13.3
Impact Factor
Theranostics 2022; 12(17):7624-7639. doi:10.7150/thno.72853 This issue Cite
Research Paper
The landscape of cancer-associated fibroblasts in colorectal cancer liver metastases
1. Institut régional du Cancer de Montpellier (ICM)-Val d'Aurelle, Montpellier, France.
2. Université de Montpellier, Montpellier, France.
3. Cancer Bioinformatics and Systems Biology Team, INSERM U1194, Montpellier, France.
4. Tumor Microenvironment and Resistance to Treatment Lab, INSERM U1194, Montpellier, France.
5. Department of Pathology, Institut régional du Cancer de Montpellier (ICM)-Val d'Aurelle, Montpellier, France.
6. Biocampus, CNRS, INSERM, Université de Montpellier, Montpellier, France.
7. Department of Medical Oncology, Institut régional du Cancer de Montpellier (ICM)-Val d'Aurelle, Montpellier, France.
8. Department of Surgery, Institut régional du Cancer de Montpellier (ICM)-Val d'Aurelle, Montpellier, France.
9. Gunma University Initiative for Advanced Research (GIAR), Maebashi, Gunma, Japan.
* Equal contribution
Abstract
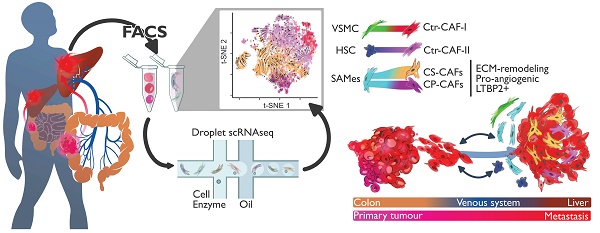
Rationale: Patients with colorectal cancer die mainly due to liver metastases (CRC-LM). Although the tumor microenvironment (TME) plays an important role in tumor development and therapeutic response, our understanding of the individual TME components, especially cancer-associated fibroblasts (CAFs), remains limited.
Methods: We analyzed CRC-LM CAFs and cancer cells by single-cell transcriptomics and used bioinformatics for data analysis and integration with related available single-cell and bulk transcriptomic datasets. We validated key findings by RT-qPCR, western blotting, and immunofluorescence.
Results: By single-cell transcriptomic analysis of 4,397 CAFs from six CRC-LM samples, we identified two main CAF populations, contractile CAFs and extracellular matrix (ECM)-remodeling/pro-angiogenic CAFs, and four subpopulations with distinct phenotypes. We found that ECM-remodeling/pro-angiogenic CAFs derive from portal resident fibroblasts. They associate with areas of strong desmoplastic reaction and Wnt signaling in low-proliferating tumor cells engulfed in a stiff extracellular matrix. By integrating public single-cell primary liver tumor data, we propose a model to explain how different liver malignancies recruit CAFs of different origins to this organ. Lastly, we found that LTBP2 plays an important role in modulating collagen biosynthesis, ECM organization, and adhesion pathways. We developed fully human antibodies against LTBP2 that depleted LTBP2+ CAFs in vitro.
Conclusion: This study complements recent reports on CRC-LM CAF heterogeneity at the single-cell resolution. The number of sequenced CAFs was more than one order of magnitude larger compared to existing data. LTBP2 targeting by antibodies might create opportunities to deplete ECM-remodeling CAFs in CRC-LMs. This might be combined with other therapies, e.g., anti-angiogenic compounds as already done in CRC. Moreover, we showed that in intrahepatic cholangiocarcinoma, in which ECM-remodeling CAF proportion is similar to that of CRC-LM, several genes expressed by ECM-remodeling CAFs, such as LTBP2, were associated with survival.
Keywords: single-cell, tumor microenvironment, CAF, liver metastasis, LTBP2.
 Global reach, higher impact
Global reach, higher impact