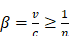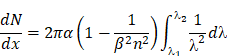13.3
Impact Factor
Theranostics 2022; 12(17):7404-7419. doi:10.7150/thno.75279 This issue Cite
Review
Cerenkov radiation-activated probes for deep cancer theranostics: a review
1. PET Center, Department of Nuclear Medicine, the First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China.
2. State Key Laboratory of Natural Medicines, Key Laboratory of Drug Quality Control and Pharmacovigilance, Department of Pharmaceutical Analysis, China Pharmaceutical University, Nanjing 210009, China.
Received 2022-5-19; Accepted 2022-9-7; Published 2022-10-24
Abstract
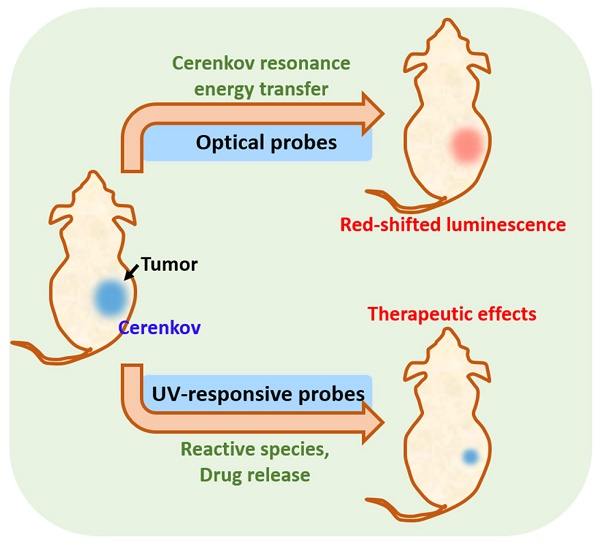
Cerenkov radiation (CR) from radionuclides and megavoltage X-ray radiation can act as an in situ light source for deep cancer theranostics, overcoming the limitations of external light sources. Despite the blue-weighted emission and low quantum yield of CR, activatable probes-mediated CR can enhance the in-vivo diagnostic signals by Cerenkov resonance energy transfer and also can produce therapeutic effects by reactive species generation/drug release, greatly promoting the biomedical applications of CR. In this review, we describe the principles and sources of CR, construction of CR-activated probes and their application to tumor optical imaging and therapy. Finally, future prospects for the design and biomedical application of CR-activated probes are discussed.
Keywords: Cerenkov radiation, probes, deep cancer, theranostics, radionuclides, X-ray radiation
1. Introduction
Light-based diagnosis and treatment methods have developed rapidly: examples include fluorescence imaging, optoacoustic imaging, photothermal therapy, and photodynamic therapy (PDT) [1-5]. However, these methods are less effective for deep tumors because of the rapid attenuation of light as it passes through tissue [6]. To overcome this limitation, Cerenkov radiation (CR) from radionuclides and megavoltage X-ray radiation can serve as an in situ excitation source within deep tissues [7]. Blue-weighted CR occurs when a charged particle travels faster than the speed of light in a given medium [8, 9]. Improvements in optical imaging technology and the increasing sensitivity of CCD cameras allow the detection of blue-weighted CR photons for functional molecular imaging [10-11]. Preclinical and clinical explorations of CR imaging have demonstrated its potential for high throughput, high sensitivity, and rapid imaging [12-17]. Nevertheless, current CR-based imaging lacks the necessary depth because the blue-weighted CR photons do not penetrate deep enough to image certain tumors [10, 18].
Activatable probes-mediated CR provides a way to enhance the diagnostic signals or produce therapeutic effects [19, 20]. These optical probes participate in Cerenkov resonance energy transfer (CRET) to red-shift emission into the near-infrared (NIR) region or red region of the visible spectrum, enabling sensitive detection from deeper inside the tissue. Besides, some activatable probes can produce therapeutic effects at greater depths: for example, ultraviolet (UV)-responsive probes can be activated by CR to produce therapeutic reactive species or to release drugs, referred to as CR-induced therapy (CRIT) [21]. The combination of activatable probes and CR expands the biomedical applications of CR.
In this review, we summarize CR-activated probes that have been reported to enhance optical imaging and therapy. CR efficiently triggers these optical probes with red-shifted emission, which include quantum dots, gold nanoclusters, lanthanide-based downconversion probes, persistent luminescence nanoparticles (PLNPs), and organic dye-associated NPs. The CR-induced PDT, photoimmunotherapy, drug release and even combinations of these therapies are also discussed in detail.
2. Cerenkov radiation
2.1 Principles of CR
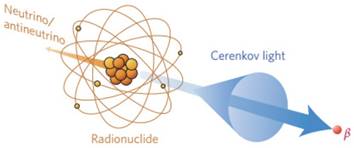
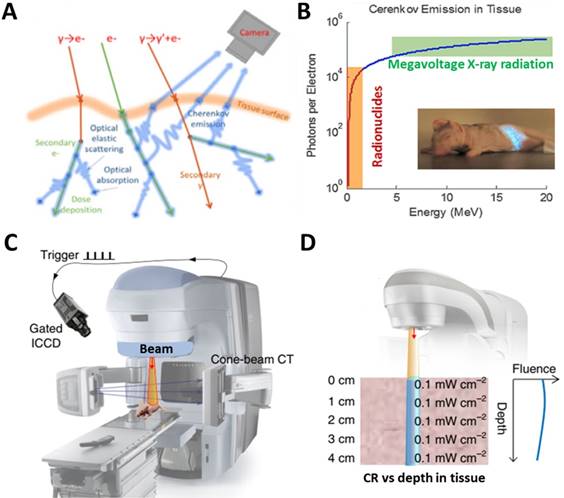
CR is generated through the interaction of charged particles and matter [9]. It can be produced from radionuclides, megavoltage X-ray radiation, cosmic events and nuclear reactors; clinical facilities may have access to radionuclides and linear accelerators [19]. The radionuclides release α, β, or γ particles during the decay, and the subatomic particles are charged when polarizing the medium. A similar process occurs in megavoltage X-ray radiation, except that the kinetic energy of the resulting electrons is 2-3 orders of magnitude greater when they pass through tissue [15].
When a charged particle travels faster than the speed of light through a dielectric medium, the surrounding molecules in the medium become locally polarized. After the particles pass through the medium, the molecules return to their ground state and release blue-weighted light called CR (Figure 1) [15, 19]. CR intensity is related to the velocity and energy of the charged particles (υ), as well as the refractive index of the medium (c). The threshold of input energy needed to generate CR can be described as [19]:
Additionally, the photon flux of CR at a given frequency can be calculated using the Frank-Tamm equation:
which demonstrates that CR is blue-weighted and decreases with 1/λ2 from the UV to infrared regions [19].
2.2 Sources of CR
2.2.1 Radionuclides
Radionuclides undergo decay through capture of positrons (β+) or electrons (β-), followed by an isomeric transition. CR arises when charged particles travel faster through the surrounding medium than the phase velocity of light [22]. Table 1 lists radioisotopes that emit energy over the CR threshold, such that they induce CR emission [23]. Positron emitters in clinical use of positron emission tomography (PET) include 18F, 64Cu, 68Ga, 74As, 89Zr, and 124I. Electron emitters in clinical use include 32P, 90Y, 131I, 177Lu, and 198Au, which can also be used for radiotherapy of cancer.
The radioactivity of each radionuclide strongly influences the intensity of the produced CR. In CR-based imaging, radionuclides with a relatively short half-life, such as 18F and 64Cu, are preferred for building theranostic nanomaterials. In CRIT, radionuclides with a long half-life, such as 90Y, 131I and 177Lu, are preferred because they can generate CR in the long term for prolonged treatment, and they can generate ionizing radiation that provides radiotherapy.
CR is produced by the medium through which charged particles propagate. Reproduced with permission [19]. Copyright 2017, Nature Publishing Group.
The properties of radionuclides used for CR applications [23, 24]
| Radionuclide | Half-life | Decay mode | Energy (keV) | Mean photo yield per disintegration | Clinical application |
|---|---|---|---|---|---|
| 18F | 109.8 min | β+ (97%) | 633.5 | 1.32 | PET |
| 64Cu | 12.7 h | β+ (17.8%) | 653 | 0.557 | PET |
| 68Ga | 67.6 min | β+ (88%) | 1899 | 33.9 | PET |
| 89Zr | 78.4 h | β+ (23%) | 2400 | 2.29 | PET |
| 124I | 4.17 d | β+ (11.7%) | 1534 | 8.97 | PET |
| 32P | 14.26 d | β- (100%) | 1710 | 28.1 | Radiotherapy |
| 90Y | 64 h | β- (99.9%) | 2280 | 47.3 | Radiotherapy |
| 131I | 8.02 d | β- (89.9%) | 606 | 0.669 | Radiotherapy |
| γ (81.7%) | 364 | ||||
| 177Lu | 6.73 d | β- (78.6%) | 498.3 | 0.141 | Radiotherapy |
| 198Au | 2.69 d | β- (98.9%) | 960.7 | Not reported | Radiotherapy |
2.2.2 Megavoltage X-ray radiation
The emission of CR needs the irradiation energy of X-rays to meet the in the high kilovoltage or megavoltage range while only 1% of secondary electrons can be delivered [25, 26]. Using X-rays to generate CR and in turn induce luminescence allows imaging at sub-millimeter resolution with nanomolar sensitivity, such as during real-time monitoring during radiotherapy in vivo [25, 26]. When X-rays pass through tissues, soft collisions during energy deposition lead to de-excitation of primary or secondary electrons, generating Cerenkov emission (Figure 2A) [7]. Megavoltage X-ray radiation is an efficient CR source because the X-ray beams contain far more Cerenkov photons per electron than what traditional radionuclides generate (Figure 2B) [7]. An experimental scanning imaging system to detect CR-excited luminescence is shown in Figure 2C. The system includes an accelerator beam and an intensified charge-coupled device. The CR shows minimal fluence loss as it propagates from deep within tissue to the detector (Figure 2D) [27].
3. Construction of CR-activated probes
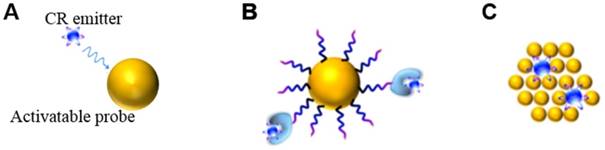
CR can be combined in space and time with activatable probes in two ways. One way is the unbound CR emitter with probes. Megavoltage X-ray radiation or clinical radiopharmaceuticals such as 18F-fluorodeoxyglucose (18F-FDG) can be used, which requires co-localizing the CR and probes in time and space in vivo. The other way is to use probes already labeled with radionuclides, mainly through (1) extrinsic chelation or (2) intrinsic radiolabelling (Figure 3) [28]. The most appropriate radiolabeling chelator depends on the coordination chemistry of the radiometal ion and the surface chemistry of the probe [29]. Commonly used chelators include diethylene triamine pentaacetic acid (DTPA), desferrioxamine (Df), 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA), and 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA). The chelation should be carefully designed in order to avoid altering the probe's pharmacokinetics. This approach carries risk of radionuclide detachment, leading to nonspecific biodistribution that leads to “noise” in the images. Intrinsic radiolabeling means incorporating radionuclides directly into nanoprobes, through such methods as “hot-plus-cold precursors”, cation exchange, and specific trapping [29]. For example, radioactive and non-radioactive precursor components can be mixed during NPs synthesis, or the radiochemical can be generated after nanoformulations through surface elemental exchange or radionuclide deposition. Intrinsic radiolabeling ensures a highly stable label that does not alter the probes' intrinsic behavior.
4. Probes for imaging based on CRET
A series of optical probes have been developed to minimize signal attenuation as it passes through tissue, yet tissue autofluorescence still generates sufficient background to severely degrade image quality [30]. CR can act as an in situ light source to activate fluorescent probes with large Stokes shifts. In this way, CR overcomes the limited penetration depth of external light, and it avoids tissue autofluorescence. At the same time, the excited fluorescent probe shifts blue-weighted CR to the NIR region or the red visible region, resulting in deeper tissue penetration. Below, we summarize different types of CR-excited fluorescent probes for optical imaging of tumors (Table 2).
(A) X-rays induce CR through soft collisions of electrons. (B) Numbers of Cerenkov photons per electron generated from radionuclides or a linear accelerator. Reproduced with permission [7]. Copyright 2021, Springer-Verlag Wien. (C) Experimental setup for scanning imaging based on Cerenkov-excited luminescence. (D) Fluence of X-ray induced CR from different depths. Reproduced with permission [27]. Copyright 2018, Nature Publishing Group.
Interactions between a CR emitter and activatable probe in the case of (A) an unbound Cerenkov emitter, (B) chelator-bound CR emitter, or (C) intrinsic radiolabelling. Reproduced with permission [28]. Copyright 2015, American Chemical Society.
Summary of CR-activated optical probes for imaging applications
| Activatable probe | CR emitter | Combination way | CRET emission | Imaging subject | Ref. |
|---|---|---|---|---|---|
| Qtracker705 | 64Cu and 18F | Unbound | 705nm | Subcutaneous pseudotumors | [33] |
| three CdSe/ZnS QDs | 131I | Unbound | 665nm, 705nm, 800nm | Subcutaneous pseudotumors | [34] |
| cRGD-QD605 | 89Zr-labeled deferoxamine-trastuzumab | Unbound | 605nm | HER2/neu-expressing xenografts | [35] |
| CdSeTe/CdSe/CdZnS | 89Zr | Extrinsic chelation | 710nm | sentinel lymph nodes and prostate cancer | [36] |
| CdSe/ZnS | 64Cu | Intrinsic radiolabelling | 526nm, 580nm, 636nm | U87MG tumor | [37] |
| CuInS/ZnS | 64Cu | Intrinsic radiolabelling | 680nm | U87MG tumor | [38] |
| PdSe QDs | Megavoltage X-ray radiation | Unbound | 1030nm | Subcutaneous pseudotumors | [39] |
| Ba0.55Y0.3F2:Eu3+ | 18F-FDG | Unbound | 597nm, 615nm, 692nn | Subcutaneous pseudotumors | [43] |
| Y2O2S:Eu3+ | 68Ga | Unbound | 660nm | Subcutaneous pseudotumors | [44] |
| Gd2O3:Eu3+ | 18F-FDG | Unbound | 620nm, 700nm | 4T1 tumor | [45] |
| Eu2O3 | 18F-FDG | Unbound | 620nm, 700nm | Subcutaneous 4T1 tumor and orthotropic HCC tumor | [46-49] |
| Eu2O3 | 89Zr | Intrinsic radiolabelling | 620nm, 700nm | Lymph node and CT26-tumor | [50] |
| Tb/Eu complexes | 18F, 89Zr | Extrinsic chelation | 490, 545nm/ 620nm | Phantoms | [51] |
| Tb(DO2Apic)-DUPA[Eu(DO2Aphen)-DUPA] | 18F-FDG | Unbound | 490, 545nm/ 620nm | PC3-PIP tumor (intratumoral treatment, IT) | [52] |
| Gold nanoclusters | 18F-FDG | Unbound | 680-700 nm | subcutaneous breast carcinomas | [54] |
| Gold nanoclusters | 64Cu | Intrinsic radiolabelling | 667nm | U87MG tumor | [55] |
| ZnGa2O4:Cr3+ | 18F-FDG | Unbound | 695nm | 4T1 tumor | [61] |
| Fluorescein sodium | 18F-FDG, 11C-CHO | Unbound | 520-530nm | subcutaneous 4T1 tumor and orthotropic HCC tumor | [64] |
| Fluorescein, rhodamine 6G, rhodamine 101, gresyl violet, cyanine 5, and indocyanine green | 90YCl3 | Unbound | 710nm | EMT6-tumor (IT) | [65] |
| Phthalocyanine-pyranine conjugates | 18F-FDG | Unbound | 700nm | Subcutaneous pseudotumors | [67] |
| Naphthofluorescein derivatives | 18F | Intrinsic radiolabelling | pH-sensitive | Normal mice | [68] |
| aluminum phthalocyanine, platinum II G4 (PtG4) | Megavoltage X-ray radiation | Unbound | 780nm | Various tumor | [69-72] |
| [Ir(pq)2(bpy)]Cl liposome | 18F-FDG | Unbound | 570nm | 4T1 tumor (IT) | [74] |
| Pluronic five different dyes-doped F127 silica nanoparticles | 32P-ATP | Unbound | 840nm | Phantoms | [75] |
4.1 Quantum dots
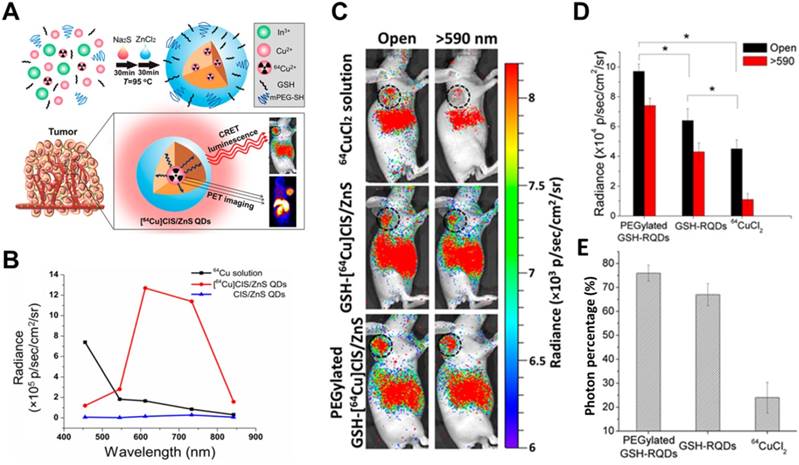
Quantum dots (QDs) are nanoscale crystalline clusters (1-10 nm) with high quantum yields, large spectral shifts, and tunable emission colors. They have attracted substantial attention for in vivo fluorescence imaging [31, 32]. They absorb broadly throughout the UV-visible range, overlapping with blue-weighted CR. 64Cu and 18F have been used to stimulate Qtracker705 QDs, and high CRET from the enhanced photons in the >590 nm filter was achieved during imaging of phantoms and tissues in vivo [33]. Na131I has been used as an unbound CR emitter to activate three CdSe/ZnS QDs with emission peaks of 665, 705, and 800 nm, thereby achieving multiplexed optical imaging [34]. In an in vivo study, 89Zr-labeled deferoxamine-trastuzumab actively targeted BT-474 tumors, and intravenously injected cRGD-QD605 accumulated in the tumor region, allowing tumors to be imaged through secondary Cerenkov-induced fluorescence [35]. Nevertheless, that work found that the radionuclides did not completely co-localize with the QDs. To improve co-localization, CdSeTe, CdSe, and CdZnS were modified with 89Zr-labeled chelator, and the resulting self-illuminating systems enabled good mapping of sentinel lymph nodes and prostate cancer [36].
To avoid radionuclide detachment, we developed two chelator-free methods to generate 64Cu-labeled QDs [37, 38]. One is to introduce 64Cu into QDs by cation exchange [37], and the other is to use 64CuCl2 as a precursor when synthesizing CuInS/ZnS (Figure 4) [38]. These two methods lead to high radiostability. The two types of 64Cu-labeled QDs exhibited higher tumor uptake and signal-to-background ratio in CRET imaging in vivo. CR was induced by X-rays to excite PdSe QDs, which emitted in the short-wave infrared region and CRET allowed sensitive depth imaging [39].
4.2 Lanthanide-based downconversion probes
Lanthanide-based downconversion probes possess good characteristics for optical imaging, such as narrow emission bandwidths, large Stokes shifts, and good photostability [40, 41]. They are usually synthesized by doping Tb3+, Eu3+, and Dy3+ to achieve the traditional Stokes luminescence, and they are activated by UV light to generate visible-NIR emission [42]. Therefore, lanthanide-based downconversion probes may be good CRET mediators to harness CR energy. Below we summarize the two groups of probes, lanthanide nanoparticles (NPs) and lanthanide complexes.
Lanthanide NPs with hard-core structures are usually synthesized by high-temperature chemical methods. Eu3+-doped Ba0.55Y0.3F2 nanophosphors with emission peaks at 597, 615, 692 nm have been prepared by a solvent-thermal method. Through CRET, excitation by 18F-FDG enhanced nanophosphor radiance at 700 nm for phantom and animal imaging [43]. Eu3+-doped Y2O3 NPs have also used as CR mediators for CRET imaging [44]; CR, rather than γ rays from 68Ga, was confirmed to enhance luminescence [44]. CR from 18F-FDG as well as β and γ radiation efficiently activated Eu3+-doped Gd2O3 NPs, enhancing optical imaging and intraoperatively guiding tumor surgery [45]. To improve the efficiency of photon leap in lanthanide NPs, Eu2O3 NPs, which have multiple absorption peaks in the UV range, have been proposed to show more efficient photon leap in lanthanide NPs after activation by ionizing radiation including CR, β, and γ scintillation. This reflects the fact that β interactions significantly contribute to activation of Eu2O3 [46-49]. Indeed, this approach allowed radiopharmaceutical-excited fluorescence imaging of phantoms as well as Bcap-37, U87MG, and 4T1-luc2 tumor models in vivo with high signal-to-background ratio. Also, Ultra-small 89Zr-labelled Eu2O3 NPs have been developed for enhanced Cerenkov imaging, allowing clear imaging of lymph nodes and tumors (Figure 5) [50].
Other lanthanide complexes have been designed to be excited in situ by radionuclides. 18F- or 89Zr-excited terbium(III) complexes of a macrocyclic polyaminocarboxylate ligand achieved a detection limit of 2.5 nmol in CRET luminescence [51]. Peptide-functionalized Tb(III) and Eu(III) complexes have been constructed for radiopharmaceutical-activated imaging in vivo. Combining these two probes with 18F-FDG led to emission at 570 and 620 nm, allowing multiplexed optical imaging of tumors [52].
(A) Schematic of chelator-free synthesis of radiolabeled QDs and application to PET and CRET imaging. (B) Photon flux of different samples obtained with different filters. (C) CRET images of U87MG tumor-bearing mice after 6 h of different treatments. (D,E) Total photon fluxes and red filter ratios in the tumor region. Reproduced with permission [38]. Copyright 2015, American Chemical Society.
(A, B) TEM image, excitation and emission spectra of ultrasmall Eu2O3 NPs. (C, D) CRET images of CT26 tumor-bearing mice and ex vivo organ imaging after 48h's injection of 89Zr- Eu2O3. Red arrow, tumor area. Reproduced with permission [50]. Copyright 2021, American Chemical Society.
4.3 Gold nanoclusters
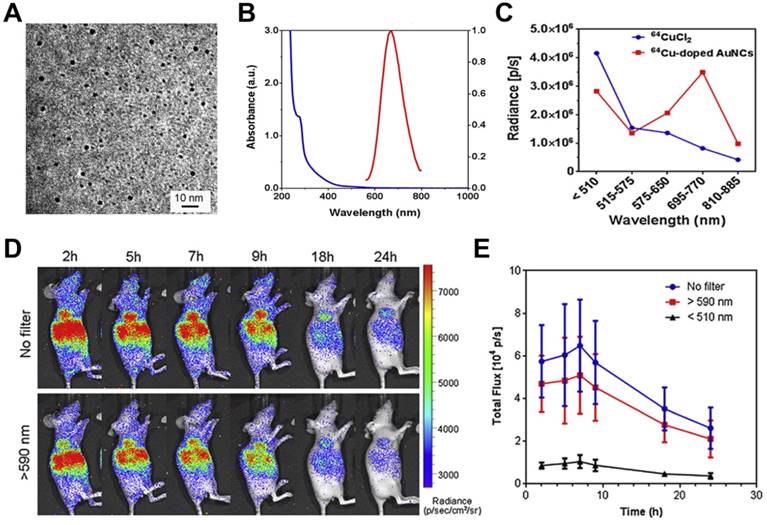
Gold nanoclusters (AuNCs) have been extensively developed as fluorescent probes for biomedical applications owing to their advantages of high compatibility, strong fluorescence, superior photostability, and excellent water solubility [53]. AuNCs are usually biomineralized with naturally functional macromolecules, which provide good colloidal stability and facilitate surface functionalization. AuNCs can fluoresce across a broad region extending from visible to near-infrared regions with a large Stokes shift. 64Cu-doped AuNCs with a diameter of 2.54 nm have been synthesized using an approach directed by human serum albumin, leading to AuNCs with an absorption peak at 280 nm and an emission peak at 667 nm (Figure 6A, B) [54]. During imaging of phantoms and a U87MG tumor model, 64Cu-doped AuNCs showed 4.3-fold greater optical intensity than free 64CuCl2 with an optical filter of 695-770 nm but lower intensity below 510 nm, demonstrating CRET from 64Cu to AuNCs (Figure 6C, D, E).
To verify the mechanism of efficient radioisotope energy transfer by AuNCs, they were excited using 18F-FDG, 90Y, or 99mTc (γ-emitter). Activation of 99mTc did not induce optical signal, while activation of 18F-FDG and 90Y enhanced optical emission [55]. The fact that the signal enhancement was not proportional to CR implies that AuNCs were excited by CR and direct Coulombic interaction [55].
4.4 PLNPs
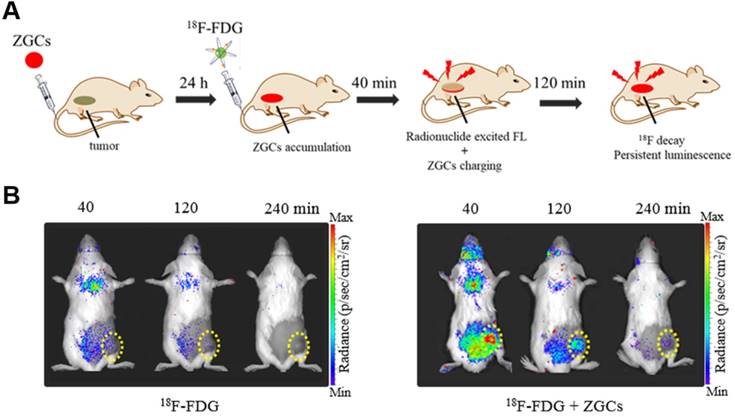
PLNPs maintain their luminescence after excitation has stopped, so they have been exploited for autofluorescence-free optical imaging with different excitation sources [56-58]. Cr3+-doped zinc gallates (ZGCs) are NIR-emitting PLNPs commonly used for tumor diagnosis, with three main excitation bands with peaks at 260, 465, and 570 nm [59, 60]. For the first time, we report that both CR and γ radiation from 18F-FDG can efficiently activate ZGCs to emit in the NIR region and persistently release photons for long-term imaging (Figure 7A) [61]. In fact, 18F-FDG excited ZGCs in 4T1 tumors in mice, maintaining luminescence for longer than 3 h (Figure 7B). Multiple injections of 18F-FDG also allowed for long-term real-time observation of tumor status, which may facilitate image-guided surgery in the future.
4.5 Organic dye or associated NPs
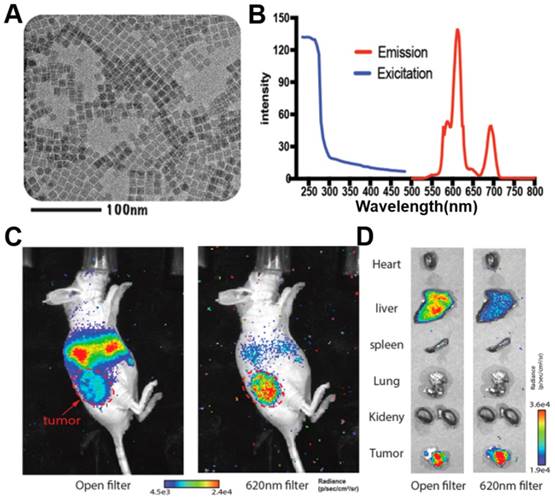
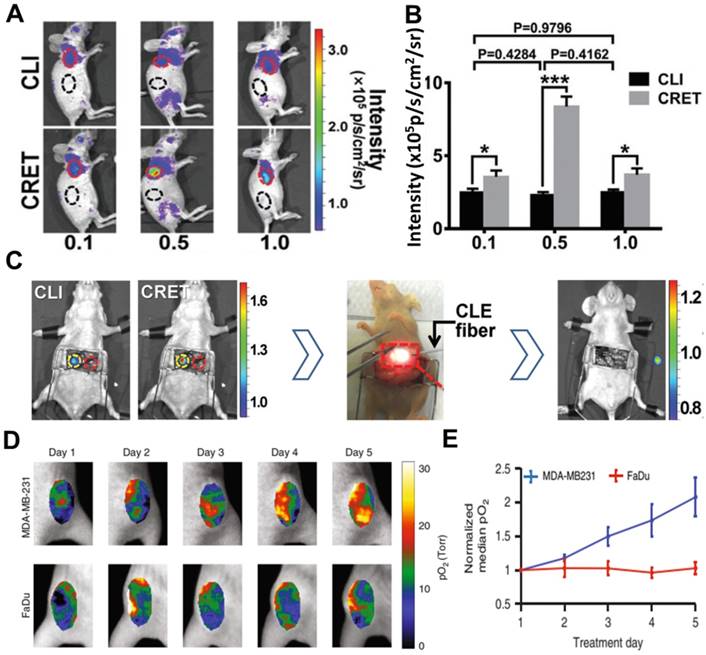
Inorganic fluorescent probes have excellent optical properties and can take full advantage of CR to undergo highly efficient CRET with large Stokes shifts. However, their potential toxicity and non-biodegradability have prevented their extensive development for clinical use. Organic compounds, in contrast, show good biodegradability and low toxicity [62]. For example, fluorescein sodium is clinically used to image retinal blood vessels; it absorbs strongly at 465-490 nm and emits at 520-530 nm [63, 64]. Despite its short Stokes shift, fluorescein sodium can be activated by 18F-FDG or 11C-CHO to show efficient CRET, as demonstrated in a subcutaneous 4T1 tumor model and orthotopic hepatocellular carcinoma tumor model (Figure 8A, B,C) [64]. In that study, tumors were precisely and completely resected through intraoperative CRET and confocal laser endomicroscopy imaging [64].
(A) TEM image of Cu-doped AuNCs (1%Cu). (B) The absorption and emission spectrum of Cu-doped AuNCs (1% Cu). (C) Intensity changes of 64CuCl2 and 64Cu-doped AuNCs at different optical filter sets. (D, E) Representative CRET images and photon flux of 64Cu-doped AuNCs on the U87MG tumor model. Reproduced with permission [54]. Copyright 2014, Elsevier.
Various fluorophores have been used to convert CR to NIR emission. Excitation of a mixture of fluorophores (fluorescein, rhodamine 6G, rhodamine 101, gresyl violet, cyanine 5, and indocyanine green) with 90YCl3 led to a CRET process, followed by several rounds of Förster resonance energy transfer (FRET), culminating in emission at 710 nm [65, 66]. All these fluorophores overlapped well in spectra, emitting twice as many photons as the radionuclide on its own. Featuring larger Stokes shifts, water-soluble phthalocyanine-pyranine conjugates have been developed with absorption at 250-450 nm and emission at 700 nm. They can be activated by 18F-FDG, leading to strong CRET using a filter of 710 nm as well as a photodynamic effect [67]. Interestingly, 18F-labeled naphthofluorescein derivatives have been developed that show selective bandwidth quenching under alkaline conditions but full-spectrum CR under acidic conditions [68].
Comparison of different CR-excited fluorophores induced by X-rays [69-72] suggests that aluminum phthalocyanine may be better for molecular luminescence [69], while platinum II G4 (PtG4), with absorption peaks at 435 and 624 nm and an emission peak at 780 nm, may be better for in vivo sensing [70]. PtG4 and X-ray induced CR have been used to detect spatial distribution of oxygen in tumors based on phosphorescence lifetimes [27, 73]. This approach revealed much higher partial pressure of oxygen (pO2) in MDA-MB-231 tumors than in FaDu tumors in animal models, suggesting that MDA-MB-231 tumors are more susceptible to radiotherapy (Figure 8D, E).
Organic dye-based NPs can enhance the accumulation of contrast agents in tumors while preserving the optical property of those agents. [Ir(pq)2(bpy)]Cl has been encapsulated into liposomes to formulate Ir@liposome, then irradiated with 18F-FDG, leading to imaging of deep tissue with a high signal-to-noise ratio [74]. Pluronic F127 silica NPs doped with five dyes have been constructed to absorb strongly in the visible range, leading to the efficient shift of CR towards NIR emission with a quantum yield of 0.12 [75]. The highly efficient energy transfer allowed the NPs to be detected from muscle as thick as 1.0 cm.
5. Probes for CR-induced therapy
CRIT refers to therapeutic effects that occur when CR-activated probes directly or indirectly produce reactive molecules that kill tumor cells. The efficiency of CRIT depends on delivery of adequate CR to target tissue, and proper stimulation of the probe. Therefore, the CR emitter and activatable probe must be selected carefully. Of course, effective tumor suppression also requires co-localization of emitter and probe. In addition, toxic effects that arise when the two inevitably co-localize in normal tissues must also be considered.
Below we provide an overview of probe-based CRIT, including CR-induced PDT, CR-induced photoimmunotherapy, CR-triggered drug release, and combination therapy (Table 3).
(A) An illustration of in vivo 18F-FDG activated persistent luminescence imaging of ZGCs. (B) Representative luminescence images of 4T1 tumor-bearing mice after administration of only 200 μCi 18F-FDG or 200 μg ZGCs injection before 24 h and following with 200 µCi 18F-FDG. Reproduced with permission [61]. Copyright 2020, Wiley-VCH.
Summary of CR-activated probes for therapeutic applications
| Activatable probe | CR emitter | Combination way | CRIT | Treated subject | Ref. |
|---|---|---|---|---|---|
| TiO2-Tf-Tc | 18F-FDG | Unbound | PDT | HT1080 tumor | [79] |
| TiO2-Tf | 89Zr | Intrinsic radiolabelling | PDT | Multiple myeloma | [80] |
| Titanocene-loaded nanoparticles | 18F-FDG | Unbound | PDT | Metastatic breast cancer and disseminated multiple myeloma | [81] |
| TiO2 | 68Ga-BSA | Unbound | PDT | 4T1 tumor (IT) | [82] |
| Chlorin e6 loaded hollow mesoporous silica NPs | 89Zr | Intrinsic radiolabelling | PDT | 4T1 tumor (IT) | [83] |
| Porphyrin surface-modified magnetic NPs | 89Zr | Intrinsic radiolabelling | PDT | 4T1 tumor | [84] |
| Df-PPN | 89Zr-labeled Df-PPN | Unbound | PDT | 4T1 tumor | [85] |
| IRDye700DX | 18F-FDG | Unbound | Photoimmunotherapy | A431-luc tumor | [88] |
| Folate-modified DOX/nanomicelles | Megavoltage X-ray radiation | Unbound | Chemotherapy | Hela tumor | [92] |
| ZGCs-ZnPcC4 | 131I | Extrinsic chelation | PDT-radiotherapy | 4T1 tumor (IT) | [95] |
| sPS NPs | 131I | Extrinsic chelation | PDT-radiotherapy | 4T1 tumor-bearing mice and VX2 liver tumor-bearing rabbit | [96] |
| EM@ALA | 131I | Extrinsic chelation | PDT-radiotherapy | 4T1 tumor-bearing mice | [97] |
| Titanium-Oxo nanoclusters | 18F-FDG | Unbound | Photo/chemodynamic therapy | HepG2 subcutaneous tumor | [98] |
| Single-layer 2D nanosheets | 32P | Intrinsic radiolabelling | Radioisotope-immunotherapy | 4T1 tumor (IT) | [100] |
(A) CR and CRET imaging of subcutaneous 4T1 tumor model after injection of 300µCi 18F-FDG (CRET group was treated with different doses of fluorescein). (B) The signal intensities and TNR of CLI and CRET. (C) Intraoperative CRI and CRET imaging of orthotropic HCC tumor model, and the process of image-guided surgery. Reproduced with permission [64]. Copyright 2019, Wiley-VCH. (D, E) pO2 images and the corresponding pO2 changes during each day of radiation (6MV X-ray beam with 5 Gy/fraction), PtG4 was iv injected 24h before the first treatment. Reproduced with permission [73]. Copyright 2019, Nature Publishing Group.
5.1. CR-induced PDT
PDT has been widely used to treat many solid tumors because of its non-invasiveness and dual selectivity [76]. In PDT, external light activates photosensitizers to generate reactive oxygen species (ROS), which damage cancer cells. A major drawback is that PDT requires direct irradiation of tissue with visible or even UV light in order to stimulate photosensitizers. This limits penetration depth, making the approach ineffective against large or deep-seated tumors [6, 77]. Blue-weighted CR can overcome this problem by acting as an in situ light source to activate photosensitizers continuously [21].
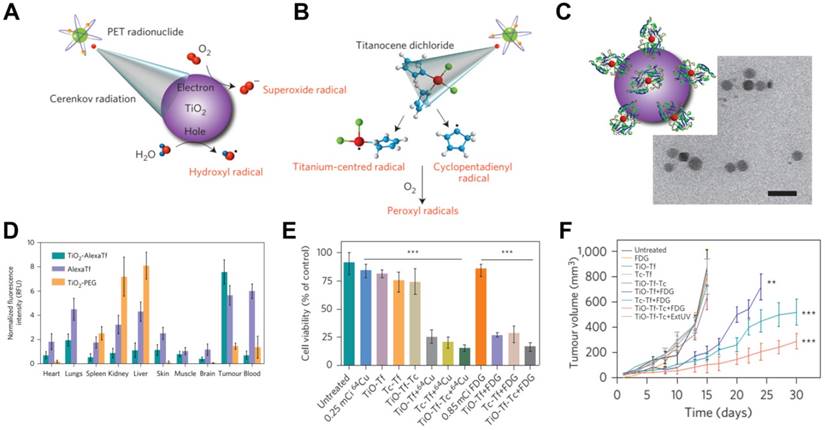
For example, TiO2 NPs act as photocatalysts that can be regenerated to efficiently absorb UV light at 275-390 nm and to produce cytotoxic superoxide radicals and hydroxyl [78]. A TiO2-based CRIT-nanoplatform has been achieved for deep PDT, in which 18F-FDG excites TiO2 to continuously generate OH- by electron-hole pair generation (Figure 9A) [79]. Of course, it is worth noting that β and γ scintillation produced from radionuclides also play a role in TiO2's activation [49]. Intratumoral administration of TiO2-PEG and 64Cu led to remarkable tumor regression within 3 days and complete inhibition within 30 days. In another approach, apo-transferrin, a ligand for overexpressed Tf receptors, and titanocene, a photogenerator of peroxyl radicals, were integrated into TiO2 (TiO2-Tf-Tc) to enhance CRIT with an intravenous platform (Figure 9B,C,E). This system accumulated in tumors to a greater extent than TiO2-PEG within 24 h, based on ex vivo fluorescence imaging (Figure 9D). Intravenous injection of TiO2-Tf-Tc followed by 18F-FDG at 24 h later significantly inhibited tumor growth (Figure 9E,F). This work paves the way to treating deep-seated tumors using CR-induced PDT.
(A, B) An illumination of CR-induced PDT during the CR-excited TiO2 NPs and Tc. (C) TEM image of TiO2-Tf-Tc. (D) In vivo biodistribution of TiO2-PEG and TiO2-AlexaTf at 24h postinjection in HT1080 tumor-bearing mice. (E) Cell-viability assays on HT1080 cells with various treatments. (F) Tumor volumes of HT1080 tumor-bearing mice with various treatments. Reproduced with permission [79]. Copyright 2015, Nature Publishing Group.
To compensate for low uptake of 18F-FDG in bone, 89Zr-labeled TiO2-Tf has been developed to target bone marrow with high selectivity, leading to accumulation of 70% of injected radioactivity there. This accumulation allowed continuous ROS production that completely eliminated tumors from a multiple myeloma model and that doubled survival time [80]. Tf-mediated transport of titanocene into various types of tumor has been improved by using high-affinity VLA-4-bonded LLP2A conjugated phospholipid micelles or human serum albumin NPs [81]. Activation of the titanocene in situ by intravenously injected 18F-FDG inhibited breast cancer metastasis and growth of disseminated multiple myeloma. 68Ga-bovine serum albumin has been used instead of 18F-FDG to improve PDT efficiency with CR-excited TiO2, leading to strong tumor inhibition in vitro and in vivo [82].
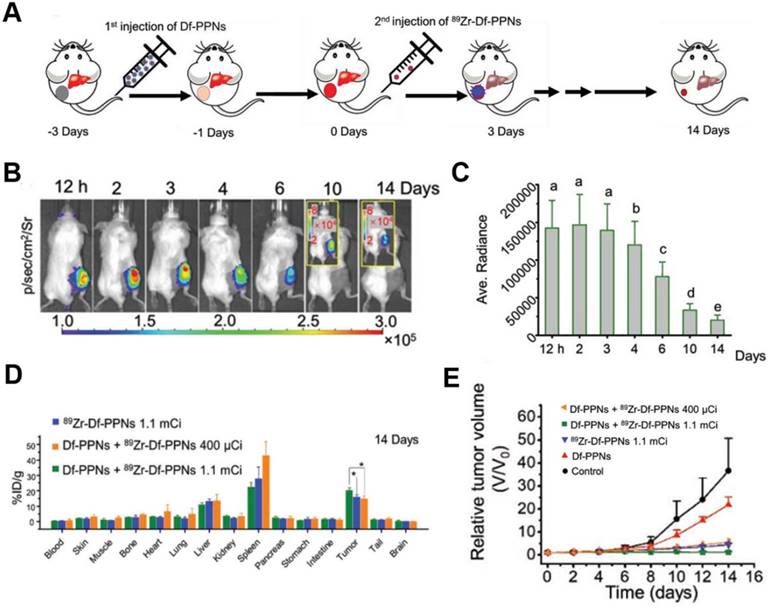
CR-induced PDT has been achieved using the photosensitizer dyes, such as chlorin e6 and tetrakis(4-carboxyphenyl)porphyrin (TCPP) [83-85]. Hollow mesoporous silica NPs were loaded with 89Zr-labeled chlorin e6, and the 89Zr provided CR to activate chlorin e6, which generated tumor-inhibiting ROS [83]. Given that intravenously injected theranostic agents do not strongly accumulate in tumors, 89Zr-labeled magnetic NPs with a modified porphyrin surface were constructed to enable accumulation of the NPs in tumors (15% ID/g) after application of a magnetic field; the accumulation was five times higher than without the magnetic field [84]. TCPP-loaded magnetic NPs significantly inhibited tumor growth through CR-induced PDT during two weeks. While promising, this strategy led to substantial NPs accumulation also in healthy organs, suggesting the potential for adverse effects. To enhance targeting in CRIT, a “missile-detonation” strategy has been developed: high-dose p-SCN-Bn-deferoxamine-porphyrin-PEG nanocomplex (Df-PPN) was administered as a CR energy receiver, and it passively targeted tumors; then, at the optimal time, low-dose 89Zr-labeled Df-PPN was injected as a CR emitter (Figure 10A) [85]. This strategy effectively mitigated toxic side effects of CRIT due to the relatively low accumulation of PPN in normal tissues. The nanocomplex persisted in the tumor in the long term, based on CR-excited fluorescence imaging (Figure 10B,C,D), and it drove continuous ROS production that significantly suppressed tumor growth (Figure 10E).
5.2 CR-induced photoimmunotherapy
NIR photoimmunotherapy is a newly targeted cancer treatment that uses antibody-photoabsorbers (IRDye700DX) to actively bind to cancer cells and then induce the selective immunogenic cell death under NIR light irradiation, resulting in the activation of anti-cancer immune system locally in the tumor microenvironment [86, 87]. However, NIR laser light cannot penetrate deep in tissue, but the CR emitter 18F-FDG can be delivered deep into tissue to deliver radiation at the absorbance peak of IRDye700DX at 350 nm, allowing photoimmunotherapy of deep-seated tumors [88]. The CR-induced photoimmunotherapy in that study inhibited tumor growth to some degree, but the effect was not consistent during the observation period. This may be because the lower CR energy stimulated a weak immune response. Megavoltage X-ray radiation may be a better CR source for activation of IRDye700DX.
(A) Schematic of the “missile-detonation” strategy. (B,C) Representative CR-excited optical images and the corresponding photon fluxes in a 4T1 tumor model on different days after treatment. (D) Biodistribution of 89Zr-Df-PPNs at 14 days after injection, based on measurement of radioactivity in each organ. (E) Relative volume of 4T1 tumors in mice after various treatments. Reproduced with permission [85]. Copyright 2019, Wiley-VCH.
5.3 CR-triggered drug release
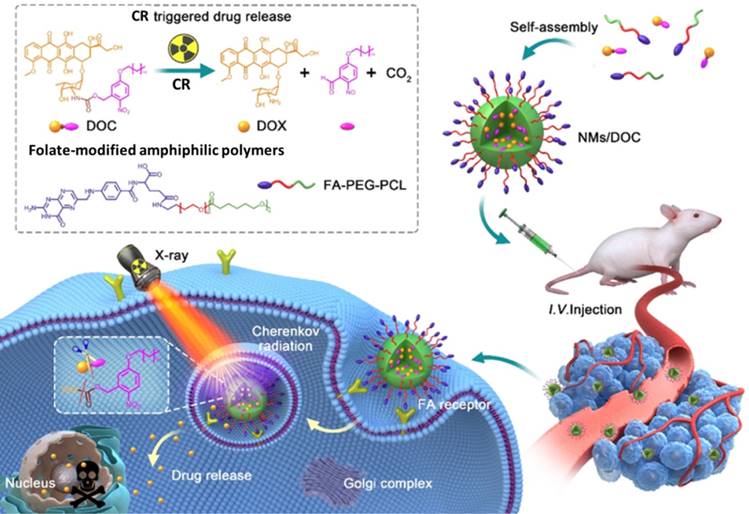
Although chemotherapeutics can be effective against cancer, delivering them to target tissue without causing toxicity in normal tissue is challenging [89]. Therefore, drug delivery systems have been developed to release drugs at specific sites in response to certain stimuli, especially light [89, 90]. However, the limited penetration of light through tissue means that external light sources cannot efficiently trigger drug release in deep tissue. Using CR as in situ light can overcome this shortcoming. UV-responsive drug delivery systems may be most appropriate for CR-activated drug release. For example, phenacyl bis-azide crosslinkers make dextran-based hydrogels photosensitive, and the co-loaded doxorubicin (DOX), BSA, and IgG are efficiently released after UV light irradiation [91]. In another approach, DOX has been caged using a photocleavable o-nitrobenzyl ester derivative and then encapsulated into nanomicelles for CR-triggered drug release and chemotherapy (Figure 11) [92]. Irradiation with X-rays induced CR, which converted the hydrophobic DOC into hydrophilic DOX, which was rapidly released and killed cancer cells. This strategy may pave the way to a new chemoradiotherapy with minimal systemic side effects.
5.4 Combination therapy
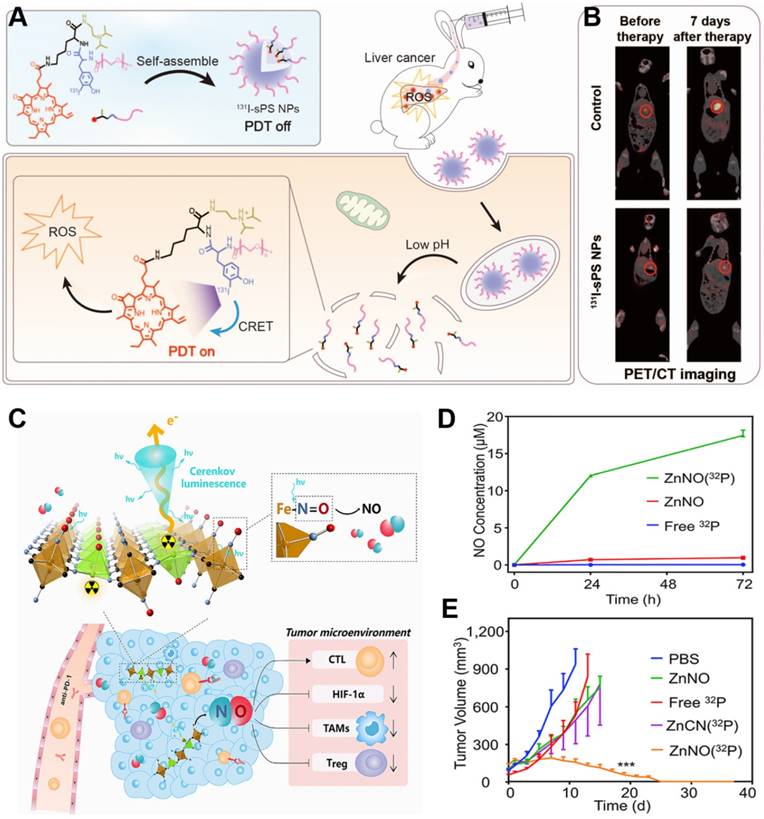
The low efficiency of CR limits its therapeutic efficacy [93], which combination therapy may improve [94]. We have developed a ZGC nanoplatform conjugated to 131I-labeled ZnPc(COOH)4 (131I-ZGCs-ZnPcC4) that can support both radiotherapy as well as continuous, radiation-induced PDT through energy transfer from the ionizing radiation to ZGCs and then to ZnPc(COOH)4 [95]. In another approach, we engineered pyropheophorbide-a containing PEG and a diisopropylamino group with an 131I-labeled tyrosine, which self-assembled into pH-sensitive NPs [96]. These NPs exhibited minimal phototoxicity in normal tissue because of the quenched photodynamic effect, and under acidic conditions, they disassembled to promote the generation of ROS to achieve PDT and radiotherapy against deep-seated tumors (Figure 12A). Their strong accumulation in tumors makes the NPs effective against 4T1 tumors in mice and VX2 liver tumors in rabbits (Figure 12B). Thus, this strategy shows promise for deep tumor therapy. Combination therapy also has been developed in which the precursor of protoporphyrin IX called 5-aminolevulinic acid (ALA) and 131I are co-loaded into biomimetic exosomes. In the tumor microenvironment, where mitochondria are abundant, the ALA is converted to protoporphyrin IX, minimizing CRIT side effects on normal tissues [97].
The CR-responsive folate-modified DOX/nanomicelles for folate cellular targeting and nuclear localization toxicity. Reproduced with permission [92]. Copyright 2020, American Chemical Society.
Functionalized inorganic materials can also achieve multiple therapeutic effects. Titanium-oxo nanoclusters have been reported for CR-induced photo/chemodynamic therapy of tumors [98]. CR can drive efficient production of hydroxyl radical (·OH) from titanium-oxo nanoclusters due to the enhanced separation of hole (h+)-electron (e-) pairs. The reaction of h+ and H2O provides type I PDT, while the transferred e- augmented Ti3+ provides chemodynamic therapy. Besides, 32P-labeled single-layer 2D nanosheets have been constructed by mixing Zn2+ ions and sodium nitroprusside [Na2Fe(CN)5NO]. CR from 32P can persistently stimulate nanosheets to release NO, which can modulate the tumor microenvironment to induce anti-tumor immunotherapy and improve the efficacy of radionuclide therapy (Figure 12C,D) [99-100]. This nanosystem has been extended to include immune-checkpoint blockade therapy against anti-programmed cell death protein 1 in order to achieve sustainably strong immune responses that effectively suppress tumor growth (Figure 12E).
6. Conclusion and perspectives
CR-activated probes take advantage of the ability of CR to pass through tissue without attenuation, enabling detection and treatment of deep-seated tumors. This review has focused on probes that can be activated by CR and that provide theranostics via CRET or production of reactive species/drug release. While these activatable probes can also be excited by ionizing radiation from radiotracers, especially lanthanide-based downconversion probes, the present review demonstrates the promise of approaches based on activation by CR. Further development of CRET will depend on the design of biocompatible optical probes with large Stokes shifts in the “deep NIR” to NIR-II regions. Future work should aim to overcome several limitations of CRIT, such as inefficient delivery of CR to therapeutic drugs in tumors, short duration of CR excitation, and damage to normal tissue during CRIT. In any case, suitable CR emitters and CR-responsive photosensitizers or drugs must always be chosen carefully in order to maximize tumor targeting and tumor cytotoxicity. Radionuclides with long half-lives are particularly promising as CR emitters, because they continuously generate CR to activate CR-responsive photosensitizers or drugs while also providing radiotherapy. Although CR remains in early stages of preclinical development, we believe that its use as an in situ light source will broaden the possibilities for imaging-based theranostics of cancer.
(A) Schematic of pH-activatable CRET-PDT. (B) Representative imaging of VX2 liver tumor-bearing rabbits using PET/CT before and at seven days after the indicated treatments. Reproduced with permission [96]. Copyright 2021, Wiley-VCH. (C) Schematic of 32P-triggered NO release for enhanced radioisotope-immunotherapy. (D) Concentration of NO released from ZnNO(32P) nanosheets at various time points. (E) Growth curves of CT26 tumors in mice after various treatments. Reproduced with permission [99]. Copyright 2019, Elsevier.
Acknowledgements
Su X. Acknowledges the National Natural Science Foundation of China (NSFC) (82071965) and Huadong Medicine Joint Funds of the Zhejiang Provincial Natural Science Foundation of China (LHDMZ22H300010). Sun X. acknowledges the National Key Research and Development Program of China (2016YFA0203600), National Natural Science Foundation of China (81971738, 81571743), the Project Program of State Key Laboratory of Natural Medicines, China Pharmaceutical University (SKLNMZZRC05).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Yun SH, Kwok SJJ. Light in diagnosis, therapy and surgery. Nat Biomed Eng. 2017;1:0008
2. Menon JU, Jadeja P, Tambe P, Vu K, Yuan B, Nguyen KT. Nanomaterials for photo-based diagnostic and therapeutic applications. Theranostics. 2013;3:152-166
3. Liu N, O'Connor P, Gujrati V, Gorpas D, Glasl S, Blutke A. et al. Facile Synthesis of a Croconaine-Based Nanoformulation for Optoacoustic Imaging and Photothermal Therapy. Adv Healthc Mater. 2021 2002115
4. Liu N, Gujrati V, Malekzadeh-Najafabadi J, Werner JPF, Klemm U, Tang L. et al. Croconaine-based nanoparticles enable efficient optoacoustic imaging of murine brain tumors. Photoacoustics. 2021;22:100263
5. Shi D, Liu W, Wang G, Guo Y, Li J. Small molecule fulorescence-based probes for agingdiagnosis. Acta Materia Medica. 2022;1(1):4-23
6. Fan W, Huang P, Chen X. Overcoming the Achilles' heel of photodynamic therapy. Che Soc Rev. 2016;45:6488-6519
7. Pogue BW, Cao X, Swartz HM, Vinogradov SA. Review of Tissue Oxygenation Sensing During Radiotherapy Based Upon Cherenkov-Excited Luminescence Imaging. Appl Magn Reson. 2021;52:1521-1536
8. Robertson R, Germanos MS, Li C, Mitchell GS, Cherry SR, Silva MD. Optical imaging of Cerenkov light generation from positron-emitting radiotracers. Phys Med Biol. 2009;54:N355-65
9. Jelley JV. Cerenkov radiation and its applications. Br J Appl Phys. 1955;6:227
10. Tanha K, Pashazadeh AM, Pogue BW. Review of biomedical Cerenkov luminescence imaging applications. Biomed Opt Express. 2015;6:3053-3065
11. Xu Y, Liu H, Cheng Z. Harnessing the power of radionuclides for optical imaging: Cerenkov luminescence imaging. J Nucl Med. 2011;52:2009-2018
12. Thorek DL, Riedl CC, Grimm J. Clinical Cerenkov luminescence imaging of 18F-FDG. J Nucl Med. 2014;55:95-98
13. Lewis DY, Mair R, Wright A, Allinson K, Lyons SK, Booth T. et al. [(18)F]fluoroethyltyrosine-induced Cerenkov Luminescence Improves Image-Guided Surgical Resection of Glioma. Theranostics. 2018;8:3991-4002
14. Grootendorst MR, Cariati M, Pinder SE, Kothari A, Douek M, Kovacs T. et al. Intraoperative Assessment of Tumor Resection Margins in Breast-Conserving Surgery Using 18F-FDG Cerenkov Luminescence Imaging: A First-in-Human Feasibility Study. J Nucl Med. 2017;58:891-898
15. Mc Larney B, Skubal M, Grimm J. A Review of Recent and Emerging Approaches for the Clinical Application of Cerenkov Luminescence Imaging. Front Phys. 2021;9:684196
16. Hachadorian RL, Bruza P, Jermyn M, Gladstone DJ, Pogue BW, Jarvis LA. Imaging radiation dose in breast radiotherapy by X-ray CT calibration of Cherenkov light. Nat Comm. 2020;11:2298
17. Pratt EC, Skubal M, Mc Larney B, Causa-Andrieu P, Das S, Sawan P. et al. Prospective testing of clinical Cerenkov luminescence imaging against standard-of-care nuclear imaging for tumour location. Nat Biomed Eng. 2022 https://doi.org/10.1038/s41551-022-00876-4
18. Niu G, Chen X. When radionuclides meet nanoparticles. Nat Nanotechnol. 2018;13:359-360
19. Shaffer TM, Pratt EC, Grimm J. Utilizing the power of Cerenkov light with nanotechnology. Nat Nanotechnol. 2017;12:106-117
20. Ferreira CA, Ni D, Rosenkrans ZT, Cai W. Radionuclide-Activated Nanomaterials and Their Biomedical Applications. Angew Chem Int Ed. 2019;58:13232
21. Li B, Lin L. Internal light source for deep photodynamic therapy. Light Sci Appl. 2022;11:85
22. Luig H, Keller C, Wolf W, Shani J, Miska H, Zyball A. et al. Radionuclides. Radionuclides, Ullmann's Encyclopedia of Industrial Chemistry. 2000
23. Ma X, Wang J, Cheng Z. Cerenkov radiation: a multi-functional approach for biological sciences. Front Phys. 2014;2:4
24. Gill RK, Mitchell GS, Cherry SR. Computed Cerenkov luminescence yields for radionuclides used in biology and medicine. Phys. Med. Biol. 2015;60:4263-4280
25. Grimm J. High-resolution Cherenkov tomography in vivo. Nat Biomed Eng. 2018;2:205-206
26. Pogue BW, Zhang R, Cao X, Jia JM, Petusseau A, Bruza P. et al. Review of in vivo optical molecular imaging and sensing from x-ray excitation. J Biomed Opt. 2021;26:010902
27. Pogue BW, Feng J, LaRochelle EP, Bruza P, Lin H, Zhang R. et al. Maps of in vivo oxygen pressure with submillimetre resolution and nanomolar sensitivity enabled by Cherenkov-excited luminescence scanned imaging. Nat Biomed Eng. 2018;2:254-264
28. Sun X, Cai W, Chen X. Positron emission tomography imaging using radiolabeled inorganic nanomaterials. Acc Chem Res. 2015;48:286-294
29. Chakravarty R, Goel S, Dash A, Cai W. Radiolabeled inorganic nanoparticles for positron emission tomography imaging of cancer: an overview. Q. J. Nucl. Med. Mol. Imaging. 2017;61:181-204
30. Zhao J, Zhong D, Zhou S. NIR-I-to-NIR-II fluorescent nanomaterials for biomedical imaging and cancer therapy. J Mater Chem B. 2018;6:349-365
31. Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ. et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538-544
32. Wegner KD, Hildebrandt N. Quantum dots: bright and versatile in vitro and in vivo fluorescence imaging biosensors. Chem Soc Rev. 2015;44:4792-4834
33. Dothager RS, Goiffon RJ, Jackson E, Harpstrite S, Piwnica-Worms D. Cerenkov radiation energy transfer (CRET) imaging: a novel method for optical imaging of PET isotopes in biological systems. PloS One. 2010;5:e13300
34. Liu H, Zhang X, Xing B, Han P, Gambhir SS, Cheng Z. Radiation-luminescence-excited quantum dots for in vivo multiplexed optical imaging. Small. 2010;6:1087-1091
35. Thorek DL, Ogirala A, Beattie BJ, Grimm J. Quantitative imaging of disease signatures through radioactive decay signal conversion. Nat Med. 2013;19:1345-1350
36. Zhao Y, Shaffer TM, Das S, Perez-Medina C, Mulder WJ, Grimm J. Near-Infrared Quantum Dot and 89Zr Dual-Labeled Nanoparticles for in vivo Cerenkov Imaging. Bioconjug Chem. 2017;28:600-608
37. Sun X, Huang X, Guo J, Zhu W, Ding Y, Niu G. et al. Self-illuminating 64Cu-doped CdSe/ZnS nanocrystals for in vivo tumor imaging. J Am Chem Soc. 2014;136:1706-1709
38. Guo W, Sun X, Jacobson O, Yan X, Min K, Srivatsan A. et al. Intrinsically Radioactive [64Cu]CuInS/ZnS Quantum Dots for PET and Optical Imaging: Improved Radiochemical Stability and Controllable Cerenkov Luminescence. ACS Nano. 2015;9:488-495
39. Cao X, Jiang S, Jia MJ, Gunn JR, Miao T, Davis SC. et al. Cherenkov excited short-wavelength infrared fluorescence imaging in vivo with external beam radiation. J Biomed Opt. 2018;24:1-4
40. Bajgiran KR, Dorman JA, Melvin AT. Dipole-Modulated Downconversion Nanoparticles as Label-Free Biological Sensors. ACS Sens. 2020;5:29-33
41. Loo JF-C, Chien Y-H, Yin F, Kong S-K, Ho H-P, Yong K-T. Upconversion and downconversion nanoparticles for biophotonics and nanomedicine. Coord Chem Rev. 2019;400:213042
42. Escudero A, Becerro AI, Carrillo-Carrión C, Núñez NO, Zyuzin MV, Laguna M. et al. Rare earth based nanostructured materials: synthesis, functionalization, properties and bioimaging and biosensing applications. Nanophotonics. 2017;6:881-921
43. Sun C, Pratx G, Carpenter CM, Liu H, Cheng Z, Gambhir SS. et al. Synthesis and radioluminescence of PEGylated Eu(3+) -doped nanophosphors as bioimaging probes. Adv Mater. 2011;23:H195-9
44. Gao Y, Ma X, Kang F, Yang W, Liu Y, Wang Z. et al. Enhanced Cerenkov luminescence tomography analysis based on Y2O3:Eu3+ rare earth oxide nanoparticles. Biomed Opt Express. 2018;9:6091
45. Shi X, Cao C, Zhang Z, Tian J, Hu Z. Radiopharmaceutical and Eu(3+) doped gadolinium oxide nanoparticles mediated triple-excited fluorescence imaging and image-guided surgery. J Nanobiotechnology. 2021;19:212
46. Hu Z, Qu Y, Wang K, Zhang X, Zha J, Song T. et al. In vivo nanoparticle-mediated radiopharmaceutical-excited fluorescence molecular imaging. Nat Comm. 2015;6:7560
47. Hu Z, Zhao M, Qu Y, Zhang X, Zhang M, Liu M. et al. In vivo 3-Dimensional Radiopharmaceutical-Excited Fluorescence Tomography. J Nucl Med. 2017;58:169-174
48. Hu Z, Chi C, Liu M, Guo H, Zhang Z, Zeng C. et al. Nanoparticle-mediated radiopharmaceutical-excited fluorescence molecular imaging allows precise image-guided tumor-removal surgery. Nanomed: Nanotechnol Biol Med. 2017;13:1323-1331
49. Pratt EC, Shaffer TM, Zhang Q, Drain CM, Grimm J. Nanoparticles as multimodal photon transducers of ionizing radiation. Nat Nanotechnol. 2018;13:418-1426
50. Zhang Q, Pratt EC, Tamura R, Ogirala A, Hsu HT, Farahmand N. et al. Ultrasmall Downconverting Nanoparticle for Enhanced Cerenkov Imaging. Nano Lett. 2021;21:4217-4224
51. Cosby AG, Ahn SH, Boros E. Cherenkov Radiation-Mediated In situ Excitation of Discrete Luminescent Lanthanide Complexes. Angewandte Chemie. 2018;57:15496-15499
52. Martin KE, Cosby AG, Boros E. Multiplex and In vivo Optical Imaging of Discrete Luminescent Lanthanide Complexes Enabled by In situ Cherenkov Radiation Mediated Energy Transfer. J Am Chem Soc. 2021;143:9206-9214
53. Kaur N, Aditya RN, Singh A, Kuo TR. Biomedical Applications for Gold Nanoclusters: Recent Developments and Future Perspectives. Nanoscale Res Lett. 2018;13:302
54. Hu H, Huang P, Weiss OJ, Yan X, Yue X, Zhang MG. et al. PET and NIR optical imaging using self-illuminating 64Cu-doped chelator-free gold nanoclusters. Biomaterials. 2014;35:9868-76
55. Volotskova O, Sun C, Stafford JH, Koh AL, Ma X, Cheng Z. et al. Efficient Radioisotope Energy Transfer by Gold Nanoclusters for Molecular Imaging. Small. 2015;11:4002-8
56. Sun SK, Wang HF, Yan XP. Engineering Persistent Luminescence Nanoparticles for Biological Applications: From Biosensing/Bioimaging to Theranostics. Acc Chem Res. 2018;51:1131-43
57. Sun X, Song L, Liu N, Shi J, Zhang Y. Chromium-Doped Zinc Gallate Near-Infrared Persistent Luminescence Nanoparticles in Autofluorescence-Free Biosensing and Bioimaging: A Review. ACS Appl Nano Mater. 2021;4:6497-514
58. Liu N, Chen X, Sun X, Sun X, Shi J. Persistent luminescence nanoparticles for cancer theranostics application. J Nanobiotechnology. 2021;19:113
59. Li Z, Zhang Y, Wu X, Wu X, Maudgal R, Zhang H. et al. In vivo Repeatedly Charging Near-Infrared-Emitting Mesoporous SiO2/ZnGa2O4:Cr3+ Persistent Luminescence Nanocomposites. Adv Sci. 2015;2:1500001
60. Li Z, Zhang Y, Wu X, Huang L, Li D, Fan W. et al. Direct Aqueous-Phase Synthesis of Sub-10 nm "Luminous Pearls" with Enhanced in vivo Renewable Near-Infrared Persistent Luminescence. J Am Chem Soc. 2015;137:5304-5307
61. Liu N, Shi J, Wang Q, Guo J, Hou Z, Su X. et al. In vivo Repeatedly Activated Persistent Luminescence Nanoparticles by Radiopharmaceuticals for Long-Lasting Tumor Optical Imaging. Small. 2020:e2001494
62. Cai Y, Si W, Huang W, Chen P, Shao J, Dong X. Organic Dye Based Nanoparticles for Cancer Phototheranostics. Small. 2018;14:e1704247
63. Dip FD, Nahmod M, Anzorena FS, Moreira A, Sarotto L, Ampudia C. et al. Novel technique for identification of ureters using sodium fluorescein. Surg Endosc. 2014;28:2730-2733
64. Zheng S, Zhang Z, Qu Y, Zhang X, Guo H, Shi X. et al. Radiopharmaceuticals and Fluorescein Sodium Mediated Triple-Modality Molecular Imaging Allows Precise Image-Guided Tumor Surgery. Adv Sci. 2019:1900159
65. Bernhard Y, Collin B, Decreau RA. Redshifted Cherenkov Radiation for in vivo Imaging: Coupling Cherenkov Radiation Energy Transfer to multiple Forster Resonance Energy Transfers. Sci Rep. 2017;7:45063
66. Bernhard Y, Collin B, Decreau RA. Inter/intramolecular Cherenkov radiation energy transfer (CRET) from a fluorophore with a built-in radionuclide. Chem Comm. 2014;50:6711-6713
67. Lioret V, Bellaye PS, Arnould C, Collin B, Decreau RA. Dual Cherenkov Radiation-induced Near-Infrared Luminescence Imaging and Photodynamic Therapy towards Tumor Resection. J Med Chem. 2020;63:9446-9456
68. Czupryna J, Kachur AV, Blankemeyer E, Popov AV, Arroyo AD, Karp JS. et al. Cerenkov-specific contrast agents for detection of pH in vivo. J Nucl Med. 2015;56:483-488
69. Petusseau AF, Bruza P, Pogue BW. Survey of X-ray induced Cherenkov excited fluorophores with potential for human use. J Radiat Res. 2021;62:833-840
70. Shell J, LaRochelle EP, Bruza P, Gunn J, Jarvis L, Gladstone D. et al. Comparison of phosphorescent agents for noninvasive sensing of tumor oxygenation via Cherenkov-excited luminescence imaging. J Biomed Opt. 2019;24:1-8
71. Axelsson J, Davis SC, Gladstone DJ, Pogue BW. Cerenkov emission induced by external beam radiation stimulates molecular fluorescence. Med Phys. 2011;38:4127-32
72. Lin H, Zhang R, Gunn JR, Esipova TV, Vinogradov S, Gladstone DJ. et al. Comparison of Cherenkov excited fluorescence and phosphorescence molecular sensing from tissue with external beam irradiation. Phys Med Biol. 2016;61:3955-68
73. Cao X, Rao Allu S, Jiang S, Jia M, Gunn JR, Yao C. et al. Tissue pO2 distributions in xenograft tumors dynamically imaged by Cherenkov-excited phosphorescence during fractionated radiation therapy. Nat Comm. 2020;11:573
74. Hou Y, Wang C, Chen M, Wang M, Deng G, Yang H. et al. Iridium complex nanoparticle mediated radiopharmaceutical-excited phosphorescence imaging. Chem Comm. 2019;55:14442-14445
75. Genovese D, Petrizza L, Prodi L, Rampazzo E, De Sanctis F, Spinelli AE. et al. Tandem Dye-Doped Nanoparticles for NIR Imaging via Cerenkov Resonance Energy Transfer. Front Chem. 2020;8:71
76. Dolmans DEJGJ, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380
77. Lovell JF, Liu TWB, Chen J, Zheng G. Activatable Photosensitizers for Imaging and Therapy. Chem Rev. 2010;110:2839-2857
78. Zhou Z, Song J, Nie L, Chen X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chemical Society reviews. 2016;45:6597-626
79. Kotagiri N, Sudlow GP, Akers WJ, Achilefu S. Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers. Nat Nanotechnol. 2015;10:370-9
80. Tang R, Zheleznyak A, Mixdorf M, Ghai A, Prior J, Black KCL. et al. Osteotropic Radiolabeled Nanophotosensitizer for Imaging and Treating Multiple Myeloma. ACS Nano. 2020;14:4255-4264
81. Kotagiri N, Cooper ML, Rettig M, Egbulefu C, Prior J, Cui G. et al. Radionuclides transform chemotherapeutics into phototherapeutics for precise treatment of disseminated cancer. Nat Comm. 2018;9:275
82. Duan D, Liu H, Xu Y, Han Y, Xu M, Zhang Z. et al. Activating TiO2 Nanoparticles: Gallium-68 Serves as a High-Yield Photon Emitter for Cerenkov-Induced Photodynamic Therapy. ACS Appl Mater Interfaces. 2018;10:5278-86
83. Kamkaew A, Cheng L, Goel S, Valdovinos HF, Barnhart TE, Liu Z. et al. Cerenkov Radiation Induced Photodynamic Therapy Using Chlorin e6-Loaded Hollow Mesoporous Silica Nanoparticles. ACS Appl Mater Interfaces. 2016;8:26630-26637
84. Ni D, Ferreira CA, Barnhart TE, Quach V, Yu B, Jiang D. et al. Magnetic Targeting of Nanotheranostics Enhances Cerenkov Radiation-Induced Photodynamic Therapy. J Am Chem Soc. 2018;140:14971-14979
85. Yu B, Ni D, Rosenkrans ZT, Barnhart TE, Wei H, Ferreira CA. et al. A "Missile-Detonation" Strategy to Precisely Supply and Efficiently Amplify Cerenkov Radiation Energy for Cancer Theranostics. Adv Mater. 2019:e1904894
86. Kobayashi H, Furusawa A, Rosenberg A, Choyke PL. Near-infrared photoimmunotherapy of cancer: a new approach that kills cancer cells and enhances anti-cancer host immunity. Int Immunol. 2020;33:7-15
87. Kobayashi H, Choyke PL. Near-Infrared Photoimmunotherapy of Cancer. Acc Chem Res. 2019;52:2332-9
88. Nakamura Y, Nagaya T, Sato K, Okuyama S, Ogata F, Wong K. et al. Cerenkov Radiation-Induced Photoimmunotherapy with 18F-FDG. J Nucl Med. 2017;58:1395-400
89. Rahim MA, Jan N, Khan S, Shah H, Madni A, Khan A. et al. Recent Advancements in Stimuli Responsive Drug Delivery Platforms for Active and Passive Cancer Targeting. Cancers (Basel). 2021;13:670
90. Mi P. Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics. Theranostics. 2020;10:4557-4588
91. Breve TG, Filius M, Weerdenburg S, van der Griend SJ, Groeneveld TP, Denkova AG. et al. Light-Sensitive Phenacyl Crosslinked Dextran Hydrogels for Controlled Delivery. Chemistry. 2022;28:e202103523
92. Yao C, Li J, Cao X, Gunn JR, Wu M, Jiang S. et al. X-ray-Induced Cherenkov Optical Triggering of Caged Doxorubicin Released to the Nucleus for Chemoradiation Activation. ACS Appl Mater Interfaces. 2020;12:44383-44392
93. Daouk J, Dhaini B, Petit J, Frochot C, Barberi-Heyob M, Schohn H. Can Cerenkov Light Really Induce an Effective Photodynamic Therapy? Radiation. 2020;1:5-17
94. Bayat Mokhtari R, Homayouni TS, Baluch N, Morgatskaya E, Kumar S, Das B. et al. Combination therapy in combating cancer. Oncotarget. 2017;8:38022-38043
95. Wang Q, Liu N, Hou Z, Shi J, Su X, Sun X. Radioiodinated Persistent Luminescence Nanoplatform for Radiation-Induced Photodynamic Therapy and Radiotherapy. Adv Healthc Mater. 2020:e2000802
96. Guo J, Feng K, Wu W, Ruan Y, Liu H, Han X. et al. Smart 131I labeled self-illuminating photosensitizers for deep tumor imaging guided therapy. Angew Chem Int Ed. 2021;60:21884
97. Qian R, Wang K, Guo Y, Li H, Zhu Z, Huang X. et al. Minimizing adverse effects of Cerenkov radiation induced photodynamic therapy with transformable photosensitizer-loaded nanovesicles. J. Nanobiotechnology. 2022;20:203
98. Li J, Dai S, Qin R, Shi C, Ming J, Zeng X. et al. Ligand Engineering of Titanium-Oxo Nanoclusters for Cerenkov Radiation-Reinforced Photo/Chemodynamic Tumor Therapy. ACS applied materials & interfaces. 2021;13:54727-54738
99. Han X, Nie G. Say No to Tumors: NO Matters. Matter. 2019;1:794-796
100. Tian L, Wang Y, Sun L, Xu J, Chao Y, Yang K. et al. Cerenkov Luminescence-Induced NO Release from 32P-Labeled ZnFe(CN)5NO Nanosheets to Enhance Radioisotope-Immunotherapy. Matter. 2019;1:1061-1076
Author contact
![]() Corresponding authors: E-mail: liuniancheercom; suxinhuiedu.cn; xiaolian_sunedu.cn.
Corresponding authors: E-mail: liuniancheercom; suxinhuiedu.cn; xiaolian_sunedu.cn.
 Global reach, higher impact
Global reach, higher impact