13.3
Impact Factor
Theranostics 2022; 12(16):7180-7190. doi:10.7150/thno.79144 This issue Cite
Research Paper
Synthesis, preclinical evaluation and radiation dosimetry of a dual targeting PET tracer [68Ga]Ga-FAPI-RGD
1. Department of Nuclear Medicine, the First Affiliated Hospital, Fujian Medical University, No. 20 Chazhong Road, Taijiang District, Fuzhou 350005, Fujian Province, China.
2. State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics & Center for Molecular Imaging and Translational Medicine, School of Public Health, Xiamen University, 4221-116 Xiang'An South Rd, Xiamen 361102, China.
3. Departments of Diagnostic Radiology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, 119074, Singapore.
4. Nanomedicine Translational Research Program, NUS Center for Nanomedicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore 117597, Singapore.
5. College of Nuclear Science and Technology, Harbin Engineering University, Harbin 150001, China.
6. Fujian Key Laboratory of Precision Medicine for Cancer, the First Affiliated Hospital, Fujian Medical University, Fuzhou 350005, Fujian Province, China.
7. Institute of Clinical Pharmacy & Pharmacology, Jining First People's Hospital, Jining Medical University, Jining 272000, China.
8. Clinical Imaging Research Centre, Centre for Translational Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore 117599, Singapore.
9. Departments of Surgery, Chemical and Biomolecular Engineering, and Biomedical Engineering, College of Design and Engineering, National University of Singapore, Singapore 117597, Singapore.
10. Institute of Molecular and Cell Biology, Agency for Science, Technology, and Research (A*STAR), 61 Biopolis Drive, Proteos, Singapore, 138673, Singapore.
#These authors contributed equally to this work.
Abstract
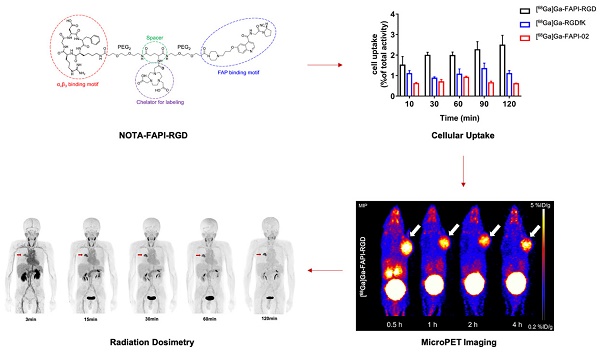
To enhance tumor uptake and retention, we designed and developed bi-specific heterodimeric radiotracers targeting both FAP and αvβ3, [68Ga]Ga-FAPI-RGD. The present study aimed to evaluate the specificity, pharmacokinetics, and dosimetry of [68Ga]Ga-FAPI-RGD by preclinical and preliminary clinical studies.
Methods: FAPI-RGD was designed and synthesized with the quinoline-based FAPI-02 and the cyclic RGDfK peptide. Preclinical pharmacokinetics were determined in Panc02 xenograft model using microPET and biodistribution experiments. The safety and effective dosimetry of [68Ga]Ga-FAPI-RGD was evaluated in 6 cancer patients, and compared with 2-[18F]FDG imaging.
Results: The [68Ga]Ga-FAPI-RGD had good stability in saline for at least 4 h, and showed favorable binding affinity and specificity in vitro and in vivo. Compared to [68Ga]Ga-FAPI-02 and [68Ga]Ga-RGDfK, the tumor uptake and retention of [68Ga]Ga-FAPI-RGD were very much enhanced than its monomeric counterparts at all the time points examined by microPET imaging. A total of 6 patients with various malignant tumors were prospectively enrolled. The effective dose of [68Ga]Ga-FAPI-RGD was 1.94E-02 mSv/MBq. The biodistribution of [68Ga]Ga-FAPI-RGD from 0 to 2 h after injection demonstrated rapid and high tumor uptake, prolonged tumor retention, and high tumor-to-background ratios (TBRs) which further increased over time. No significant difference in mean SUVmax of [68Ga]Ga-FAPI-RGD and 2-[18F]FDG was present in primary tumors (8.9±3.2 vs. 10.3 ± 6.9; p = 0.459).
Conclusion: The dual targeting PET tracer [68Ga]Ga-FAPI-RGD showed significantly improved tumor uptake and retention, as well as cleaner background over 68Ga-labeled FAPI and RGD monospecific tracers. The first-in-human biodistribution study showed high TBRs over time, suggesting high diagnostic performance and favorable tracer kinetics for potential therapeutic applications.
Keywords: FAPI-RGD, heterodimer, fibroblast activation protein, integrin αvβ3, 68Ga
 Global reach, higher impact
Global reach, higher impact