13.3
Impact Factor
Theranostics 2022; 12(14):6057-6068. doi:10.7150/thno.72328 This issue Cite
Research Paper
Integrin β3-PKM2 pathway-mediated aerobic glycolysis contributes to mechanical ventilation-induced pulmonary fibrosis
Department of Critical Care Medicine, Renji Hospital, School of Medicine, Shanghai Jiaotong University, 200127 Shanghai, China.
*These authors contributed equally to this work and should be considered as co-first authors.
Abstract
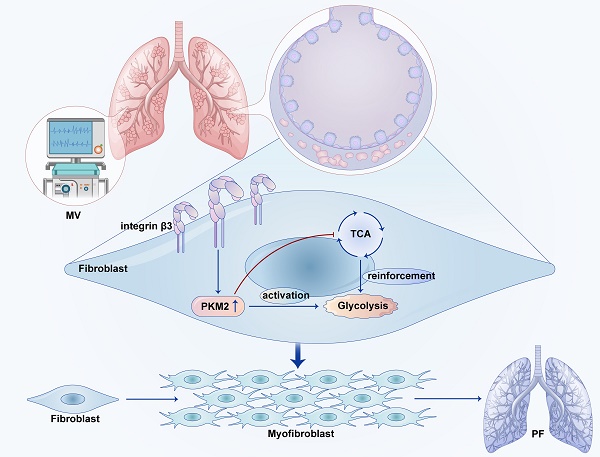
Background: Mechanical ventilation (MV) can induce pulmonary fibrosis. This study aims to investigate whether MV-induced pulmonary fibrosis is associated with aerobic glycolysis and seeks to uncover the underlying mechanisms mediated by integrin β3-pyruvate kinase M2 (PKM2) pathway.
Methods: PKM2 knockdown or inhibition, integrin β3 knockout or inhibition and wild-type mice were exposed to MV (20 mL/kg) for 2 h.
Results: Mice exposed to MV exhibited increased expression of collagen deposition, and upregulation of α-smooth muscle actin and collagen I in lung tissues. Single cells analysis showed that MV-induced pulmonary fibrosis was associated with increased gene expression of integrin and glycolysis in pulmonary fibroblasts, as well as upregulation of glycolytic products tested by metabolomics. Meanwhile, increased protein level of integrin β3 and PKM2 was confirmed by western blot and immunohistochemistry. Double immunofluorescence staining and flow cytometric analysis showed increased number of fibronectin+/integrin β3+ and fibronectin+/PKM2+ fibroblasts in lung tissues. Furthermore, MV-induced aerobic glycolysis and pulmonary fibrosis were ameliorated after treatment with PKM2 knockdown-AAV and inhibition, or in integrin β3 knockout and inhibition mice.
Conclusions: Integrin β3-PKM2 pathway-mediated aerobic glycolysis contributes to MV-induced pulmonary fibrosis. The inhibition of aerobic glycolysis targeting integrin β3-PKM2 pathway may be a promising treatment for MV-induced pulmonary fibrosis.
Keywords: integrin β3, pyruvate kinase M2, aerobic glycolysis, mechanical ventilation, pulmonary fibrosis
 Global reach, higher impact
Global reach, higher impact