13.3
Impact Factor
Theranostics 2022; 12(17):7351-7370. doi:10.7150/thno.74753 This issue Cite
Research Paper
Cancer-associated fibroblasts facilitate premetastatic niche formation through lncRNA SNHG5-mediated angiogenesis and vascular permeability in breast cancer
1. Key Laboratory of Laboratory Medical Diagnostics, Chinese Ministry of Education, Chongqing Medical University, Chongqing 400016, China.
2. Experimental Teaching & Lab Management Center, Chongqing Medical University, Chongqing 400016, China.
3. Department of Breast and Thyroid Surgery, the Second Affiliated Hospital of Chongqing Medical University, Chongqing 400010, China.
4. Department of Cell Biology and Medical Genetics, Basic Medical School, Chongqing Medical University, #1 Yi-Xue-Yuan Rd., Yu-zhong District, Chongqing 400016, China.
*These authors contributed equally to this work.
Abstract
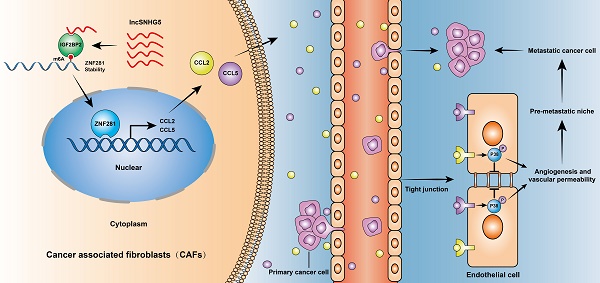
Background: Metastasis is the leading cause of death in patients with breast cancer (BC). Primary tumors create a premetastatic niche (PMN) in secondary organs for subsequent metastases. Cancer-associated fibroblasts (CAFs) are a predominant stromal component in the tumor microenvironment and serve as a major contributor to tumor metastasis. However, the function and mechanism of primary CAFs in the premetastatic niche of secondary organs remain unclear in BC.
Methods: We investigated the expression profiles of lncRNAs in pairs of CAFs and NFs derived from breast tumor tissues using lncRNA microarray. The expression levels of lncSNHG5, ZNF281, IGF2BP2, CCL2 and CCL5 were assessed by qRT-PCR; the protein levels of related genes (e.g., ZNF281, IGF2BP2, CCL2, and CCL5) were analyzed using western blotting and/or ELISA in primary and immortalized CAFs and clinical samples. Tubule formation and three-dimensional sprouting assays and tissue fluorescence staining were conducted to investigate angiogenesis. In vitro permeability assays, trans-endothelial invasion assays, in vivo permeability assays and tissue fluorescence staining were conducted to examine vascular permeability. The regulatory mechanism of lncSNHG5 was investigated by RNA sequencing, fluorescent in situ hybridization, cellular fractionation assay, mass spectrometry, RNA pull-down, RNA immunoprecipitation, gene-specific m6A assay, chromatin immunoprecipitation, dual luciferase reporter assay and actinomycin D treatment in CAFs and NFs.
Results: LncSNHG5 was highly expressed in breast CAFs and played an essential role in premetastatic niche formation by promoting angiogenesis and vascular leakiness through regulation of ZNF281 in CAFs. lncSNHG5 enhanced ZNF281 mRNA stability by binding with the m6A reader IGF2BP2. Enhanced ZNF281 transcriptionally regulated CCL2 and CCL5 expression to activate P38 MAPK signaling in endothelial cells. High CCL2 and CCL5 expression was associated with tumor metastasis and poor prognosis in BC patients. The inhibitors RS102895, marasviroc and cenicriviroc inhibited angiogenesis and vascular permeability in the PMN by blocking the binding of CCL2/CCR2 and CCL5/CCR5. The lncSNHG5-ZNF281-CCL2/CCL5 signaling axis plays an essential role in inducing premetastatic niche formation to promote BC metastasis.
Conclusions: Our work demonstrates that lncSNHG5 and its downstream signaling ZNF281-CCL2/CCL5 in CAFs play a crucial role in premetastatic niche formation in breast cancer and may serve as potential targets for the diagnosis and treatment of BC metastasis.
Keywords: Cancer-associated fibroblasts, premetastatic niche, angiogenesis, vascular permeability, lncSNHG5
 Global reach, higher impact
Global reach, higher impact