13.3
Impact Factor
Theranostics 2022; 12(17):7450-7464. doi:10.7150/thno.75936 This issue Cite
Research Paper
Hippocalcin-Like 1 blunts liver lipid metabolism to suppress tumorigenesis via directly targeting RUVBL1-mTOR signaling
1. United International Laboratory, Department of Clinical Laboratory, Sixth People's Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China.
2. Department of Biliary-Pancreatic Surgery, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China.
3. Department of Hepatobiliary Surgery, First Affiliated Hospital of Bengbu Medical College, Bengbu, 233000, Anhui Province, China.
4. Department of Pathology Affiliated Tongren Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai, China.
5. Institutes of Biomedical Sciences of Shanghai Medical School, Fudan University, Shanghai, China.
6. Renji-Med-X Clinical Stem Cell Research Center, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China.
*These authors contributed equally to this work.
Abstract
Rationale: Hepatocellular carcinoma (HCC) is one of the most severe cancers worldwide, with few effective targeted therapies for HCC. Lipid metabolic reprogramming is emerged as a hallmark of cancer metabolism that guides response to antitumoral therapies. Such lipid metabolic alteration in cancers is critically regulated by the mammalian target of rapamycin mTOR, which is considered as a promising therapeutic target. Despite efforts, mTOR inhibitors (mTORi) have produced limited response clinically, partly due to incomplete knowledge of mTORC1 addiction in cancers.
Methods: CRISPR-Cas9 system was used to establish Hpcal1 null mice. The liver cancer model in mice was generated using Hpcal1-deficient mice with diethylnitrosamine (DEN) /CCL4 or MYC/Trp53-/- via hydrodynamic tail-vein injection. RNA-sequencing (RNA-seq) was used to identify potential signaling pathways. The expression of HPCAL1 and mTOR signaling were determined using quantitative polymerase chain reaction (qPCR), western blot and immunohistochemistry. The role of Hpcal1 in liver tumorigenesis and its response to mTORi was assessed by CCK-8 measurements, colony formation assay and in mouse model.
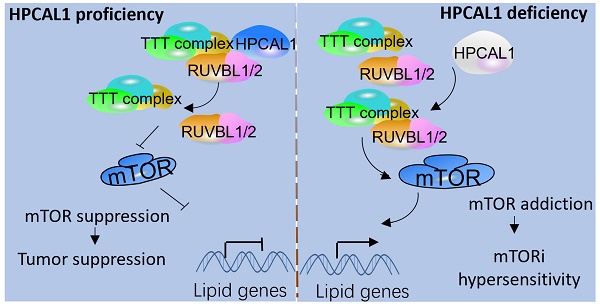
Results: In this study, we identified hippocalcin-like protein 1 (HPCAL1) as an important negative regulator of de novo lipid biosynthesis and mTOR signaling activation, limiting liver tumorigenesis and establishing a metabolic vulnerability of HCC in mice. Genetic loss of HPCAL1 rendered HCC mTORC1-addicted and sensitive to mTORi AZD-8055 in vitro and in vivo. Importantly, HPCAL1 expression was inversely correlated with the levels of mTOR phosphorylation and several critical lipid biosynthesis enzymes in human specimens. Mechanistically, HPCAL1 directly bound to RuvB Like AAA ATPase 1 (RUVBL1), inhibiting the assembly of TEL2-TTI1-TTI2 (TTT)-RUVBL complex and subsequent leading the mTOR signaling suppression.
Conclusion: We uncover a metabolic vulnerability and mTOR addiction in HCC with HPCAL1 loss that provides a selective therapeutic window for HCC with mTORC1 hyperactivation using mTORi.
Keywords: Hippocalcin-Like 1, RUVBL1, mTOR addiction, fatty acid biosynthesis, Cholesterol synthesis
 Global reach, higher impact
Global reach, higher impact