Good scalability and absorption properties are achieved, and the materials are promising as alternative testing products to endoscopy that can be used to collect a large number of biological samples to help diagnose numerous diseases in a timely and accurate manner [187].
Molecular techniques are primarily used to prepare single- or multi-tube carbon nanotube (CNT) bundles. The three main methods used for the preparation of CNT bundles are arc discharge [188], laser ablation, and chemical vapor deposition [189]. Among these, the chemical vapor deposition method is the most popular as it is cost-efficient. This method can be used to produce materials with high yields, and the process can be executed under controlled experimental conditions. The chemical vapor deposition method is used to deposit catalysts on a flat substrate such as quartz. The method involves the passage of a carbon-containing gas at a high temperature to decompose, precipitate, and grow CNTs on the catalyst particles. This method can be used to produce ordered parallel/perpendicularly aligned CNTs that are characterized by high specific surface areas [189]. Patel et al. modified the surface of polymeric nanofibers with CNT and investigated the role of the composites in modulating inflammation, angiogenesis, and bone repair (in vivo). The CNT-coated nanofibers exhibited a reduced inflammatory response and enhanced angiogenic and angiogenic marker expression levels when injected subcutaneously into rats [35]. The electrical conductivity of CNTs can also induce osteoblast differentiation and cell proliferation through electrical stimulation, and these materials are frequently used to prepare synthetic bone matrices [190]. The major obstacle that hinders the further application of CNTs in biomedical fields is their toxicity toward tissue cells [191].
The nanofibers of natural or synthetic polymers prepared following the above methods can effectively adhere to and activate platelets to promote the processes of hemostasis and wound healing in cases of serious or irregular injuries. However, hemostatic materials are difficult to remove, and this problem is yet to be addressed.
Gels
Hydrogels
Hydrogel presents a three-dimensional network consisting of a macromolecular backbone and side chains containing hydrophilic or hydrophobic groups. It can absorb and retain large amounts of water by exploiting the cross-linked network. Its application in the field of hemostasis and wound healing can increase the concentration of local platelets and clotting factors and shorten the clotting time [192]. Most existing hemostatic materials adhere weakly to wet tissues and cannot be effectively used to treat heavy-bleeding wounds. Hydrogels can effectively adhere to tissues, keeping the wound site moist to stop bleeding and promote healing. The hydrophilic groups in the hydrogel can bind tightly to the local protein or peptide units in tissues. The hydrogel adheres firmly to damaged tissues under these conditions [193]. Hydrogels can be classified according to the preparation method, source of the raw material, size of the material, and shape of the material. Herein, we discuss hydrogel-based hemostatic materials that are categorized based on the preparation method.
Hydrogels can be classified into physical and chemical hydrogels based on the hydrogel synthesis method followed and the nature of network bonding. Physical hydrogels are formed under the influence of physical forces such as electrostatic interactions, hydrogen bonding, and chain entanglement [194]. Physically prepared hydrogels are prepared in the absence of chemical cross-linking agents. They are readily degradable and can be used extensively used in the biomedical field. These materials are characterized by good degradability and thixotropy. Physical hydrogels can be converted to solutions when heated; therefore, these are also known as pseudogels or thermally reversible gels [195]. The PVA hydrogel obtained following the repeated freezing-thawing process is a physical hydrogel. The distribution of the PVA molecular chain in the homogeneous aqueous solution of PVA is highly disordered. The mutual contact between the chains is limited, and this makes the formation of cross-links difficult [196]. The freezing process helps freeze the movement of the PVA molecular chains for a moment. This helps increase the contact time. The van der Waals forces and hydrogen bonds are exploited to form tightly bound local physical cross-linking points. The repeated freezing-thawing process results in the formation of a plurality of physical cross-linking points in the PVA hydrogel. Heating destroys the physical cross-linking point between the molecules, and the PVA molecular chains revert to the disordered state. The hydrogel changes to the liquid state under these conditions. The heating method is one of the most common methods used for the preparation of thermosensitive polymer gels. Xu et al. prepared self-assembled graphene hydrogels (SGH) by heating aqueous dispersions of GO in a Teflon-lined autoclave. The samples were heated at 180 °C for 12 h [197]. The SGH obtained following this method is characterized by excellent mechanical strength and thixotropy. It can be easily prepared. It is noteworthy that the high-heat reaction is hazardous. Electrostatic interactions can stabilize hydrogel networks. Metal cations (Ca2+, Mg2+, and Fe3+) can interact with negatively charged polysaccharide carboxylate groups to form tight networks. This results in the generation of mechanically sound metal-polysaccharide hydrogels. Polysaccharide polymers capable of forming cross-linked hydrogels with metal ions include chitosan [19, 198], pectin [199], and cellulose [200]. The hemostatic effect of the polysaccharide moieties helps maintain moisture in wounds and promotes the process of wound healing. The physical methods used to prepare hydrogels are reversible, and the hydrogels are formed under mild conditions. It has been observed that the stability and mechanical strength of such hydrogels are poorer than those of the hydrogels prepared following other methods.
Hydrogels prepared following chemical methods (for example, those prepared in the presence of cross-linking agents or those synthesized by accelerating the reaction of functional groups in polymer chains) present a stable structure. These are widely used in the field of biomedicine [113]. PVA-based hydrogels can be synthesized using cross-linking agents and following the freezing-thawing method described in the previous section. The addition of glutaraldehyde to the aqueous solution of PVA induces the alcohol-formaldehyde condensation reaction that results in the cross-linking of PVA to form a network of polymer hydrogels [201]. Such cross-linking agents are integrated into the hydrogel cross-linking network. The use of a type of zero-length cross-linker allows the formation of covalent bonds between polymer chains. Integration of the system into the hydrogel network is avoided under these conditions. For example, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) can react with a carboxylic acid group on the polymer chain to form an o-acyl isourea unit. Urea is formed as the by-product, and it is subsequently released when a primary amine attacks the reactive ester intermediate on the adjacent polymer chain. The reaction results in the formation of a hydrogel network that is stabilized by covalent amide crosslinks [202]. Such cross-linking agents are primarily used to prepare gelatin- [203], collagen- [202], and fibrin-based hydrogels. The mixing of nanoparticles and polymers induced the formation of hydrogel cross-linked networks. The interaction between hydrophobically modified carboxymethylcellulose-based polymer chains and polystyrene or PEG-based nanoparticle surfaces facilitates the formation of polymer-nanoparticle hydrogel networks. The reversible hydrophobic forces under action allow the hydrogel to flow under the influence of the applied shear stress. When the stress is relaxed, the hydrogel regains its original properties [204]. The hydrogel produced from the nanoparticle blend can be effectively used for drug delivery, and it is currently used for the treatment of subcutaneous melanoma in a mouse model [205]. Some enzymes facilitate the hydrogel cross-linking reaction. Transglutaminase catalyzes the formation of isopeptide bonds between lysine and glutamine side chain amides. This reaction can be used to produce intermolecular crosslinks between soluble fibrinogen molecules, resulting in the formation of fibrinogen- and fibrin-based hydrogels [206]. Lysyl oxidase, a copper-dependent enzyme associated with the formation of collagen fibrils, allows the formation of aldehydes from lysine side chain residues under conditions of enzymatic reduction that is followed by the process of interpeptide cross-linking (such as Schiff base reaction). The process results in the production of covalent hydrogels [207]. UV light triggers the process of gelation through the action of photoinitiator molecules which absorb radiation and form substances that can mediate polymerization reactions [208]. The photo-initiator is irradiated by UV light, and this results in the formation of free radicals which attack the vinyl group. Under these conditions vinyl free radicals are formed and the polymerization of the vinyl group proceeds under conditions of free radical polymerization. Therefore, most of the base materials commonly used to prepare photo-cross-linked hydrogels are vinyl-functionalized polymers, such as PEGDA [209], gelatin methacrylate (GelMA) [210], and methacrylated HA (HAMA) [211]. The cross-linked network of hydrogels formed in the presence of photo-initiators is uncontrolled, and heterogeneous networks are produced under these conditions.
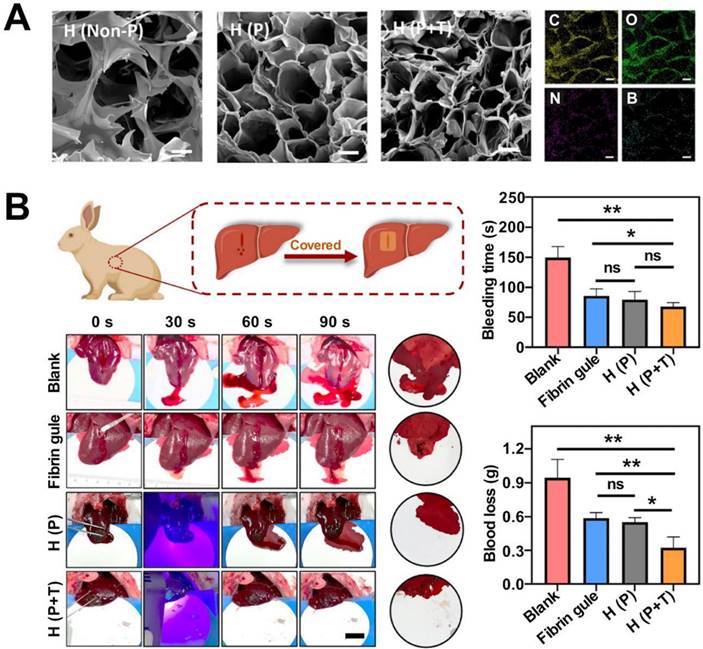
Hydrogel, as a hemostatic material, adheres strongly to tissues and can be used to treat wounds with high blood flow. The Schiff base reaction is a nucleophilic addition reaction between primary amine-bearing compounds and carbonyl-containing aldehydes and ketones [212]. The adhesive formed from cross-linked hydrogels produced following the Schiff base reaction presents a network-like structure that is characterized by a high degree of porosity and water content. These materials are biocompatible and readily bind to tissues. The adhesive strength of the hydrogel is high, and the material can be potentially used to heal wounds [213]. Yu et al. attached O-nitrobenzyl alcohol (NB) units (modified under photosensitive conditions) to the backbone of water-soluble carboxymethyl chitosan (CMC), exploiting amide bonds to synthesize the NB-CMC composite. Under conditions of UV irradiation, the NB units in the NB-CMC composite were converted to the O-nitrosobenzaldehyde group, and the formaldehyde group reacted with the amino group in CMC to form a stable cross-linked network. Photoluminescent aldehydes react with amino groups present on the tissue surface to form covalent bonds at the hydrogel-tissue interface. Rapid tissue integration is achieved under these conditions, the adhesive strength realized is high. Experimental results revealed that the adhesive strength of the composite hydrogels is significantly higher than that of commercially available bioadhesives used in clinical practice. The former exhibits excellent blood cell adhesion and coagulation ability and can be used to exert excellent hemostatic effects in vitro and in vivo [20]. The multifunctional bioadhesive hydrogels (containing carboxymethyl chitosan (CMCS), sodium alginate (SA), and tannic acid (TA)) prepared exploiting dynamic covalent bonds (borate ester bonds and Schiff base bonds) helped optimize the mechanical, antibacterial, and antioxidant properties of the hydrogels. The improved adhesion ability of the material was obtained using a rabbit liver hemorrhage model [214] (Figure 2). Gelatin hydrogels are cross-linked via amide bonds, and the cross-linking process usually proceeds following the EDC-based coupling method. The amide bond is formed between the primary amine and the carboxylic acid group on the side chain of the adjacent gelatin chain. Ya et al. introduced cyclized succinate groups into a tetra-PEG hydrogel. The residual butadiimide active ester in the compound could react with the amino group in the tissue to form stable amide bonds. This helped to seal the wounds and control bleeding [99] effectively. Experimental results revealed that the tetra-PEG hydrogel could withstand pressures of ≤294 ± 27 mmHg, and the material exerted significantly high levels of hemostatic effects on a porcine spleen hemorrhage model. The challenges lay in removing the hydrogels that exhibited strong adhesion properties.
Multifunctional biohydrogels based on carboxymethyl chitosan, sodium alginate and tannic acid and its application in rabbit liver injury model. A: scanning electron microscope images of hydrogels and elemental distribution of C, N, O and B; B: Schematic diagram of the application of hydrogel in a rabbit liver hemorrhage model, including photos of the hemostatic process, comparison of bleeding time and blood loss. Reproduced with permission from [214]. © 2022 Zou Chen-Yu et al.
Hydrogels are a popular form of hemostatic material. The residual aldehyde group on the surface of hydrogels prepared following the Schiff base reaction is potentially cytotoxic, and the strong adhesion ability of the hydrogel makes the safe removal (without causing pain to the patient) of the hydrogels challenging.
Aerogels
Aerogel is a nanoscale porous solid material formed by replacing the liquid phase in the gel with gas following a drying method. Aerogel is the least dense solid in the world and is characterized by high porosity, specific surface area, and adsorption power. Aerogel accelerates hemostasis by absorbing local water from wounds. The porous structure of the aerogel and an increase in the concentration of clotting factors and platelets promote water absorption. A substrate for clot attachment is formed when the aerogel comes in contact with blood. Aerogels can be classified into three categories based on the backbone constituents: inorganic aerogels, organic aerogels, and carbon aerogels.
Inorganic aerogels are currently being researched for hemostasis, and the most common material being studied is silica. The sources of raw materials used to prepare SiO2-based aerogel are abundant. The preparation process followed is simple and good controllability is achieved. These aerogels are characterized by high porosity and high specific surface area. The freeze-drying method is the most common method that is followed to prepare aerogels. The microscopic pore structure of aerogels can be modified by controlling the cooling rate and temperature gradient. Yin et al. reported that an increase in the cooling rate results in an increase in the degree of refinement of ice crystals. This eventually results in the formation of a fine and homogeneous pore structure [215]. Chen et al. prepared chitosan/diatom biomineralized silica-based aerogel systems following the freeze-drying method. The pore size of the material could be controlled. The composite aerogel presents a hierarchical porous structure. The ice crystals formed using tert-butyl alcohol during the freeze-drying process can maintain the porosity of biomineralized silica in the aerogel. Under these conditions, a composite aerogel with a high specific surface area (74.441 m2/g) and excellent water absorption ability (316.83 ± 2.04%) is formed. The hemostatic time recorded for the composite aerogel was less than 70 s (in vitro), and the material outperformed similar hemostatic products in terms of hemostatic time and bleeding volume indices. The results were arrived at by studying the rat severed tail model and the femoral arterial vein dissection model [21]. Metal oxides also constitute an inorganic skeleton of aerogel. Oxide-based aerogels such as TiO2, ZrO2, and Al2O3 are used primarily to prevent air and water pollution. Electrically conductive oxide-based aerogels have also been developed [216, 217].
Organic aerogels can be classified into two categories: biomass-based and polymer-based. Biomass-based aerogels are biocompatible, biodegradable, and widely used in the pharmaceutical and food industries. Various plant and animal biopolymers have been used to prepare aerogels. Saini et al. prepared a biomass-based organic aerogel by freeze-drying a viscous gel obtained by mixing microprotofibrillated cellulose (MFC; prepared from cinnamon extracts) and carboxylated cellulose nanocrystals (cCNCs). The compressive strength of this aerogel was approximately 106% higher than the compressive strength recorded for MFC. The modulus of the former was 175% higher than that of the latter [218]. Aerogels based on carbon fibers (CNFs) can be prepared following the processes of freeze-drying, supercritical drying (SCD), spray drying (SD), and oven drying (OD) [219, 220]. Freeze-drying at -80 °C and 15 Pa for approximately 72 hours helps obtain a mixture of poly(amidoamide) epichlorohydrin resin and CNF. The SD method is used to concentrate and extract liquid from the CNF-based hydrogel. The sample was dehydrated in hot gas. The primary advantage of using this preparation method lies in the fact that the size of the aerogel pores can be controlled effectively using the SD method, and the preparation process is cost-effective. CNF-based aerogels exhibit excellent water absorption and adhesion properties. Wang et al. prepared a composite aerogel consisting of CNF and copper-containing mesoporous bioactive glass. The prepared aerogel exhibited excellent antimicrobial properties and could inhibit the growth of E. coli and other inflammatory bacteria. This could be potentially used to treat chronic wounds [42]. Polymer-based organic aerogels are polymer molecules with porous network structures. These aerogels are prepared by combining colloidal particles by exploiting hydrogen bonding or van der Waals forces. Yan et al. prepared an antibacterial aerogel-based dressing using PVA and oxidized betaine polysaccharide Schiff base (ORBPS). The material was fabricated following the freeze-drying method, and a mouse model of total skin defect was studied. The dressing was found to reduce inflammation, promote angiogenesis, and facilitate the wound-healing process [32] (Figure 3).
Antimicrobial and wound healing properties of oxidized Bletilla rhizome polysaccharide-based aerogel. A: scanning electron microscopy images of porous aerogels; B: antibacterial activity of aerogels against E. coli and S. aureus; C: application to a mouse model of total skin defect to promote wound healing and D wound healing rate statistics (comparison with nano silver dressing). Reproduced with permission from [32]. ©2022 Elsevier Ltd. All rights reserved.
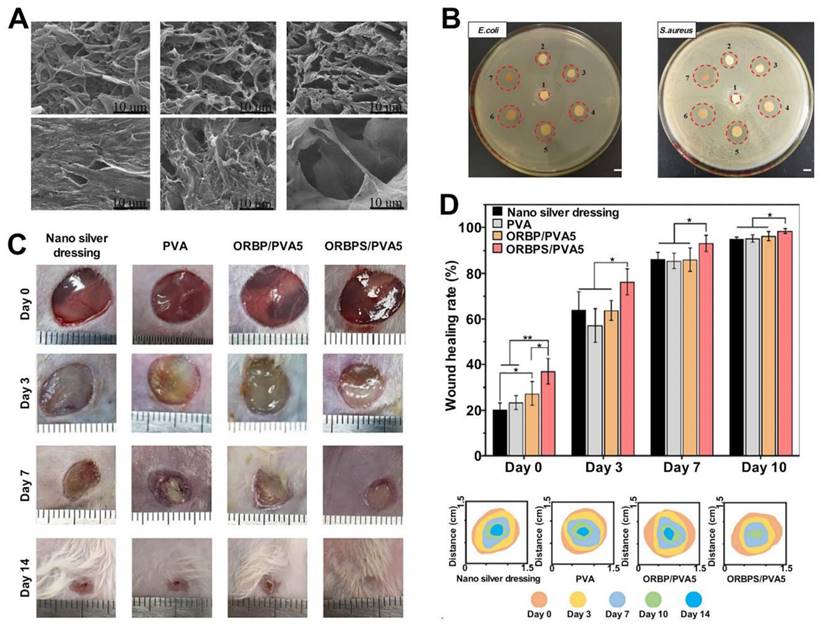
Carbon aerogels are chemically stable materials characterized by high hardness and melting points. The carbon skeleton structure is solely retained under an inert atmosphere and high-temperature conditions. These aerogel systems are primarily used in fields operating under high-temperature conditions. Carbon aerogels find their use in the field of aerospace engineering and high-temperature furnaces. These are also used to produce nuclear energy. Graphene aerogel is a special type of carbon aerogel. GO is usually reduced and treated to obtain graphene gel, which is freeze-dried or supercritically dried using CO2 to obtain graphene aerogel. GO presents a 2D lamellar structure and contains numerous oxygen-containing functional groups on its surface. It can activate platelets and trigger a strong aggregation reaction, promoting hemostasis. Jessica et al. prepared aerogels based on GO and gelatin and experimentally demonstrated that the sample could absorb 50 times its weight when in contact with liquid media under physiological conditions. Analysis of the SEM images revealed that red blood cells adhered well to the sample surface [40]. The use of highland anthocyanin (concentration: 5%) can effectively improve the blood absorption and adhesion capacity of the composite aerogel, and the negative charge on the surface of highland anthocyanin can promote the process of platelet and erythrocyte aggregation exploiting electrostatic interactions.
The highly porous structure of aerogels has attracted the attention of researchers working in the field of drug delivery [221]. The unique structure enables the rapid loading of small-molecule drugs and provides a low degree of restriction to the internal regions of the matrix. Site specificity, stimulus responsiveness, and prolonged drug release properties are also achieved.
Sponges
Sponges exploit their dense porous structure to absorb local moisture from wounds. This results in an increase in the concentration of platelets and clotting factors. The platelets adhere and aggregate under this condition, forming a thrombus that stops bleeding. Absorbent gelatin sponges are the most frequently used hemostatic material. Commercial gelatin sponges (such as Gelatin Sponge®) are cheaper than other materials and exert a hemostatic effect. These materials can be used effectively to treat deep wounds and trauma. These are also used during surgery, but the poor mechanical properties of the material limit their application prospects. The material is prone to breakage when it encounters blood. It is difficult to stop large wounds from bleeding as it is easy to stretch fragile blood cells and platelets. The freeze-drying method is used to lyophilize the solution and form porous sponges. Alkylated chitosan promotes blood clotting when inserted into blood cell membranes [59]. A composite sponge characterized by a porous structure can be formed by lyophilizing a mixture of alkylated chitosan and silica [14]. The introduction of different amounts of alkyl groups on the chitosan surface and the different concentrations of alkylated chitosan affect the porosity of the sponge samples. The solution of 1% alkylated chitosan dissolved in acetic acid mixed with silica was lyophilized to obtain a sponge with a porosity of approximately 79%. A high compressive strength was recorded for the sponge sample, and it was observed that the sponge exhibited excellent hemostatic ability when used to study a mouse tail break model.
Peptide immobilized starch/PEG sponge for hemostasis in a rat liver defect model. A: schematic diagram of the synthesis of the receptor agonist peptide (TRAP)/PEG sponge; B: schematic diagram of the procedure for hemostasis experiments in liver defects; C: hemostasis time and D: blood loss of the composite sponge in all samples of the rat liver defect model. Reproduced with permission from [29]. ©2019 Acta Materialia Inc. Published by Elsevier Ltd. PEG: polyethylene glycol; TRAP: the synthesis of the receptor agonist peptide.
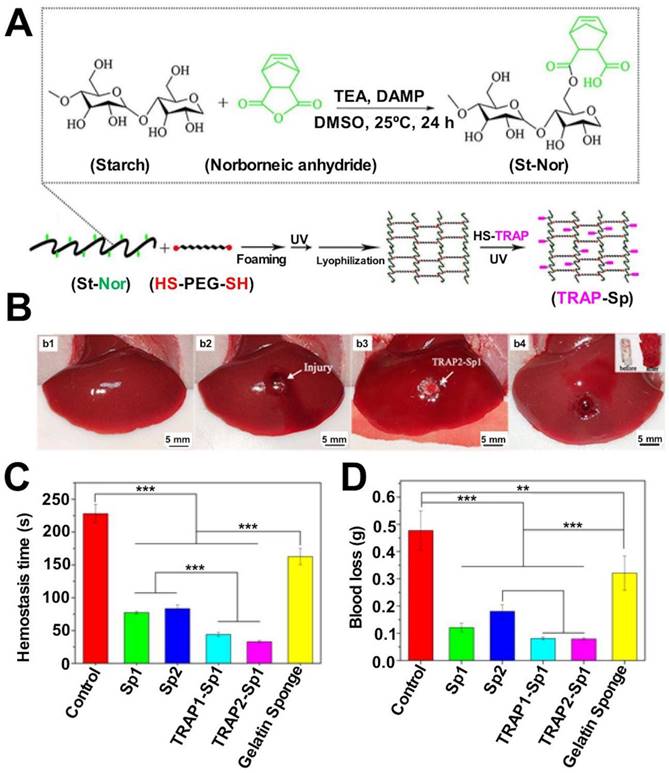
The pore structure of the sponge also offers the possibility of loading other hemostatic active ingredients. Goncharuk et al. prepared poly (vinyl formal) (PVF) solutions by condensing PVA with formaldehyde. Multivacancy sponges were obtained by freeze-drying the mixed solutions. It was observed that the pore size of the samples was affected by the concentration of PVA in the solution [33]. PVF sponges consist of large, interconnected pores, the walls of which consist of fine pores of small radii (1-3 μm). The radius of the coarse pore is approximately 430 μm, and that of the fine pores is in the range of 195-225 μm. Chitosan hydrogel, CaCO3 particles, and silica particles were added into the coarse and fine pores of the PVF sponge to compare their mechanical strengths, swelling performances, and hemostatic effects. It was demonstrated that the chitosan hydrogel-modified PVF sponge containing fine pores exhibited excellent water absorption ability, and the hemostatic efficiency of the PVF sponge containing fine pores was 92.7% higher than that recorded for Celoxin®. The mouse tail break model was used to arrive at the results. However, the application of functional fillers did not significantly improve the hemostatic efficacy of the PVF sponges. This can be potentially attributed to the deactivation of the aldehyde group on the PVF surface. The deactivation could be attributed to the interaction of the aldehyde group with the amino group of the active ingredient. Besides, the potential cytotoxic effect of the residual aldehyde groups should be considered in practical applications.
Hemostatic active ingredients such as thrombin receptor agonist peptides (TRAP) can be immobilized on the surface of the sponge to accelerate hemostasis. A modified starch solution and the dithiol-functionalized PEG solution are mixed in a certain ratio, a cross-linked network is established using UV light, and the composite solution is lyophilized to obtain a starch-based sponge [29] (Figure 4). TRAP was immobilized on the sponge surface through thiol-ene click chemistry exploiting sulfhydryl groups. Experimental results revealed that the composite sponge was characterized by a high degree of porosity, and the property of rapid water-triggered shape recovery was also observed. The hemostatic ability of the gelatin sponges and the TRAP-composite sponges were compared using the rat femoral artery and liver defect models. The results revealed that the TRAP-composite sponges could significantly reduce the bleeding volume and accelerate the clotting time in incompressible and arterial bleeding cases. Deep irregular wounds (such as those caused by liver injury) were studied, and it was observed that the TRAP-composite sponge could rapidly expand and close the wounds after absorbing water. The samples were characterized by high mechanical strength and were not significantly toxic to tissue cells.
Sponges could increase the local concentration of platelets and clotting factors in the wounds, and it could be attributed to the high porosity of the sponges and the excellent water absorption properties of the samples. A hemostatic sponge can compress the local area when it swells with water. However, hemostatic sponges are not suitable for the treatment of deep and irregular wounds, and these may cause pain and exert localized pressure on nerves.
Nanoparticles
Nanoparticles can be potentially used to treat deep and irregular wounds. These can also be used to load other hemostatic active ingredients or drugs. The use of nanoparticles can ensure the stability and solubility of the loaded ingredients or drugs, promote transmembrane transport, and significantly improve hemostatic efficacy. Nanoparticles formed from natural and synthetic polymers exhibit good drug-loading stability and are easily surface-modified. Controlled release and targeting of drugs can be achieved by modulating the properties of polymers and by realizing surface modifications. Nanoparticles are classified into several categories according to their chemical composition: liposome-based nanoparticles (LNPs), polymeric nanoparticles, and inorganic nanoparticles.
LNPs are extensively studied as drug delivery carriers. These nanoparticles generally comprise ionized cationic lipids, phospholipids, cholesterol, and polyethylene glycol lipids. LNPs exploit the properties of phospholipid molecules to penetrate cell membranes, laying the foundation for the development of drug delivery carriers. Chen et al. used LNPs to wrap hemostatic macromolecules such as thrombin and studied the activation of platelets in the presence of LNPs loaded with thrombin. They conducted in vitro platelet contraction assays and thromboelastography tests to arrive at the results [222]. It was observed that platelet endocytosis of LNPs loaded with thrombin significantly increased the sensitivity of platelets toward collagen and significantly accelerated the time to clot formation. An increase in clot stiffness was also observed. Its introduction into the plasma of patients suffering from coagulation disorders also markedly accelerated the time of formation of blood clots. Attention should be paid to the fact that thrombin not fully encapsulated by LNPs may activate platelets prematurely, resulting in rapid platelet activation and thrombosis. LNPs equipped with ADP could also effectively activate and aggregate platelets, thus significantly shortening the clotting time in a rabbit liver defect model. Thrombus formation in arteries is avoided under these conditions [222].
Polymer-based nanoparticles can be primarily classified into two categories: natural polymer-based nanoparticles and synthetic polymer-based nanoparticles. The chitosan surface contains a positive charge and can be self-assembled with negatively charged polyanions (such as sodium tripolyphosphate) to synthesize chitosan nanoparticles exploiting electrostatic interactions [223, 224]. The size, diameter, and hydrodynamic characteristics of chitosan nanoparticles are influenced by the chitosan concentration, the ratio of chitosan to anionic crosslinker, and the pH of the reflective system. Small chitosan nanoparticles are characterized by better antibacterial properties than large chitosan nanoparticles. The former exhibit better membrane penetration ability than the latter [225]. Nanoparticles are smaller than chitosan nanofibers, chitosan hydrogels, and chitosan sponges, and the degrees of dispersion realized for the nanoparticles are higher than the degrees of dispersion realized for the other systems. It has been observed that nanoparticles can effectively treat deep and irregular wounds. These can also function as drug delivery vehicles to realize the precise release of antitumor drugs [226] and insulin [227] to target areas. Some researchers have reported the cytotoxicity of chitosan nanoparticles. Nanoparticles can also be used to treat fragile brain tissues for hemostasis as they exhibit good membrane penetration ability. Moreover, they can also cross the blood-brain barrier. These also exhibit anti-inflammation properties. PU nanoparticles, which differ from most nanoparticles in terms of stimulating immune responses, reduce the gene expression levels of pro-inflammatory factors in macrophages and exhibit the suppressive properties of immune responses [228]. Abel et al. blended a commercial gelatin granule formulation (Spongostan®) with PU nanoparticles and applied it to a rat neurosurgical model. It was observed that the sample significantly accelerated the clotting time and reduced bleeding [229] (Figure 5). Results obtained using the MRI technique revealed that the application of mixed nanoparticles significantly reduced brain edema and neuroinflammation in rats. It is necessary to pay attention to the possible cytotoxicity of the organic solvents used during the preparation process of polymeric nanoparticles.
PU NPs allow for hemostasis of fragile brain tissue. A: structure and properties of polyurethane NPs; B: comparison of brain edema formation after implantation of gelatin soaked with saline or PU NPs in a rat neurosurgical model; C: measurement of T2WI signals in the prefrontal lobe and comparison of signal intensity between the ipsilateral and contralateral prefrontal cortex on day 1 and day 7 after implantation. Reproduced with permission from [229]. ©2020 Elsevier Ltd. All rights reserved. PU: polyurethane.
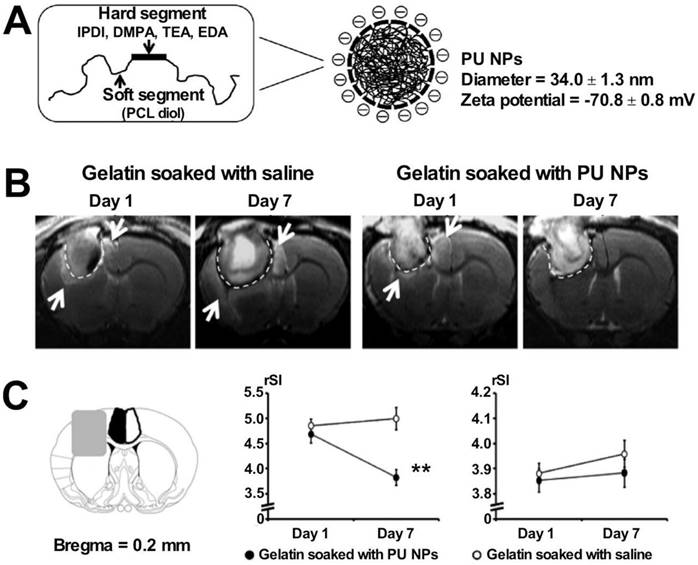
Most inorganic nanoparticles are easily synthesized. These exhibit good biocompatibility and are compatible with various ligands and biomolecules. Silver ions have antibacterial properties. Małgorzata et al. deposited Ag nanoparticles on the surface of chitosan nanofibers prepared following the electrostatic spinning technique. They studied the properties of these fibrous scaffolds without Ag nanoparticles and tested the E. coli and S. aureus inhibiting abilities [171]. Silicon dioxide-based nanoparticles (SNPs) have been the focus of research in the field of drug delivery as they exhibit excellent biocompatibility and are readily functionalized. SNPs could increase the concentration of platelets and coagulation factors by absorbing local water from wounds, and the negative charge on their surface could activate Factor XII to accelerate the coagulation cascade [157]. Chen et al. prepared functionalized SNPs following the sol-gel method using chitosan and hydrocaffeic acid. They demonstrated that the composite SNPs could effectively accelerate the formation of blood clots and help in hemostasis. They studied the rat femoral artery hemorrhage and the liver injury models to arrive at the results. Results from in vitro experiments indicated that the chitosan-modified SNPs could effectively adhere to erythrocytes and connect with fibrin to form blood clots [39].
Chen et al. exploited E. coli cytoplasmic membranes (EMs) and tumor cell membranes (TMs) from autologous tumor tissues to wrap polymeric PLGA nanoparticles on the surface to study the applicability of the samples in the field of development of tumor vaccines. It was observed that the samples could specifically elicit good anti-tumor reactivity, and significantly high levels of adverse effects were not observed [230]. Tests were conducted in mice models inoculated with CT26 colon cancer cells, B16-F10 melanoma, and EMT6 mammary tumors, and the results revealed that the composite nanoparticles could effectively inhibit the recurrence of tumor cells. These were also found to be hypersensitive to immunotherapy.
Hemostatic materials in the form of nanoparticles can be used to stop bleeding from deep and irregular wounds. These materials are portable and exhibit temperature-independent properties. It is noteworthy that nanoparticles are not easily removable. However, substances that are difficult to metabolize and degrade are potentially toxic and may obstruct blood vessels.
Others
In addition to the single forms of hemostatic materials discussed in the preceding sections, composite hemostatic materials have been developed to take advantage of the strengths of the single forms of hemostatic materials. The disadvantages of the single forms can be negated using composite hemostatic materials. The sponge deposited using gelatin nanofibers is an example of an efficient hemostatic material. Gelatin is spun electrostatically to produce nanofibers, and the continuous built up of the nanofibers results in the formation of a fluffy nanofiber sponge [25]. This highly fluffy nano-fiber sponge is ultra-lightweight and is characterized by excellent compressibility. The degree of compressibility is higher than the degree of compressibility recorded for the normal gelatin sponges. The preparation method is easy to execute, relatively cost-effective, and suitable for industrial production. The gelatin nanofiber-based sponge can remarkably reduce bleeding volume and shorten the clotting time in rabbit ear artery bleeding and liver injury models. It can rapidly fill the deep part of the wound attributable to incompressible injury (e.g., liver injury) to plug the bleeding site.
Cryogel presents an interconnected macroporous structure, and it is a shape memory hemostatic material. However, the mechanical strength of cryogels formed at low temperatures is lower than the mechanical strength of the cryogels formed at high temperatures. The introduction of CNT as an additive to cryogels can effectively improve their mechanical properties and the antibacterial ability of the systems [22]. CNT was dispersed into the quaternized chitosan solution under the action of ultrasonic waves, and the cryogel with shape memory was obtained under conditions of low-temperature polymerization at -20 ℃ [22]. The excellent hemostatic effect was observed in the mouse liver injury and rabbit liver injury models, and the sample could effectively promote wound healing in the whole skin defect model.
Injuries, especially battle wounds or trauma, hemorrhage, and these frequently get infected. Ci et al. synthesized biocompatible antimicrobial hybrid nanofibers using MoS2/ZnS and PCL following the electrostatic spinning method. The fabricated material exhibited promising antimicrobial activity under photocatalysis and could be potentially used to manufacture wearable antimicrobial hemostatic fabrics [231]. Luo et al. co-doped GO and Pt nanoparticles into a metal-organic framework (MOF, NH2-MIL-125), and this complex presents a promising photothermal effect under conditions of white light irradiation. The complex can be used to develop effective bactericides. It was shown that the antibacterial efficiency of the complexes against S. aureus and E. coli was as high as 99.94 and 99.12%, respectively. The results were achieved within 20 min of white light irradiation [232]. Wang et al. prepared a composite hybrid material containing Zn nanosheets and biodegradable MOF that can effectively kill bacteria and promote wound healing in bacterial infections [233]. Hybrid nanosheets of Zn and GO exhibit excellent antibacterial properties and accelerate the process of wound healing [234]. The modification of MOFs with metal ions and poly(dopamine) imparted excellent antimicrobial properties to the materials [235]. Metal-based MOF particles can be further polymerized with quaternary ammonium chitosan to synthesize photosensitive hydrogels, which exhibit excellent photothermal properties when irradiated with near-infrared light. The materials inhibit bacterial metabolism and respiration by charge transfer, resulting in bacterial death [236]. This hybrid material can be used to effectively treat bacterially infected open wounds in pathogenic bacteria-contaminated environments.
Composite materials can effectively integrate the advantages of individual forms. These materials exhibit antibacterial properties, boast of accelerated wound healing ability, and are characterized by shape memory properties. These materials are the focus of current research and are used to develop hemostatic materials.
Medical applications
Hemostatic materials are applied in various medical fields based on their properties, and currently, the wound healing and antimicrobial characteristics of these materials are the focus of research. Corneal healing and bone repair require the assistance of hemostatic materials. In addition, hemostatic materials can effectively reduce the degree of dissemination of tumor cells caused by bleeding. These materials are being extensively researched to develop improved materials with surgical applications.
Wound healing
Uncontrollable bleeding is frequently associated with patient death. Various hemostatic materials have been used to develop commercial formulations that can be used to realize hemostasis in humans. For example, Hemcon® (based on chitosan), TraumaDEX® (potato starch-based microparticles), and QuickClot® (based on zeolite), etc. are examples of such materials. However, the existing commercial materials exhibit poor biodegradability, cannot be used to treat complex wounds, and are difficult to remove. Hence, researchers are constantly trying to develop improved hemostatic materials.
Natural polymers are biocompatible and degradable. Chitosan, a positively charged natural polysaccharide, exhibits excellent hemostatic and antibacterial properties. Hence, it is being widely researched as a hemostatic material. Chitosan-based hemostatic materials exist in the forms of nanofibers, hydrogels, and nanoparticles. Composite materials can also be formed using chitosan. The diameters of the chitosan-based nanofibers prepared following the electrostatic spinning method are in the range of 9.2±3.7 nm. Thus, the materials boast of a high surface area that is in contact with wounds. These exhibit excellent hemostatic effects in hepatic perforation models in rats, rabbits, and pigs [237]. The chitosan-based hydrogel can moisten wounds to promote healing and stop bleeding. These hydrogels exhibit certain antibacterial properties to prevent wound infection [238]. Biranje et al. assembled chitosan nanoparticles into a chitosan dressing under conditions of lyophilization. They used it to study an in vitro model of human skin fibroblasts, and the results revealed that the sample could be used to accelerate the process of thrombin formation and effectively promote wound healing [239]. Composite materials integrate the advantages of multiple components. A composite sponge was prepared from cellulose and chitosan. Cellulose imparted good water absorption and rapid shape recovery properties, and chitosan imparted good antibacterial properties to the composite sponge. Fan et al. used composite sponges to study the mouse severed tail model, the rat liver injury model, and the rat femoral artery trauma model. The properties of the prepared sponge were compared with the properties of the gelatin sponges. It was observed that the composite sponges significantly shortened clotting time and reduced bleeding [23] (Figure 6).
Synthetic polymers are stable systems that can be easily prepared in large quantities and functionalized. Most synthetic polymers are not bioactive and thus cannot promote blood clotting. These cannot be considered as substrates or cross-linking agents for hemostatic materials. Hemostatic materials based on synthetic polymers are prepared as nanofibers, hydrogels, nanoparticles, and sponges. PCL nanofibers exhibit excellent biocompatibility and degradability and are FDA-approved medical materials that are being used in the field of hemostasis research. PCL nanofibers loaded with carmine can effectively accelerate the process of wound healing. These reduce scar tissue areas in diabetic mice wound models [240]. Self-assembling peptides can be used to treat various types of wounds. The short peptide RADA16 can rapidly self-assemble into nanofibers in an ion-rich environment, and the nanofiber barrier can rapidly stop bleeding when the material is used locally in the brain, liver, and femoral artery hemorrhage models. The peptide breaks down into amino acids and can further promote wound healing at the damaged site [241]. The injectable hydrogels based on polyglutamic acid and tetra PEG can be gelated rapidly in situ. These materials exhibit high mechanical strength and can be used to close large arterial bleeding sites to significantly shorten the hemostasis time and reduce blood loss [242]. PVA sponge is characterized by excellent water absorption ability. These can be used for effective hemostasis, and the materials function through expansion-local physical compression cycles [132].
Cellulose/chitosan sponges are available for deep wound hemostasis. A: schematic diagram of the process of preparing cellulose sponges with porogenic agents and surfactants; B: evaluation of the hemostatic effect of composite sponges applied to a rat liver trauma model. Reproduced with permission from [23]. ©2020 Elsevier Ltd. All rights reserved.
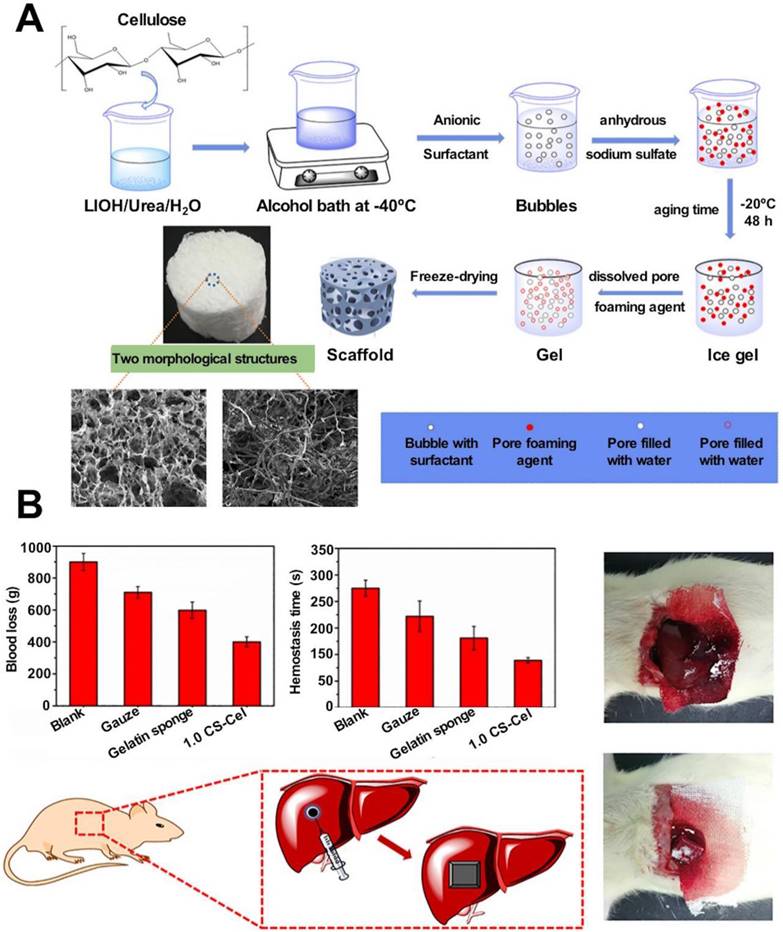
The first inorganic material to be used clinically to control bleeding was zeolite. QuickClot® was carried by U.S. troops in 2002. Zeolites can rapidly absorb fluid from the blood. These help to concentrate local clotting factors and platelets and promote the formation of blood clots. However, heat is released during the process, which can potentially damage tissues [243]. Liang et al. cross-linked zeolite with graphene sponges and controlled the process of heat release by exploiting the heat conduction ability of graphene to eliminate the thermal effect of zeolite. The process helped to control the wound temperature below 42 °C effectively. Rapid hemostasis was observed in the rat arterial injury model, and the use of the material shortened the bleeding time significantly. The bleeding time was shorter than the bleeding time recorded when the control group was treated with Quikclot® [155]. CNF can promote fibrin growth and blood clot formation, and its hydrophobic surface reduces the possibility of secondary bleeding when the dressing is removed. The deposition of CNfs on the surface of a gauze or sponge can significantly increase its surface area [244].
Metal ions such as silver, zinc, and copper exhibit excellent broad-spectrum antibacterial properties, heat resistance, and dispersibility. Metal ions are commonly used to prepare composite hemostatic materials. These are combined with other hemostatic active ingredients to prepare hemostatic materials. It is difficult to heal wounds in diabetic patients, and these wounds are associated with a high incidence of bacterial infections. Chronic nerve and vascular damage are also observed in diabetic patients. Nosheen Masood et al. blended silver nanoparticles with antimicrobial properties with a solution consisting of PEG and chitosan to form a hydrogel in the presence of glutaraldehyde (a cross-linking agent). They used the material to treat a diabetic rabbit wound model as the material exerted antioxidant and antimicrobial effects and promoted wound healing [245].
Schematic diagram of the synthesis of cryogels and their application in the prevention of tumor recurrence. A: schematic diagram of the synthesis of QCSG/HA-DA/ZDH composite cryogel; B: the role of composite cryogel in hemostasis and prevention of tumor recurrence. Reproduced with permission from [251]. © 2021 Wiley‐VCH GmbH. QCSG: glycidyl methacrylate-functionalized quaternized chitosan; HADA: dopamine-modified hyaluronic acid; HMME: hematoporphyrin monomethyl ether; ZDH: ZIF-8/DA/HMME.
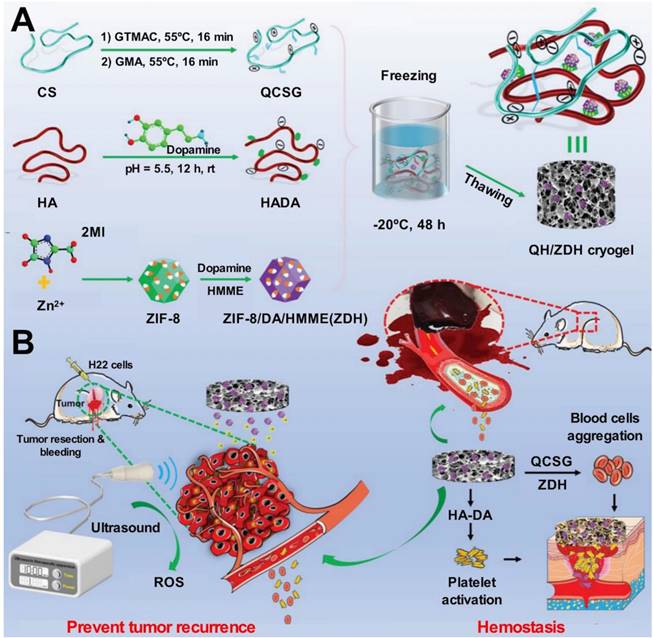
Electrospun PCL/MoS2 nanofiber membranes could accelerate bone regeneration. A: schematic diagram of PCL/MoS2 nanofibrous membrane preparation and promotion of osteogenesis in vivo; B: microCT images and bone volume/total volume (BV/TV) ratio analysis after implantation of PCL and PCL/1% MoS2 in a rat tibial defect model. Reproduced with permission from [258]. © 2021 Wiley-VCH GmbH. PCL: poly(ε-caprolactone)
Prevention of tumor recurrence
Hemostatic materials are regularly used in surgical procedures. Surgery is the preferred and most effective way to treat common solid tumors (those associated with lung, breast, and colorectal cancers). Tumor tissues have an abundant local blood supply and contain multiple abnormal neovascularizations. Thus, bleeding inevitably occurs during surgery, posing difficulties during surgical procedures. However, it adversely affects disease prognosis [246]. Intraoperative bleeding significantly increases the risk of tumor recurrence attributable to the metastasis of cancer cells [247]. Although some commercial hemostatic materials such as QuickClot®, glutaraldehyde-cross-linked albumin [248], and fibrin bandages [249] are used to treat superficial bleeding conditions, simple gauze tamponade is primarily used during surgery in the cases of malignant tumors. This significantly increases surgical risks. Rapid hemostasis should be achieved using hemostatic materials. Therefore, the hemostatic materials should be able to improve patient prognosis and prevent the recurrence of postoperative tumors.
It is important to develop advanced integrated therapeutic solutions (hemostatic materials) that can rapidly stop bleeding and load anti-tumor drugs to advance the field of oncology treatment. In addition to exerting hemostatic effects, hemostatic gelatin sponges loaded with 5-FU can release drugs continuously and target local tumors. These materials exhibit significantly high degrees of anti-tumor effects when used to treat (in situ) a mouse colorectal cancer model [250]. The applicability of gelatin sponges is limited by the low strength and rapid degradation rate of the materials. These materials exert a limited effect on extensively bleeding surgical sites. Hence, various composites have been developed. Guo et al. combined the advantages of multiple hemostatic activities to prepare zeolite nanoparticle-enhanced multi-network cryogels. Materials based on quaternized chitosan, dopamine-modified HA, and ultrasound-sensitizer-loaded dopamine-modified ZIF-8 were developed, and the cross-linking between the components was realized at -20 ℃ following the cryogelation technique. The crystalloid network exhibits excellent water absorption performance, effectively concentrates local blood cells and platelets, and improves the clotting effect. These properties can be attributed to the high-density through-pore structure of the material. Rapid and efficient hemostasis could be achieved when it was used to address large-volume defects associated with the liver. Reactive oxygen species (ROS) were produced under the action of ultrasound, and acoustodynamic anti-cancer effects were recorded. It was used to study the mouse ectopic liver tumor model, and it was observed that it could significantly improve the survival rate of mice after surgery [251] (Figure 7). Combinations of sponges and nanofibers could also be used to achieve intraoperative hemostasis and generate antitumor effects. Chen et al. used alginate and gelatin sponges cross-linked following the interpenetrating polymer network (IPN) strategy to exert hemostatic synergy. The system was combined with the curcumin-loaded PCL-PEG-PCL nanofibers to control the accumulation of curcumin at the tumor surgical sites. The experiments were conducted using a mouse subcutaneous tumor recurrence model. The results revealed that the material could significantly inhibit tumor recurrence [252]. The chitosan/gelatin sponge prepared following the freeze-drying method was characterized by the presence of a sandwich-like layer of adriamycin-regalactone fibers in the middle. The sponge could safely and efficiently implant the composite material locally into the surgical resection cavity to prevent bleeding at the surgical site. These could release drugs to inhibit tumor recurrence. Postoperative patient mortality could be reduced, and patient prognosis could be improved [253].
The appropriate selection and application of hemostatic materials can effectively reduce intraoperative bleeding. Hemostatic materials can help clear the surgical field clear and reduce the difficulty of surgery. The materials can also be used to reduce tumor metastasis attributable to blood. In addition, the extent of postoperative recurrence can be reduced, and the prognosis can be improved. Thus, developing effective hemostatic materials with a clinical application is important.
Bone repair
Bone defects are a common complication of trauma, inflammation, infection, and bone tumor resection. The bleeding caused by bone defects can be attributed to the injury of cancellous bone, which is loose in structure and rich in blood flow. In most cases, blood oozes out from cancellous bones, and hemostasis by vasoconstriction is difficult. It is difficult to stop bleeding using hemostatic gauze or following conventional methods such as electrocoagulation, clamping, and collagen sponge filling [254]. Liu et al. assembled quaternized branched-chain starch and tannic acid onto the surface of porous starch to construct multilayer microspheres to treat hemorrhage in cancellous bone defects. The composite microspheres consisting of branched starch and tannic acid could promote platelet adhesion, activation, and aggregation of RBCs, exhibiting optimal hemostatic properties. Inadequate inflammatory response and pro-bone repair properties were observed when the material was used to study a mouse cancellous bone defect model. Further proof-of-concept models in Beagle dogs confirmed the positive effect of composite microspheres, and it was observed that the composites could be used to treat bleeding bone defects in large mammals [255]. Loading calcium phosphate and chitosan layers on the surface of electrospun gelatin could provide rapid hemostasis. Application of the material in a rat skull defect model revealed that it could significantly promote the process of bone regeneration [256].
Severe infections accompany bone defects resulting from trauma. Wang et al. prepared a bone defect-filling material with a sequential release system that rapidly releases hydroxypropyltrimethyl ammonium chloride chitosan, a potent antimicrobial agent, to kill potentially infected bacteria. The bone morphogenetic protein-2 (BMP2) is gradually released, and the process of bone repair is promoted [257].
A combination of PCL nanofibers and molybdenum disulfide significantly promotes the growth of bone mesenchymal stem cells in vitro. The composite could accelerate osteogenesis and the process of bone healing when applied to a rat tibial bone defect model [258] (Figure 8). The introduction of polyacrylic acid into PVA-based hydrogels can significantly enhance the mechanical strength of the material and improve the cell adhesion property. It can be used as a cartilage tissue replacement that can maintain the cell growth morphology and promote the processes of bone regeneration and repair [196]. Hydroxyapatite, alumina ceramics, calcium carbonate, and other inorganic materials are also used as scaffolds in the field of bone tissue engineering [259]. In addition to promoting bone repair, some hemostatic materials with antimicrobial properties can be used to treat osteomyelitis. For example, magnetic composites prepared from molybdenum disulfide and iron oxide have been used to treat bacterially infected osteomyelitis effectively [260].
Eye diseases
Hemostatic materials should be carefully chosen to treat special areas of the body. The transparent cornea is the first line of defense of the eye against external forces. It forms the foundation of the visual function. Corneal damage is a common cause of visual impairment. The usage of biomaterials for corneal repair can effectively reduce patient costs as the process is cheaper than the transplantation process, which suffers from a severe donor shortage [261, 262]. The selection of hemostatic materials for the treatment of corneal injury should take into account the following relevant properties: 1) excellent biocompatibility and biodegradability; 2) high transparency; 3) mechanical stability and support; 4) high adhesion to tissue; 5) ability to promote endogenous tissue regeneration; and 6) stable physicochemical properties and ease of application. Multiple natural and synthetic polymers have been developed as tissue adhesives for corneal sealing and repair. The natural polymer chitosan exhibits excellent mucosal adhesion and antimicrobial properties. Moreover, it promotes corneal wound healing. Therapeutic drugs can be incorporated into chitosan gels to protect them from degradation and improve the extent of absorption [263]. Sani et al. designed a gelatin-based adhesive consisting of gelatin and a photo-initiator that can be photo-crosslinked following a short exposure to visible light to form a hydrogel that adheres firmly to the corneal surface. The researchers used the adhesive to study a rabbit corneal stromal wound model and demonstrated that it could effectively seal corneal defects and induce stromal regeneration. The combination of gelatin and photoinitiator is facile to apply. The composite significantly improves the contact time of the adhesive with the cornea. This helps reduce the frequency of administration and improves patient compliance [264] (Figure 9). Collagen, a natural polymer, is the equivalent of type I collagenous protofibrils present in human connective tissues. It is biocompatible and permeable and promotes the processes of corneal epithelial and stromal regeneration [265]. Chae et al. used collagen vitrigel to study a rabbit corneal stromal wound model and revealed that collagen vitrigel effectively promoted corneal repair. The material could be used to maintain the original tissue transparency. It could also be used to reduce the degree of inflammatory responses generated and inhibit neovascularization [266]. Natural polymers are biocompatible. However, the degree of mechanical stability is low. ReSure Sealant®, a PEG-based adhesive, was the first to be approved by the FDA in 2014 for the treatment of corneal incisions following IOL placement for cataract surgery [267]. Shehata et al. tested the ability of ReSure® to withstand elevated intraocular pressure in a rabbit corneal wound model [268]. It was observed that ReSure® could not adhere strongly to ocular surfaces, and it could easily get detached from the surfaces in wet ocular environments. It also could not promote the processes of corneal epithelial and stromal regeneration. Various composite materials are being prepared for application in the field of managing corneal wounds. Chitosan-based thermosensitive in situ prepared hydrogels remain in the liquid state at room temperature. They get converted to the gel state as the hydrogen bonds break with an increase in temperature. Gratieri et al. prepared (in situ) a hydrogel based on PEO-PPO-PEO and chitosan. The experimental results revealed that 50-60% of the hydrogel remained in contact with the corneal surface after 10 min of dropping them into the human eye. This significantly increased the retention time [269]. The addition of chitosan nanoparticles to chitosan/polycaprolactone gels can effectively improve the mechanical properties and antibacterial ability of the composites. This also helped maintain the corneal shape and surface-wetting properties. Matrix regeneration was also promoted [270].
Tooth Repair
Tooth extraction is a common surgical procedure. Oral and maxillofacial surgeries are performed to extract incurable affected teeth and pathogenic teeth that cause other diseases. Surgeries are also performed to extract teeth to introduce orthodontics or prosthetics. A surgeon chooses an appropriate hemostatic material to fill the bleeding wound to prevent secondary bleeding or bacterial infection. The oral cavity is the second largest microbial habitat in the body after the intestinal tract. Thus, with its antimicrobial properties, chitosan can be effectively used for oral hemostasis. Chang et al. prepared a composite hydrogel from chitosan and polyglutamic acid and used it to treat the Wistar rat incisor extraction model in a controlled group. The effects exerted by gelatin sponges, pure chitosan, and composite hydrogels were compared. The results indicated that the composite hydrogel could effectively promote the process of formation of new bones in the alveolar sockets after tooth extraction. The use of hydrogel could reduce the occurrence of infection [271]. Chitosan exhibits anti-bacterial and anti-fungal properties and the application of chitosan hydrogel can effectively reduce pain and inflammation following dental procedures [272]. Fibrin hydrogels can also be used for hemostasis after tooth extraction. Sarkar et al. conducted a clinical randomized controlled trial that included 60 patients with extracted teeth. The patients were subjected to conditions of oral antiplatelet therapy and randomized to apply either fibrin-based hydrogels or chitosan-based hydrogels for hemostasis. The trial revealed that chitosan hydrogels were more effective than fibrin-based hydrogels for hemostasis. These significantly reduced clotting time. However, the fibrin-based hydrogels were better at promoting the process of wound healing [273].
Periodontal disease is a common oral disease and is one of the leading causes of tooth loss in adults. It is primarily manifested by inflammation and bleeding of gums, resorption of alveolar bone, loosening and displacement of teeth, and tooth loss [274]. Chitosan promotes tissue healing and induces alveolar bone formation. It plays a vital role in guided tissue regeneration techniques [275]. Liu et al. prepared a thermosensitive responsive hydrogel loaded with stromal cell-derived factor-1 (SDF-1) and used it to study a rat periodontitis model. The results revealed that the material could inhibit inflammation and promote the regeneration of alveolar bone [276] (Figure 10). Zhang et al. prepared a sandwich-like composite scaffold consisting of chitosan/PCL/gelatin. The scaffold was biocompatible and mechanically stable. It swelled and adhered well to cells. Sufficient oxygen and energy for the cell and tissue regeneration processes were obtained via the pores [100].
Gelatin-based naturally adherent hydrogels (GelCORE) can be a suitable tool for repairing corneal damage. A: schematic diagram of GelCORE synthesis; B: schematic diagram of GelCORE for rapid and long-term repair of corneal damage; anterior segment optical coherence tomography images of a rabbit corneal defect model C before and after application of GelCORE. Reproduced with permission from [264]. ©2019 Authors.
PEG-DA-based thermosensitive hydrogels are available for therapeutic use in periodontal disease. A: schematic diagram of the synthesis of in situ thermosensitive hydrogel; B: micro-CT analysis of the effect of in situ hydrogel injection on periodontal bone regeneration after one week. Reproduced with permission from [276]. ©2021 American Chemical Society. PEGD: polyethylene glycol diacrylate; SDF-1: stromal cell derived factor-1
Conclusion and Perspectives
In this review, we have discussed the advantages and disadvantages of using different components or materials used to develop hemostatic materials. Three aspects have been primarily considered: active hemostatic ingredients, material forms, and medical applications. Natural polymers, synthetic polymers, inorganic materials, and materials containing metals are the four main categories of materials used to realize hemostasis. Chitosan-based nanofibers, hemostatic sponges, and hydrogels have been used to obtain satisfactory results in animal models characterized by low bleeding volumes. However, excellent performance could not be achieved for the cases of high-bleeding wounds. Collagen, gelatin, and other natural polysaccharides can potentially cause allergic reactions. Synthetic polymers are readily available and can be conveniently and functionally modified. The poor bioactivity of synthetic polymers, which can be produced in bulk, hinders their practical application. Inorganic materials have shown long-term physical and chemical stability without bio-toxicity. Zeolite emits a lot of heat when it encounters water or blood. This results in an increase in the local temperature of the wound. This can potentially cause skin burns and hinder the process of wound healing. The various metal ions used to prepare the hemostatic materials impart excellent antibacterial properties to the materials. The metal ion also improves the coagulation efficiency of the materials. There is a growing trend to design smart hemostatic materials that can mimic natural hemostatic processes. For example, platelet mimics that promote thrombus contraction and fibrin-structured nanofibers that facilitate clot formation may point toward the development of novel hemostatic materials.
At present, most commercially available hemostatic materials are designed for large, flat wounds, and materials to treat deep, irregular wounds should be developed. Flexible hemostatic materials that adhere tightly to the surrounding tissue should be developed to treat wounds near joints to avoid dressing dislodgement. Excessive bleeding hinders adhesion. Hemostatic materials prepared in the form of hydrogels and sponges adhere well to tissues. Methods to safely remove or degrade the materials following use should be researched. Hemostatic materials should be removed safely without causing pain and secondary bleeding (due to exfoliation). For degradable materials, the relation between the rate of degradation and the pace of hemostasis and tissue repair needs to be considered. The degradation products should not induce inflammatory responses or obstruct blood vessels in vivo. An ideal hemostatic material should have the following characteristics: a) it should have the ability to be used directly at bleeding sites, and the hemostatic time should be short; b) it should be inexpensive and convenient to apply; c) it should have the potential to be mass-produced, there should not be differences in the properties of the materials produced in different batches; d) it should be stable and portable and should be characterized by a prolonged storage time; e) it should be absorbable, should not require cleaning, and should not cause tissue damage or infection.
Abbreviations
HA: hyaluronic acid; PEG: polyethylene glycol; PU: polyurethane; PVA: polyvinyl alcohol; PCL: polycaprolactone; PEO: poly (ethylene oxide); EDTA: ethylenediaminetetraacetic acid; OC: oxidized cellulose; TEMPO: 2,2,6,6-tetramethyl-1-piperidinyloxy; DTP: 3,3'-dithiopropionic acid; HA-DTPH: mercapto-hyaluronic acid derivative; HA-tyr: HA-tyrosine; HA-Ph: phenolic hydroxy HA; AOS: alginate oligomers; PEGDA: poly(ethylene glycol)diacrylate; FDA: Food and Drug Administration; PMMA: polymethylmethacrylate; PPO: poly(propylene oxide); GO: graphene oxide; GMCS: graphene/montmorillonite composite sponges; MMT: montmorillonite; ALP: alkaline phosphatase; PLLA: poly(lactic acid); FMP: fibrinogen mimetic peptide; PLGA-PLL: poly(lactic-co-glycolic acid)-poly-L-lysine; CNT: carbon nanotube; SGH: self-assembled graphene hydrogel; EDC: 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide; GelMA: gelatin methacrylate; HAMA: methacrylated HA; CMC: carboxymethyl chitosan; SA: sodium alginate; TA: tannic acid; MFC: microprotofibrillated cellulose; cCNCs: carboxylated cellulose nanocrystals; SCD: supercritical drying; SD: spray drying; OD: oven drying; PVF: poly(vinyl formal); TRAP: thrombin receptor agonist peptides; LNPs: liposome-based nanoparticles; SNPs: silicon dioxide-based nanoparticles; ROS: reactive oxygen species; IPN: interpenetrating polymer network; BMP2: bone morphogenetic protein-2; SDF-1: stromal cell-derived factor-1.
Acknowledgements
We would like to thank KetengEdit (www.ketengedit.com) for linguistic assistance in the preparation of this manuscript.
Funding
This study was supported by National Natural Science Foundation of China (Grant No. 32000953), Science and Technology Department of Jilin Province (Grant No. YDZJ202201ZYTS004), Science and Technology Department of Changchun (Grant No. 3D5220102429).
Consent for publication
All the authors reviewed the manuscript and agreed to the publication.
Author contributions
Yu Guo and Min Wang drafted the manuscript, Guoliang Liu and Qi Liu revised and produced the pictures, and Shuang Wang and Jiannan Li reviewed and revised the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Blackbourne LH, Baer DG, Eastridge BJ, Kheirabadi B, Bagley S, Kragh JF Jr. et al. Military medical revolution: Prehospital combat casualty care. J Trauma Acute Care Surg. 2012;73(6 Suppl 5):S372-77
2. Mabry R, McManus JG. Prehospital advances in the management of severe penetrating trauma. Crit Care Med. 2008;36(7 Suppl):S258-66
3. Dries DJ. The contemporary role of blood products and components used in trauma resuscitation. Scand J Trauma Resusc Emerg Med. 2010;18:63
4. Dudaryk R, Hess AS, Varon AJ, Hess JR. What is new in the blood bank for trauma resuscitation. Curr Opin Anaesthesiol. 2015;28(2):206-09
5. Mecwan M, Li J, Falcone N, Ermis M, Torres E, Morales R. et al. Recent advances in biopolymer-based hemostatic materials. Regen Biomater. 2022;9:rbac063
6. O'Donnell JS, O'Sullivan JM, Preston RJS. Advances in understanding the molecular mechanisms that maintain normal haemostasis. Br J Haematol. 2019;186(1):24-36
7. Puri RN, Colman RW. ADP-induced platelet activation. Crit Rev Biochem Mol Biol. 1997;32(6):437-502
8. Grover SP, Mackman N. Intrinsic pathway of coagulation and thrombosis. Arterioscler Thromb Vasc Biol. 2019;39(3):331-38
9. Winter WE, Flax SD, Harris NS. Coagulation testing in the core laboratory. Lab Med. 2017;48(4):295-313
10. Brière JB. Essential thrombocythemia. Orphanet J Rare Dis. 2007;2:3
11. Wilkins RG, Unverdorben M. Wound cleaning and wound healing: A concise review. Adv Skin Wound Care. 2013;26(4):160-63
12. Bogdan Y, Helfet DL. Use of tourniquets in limb trauma surgery. Orthop Clin North Am. 2018;49(2):157-65
13. Burduk PK, Wierzchowska M, Grześkowiak B, Kaźmierczak W, Wawrzyniak K. Clinical outcome and patient satisfaction using biodegradable (nasopore) and non-biodegradable packing, a double-blind, prospective, randomized study. Braz J Otorhinolaryngol. 2017;83(1):23-28
14. Sun X, Li J, Shao K, Su C, Bi S, Mu Y. et al. A composite sponge based on alkylated chitosan and diatom-biosilica for rapid hemostasis. Int J Biol Macromol. 2021;182:2097-107
15. Slezak P, Keibl C, Redl H, Labahn D, Gulle H. An efficacy comparison of two hemostatic agents in a porcine liver bleeding model: Gelatin/thrombin flowable matrix versus collagen/thrombin powder. J Invest Surg. 2020;33(9):828-38
16. Chen SX, Li RQ, Li XR, Xie JW. Electrospinning: An enabling nanotechnology platform for drug delivery and regenerative medicine. Adv Drug Deliv Rev. 2018;132:188-213
17. Chen SX, John JV, McCarthy A, Xie JW. New forms of electrospun nanofiber materials for biomedical applications. J Mater Chem B. 2020;8(17):3733-46
18. Ahmed R, Tariq M, Ali I, Asghar R, Noorunnisa Khanam P, Augustine R. et al. Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. Int J Biol Macromol. 2018;120(Pt A):385-93
19. Eivazzadeh-Keihan R, Radinekiyan F, Aliabadi HAM, Sukhtezari S, Tahmasebi B, Maleki A. et al. Chitosan hydrogel/silk fibroin/Mg(OH)(2) nanobiocomposite as a novel scaffold with antimicrobial activity and improved mechanical properties. Sci Rep. 2021;11(1):650
20. Ma Y, Yao J, Liu Q, Han T, Zhao J, Ma X. et al. Liquid bandage harvests robust adhesive, hemostatic, and antibacterial performances as a first-aid tissue adhesive. Adv Funct Mater. 2020;30:2001820
21. Li J, Sun X, Zhang K, Yang G, Mu Y, Su C. et al. Chitosan/diatom-biosilica aerogel with controlled porous structure for rapid hemostasis. Adv Healthc Mater. 2020;9(21):e2000951
22. Zhao X, Guo B, Wu H, Liang Y, Ma PX. Injectable antibacterial conductive nanocomposite cryogels with rapid shape recovery for noncompressible hemorrhage and wound healing. Nat Commun. 2018;9(1):2784
23. Fan X, Li M, Yang Q, Wan G, Li Y, Li N. et al. Morphology-controllable cellulose/chitosan sponge for deep wound hemostasis with surfactant and pore-foaming agent. Mater Sci Eng C Mater Biol Appl. 2021;118:111408
24. Hong Y, Zhou F, Hua Y, Zhang X, Ni C, Pan D. et al. A strongly adhesive hemostatic hydrogel for the repair of arterial and heart bleeds. Nat Commun. 2019;10(1):2060
25. Xie X, Li D, Chen Y, Shen Y, Yu F, Wang W. et al. Conjugate electrospun 3D gelatin nanofiber sponge for rapid hemostasis. Adv Healthc Mater. 2021;10(20):e2100918
26. Yang X, Zhang Y, Huang C, Lu L, Chen J, Weng Y. Biomimetic hydrogel scaffolds with copper peptide-functionalized rada16 nanofiber improve wound healing in diabetes. Macromol Biosci. 2022 e2200019
27. Nie W, Yuan X, Zhao J, Zhou Y, Bao H. Rapidly in situ forming chitosan/ε-polylysine hydrogels for adhesive sealants and hemostatic materials. Carbohydr Polym. 2013;96(1):342-48
28. Bu Y, Zhang L, Sun G, Sun F, Liu J, Yang F. et al. Tetra-PEG based hydrogel sealants for in vivo visceral hemostasis. Adv Mater. 2019;31(28):e1901580
29. Yang X, Liu W, Shi Y, Xi G, Wang M, Liang B. et al. Peptide-immobilized starch/PEG sponge with rapid shape recovery and dual-function for both uncontrolled and noncompressible hemorrhage. Acta Biomaterialia. 2019;99:220-35
30. Huang AP, Lai DM, Hsu YH, Tsai HH, Su CY, Hsu SH. An anti-inflammatory gelatin hemostatic agent with biodegradable polyurethane nanoparticles for vulnerable brain tissue. Mater Sci Eng C Mater Biol Appl. 2021;121:111799
31. Schulten L, Spillner J, Kanzler S, Teubner A, Jockenhoevel S, Apel C. A polyurethane-based surgical adhesive for sealing blood vessel anastomoses-a feasibility study in pigs. J Biomed Mater Res B Appl Biomater. 2022;110(8):1922-31
32. Yan Q, Long X, Zhang P, Lei W, Sun D, Ye X. Oxidized bletilla rhizome polysaccharide-based aerogel with synergistic antibiosis and hemostasis for wound healing. Carbohydr Polym. 2022;293:119696
33. Goncharuk O, Korotych O, Samchenko Y, Kernosenko L, Kravchenko A, Shtanova L. et al. Hemostatic dressings based on poly(vinyl formal) sponges. Mater Sci Eng C Mater Biol Appl. 2021;129:112363
34. Adeli-Sardou M, Yaghoobi MM, Torkzadeh-Mahani M, Dodel M. Controlled release of lawsone from polycaprolactone/gelatin electrospun nano fibers for skin tissue regeneration. Int J Biol Macromol. 2019;124:478-91
35. Patel KD, Kim TH, Mandakhbayar N, Singh RK, Jang JH, Lee JH. et al. Coating biopolymer nanofibers with carbon nanotubes accelerates tissue healing and bone regeneration through orchestrated cell- and tissue-regulatory responses. Acta Biomater. 2020;108:97-110
36. Daristotle JL, Zaki ST, Lau LW, Torres L Jr, Zografos A, Srinivasan P. et al. Improving the adhesion, flexibility, and hemostatic efficacy of a sprayable polymer blend surgical sealant by incorporating silica particles. Acta Biomater. 2019;90:205-16
37. Deineka V, Sulaieva O, Pernakov M, Korniienko V, Husak Y, Yanovska A. et al. Hemostatic and tissue regeneration performance of novel electrospun chitosan-based materials. Biomedicines. 2021;9(6):588
38. Wang C, Zhou H, Niu H, Ma X, Yuan Y, Hong H. et al. Tannic acid-loaded mesoporous silica for rapid hemostasis and antibacterial activity. Biomater Sci. 2018;6(12):3318-31
39. Chen J, Ai J, Chen S, Xu Z, Lin J, Liu H. et al. Synergistic enhancement of hemostatic performance of mesoporous silica by hydrocaffeic acid and chitosan. INT J BIOL MACROMOL. 2019;139:1203-11
40. Borges-Vilches J, Figueroa T, Guajardo S, Aguayo C, Fernández K. Improved hemocompatibility for gelatin-graphene oxide composite aerogels reinforced with proanthocyanidins for wound dressing applications. Colloids Surf B Biointerfaces. 2021;206:111941
41. Li G, Quan K, Liang Y, Li T, Yuan Q, Tao L. et al. Graphene-montmorillonite composite sponge for safe and effective hemostasis. ACS Appl Mater Interfaces. 2016;8(51):35071-80
42. Wang X, Cheng F, Liu J, Smått JH, Gepperth D, Lastusaari M. et al. Biocomposites of copper-containing mesoporous bioactive glass and nanofibrillated cellulose: Biocompatibility and angiogenic promotion in chronic wound healing application. Acta Biomater. 2016;46:286-98
43. Jung H, Kang YY, Mok H. Platelet-derived nanovesicles for hemostasis without release of pro-inflammatory cytokines. Biomater Sci. 2019;7(3):856-59
44. Modery-Pawlowski CL, Tian LL, Ravikumar M, Wong TL, Sen Gupta A. In vitro and in vivo hemostatic capabilities of a functionally integrated platelet-mimetic liposomal nanoconstruct. Biomaterials. 2013;34(12):3031-41
45. Sandri G, Rossi S, Bonferoni MC, Miele D, Faccendini A, Del Favero E. et al. Chitosan/glycosaminoglycan scaffolds for skin reparation. Carbohydr Polym. 2019;220:219-27
46. Ye Q, Chen SH, Zhang Y, Ruan B, Zhang YJ, Zhang XK. et al. Chitosan/polyvinyl alcohol/ lauramidopropyl betaine/2D-HOF mixed film with abundant hydrogen bonds acts as high mechanical strength artificial skin. Macromol Biosci. 2021;21(12):e2100317
47. Ávila Filho SH, Dias Ferreira K, Vieira da Silva R, Vieira de Souza Silva E, Fernandes Santos Catelan B, Brianezi Dignani de Moura VM. et al. Comparison between poliglecaprone and chitosan absorbable sutures in laparorrhaphy and cecorrhaphy in rabbits. J Biomed Mater Res B Appl Biomater. 2019;107(6):2102-08
48. Yang Y, Yang SB, Wang YG, Zhang SH, Yu ZF, Tang TT. Bacterial inhibition potential of quaternised chitosan-coated vicryl absorbable suture: An in vitro and in vivo study. J Orthop Translat. 2017;8:49-61
49. Shi C, Zhu Y, Ran X, Wang M, Su Y, Cheng T. Therapeutic potential of chitosan and its derivatives in regenerative medicine. J Surg Res. 2006;133(2):185-92
50. Islam MA, Park TE, Reesor E, Cherukula K, Hasan A, Firdous J. et al. Mucoadhesive chitosan derivatives as novel drug carriers. Curr Pharm Des. 2015;21(29):4285-309
51. Turcsányi Á, Varga N, Csapó E. Chitosan-modified hyaluronic acid-based nanosized drug carriers. Int J Biol Macromol. 2020;148:218-25
52. Rodríguez-Vázquez M, Vega-Ruiz B, Ramos-Zúñiga R, Saldaña-Koppel DA, Quiñones-Olvera LF. Chitosan and its potential use as a scaffold for tissue engineering in regenerative medicine. Biomed Res Int. 2015;2015:821279
53. Rao SB, Sharma CP. Use of chitosan as a biomaterial: Studies on its safety and hemostatic potential. J Biomed Mater Res. 1997;34(1):21-28
54. Tan W, Li Q, Dong F, Chen Q, Guo Z. Preparation and characterization of novel cationic chitosan derivatives bearing quaternary ammonium and phosphonium salts and assessment of their antifungal properties. Molecules. 2017;22(9):1438
55. Li Q, Li Q, Tan W, Zhang J, Guo Z. Phenolic-containing chitosan quaternary ammonium derivatives and their significantly enhanced antioxidant and antitumor properties. Carbohydr Res. 2020;498:108169
56. Wibel R, Braun DE, Hämmerle L, Jörgensen AM, Knoll P, Salvenmoser W. et al. In vitro investigation of thiolated chitosan derivatives as mucoadhesive coating materials for solid lipid nanoparticles. Biomacromolecules. 2021;22(9):3980-91
57. Jayakumar R, Selvamurugan N, Nair SV, Tokura S, Tamura H. Preparative methods of phosphorylated chitin and chitosan-an overview. Int J Biol Macromol. 2008;43(3):221-25
58. Martău GA, Mihai M, Vodnar DC. The use of chitosan, alginate, and pectin in the biomedical and food sector-biocompatibility, bioadhesiveness, and biodegradability. Polymers (Basel). 2019;11(11):1837
59. Chen Z, Yao X, Liu L, Guan J, Liu M, Li Z. et al. Blood coagulation evaluation of n-alkylated chitosan. Carbohydr Polym. 2017;173:259-68
60. Rong L, Liu H, Wang B, Mao Z, Xu H, Zhang L. et al. Durable antibacterial and hydrophobic cotton fabrics utilizing enamine bonds. Carbohydr Polym. 2019;211:173-80
61. Zhang S, Li J, Chen S, Zhang X, Ma J, He J. Oxidized cellulose-based hemostatic materials. Carbohydr Polym. 2020;230:115585
62. Parikka K, Leppänen AS, Xu C, Pitkänen L, Eronen P, Osterberg M. et al. Functional and anionic cellulose-interacting polymers by selective chemo-enzymatic carboxylation of galactose-containing polysaccharides. Biomacromolecules. 2012;13(8):2418-28
63. Soares LP, Oliveira MG, Pinheiro AL, Fronza BR, Maciel ME. Effects of laser therapy on experimental wound healing using oxidized regenerated cellulose hemostat. Photomed Laser Surg. 2008;26(1):10-13
64. Li H, Cheng F, Chávez-Madero C, Choi J, Wei X, Yi X. et al. Manufacturing and physical characterization of absorbable oxidized regenerated cellulose braided surgical sutures. Int J Biol Macromol. 2019;134:56-62
65. Isogai T, Saito T, Isogai A. Tempo electromediated oxidation of some polysaccharides including regenerated cellulose fiber. Biomacromolecules. 2010;11(6):1593-99
66. Aydemir Sezer U, Sahin İ, Aru B, Olmez H, Yanıkkaya Demirel G, Sezer S. Cytotoxicity, bactericidal and hemostatic evaluation of oxidized cellulose microparticles: Structure and oxidation degree approach. Carbohydr Polym. 2019;219:87-94
67. Ibne Mahbub MS, Sultana T, Gwon JG, Lee BT. Fabrication of thrombin loaded tempo-oxidized cellulose nanofiber-gelatin sponges and their hemostatic behavior in rat liver hemorrhage model. J Biomater Sci Polym Ed. 2022;33(4):499-516
68. Spaziani E, Di Filippo A, Francioni P, Spaziani M, De Cesare A, Picchio M. Subhepatic mass occurrence after using oxidized and regenerated cellulose polymer in laparoscopic cholecystectomy: A case series. Acta Chir Belg. 2018;118(1):48-51
69. Franceschini G. Internal surgical use of biodegradable carbohydrate polymers. Warning for a conscious and proper use of oxidized regenerated cellulose. Carbohydr Polym. 2019;216:213-16
70. Fallacara A, Manfredini S, Durini E, Vertuani S. Hyaluronic acid fillers in soft tissue regeneration. Facial Plast Surg. 2017;33(1):87-96
71. Aya KL, Stern R. Hyaluronan in wound healing: Rediscovering a major player. Wound Repair Regen. 2014;22(5):579-93
72. Tavianatou AG, Caon I, Franchi M, Piperigkou Z, Galesso D, Karamanos NK. Hyaluronan: Molecular size-dependent signaling and biological functions in inflammation and cancer. Febs j. 2019;286(15):2883-908
73. Harrington S, Williams J, Rawal S, Ramachandran K, Stehno-Bittel L. Hyaluronic acid/collagen hydrogel as an alternative to alginate for long-term immunoprotected islet transplantation. Tissue Eng Part A. 2017;23(19-20):1088-99
74. Loebel C, Szczesny SE, Cosgrove BD, Alini M, Zenobi-Wong M, Mauck RL. et al. Cross-linking chemistry of tyramine-modified hyaluronan hydrogels alters mesenchymal stem cell early attachment and behavior. Biomacromolecules. 2017;18(3):855-64
75. Bagheri S, Bagher Z, Hassanzadeh S, Simorgh S, Kamrava SK, Nooshabadi VT. et al. Control of cellular adhesiveness in hyaluronic acid-based hydrogel through varying degrees of phenol moiety cross-linking. J Biomed Mater Res A. 2021;109(5):649-58
76. Choi YR, Kim HJ, Ahn GY, Lee MJ, Park JR, Jun DR. et al. Fabrication of dihydroxyflavone-conjugated hyaluronic acid nanogels for targeted antitumoral effect. Colloids Surf B Biointerfaces. 2018;171:690-97
77. Štrympl O, Vohlídal J, Hermannová M, Maldonado-Domínguez M, Brandejsová M, Kopecká K. et al. Oleate-modified hyaluronan: Controlling the number and distribution of side chains by varying the reaction conditions. Carbohydr Polym. 2021;267:118197
78. Browne S, Hossainy S, Healy K. Hyaluronic acid macromer molecular weight dictates the biophysical properties and in vitro cellular response to semisynthetic hydrogels. ACS Biomater Sci Eng. 2020;6(2):1135-43
79. Lee SY, Oh JH, Kim JC, Kim YH, Kim SH, Choi JW. In vivo conjunctival reconstruction using modified PLGA grafts for decreased scar formation and contraction. Biomaterials. 2003;24(27):5049-59
80. Yadav I, Purohit SD, Singh H, Das N, Roy P, Mishra NC. A highly transparent tri-polymer complex in situ hydrogel of HA, collagen and four-arm-PEG as potential vitreous substitute. Biomed Mater. 2021;16(6):065018
81. Abazari MF, Nejati F, Nasiri N, Khazeni ZAS, Nazari B, Enderami SE. et al. Platelet-rich plasma incorporated electrospun PVA-chitosan-HA nanofibers accelerates osteogenic differentiation and bone reconstruction. Gene. 2019;720:144096
82. Erikci S, Mundinger P, Boehm H. Small physical cross-linker facilitates hyaluronan hydrogels. Molecules. 2020;25(18):4166
83. Mehra TD, Ghosh K, Shu XZ, Prestwich GD, Clark RA. Molecular stenting with a crosslinked hyaluronan derivative inhibits collagen gel contraction. J Invest Dermatol. 2006;126(10):2202-09
84. Sakai S, Hirose K, Moriyama K, Kawakami K. Control of cellular adhesiveness in an alginate-based hydrogel by varying peroxidase and H(2)O(2) concentrations during gelation. Acta Biomater. 2010;6(4):1446-52
85. Khanmohammadi M, Dastjerdi MB, Ai A, Ahmadi A, Godarzi A, Rahimi A. et al. Horseradish peroxidase-catalyzed hydrogelation for biomedical applications. Biomater Sci. 2018;6(6):1286-98
86. Khanmohammadi M, Nemati S, Ai J, Khademi F. Multipotency expression of human adipose stem cells in filament-like alginate and gelatin derivative hydrogel fabricated through visible light-initiated crosslinking. Mater Sci Eng C Mater Biol Appl. 2019;103:109808
87. Khoshfetrat AB, Khanmohammadi M, Sakai S, Taya M. Enzymatically-gellable galactosylated chitosan: Hydrogel characteristics and hepatic cell behavior. Int J Biol Macromol. 2016;92:892-99
88. Yuguchi Y, Hasegawa A, Padoł AM, Draget KI, Stokke BT. Local structure of ca(2+) induced hydrogels of alginate-oligoguluronate blends determined by small-angle-X-ray scattering. Carbohydr Polym. 2016;152:532-40
89. Zhou R, Shi XY, Bi DC, Fang WS, Wei GB, Xu X. Alginate-derived oligosaccharide inhibits neuroinflammation and promotes microglial phagocytosis of β-amyloid. Mar Drugs. 2015;13(9):5828-46
90. Liu J, Yang S, Li X, Yan Q, Reaney MJT, Jiang Z. Alginate oligosaccharides: Production, biological activities, and potential applications. Compr Rev Food Sci Food Saf. 2019;18(6):1859-81
91. Li SY, Wang ZP, Wang LN, Peng JX, Wang YN, Han YT. et al. Combined enzymatic hydrolysis and selective fermentation for green production of alginate oligosaccharides from laminaria japonica. Bioresour Technol. 2019;281:84-89
92. Wang QQ, Liu Y, Zhang CJ, Zhang C, Zhu P. Alginate/gelatin blended hydrogel fibers cross-linked by Ca(2+) and oxidized starch: Preparation and properties. Mater Sci Eng C Mater Biol Appl. 2019;99:1469-76
93. Chan G, Mooney DJ. Ca(2+) released from calcium alginate gels can promote inflammatory responses in vitro and in vivo. Acta Biomater. 2013;9(12):9281-91
94. Yamamoto K, Yuguchi Y, Stokke BT, Sikorski P, Bassett DC. Local structure of Ca(2+) alginate hydrogels gelled via competitive ligand exchange and measured by small angle X-ray scattering. Gels. 2019;5(1):3
95. Zhang M, Qiao X, Han W, Jiang T, Liu F, Zhao X. Alginate-chitosan oligosaccharide-ZnO composite hydrogel for accelerating wound healing. Carbohydr Polym. 2021;266:118100
96. Manon-Jensen T, Kjeld NG, Karsdal MA. Collagen-mediated hemostasis. J Thromb Haemost. 2016;14(3):438-48
97. Farndale RW. Collagen-induced platelet activation. Blood Cells Mol Dis. 2006;36(2):162-65
98. Farndale RW, Sixma JJ, Barnes MJ, de Groot PG. The role of collagen in thrombosis and hemostasis. J Thromb Haemost. 2004;2(4):561-73
99. Böhm S, Strauß C, Stoiber S, Kasper C, Charwat V. Impact of source and manufacturing of collagen matrices on fibroblast cell growth and platelet aggregation. Materials (Basel). 2017;10(9):1086
100. Zhang L, Dong Y, Zhang N, Shi J, Zhang X, Qi C. et al. Potentials of sandwich-like chitosan/polycaprolactone/gelatin scaffolds for guided tissue regeneration membrane. Mater Sci Eng C Mater Biol Appl. 2020;109:110618
101. Gazzeri R, De Bonis C, Galarza M. Use of a thrombin-gelatin hemostatic matrix (surgiflo) in spinal surgery. Surg Technol Int. 2014;25:280-85
102. Park SM, Kang DR, Lee JH, Jeong YH, Shin DA, Yi S. et al. Efficacy and safety of a thrombin-containing collagen-based hemostatic agent in spinal surgery: A randomized clinical trial. World Neurosurg. 2021;154:e215-21
103. Wang X, Guo J, Zhang Q, Zhu S, Liu L, Jiang X. et al. Gelatin sponge functionalized with gold/silver clusters for antibacterial application. Nanotechnology. 2020;31(13):134004
104. Chung JP, Leung TY. Uses of floseal(©) in obstetric hemorrhage: Case series and literature review. Taiwan J Obstet Gynecol. 2017;56(6):827-30
105. Bonduelle Q, Biggs TC, Sipaul F. Floseal: A novel application technique for the treatment of challenging epistaxis. Clin Otolaryngol. 2020;45(6):960-62
106. Lee JM, Wu V, Faughnan ME, Lasso A, Figol A, Kilty SJ. Prospective pilot study of floseal® for the treatment of anterior epistaxis in patients with hereditary hemorrhagic telangiectasia (HHT). J Otolaryngol Head Neck Surg. 2019;48(1):48
107. Helito CP, Bonadio MB, Sobrado MF, Giglio PN, Pécora JR, Camanho GL. et al. Comparison of floseal® and tranexamic acid for bleeding control after total knee arthroplasty: A prospective randomized study. Clinics (Sao Paulo). 2019;74:e1186
108. Kamamoto D, Kanazawa T, Ishihara E, Yanagisawa K, Tomita H, Ueda R. et al. Efficacy of a topical gelatin-thrombin hemostatic matrix, floseal(®), in intracranial tumor resection. Surg Neurol Int. 2020;11:16
109. Kattula S, Byrnes JR, Wolberg AS. Fibrinogen and fibrin in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2017;37(3):e13-21
110. Takizawa K, Okazaki D, Takegawa Y, Koga Y, Sagata M, Michishita K. et al. Evaluation of the hemostatic effect of a combination of hemostatic agents and fibrin glue in a rabbit venous hemorrhage model. BMC Neurol. 2021;21(1):270
111. Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3(8):1894-904
112. Dhillon S. Fibrin sealant (evicel® [quixil®/crosseal™]): A review of its use as supportive treatment for haemostasis in surgery. Drugs. 2011;71(14):1893-915
113. Du X, Liu Y, Wang X, Yan H, Wang L, Qu L. et al. Injectable hydrogel composed of hydrophobically modified chitosan/oxidized-dextran for wound healing. Mater Sci Eng C Mater Biol Appl. 2019;104:109930
114. Carter T, Qi G, Wang W, Nguyen A, Cheng N, Ju YM. et al. Self-assembling peptide solution accelerates hemostasis. Adv Wound Care (New Rochelle). 2021;10(4):191-203
115. Shi C, He Y, Feng X, Fu D. Ε-polylysine and next-generation dendrigraft poly-l-lysine: Chemistry, activity, and applications in biopharmaceuticals. J Biomater Sci Polym Ed. 2015;26(18):1343-56
116. Man Z, Sidi L, Xubo Y, Jin Z, Xin H. An in situ catechol functionalized ε-polylysine/polyacrylamide hydrogel formed by hydrogen bonding recombination with high mechanical property for hemostasis. Int J Biol Macromol. 2021;191:714-26
117. Charbonneau S, Lemarié CA, Peng HT, Ganopolsky JG, Shek PN, Blostein MD. Surface-attached amphipathic peptides reduce hemorrhage in vivo. J Trauma Acute Care Surg. 2012;72(1):136-42
118. Wang R, Wang Z, Guo Y, Li H, Chen Z. Design of a RADA16-based self-assembling peptide nanofiber scaffold for biomedical applications. J Biomater Sci Polym Ed. 2019;30(9):713-36
119. Wang T, Zhong X, Wang S, Lv F, Zhao X. Molecular mechanisms of RADA16-1 peptide on fast stop bleeding in rat models. Int J Mol Sci. 2012;13(11):15279-90
120. Wang J, Lu Y, Li S, Wang X, Huang Y, Tang R. pH-sensitive amphiphilic triblock copolymers containing ortho ester main-chains as efficient drug delivery platforms. Mater Sci Eng C Mater Biol Appl. 2019;94:169-78
121. Li J, Qiao Y, Pan T, Zhong K, Wen J, Wu S. et al. Amphiphilic fluorine-containing block copolymers as carriers for hydrophobic PtTFPP for dissolved oxygen sensing, cell respiration monitoring and in vivo hypoxia imaging with high quantum efficiency and long lifetime. Sensors (Basel). 2018;18(11):3752
122. Lasowski F, Rambarran T, Rahmani V, Brook MA, Sheardown H. PEG-containing siloxane materials by metal-free click-chemistry for ocular drug delivery applications. J Biomater Sci Polym Ed. 2021;32(5):581-94
123. Kaldybekov DB, Filippov SK, Radulescu A, Khutoryanskiy VV. Maleimide-functionalised PLGA-PEG nanoparticles as mucoadhesive carriers for intravesical drug delivery. Eur J Pharm Biopharm. 2019;143:24-34
124. Pasut G, Sergi M, Veronese FM. Anti-cancer PEG-enzymes: 30 years old, but still a current approach. Adv Drug Deliv Rev. 2008;60(1):69-78
125. Lih E, Lee JS, Park KM, Park KD. Rapidly curable chitosan-PEG hydrogels as tissue adhesives for hemostasis and wound healing. Acta Biomater. 2012;8(9):3261-69
126. Stillman ZS, Jarai BM, Raman N, Patel P, Fromen CA. Degradation profiles of poly(ethylene glycol) diacrylate (PEGDA)-based hydrogel nanoparticles. Polym Chem. 2020;11(2):568-80
127. Della Sala F, Biondi M, Guarnieri D, Borzacchiello A, Ambrosio L, Mayol L. Mechanical behavior of bioactive poly(ethylene glycol) diacrylate matrices for biomedical application. J Mech Behav Biomed Mater. 2020;110:103885
128. Beaman HT, Shepherd E, Satalin J, Blair S, Ramcharran H, Serinelli S. et al. Hemostatic shape memory polymer foams with improved survival in a lethal traumatic hemorrhage model. Acta Biomater. 2022;137:112-23
129. Duggan M, Rago A, Sharma U, Zugates G, Freyman T, Busold R. et al. Self-expanding polyurethane polymer improves survival in a model of noncompressible massive abdominal hemorrhage. J Trauma Acute Care Surg. 2013;74(6):1462-67
130. Tamer TM, Alsehli MH, Omer AM, Afifi TH, Sabet MM, Mohy-Eldin MS. et al. Development of polyvinyl alcohol/kaolin sponges stimulated by marjoram as hemostatic, antibacterial, and antioxidant dressings for wound healing promotion. Int J Mol Sci. 2021;22(23):13050
131. Melis A, Karligkiotis A, Bozzo C, Machouchas N, Volpi L, Castiglia P. et al. Comparison of three different polyvinyl alcohol packs following functional endoscopic nasal surgery. Laryngoscope. 2015;125(5):1067-71
132. Kim JW, Woo K, Kim JM, Choi ME, Kim YM, Yang SG. et al. Effect of expanding nanocellulose sponge on nasal mucosal defects in an animal model. Regen Biomater. 2020;7(1):47-52
133. Ho TT, Doan VK, Tran NM, Nguyen LK, Le AN, Ho MH. et al. Fabrication of chitosan oligomer-coated electrospun polycaprolactone membrane for wound dressing application. Mater Sci Eng C Mater Biol Appl. 2021;120:111724
134. Yang S, Li X, Liu P, Zhang M, Wang C, Zhang B. Multifunctional chitosan/polycaprolactone nanofiber scaffolds with varied dual-drug release for wound-healing applications. ACS Biomater Sci Eng. 2020;6(8):4666-76
135. Dai NT, Fu KY, Hsieh PS, Hung YM, Fang YL, Huang NC. et al. A biodegradable hemostatic gelatin/polycaprolactone composite for surgical hemostasis. Ann Plast Surg. 2017;78(3 Suppl 2):S124-28
136. Cheng W, Zhang Z, Xu R, Cai P, Kristensen P, Chen M. et al. Incorporation of bacteriophages in polycaprolactone/collagen fibers for antibacterial hemostatic dual-function. J Biomed Mater Res B Appl Biomater. 2018;106(7):2588-95
137. Hu WW, Lin YT. Alginate/polycaprolactone composite fibers as multifunctional wound dressings. Carbohydr Polym. 2022;289:119440
138. Adlington K, Nguyen NT, Eaves E, Yang J, Chang CY, Li J. et al. Application of targeted molecular and material property optimization to bacterial attachment-resistant (meth)acrylate polymers. Biomacromolecules. 2016;17(9):2830-38
139. Li N, Cui W, Cong P, Tang J, Guan Y, Huang C. et al. Biomimetic inorganic-organic hybrid nanoparticles from magnesium-substituted amorphous calcium phosphate clusters and polyacrylic acid molecules. Bioact Mater. 2021;6(8):2303-14
140. Frazer RQ, Byron RT, Osborne PB, West KP. PMMA: An essential material in medicine and dentistry. J Long Term Eff Med Implants. 2005;15(6):629-39
141. Li C, Sun J, Shi K, Long J, Li L, Lai Y. et al. Preparation and evaluation of osteogenic nano-MgO/PMMA bone cement for bone healing in a rat critical size calvarial defect. J Mater Chem B. 2020;8(21):4575-86
142. Borie E, Rosas E, Kuramochi G, Etcheberry S, Olate S, Weber B. Oral applications of cyanoacrylate adhesives: A literature review. Biomed Res Int. 2019;2019:8217602
143. Moreno-Egea A. Is it possible to eliminate sutures in open (lichtenstein technique) and laparoscopic (totally extraperitoneal endoscopic) inguinal hernia repair? A randomized controlled trial with tissue adhesive (n-hexyl-α-cyanoacrylate). Surg Innov. 2014;21(6):590-99
144. Robles-Medranda C, Valero M, Nebel JA, de Britto Junior SR, Puga-Tejada M, Ospina J. et al. Endoscopic-ultrasound-guided coil and cyanoacrylate embolization for gastric varices and the roles of endoscopic doppler and endosonographic varicealography in vascular targeting. Dig Endosc. 2019;31(3):283-90
145. Tang J, Lv F, Li W, Zhang H, Luo Y, An L. et al. Percutaneous injection of hemostatic agents for severe blunt hepatic trauma: An experimental study. Eur Radiol. 2008;18(12):2848-53
146. Huang Y, Wang L, Liu Y, Li T, Xin B. Drug-loaded PLCL/PEO-SA bilayer nanofibrous membrane for controlled release. J Biomater Sci Polym Ed. 2021;32(18):2331-48
147. Mengistu Lemma S, Bossard F, Rinaudo M. Preparation of pure and stable chitosan nanofibers by electrospinning in the presence of poly(ethylene oxide). Int J Mol Sci. 2016;17(11):1790
148. Szymańska E, Wojasiński M, Czarnomysy R, Dębowska R, Łopianiak I, Adasiewicz K. et al. Chitosan-enriched solution blow spun poly(ethylene oxide) nanofibers with poly(dimethylsiloxane) hydrophobic outer layer for skin healing and regeneration. Int J Mol Sci. 2022;23(9):5135
149. Tiwari S, Kansara V, Bahadur P. Targeting anticancer drugs with pluronic aggregates: Recent updates. Int J Pharm. 2020;586:119544
150. Piotrowska-Kirschling A, Brzeska J. The effect of chitosan on the chemical structure, morphology, and selected properties of polyurethane/chitosan composites. Polymers (Basel). 2020;12(5):1205
151. Basadonna G. Quikclot combat gauze for hemorrhage control. Prehosp Disaster Med. 2012;27(2):217
152. Gegel BT, Austin PN, Johnson AD. An evidence-based review of the use of a combat gauze (quikclot) for hemorrhage control. Aana j. 2013;81(6):453-58
153. Kang J, Liu H, Zheng Y-M, Qu J, Chen JP. Application of nuclear magnetic resonance spectroscopy, fourier transform infrared spectroscopy, uv-visible spectroscopy and kinetic modeling for elucidation of adsorption chemistry in uptake of tetracycline by zeolite beta. J Colloid Interface Sci. 2011;354(1):261-67
154. Fathi P, Sikorski M, Christodoulides K, Langan K, Choi YS, Titcomb M. et al. Zeolite-loaded alginate-chitosan hydrogel beads as a topical hemostat. J Biomed Mater Res B Appl Biomater. 2018;106(5):1662-71
155. Liang Y, Xu C, Liu F, Du S, Li G, Wang X. Eliminating heat injury of zeolite in hemostasis via thermal conductivity of graphene sponge. ACS Appl Mater Interfaces. 2019;11(27):23848-57
156. Shang X, Chen H, Castagnola V, Liu K, Boselli L, Petseva V. et al. Unusual zymogen activation patterns in the protein corona of Ca-zeolites. Nat Catal. 2021;4(7):607-14
157. Wan MM, Li YY, Yang T, Zhang T, Sun XD, Zhu JH. In situ loading of drugs into mesoporous silica SBA-15. Chemistry. 2016;22(18):6294-301
158. Bernardos A, Piacenza E, Sancenón F, Hamidi M, Maleki A, Turner RJ. et al. Mesoporous silica-based materials with bactericidal properties. Small. 2019;15(24):e1900669
159. Rathinavel S, Korrapati PS, Kalaiselvi P, Dharmalingam S. Mesoporous silica incorporated PCL/curcumin nanofiber for wound healing application. Eur J Pharm Sci. 2021;167:106021
160. Gou K, Wang Y, Guo X, Wang Y, Bian Y, Zhao H. et al. Carboxyl-functionalized mesoporous silica nanoparticles for the controlled delivery of poorly water-soluble non-steroidal anti-inflammatory drugs. Acta Biomater. 2021;134:576-92
161. Daniyal M, Liu B, Wang W. Comprehensive review on graphene oxide for use in drug delivery system. Curr Med Chem. 2020;27(22):3665-85
162. Sattari S, Adeli M, Beyranvand S, Nemati M. Functionalized graphene platforms for anticancer drug delivery. Int J Nanomedicine. 2021;16:5955-80
163. Lin J, Chen X, Huang P. Graphene-based nanomaterials for bioimaging. Adv Drug Deliv Rev. 2016;105(Pt B):242-54
164. Chung S, Revia RA, Zhang M. Graphene quantum dots and their applications in bioimaging, biosensing, and therapy. Adv Mater. 2021;33(22):e1904362
165. Song S, Shen H, Wang Y, Chu X, Xie J, Zhou N. et al. Biomedical application of graphene: From drug delivery, tumor therapy, to theranostics. Colloids Surf B Biointerfaces. 2020;185:110596
166. Li G, Quan K, Xu C, Deng B, Wang X. Synergy in thrombin-graphene sponge for improved hemostatic efficacy and facile utilization. Colloids Surf B Biointerfaces. 2018;161:27-34
167. Koklic T, Majumder R, Lentz BR. Ca2+ switches the effect of ps-containing membranes on factor Xa from activating to inhibiting: Implications for initiation of blood coagulation. Biochem J. 2014;462(3):591-601
168. Ghilotti M, Lova P, Balduini C, Torti M. Epinephrine induces intracellular Ca2+ mobilization in thrombin-desensitized platelets: A role for GPIb-IX-V. Platelets. 2007;18(2):135-42
169. Subramaniam T, Fauzi MB, Lokanathan Y, Law JX. The role of calcium in wound healing. Int J Mol Sci. 2021;22(12):6486
170. Niamsap T, Lam NT, Sukyai P. Production of hydroxyapatite-bacterial nanocellulose scaffold with assist of cellulose nanocrystals. Carbohydr Polym. 2019;205:159-66
171. Zienkiewicz-Strzałka M, Deryło-Marczewska A, Skorik YA, Petrova VA, Choma A, Komaniecka I. Silver nanoparticles on chitosan/silica nanofibers: Characterization and antibacterial activity. Int J Mol Sci. 2019;21(1):166
172. Mishra SK, Mary DS, Kannan S. Copper incorporated microporous chitosan-polyethylene glycol hydrogels loaded with naproxen for effective drug release and anti-infection wound dressing. Int J Biol Macromol. 2017;95:928-37
173. Ahmed NS, Lopes-Pires M, Pugh N. Zinc: An endogenous and exogenous regulator of platelet function during hemostasis and thrombosis. Platelets. 2021;32(7):880-87
174. Zhang M, Chen S, Zhong L, Wang B, Wang H, Hong F. Zn(2+)-loaded tobc nanofiber-reinforced biomimetic calcium alginate hydrogel for antibacterial wound dressing. Int J Biol Macromol. 2020;143:235-42
175. Lin J, Xu D, Liu Z, Jiang Y, Ren M, Xiang H. et al. Physicochemical and biological properties of carboxymethyl chitosan zinc (CMCS-Zn)/α-calcium sulfate hemihydrate (α-CSH) composites. Mater Sci Eng C Mater Biol Appl. 2021;131:112496
176. Hosseini A, Sharifi AM, Abdollahi M, Najafi R, Baeeri M, Rayegan S. et al. Cerium and yttrium oxide nanoparticles against lead-induced oxidative stress and apoptosis in rat hippocampus. Biol Trace Elem Res. 2015;164(1):80-89
177. Liu J, Zhou X, Zhang Y, Wang A, Zhu W, Xu M. et al. Rapid hemostasis and high bioactivity cerium-containing mesoporous bioglass for hemostatic materials. J Biomed Mater Res B Appl Biomater. 2021;110(6):1255-64
178. Obata A, Ogasawara T, Kasuga T. Combinatorial effects of inorganic ions on adhesion and proliferation of osteoblast-like cells. J Biomed Mater Res A. 2019;107(5):1042-51
179. Zhang K, Jia Z, Yang B, Feng Q, Xu X, Yuan W. et al. Adaptable hydrogels mediate cofactor-assisted activation of biomarker-responsive drug delivery via positive feedback for enhanced tissue regeneration. Adv Sci (Weinh). 2018;5(12):1800875
180. Lv C, Li L, Jiao Z, Yan H, Wang Z, Wu Z. et al. Improved hemostatic effects by Fe(3+) modified biomimetic plla cotton-like mat via sodium alginate grafted with dopamine. Bioact Mater. 2021;6(8):2346-59
181. Bertram JP, Williams CA, Robinson R, Segal SS, Flynn NT, Lavik EB. Intravenous hemostat: Nanotechnology to halt bleeding. Sci Transl Med. 2009;1(11):11-22
182. Gong C, Guan W, Liu X, Zheng Y, Li Z, Zhang Y. et al. Biomimetic bacteriophage-like particles formed from probiotic extracts and no donors for eradicating multidrug-resistant staphylococcus aureus. Adv Mater. 2022: e2206134.
183. Liu HP, Li JF, Liu XM, Li ZY, Zhang Y, Liang YQ. et al. Photo-sono interfacial engineering exciting the intrinsic property of herbal nanomedicine for rapid broad-spectrum bacteria killing. ACS NANO. 2021;15(11):18505-19
184. Juncos Bombin AD, Dunne NJ, McCarthy HO. Electrospinning of natural polymers for the production of nanofibres for wound healing applications. Mater Sci Eng C Mater Biol Appl. 2020;114:110994
185. Gao Y, Zhang J, Su Y, Wang H, Wang XX, Huang LP. et al. Recent progress and challenges in solution blow spinning. Mater Horiz. 2021;8(2):426-46
186. Xue J, Xie J, Liu W, Xia Y. Electrospun nanofibers: New concepts, materials, and applications. Acc Chem Res. 2017;50(8):1976-87
187. John JV, McCarthy A, Su YJ, Ackerman DN, Shahriar SMS, Carlson MA. et al. Nanofiber capsules for minimally invasive sampling of biological specimens from gastrointestinal tract. Acta Biomater. 2022;146:211-21
188. Huang L, Wu B, Chen J, Xue Y, Liu Y, Kajiura H. et al. Synthesis of single-walled carbon nanotubes by an arc-discharge method using selenium as a promoter. Carbon. 2011;49(14):4792-800
189. Thess A, Lee R, Nikolaev P, Dai H, Petit P, Robert J. et al. Crystalline ropes of metallic carbon nanotubes. Science. 1996;273(5274):483-87
190. Newman P, Minett A, Ellis-Behnke R, Zreiqat H. Carbon nanotubes: Their potential and pitfalls for bone tissue regeneration and engineering. Nanomedicine. 2013;9(8):1139-58
191. Girardello R, Baranzini N, Tettamanti G, de Eguileor M, Grimaldi A. Cellular responses induced by multi-walled carbon nanotubes: In vivo and in vitro studies on the medicinal leech macrophages. Sci Rep. 2017;7(1):8871
192. Zhang A, Liu Y, Qin D, Sun M, Wang T, Chen X. Research status of self-healing hydrogel for wound management: A review. Int J Biol Macromol. 2020;164:2108-23
193. Zhou D, Li S, Pei M, Yang H, Gu S, Tao Y. et al. Dopamine-modified hyaluronic acid hydrogel adhesives with fast-forming and high tissue adhesion. ACS Appl Mater Interfaces. 2020;12(16):18225-34
194. Okay O. Semicrystalline physical hydrogels with shape-memory and self-healing properties. J Mater Chem B. 2019;7(10):1581-96
195. Takahashi R, Ikai T, Kurokawa T, King DR, Gong JP. Double network hydrogels based on semi-rigid polyelectrolyte physical networks. J Mater Chem B. 2019;7(41):6347-54
196. Cheng Y, Hu Y, Xu M, Qin M, Lan W, Huang D. et al. High strength polyvinyl alcohol/polyacrylic acid (PVA/PAA) hydrogel fabricated by cold-drawn method for cartilage tissue substitutes. J Biomater Sci Polym Ed. 2020;31(14):1836-51
197. Xu Y, Sheng K, Li C, Shi G. Self-assembled graphene hydrogel via a one-step hydrothermal process. ACS Nano. 2010;4(7):4324-30
198. Chronopoulou L, Cacciotti I, Amalfitano A, Di Nitto A, D'Arienzo V, Nocca G. et al. Biosynthesis of innovative calcium phosphate/hydrogel composites: Physicochemical and biological characterisation. Nanotechnology. 2021;32(9):095102
199. Lopez-Sanchez P, Martinez-Sanz M, Bonilla MR, Wang D, Walsh CT, Gilbert EP. et al. Pectin impacts cellulose fibre architecture and hydrogel mechanics in the absence of calcium. Carbohydr Polym. 2016;153:236-45
200. Tuan Mohamood NFA, Abdul Halim AH, Zainuddin N. Carboxymethyl cellulose hydrogel from biomass waste of oil palm empty fruit bunch using calcium chloride as crosslinking agent. Polymers (Basel). 2021;13(23):4056
201. Thakur K, Rajhans A, Kandasubramanian B. Starch/PVA hydrogels for oil/water separation. Environ Sci Pollut Res Int. 2019;26(31):32013-28
202. Hwang YJ, Granelli J, Lyubovitsky J. Effects of zero-length and non-zero-length cross-linking reagents on the optical spectral properties and structures of collagen hydrogels. ACS Appl Mater Interfaces. 2012;4(1):261-67
203. Van Vlierberghe S. Crosslinking strategies for porous gelatin scaffolds. J Mater Sci. 2016;51(9):4349-57
204. Appel EA, Tibbitt MW, Webber MJ, Mattix BA, Veiseh O, Langer R. Self-assembled hydrogels utilizing polymer-nanoparticle interactions. Nat Commun. 2015;6:6295
205. Park J, Wrzesinski SH, Stern E, Look M, Criscione J, Ragheb R. et al. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat Mater. 2012;11(10):895-905
206. Teixeira LS, Feijen J, van Blitterswijk CA, Dijkstra PJ, Karperien M. Enzyme-catalyzed crosslinkable hydrogels: Emerging strategies for tissue engineering. Biomaterials. 2012;33(5):1281-90
207. Herchenhan A, Uhlenbrock F, Eliasson P, Weis M, Eyre D, Kadler KE. et al. Lysyl oxidase activity is required for ordered collagen fibrillogenesis by tendon cells. J Biol Chem. 2015;290(26):16440-50
208. Brown TE, Anseth KS. Spatiotemporal hydrogel biomaterials for regenerative medicine. Chem Soc Rev. 2017;46(21):6532-52
209. Nguyen QT, Hwang Y, Chen AC, Varghese S, Sah RL. Cartilage-like mechanical properties of poly (ethylene glycol)-diacrylate hydrogels. Biomaterials. 2012;33(28):6682-90
210. Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010;31(21):5536-44
211. Bian L, Guvendiren M, Mauck RL, Burdick JA. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance msc chondrogenesis. Proc Natl Acad Sci USA. 2013;110(25):10117-22
212. Mo C, Xiang L, Chen Y. Advances in injectable and self-healing polysaccharide hydrogel based on the schiff base reaction. Macromol Rapid Commun. 2021;42(10):e2100025
213. Xu J, Liu Y, Hsu SH. Hydrogels based on schiff base linkages for biomedical applications. Molecules. 2019;24(16):3005
214. Zou C-Y, Lei X-X, Hu J-J, Jiang Y-L, Li Q-J, Song Y-T. et al. Multi-crosslinking hydrogels with robust bio-adhesion and pro-coagulant activity for first-aid hemostasis and infected wound healing. Bioactive Materials. 2022;16:388-402
215. Yin TJ, Jeyapalina S, Naleway SE. Characterization of porous fluorohydroxyapatite bone-scaffolds fabricated using freeze casting. J Mech Behav Biomed Mater. 2021;123:104717
216. Li K, Zhang X, Qin Y, Li Y. Construction of the cellulose nanofibers (CNFs) aerogel loading TiO(2) NPs and its application in disposal of organic pollutants. Polymers (Basel). 2021;13(11):1841
217. Hou X, Ding R, Yan S, Zhao H, Yang Q, Wu W. ZrO(2) nanoparticles and poly(diallyldimethylammonium chloride)-doped graphene oxide aerogel-coated stainless-steel mesh for the effective adsorption of organophosphorus pesticides. Foods. 2021;10(7):1616
218. Saini A, Yadav C, Sethi SK, Xue BL, Xia Y, Li K. et al. Microdesigned nanocellulose-based flexible antibacterial aerogel architectures impregnated with bioactive cinnamomum cassia. ACS Appl Mater Interfaces. 2021;13(4):4874-85
219. Peng Y, Gardner DJ, Han Y. Drying cellulose nanofibrils: In search of a suitable method. Cellulose. 2012;19(1):91-102
220. Beck S, Bouchard J, Berry R. Dispersibility in water of dried nanocrystalline cellulose. Biomacromolecules. 2012;13(5):1486-94
221. García-González CA, Sosnik A, Kalmár J, De Marco I, Erkey C, Concheiro A. et al. Aerogels in drug delivery: From design to application. J Control Release. 2021;332:40-63
222. Chan V, Sarkari M, Sunderland R, St John AE, White NJ, Kastrup CJ. Platelets loaded with liposome-encapsulated thrombin have increased coagulability. J Thromb Haemost. 2018;16(6):1226-35
223. Kamat V, Marathe I, Ghormade V, Bodas D, Paknikar K. Synthesis of monodisperse chitosan nanoparticles and in situ drug loading using active microreactor. ACS Appl Mater Interfaces. 2015;7(41):22839-47
224. Sreekumar S, Goycoolea FM, Moerschbacher BM, Rivera-Rodriguez GR. Parameters influencing the size of chitosan-TPP nano- and microparticles. Sci Rep. 2018;8(1):4695
225. Barua S, Mitragotri S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: A review of current status and future prospects. Nano Today. 2014;9(2):223-43
226. Deng X, Cao M, Zhang J, Hu K, Yin Z, Zhou Z. et al. Hyaluronic acid-chitosan nanoparticles for co-delivery of miR-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials. 2014;35(14):4333-44
227. Abbad S, Zhang Z, Waddad AY, Munyendo WL, Lv H, Zhou J. Chitosan-modified cationic amino acid nanoparticles as a novel oral delivery system for insulin. J Biomed Nanotechnol. 2015;11(3):486-99
228. Huang YJ, Hung KC, Hsieh FY, Hsu SH. Carboxyl-functionalized polyurethane nanoparticles with immunosuppressive properties as a new type of anti-inflammatory platform. Nanoscale. 2015;7(48):20352-64
229. Huang AP-H, Lai D-M, Hsu Y-H, Tsai H-H, Su C-Y, Hsu S-h. An anti-inflammatory gelatin hemostatic agent with biodegradable polyurethane nanoparticles for vulnerable brain tissue. Mater Sci Eng C Mater Biol Appl. 2021;121:111799
230. Chen L, Qin H, Zhao R, Zhao X, Lin L, Chen Y. et al. Bacterial cytoplasmic membranes synergistically enhance the antitumor activity of autologous cancer vaccines. Sci Transl Med. 2021;13(601):eabc2816
231. Ci H, Ma L, Liu X, Liang Y, Zheng Y, Li Z. et al. Photo-excited antibacterial poly(ɛ-caprolactone)@MoS2/ZnS hybrid nanofibers. J Chem Eng. 2022;434:134764
232. Luo Y, Li B, Liu X, Zheng Y, Wang E, Li Z. et al. Simultaneously enhancing the photocatalytic and photothermal effect of NH2-MIL-125-GO-Pt ternary heterojunction for rapid therapy of bacteria-infected wounds. Bioact Mater. 2022;18:421-32
233. Wang C, Luo Y, Liu X, Cui Z, Zheng Y, Liang Y. et al. The enhanced photocatalytic sterilization of mof-based nanohybrid for rapid and portable therapy of bacteria-infected open wounds. Bioact Mater. 2022;13:200-11
234. Li Y, Liu X, Tan L, Cui Z, Yang X, Zheng Y. et al. Rapid sterilization and accelerated wound healing using Zn2+ and graphene oxide modified g-C3N4 under dual light irradiation. Adv Funct Mater. 2018;28(30):1800299
235. Liu H, Li J, Liu X, Li Z, Zhang Y, Liang Y. et al. Photo-sono interfacial engineering exciting the intrinsic property of herbal nanomedicine for rapid broad-spectrum bacteria killing. ACS Nano. 2021;15(11):18505-19
236. Han DL, Li Y, Liu XM, Li B, Han Y, Zheng YF. et al. Rapid bacteria trapping and killing of metal-organic frameworks strengthened photo-responsive hydrogel for rapid tissue repair of bacterial infected wounds. Chem Eng J. 2020;396:125194
237. Leonhardt EE, Kang N, Hamad MA, Wooley KL, Elsabahy M. Absorbable hemostatic hydrogels comprising composites of sacrificial templates and honeycomb-like nanofibrous mats of chitosan. Nat Commun. 2019;10(1):2307
238. Hemmingsen LM, Julin K, Ahsan L, Basnet P, Johannessen M, Škalko-Basnet N. Chitosomes-in-chitosan hydrogel for acute skin injuries: Prevention and infection control. Mar Drugs. 2021;19(5):269
239. Biranje SS, Madiwale PV, Patankar KC, Chhabra R, Dandekar-Jain P, Adivarekar RV. Hemostasis and anti-necrotic activity of wound-healing dressing containing chitosan nanoparticles. Int J Biol Macromol. 2019;121:936-46
240. Pinzón-García AD, Cassini-Vieira P, Ribeiro CC, de Matos Jensen CE, Barcelos LS, Cortes ME. et al. Efficient cutaneous wound healing using bixin-loaded pcl nanofibers in diabetic mice. J Biomed Mater Res B Appl Biomater. 2017;105(7):1938-49
241. Ellis-Behnke RG, Liang YX, Tay DK, Kau PW, Schneider GE, Zhang S. et al. Nano hemostat solution: Immediate hemostasis at the nanoscale. Nanomedicine. 2006;2(4):207-15
242. Ye H, Xian Y, Li S, Zhang C, Wu D. In situ forming injectable γ-poly(glutamic acid)/PEG adhesive hydrogels for hemorrhage control. Biomater Sci. 2022;10(15):4218-27
243. Yu P, Zhong W. Hemostatic materials in wound care. Burns Trauma. 2021;9:tkab019
244. Abdo GG, Zagho MM, Al Moustafa AE, Khalil A, Elzatahry AA. A comprehensive review summarizing the recent biomedical applications of functionalized carbon nanofibers. J Biomed Mater Res B Appl Biomater. 2021;109(11):1893-908
245. Masood N, Ahmed R, Tariq M, Ahmed Z, Masoud MS, Ali I. et al. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int J Pharm. 2019;559:23-36
246. Viallard C, Larrivée B. Tumor angiogenesis and vascular normalization: Alternative therapeutic targets. Angiogenesis. 2017;20(4):409-26
247. Pretzsch E, Bösch F, Renz B, Werner J, Angele M, Chaudry IH. Operative trauma and blood loss - impact on tumor growth and recurrence. Shock. 2021;55(4):455-64
248. Bhamidipati CM, Coselli JS, LeMaire SA. Bioglue in 2011: What is its role in cardiac surgery? J Extra Corpor Technol. 2012;44(1):P6-12
249. Alston SM, Solen KA, Sukavaneshvar S, Mohammad SF. In vivo efficacy of a new autologous fibrin sealant. J Surg Res. 2008;146(1):143-48
250. Sun W, Chen Y, Yuan W. Hemostatic absorbable gelatin sponge loaded with 5-fluorouracil for treatment of tumors. Int J Nanomedicine. 2013;8:1499-506
251. Liang Y, Li M, Huang Y, Guo B. An integrated strategy for rapid hemostasis during tumor resection and prevention of postoperative tumor recurrence of hepatocellular carcinoma by antibacterial shape memory cryogel. Small. 2021;17(38):e2101356
252. Chen K, Pan H, Yan Z, Li Y, Ji D, Yun K. et al. A novel alginate/gelatin sponge combined with curcumin-loaded electrospun fibers for postoperative rapid hemostasis and prevention of tumor recurrence. Int J Biol Macromol. 2021;182:1339-50
253. Wang H, Jin Y, Chen Y, Luo Y, Lv S, Li M. et al. Multifunctional hybrid sponge for in situ postoperative management to inhibit tumor recurrence. Biomater Sci. 2021;9(11):4066-75
254. Sandberg OH, Aspenberg P. Inter-trabecular bone formation: A specific mechanism for healing of cancellous bone. Acta Orthop. 2016;87(5):459-65
255. Liu JY, Hu Y, Li L, Wang C, Wang J, Li Y. et al. Biomass-derived multilayer-structured microparticles for accelerated hemostasis and bone repair. Adv Sci (Weinh). 2020;7(22):2002243
256. Padalhin AR, Lee BT. Hemostasis and bone regeneration using chitosan/gelatin-BCP bi-layer composite material. Asaio j. 2019;65(6):620-27
257. Wang D, Liu Y, Liu Y, Yan L, Zaat SAJ, Wismeijer D. et al. A dual functional bone-defect-filling material with sequential antibacterial and osteoinductive properties for infected bone defect repair. J Biomed Mater Res A. 2019;107(10):2360-70
258. Ma K, Liao C, Huang L, Liang R, Zhao J, Zheng L. et al. Electrospun PCL/MoS(2) nanofiber membranes combined with NIR-triggered photothermal therapy to accelerate bone regeneration. Small. 2021;17(51):e2104747
259. Aguiar AE, De OSM, Rodas ACD, Bertran CA. Mineralized layered films of xanthan and chitosan stabilized by polysaccharide interactions: A promising material for bone tissue repair. Carbohydr Polym. 2019;207:480-91
260. Jin LG, Zheng YF, Liu XM, Zhang Y, Li ZY, Liang YQ. et al. Magnetic composite rapidly treats staphylococcus aureus-infected osteomyelitis through microwave strengthened thermal effects and reactive oxygen species. SMALL. 2022;18(41):e2204028
261. Gain P, Jullienne R, He Z, Aldossary M, Acquart S, Cognasse F. et al. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol. 2016;134(2):167-73
262. Islam MM, Buznyk O, Reddy JC, Pasyechnikova N, Alarcon EI, Hayes S. et al. Biomaterials-enabled cornea regeneration in patients at high risk for rejection of donor tissue transplantation. NPJ Regen Med. 2018;3:2
263. Irimia T, Dinu-Pîrvu CE, Ghica MV, Lupuleasa D, Muntean DL, Udeanu DI. et al. Chitosan-based in situ gels for ocular delivery of therapeutics: A state-of-the-art review. Mar Drugs. 2018;16(10):373
264. Shirzaei Sani E, Kheirkhah A, Rana D, Sun Z, Foulsham W, Sheikhi A. et al. Sutureless repair of corneal injuries using naturally derived bioadhesive hydrogels. Sci Adv. 2019;5(3):eaav1281
265. Takezawa T, Takeuchi T, Nitani A, Takayama Y, Kino-Oka M, Taya M. et al. Collagen vitrigel membrane useful for paracrine assays in vitro and drug delivery systems in vivo. J Biotechnol. 2007;131(1):76-83
266. Chae JJ, Ambrose WM, Espinoza FA, Mulreany DG, Ng S, Takezawa T. et al. Regeneration of corneal epithelium utilizing a collagen vitrigel membrane in rabbit models for corneal stromal wound and limbal stem cell deficiency. Acta Ophthalmol. 2015;93(1):e57-66
267. Tan J, Foster LJR, Watson SL. Corneal sealants in clinical use: A systematic review. Curr Eye Res. 2020;45(9):1025-30
268. Shehata AEM, Kambhampati SP, Wang J, Soiberman US. Burst pressures in eyes with clear corneal incisions treated with resure glue. J Ophthalmol. 2021;2021:6691489
269. Gratieri T, Gelfuso GM, Rocha EM, Sarmento VH, de Freitas O, Lopez RF. A poloxamer/chitosan in situ forming gel with prolonged retention time for ocular delivery. Eur J Pharm Biopharm. 2010;75(2):186-93
270. Tayebi T, Baradaran-Rafii A, Hajifathali A, Rahimpour A, Zali H, Shaabani A. et al. Biofabrication of chitosan/chitosan nanoparticles/polycaprolactone transparent membrane for corneal endothelial tissue engineering. Sci Rep. 2021;11(1):7060
271. Chang HH, Wang YL, Chiang YC, Chen YL, Chuang YH, Tsai SJ. et al. A novel chitosan-γpga polyelectrolyte complex hydrogel promotes early new bone formation in the alveolar socket following tooth extraction. PLoS One. 2014;9(3):e92362
272. Cicciù M, Fiorillo L, Cervino G. Chitosan use in dentistry: A systematic review of recent clinical studies. Mar Drugs. 2019;17(7):417
273. Sarkar S, Prashanth NT, Shobha ES, Rangan V, Nikhila G. Efficacy of platelet rich fibrin versus chitosan as a hemostatic agent following dental extraction in patients on antiplatelet therapy. J Oral Biol Craniofac Res. 2019;9(4):336-39
274. Kinane DF. Causation and pathogenesis of periodontal disease. Periodontol 2000. 2001;25:8-20
275. Xu C, Lei C, Meng L, Wang C, Song Y. Chitosan as a barrier membrane material in periodontal tissue regeneration. J Biomed Mater Res B Appl Biomater. 2012;100(5):1435-43
276. Liu S, Wang YN, Ma B, Shao J, Liu H, Ge S. Gingipain-responsive thermosensitive hydrogel loaded with sdf-1 facilitates in situ periodontal tissue regeneration. ACS Appl Mater Interfaces. 2021;13(31):36880-93
Author contact
![]() Corresponding authors: Shuang Wang, E-mail: jdeywangshuangcom, Department of the Dermatology, Jilin University Second Hospital, Changchun, China. Jiannan Li, E-mail: jnliac.cn, Department of the General Surgery, Jilin University Second Hospital, Changchun, China.
Corresponding authors: Shuang Wang, E-mail: jdeywangshuangcom, Department of the Dermatology, Jilin University Second Hospital, Changchun, China. Jiannan Li, E-mail: jnliac.cn, Department of the General Surgery, Jilin University Second Hospital, Changchun, China.
 Global reach, higher impact
Global reach, higher impact