13.3
Impact Factor
Theranostics 2022; 12(16):6883-6897. doi:10.7150/thno.77457 This issue Cite
Research Paper
A novel afterglow nanoreporter for monitoring cancer therapy
1. State Key Laboratory of Chemo/Bio-Sensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, P. R. China.
2. Department of Hepatobiliary Surgery, Hunan Provincial People's Hospital/The First Affiliated Hospital of Hunan Normal University, Changsha, Hunan Province, People's Republic of China.
*These authors contributed equally to this work.
Abstract
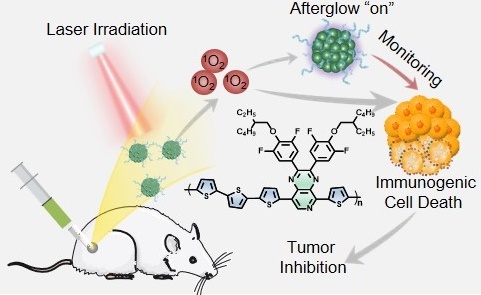
Rationale: Immunogenic cell death (ICD)-associated immunogenicity evoked through reactive oxygen species (ROS) is an efficient way to fight against the immune-dysfunctional microenvironment, so as to provoke potent anti-tumor immunity. However, the unknown ROS dose during cancer therapies may induce adverse immune responses (e.g., insufficient ICD, toxicity toward normal tissues or immune system).
Methods: Herein, we developed a pyrido pyrazine - thiophene based semiconducting polymer as novel near-infrared (NIR) organic afterglow nanoparticles for the real-time visualization of self-generated ROS, during photodynamic-mediated immunogenic cell death. Specifically, we introduced the strong “acceptor” (pyrido pyrazine) into thiophene based semiconducting polymer to redshift emission wavelength, and further modulate the “donor” to afford more afterglow reaction sites and reducing ΔEst, so as to enhance luminescence intensity.
Results: The semiconducting polymer-based afterglow nanoparticles exhibit strong afterglow emission with longer-wavelength emission (> 800 nm), compared with the reported organic afterglow nanoparticles (e.g., MEHPPV, PFODBT or Chlorin, < 690 nm), which endows this afterglow nanoparticles with a greatly improvement of signal to noise ratio. Moreover, the photodynamic effect of this afterglow nanoparticles can induce immunogenic cell death of cancer cells and further cause immune responses in mice.
Conclusions: The NIR afterglow signal presents a good relationship with ROS generation, immunogenic cell death and outcome of treatment. Therefore, it was able to provide a non-invasive tool for predicting the degree of ICD that occurs during ROS-mediated cancer therapy and may contribute to precise immunotherapy.
Keywords: Molecular engineering, Semiconducting polymer nanoparticle, Afterglow imaging, Immunogenic cell death, Therapy monitoring
 Global reach, higher impact
Global reach, higher impact