13.3
Impact Factor
Theranostics 2022; 12(14):6189-6206. doi:10.7150/thno.72134 This issue Cite
Research Paper
ARHGAP24 represses β-catenin transactivation-induced invasiveness in hepatocellular carcinoma mainly by acting as a GTPase-independent scaffold
1. Department of Laboratory Medicine, Zhongshan Hospital, Fudan University, Shanghai 200032, P. R. China.
2. Department of Liver Surgery & Transplantation, Liver Cancer Institute, Zhongshan Hospital, Fudan University; Key Laboratory of Carcinogenesis and Cancer Invasion, Ministry of Education, Shanghai 200032, P. R. China.
3. Cancer Center, Zhongshan Hospital, Fudan University, Shanghai 200032, P. R. China.
4. Department of Laboratory Medicine, Xiamen Branch, Zhongshan Hospital, Fudan University, Xiamen 361015, P. R. China.
5. Department of Laboratory Medicine, Wusong Branch, Zhongshan Hospital, Fudan University, Shanghai 200940, P. R. China.
6. Shanghai Geriatric Institute of Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai 200031, P. R. China.
*These authors contributed equally to this work.
Abstract
Rationale: Accumulating evidence shows that Rho-GTPase-activating proteins (RhoGAPs) exert suppressive roles in cancer cell proliferation and metastasis. However, no study has systematically investigated the clinical significance of RhoGAPs and analyzed the functions of ARHGAP24 in hepatocellular carcinoma (HCC).
Methods: The relationship between RhoGAP expression and HCC prognosis was investigated via using The Cancer Genome Atlas and Gene Expression Omnibus databases. ARHGAP24 expression was detected by reverse transcription-polymerase chain reaction, western blot and immunohistochemistry staining assays. Moreover, in vitro assays including cell counting kit-8, colony formation, wound healing and Transwell assays, and in vivo tumor growth and pulmonary metastases evaluations were conducted to evaluate the biological function of ARHGAP24 in HCC. Liquid chromatography-tandem mass spectrometry, co-immunoprecipitation, GTPase activation, ubiquitination, and luciferase reporter assays and bioinformatics analysis were carried out to gain insights into the mechanisms underlying the tumor-suppressive function of ARHGAP24.
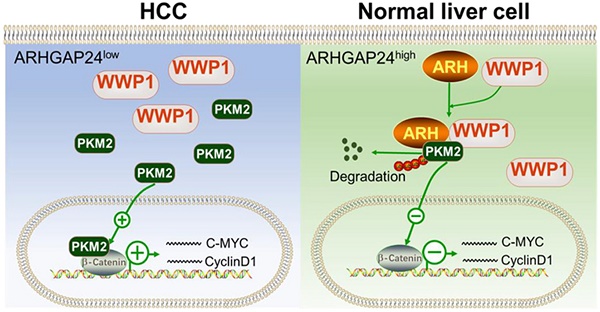
Results: ARHGAP24 expression was dramatically decreased in HCC tissues, and low ARHGAP24 expression was an independent poor prognostic indicator for progression-free survival in HCC patients. ARHGAP24 overexpression significantly inhibited cell proliferation, migration and invasion, while knockdown of ARHGAP24 exerted the opposite effects. Through Gene Set Enrichment Analysis (GSEA), we found ARHGAP24 mainly suppressed HCC cell proliferation and invasion by attenuating β-catenin transactivation and blocking β-catenin signaling could effectively abolish the promotional effects of ARHGAP24 knockdown in HCC cells. Notably, GAP-deficient mutant of ARHGAP24 exerted similar inhibitory effects as the wild-type did, indicating suppressive function of ARHGAP24 was independent of its RhoGAP activity. Moreover, we identified pyruvate kinase M2 (PKM2) as a new binding partner of ARHGAP24, which recruited a novel E3 ligase (WWP1) and subsequently promoted PKM2 degradation. WWP1 knockdown significantly reduced the inhibitory function of ARHGAP24, and the C-terminal fragments of ARHGAP24 (amino acids 329 - 430 and 631 - 748) bound directly to WWP1 and PKM2 (amino acids 388 - 531), respectively.
Conclusions: Our data indicate that ARHGAP24 may be an independent prognostic indicator for HCC. It is a critical suppressor of HCC that recruits WWP1 for PKM2 degradation. Targeting the ARHGAP24/WWP1/PKM2/β-catenin axis may provide new insights into HCC prevention and treatment.
Keywords: HCC, invasion, ARHGAP24, PKM2 degradation, β-catenin transactivation
 Global reach, higher impact
Global reach, higher impact