13.3
Impact Factor
Theranostics 2022; 12(13):5995-6020. doi:10.7150/thno.73681 This issue Cite
Review
Emerging nanozyme-based multimodal synergistic therapies in combating bacterial infections
1. College of Life Science, Dalian Minzu University, Economical and Technological Development Zone, Dalian, 116600, China.
2. State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Science, P. O. Box 110, Dalian 116023, China.
3. Key Laboratory of Biotechnology and Bioresources Utilization (Dalian Minzu University), Ministry of Education, China.
Received 2022-4-6; Accepted 2022-7-22; Published 2022-8-8
Abstract
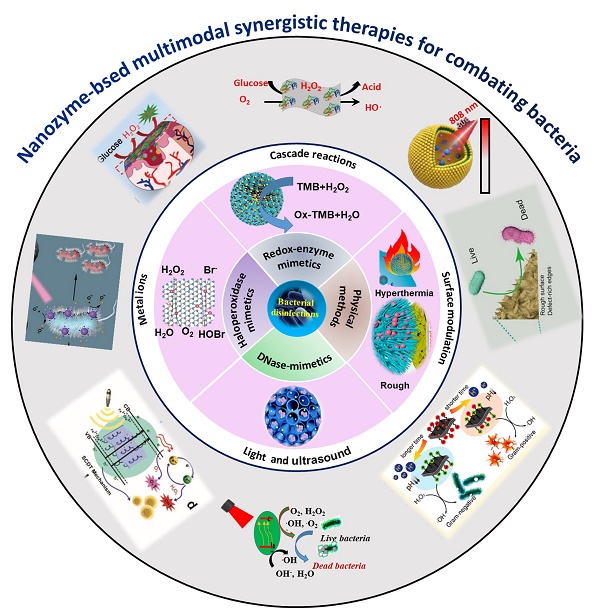
Pathogenic infections have emerged as major threats to global public health. Multidrug resistance induced by the abuse of antibiotics makes the anti-infection therapies to be a global challenge. Thus, it is urgent to develop novel, efficient and biosafe antibiotic alternatives for future antibacterial therapy. Recently, nanozymes have emerged as promising antibiotic alternatives for combating bacterial infections. More significantly, the multimodal synergistic nanozyme-based antibacterial systems open novel disinfection pathways. In this review, we are mainly focusing on the recent research progress of nanozyme-based multimodal synergistic therapies to eliminate bacterial infections. Their antibacterial mechanism, the synergistic antibacterial systems are systematically summarized and discussed according to the combination of mechanisms and the purpose to improve their antibacterial efficiency, biosafety and specificity. Finanly, the current challenges and prospects of the multimodal synergistic antibacterial systems are proposed.
Keywords: Multidrug resistance, nanozyme, peroxidase, oxidase, reactive oxygen radical, photothermal effect, selective, integrated platform, detection
1. Introduction
In recent decade, bacterial infections are being recognized as a serious public threat to public health. Many conventional natural or chemically synthesized antibiotics can selectively inhibit or kill bacterium. However, pathogens can adapt or become resistant to almost all available traditional antibiotics and evolved into multi drug-resistant strains even super bacteria, threatening the survivals of millions of people in the global word. The emergence of “super bacteria” not only greatly reduced the clinical effect of bacterial infection treatment, but also caused the dilemma of “no medicine available” [1,2]. In addition, the clinical treatment of biofilm-related infections is more difficult because biofilm is a three-dimensional structure formed by microorganisms attached to biological or non-biological surfaces in which cells are surrounded by extracellular polymers produced by themselves. Protective-biofilm formation is considered as a self-defense mechanism produced by microorganisms in response to biological and non-biological stresses. Thus, it is difficult for common drugs to kill bacteria encapsulated in biofilm by extracellular matrix, which leads to higher resistance of bacteria in biofilm form. Compared with planktonic-bacteria without biofilm, biofilm-bacteria are much more recalcitrant to antibiotics, hydrogen peroxide (H2O2) and other antimicrobial agents [3]. Therefore, it is highly desirable to develop new antimicrobial agents or strategies with high efficiency, environmental friendliness and without inducing drug-resistance for combating planktonic-bacteria and biofilms.
To eliminate the increasing threat of drug-resistance bacterial infections, extensive interdisciplinary efforts have been devoted to discovery and design of novel and more efficient antimicrobial agents. The rapid development of nanozymes provides us new opportunities to solve the problems [4-10]. Nanozymes are refer to artificial nanomaterials with intrinsic enzyme-like catalytic performance and have arouse wide concerns in the recent decades. Unlike natural enzymes, nanozymes are low cost, more stable and activity-tunable, which are considered to be promising alternatives to natural enzymes for applications in industrial, biological, and medical fields [4-19]. Compared with traditional antibiotics, nanozymes have higher membrane permeability and can be used as an inhibitor of efflux pump. Moreover, it is not easy to induce drug resistance because they can achieve multiple antibacterial functions. Importantly, the antibacterial performance is tunable by controlling the size, structure, surface morphology, components, surface charge and other factors of nanomaterials, and can be further optimized by combining more than one functional mechanism in one [4-40]. In particular, in addition to their own antibacterial ability, many nanozyme-based antibacterial agents have the characteristics of magnetism, photothermal, fluorescence [41-44]. Therefore, nanozymes-based multimodal synergistic platforms for combating bacterial infections can be constructed by combining them with their antibacterial activities, and thus realize the separation, detection and killing of bacteria in one [21,24-26]. To date, much attractive progress in this regard has been reported, thus comprehensive reviews are highly desirable towards their current progress. Herein, in this comprehensive review, we have mainly focused on summary of the recent advances of multimodal synergistic antibacterial strategies (Figure 1). Finally, the current challenges and prospect of nanozyme-based synergistic antibacterial strategies in practical antibacterial applications are discussed. This review is intended to provide new insights into developing new safe antibacterial therapeutics for clinical applications.
Schematic diagram of nanozyme-based synergistic antibacterial strategies for improved antimicrobial performances and biosafety. Partial images were adapted with permission from ref. [29], copyright 2022, ref. [32], copyright 2019, ref. [33], copyright 2019, ref. [34], copyright 2020, ref. [38], copyright 2021, ref. [39], copyright 2019, WILEY-VCH. Ref. [30], copyright 2021, ref. [31], copyright 2019, ref. [36], copyright 2020, ref. [37], copyright 2018, the American Chemical Society. Ref. [35], copyright 2020, ref. [40], copyright 2019, the Royal Society of Chemistry.
2. Antibacterial Mechanisms of nanozymes
In recent decade, nanozyme-based antibacterial agents have become a hotspot in antibacterial field. Compared with traditional antibiotics, they can execute multiple bactericidal pathways thus is not easy to induce drug-resistance for combating bacteria and biofilms [45-50]. Up to now, a significant number of nanomaterials such as carbons [51,52], noble metal nanoparticles [53-55], metal organic frameworks (MOFs) [31, 33,46], covalent organic frameworks (COFs) [38,56,57] and metal oxides [58-61] have been discovered and designed that exhibit peroxidase (POD) [62-65], oxidase (OXD) [55,65-68], deoxyribonuclease (DNase) [69,70] and haloperoxidase (HPO) [71-74] mimetic activities. Currently, by utilizing their superior performances, the nanozyme-based antibacterial agents have emerged as new powerful tools for combating bacterial infections. Their antibacterial mechanisms are summarized as follows. In particular, in this review, we also termed the nanomaterials that can activate molecular oxygen especially under light or ultrasound into reactive oxygen species as redox nanozymes, even though the authors didn't refer to their biomimetic properties in their articles.
2.1 Combating bacterial infections by redox enzyme mimetics
Due to the super oxidation capability of high level of reactive oxygen species (ROS) which can damage the lipids, proteins and DNA in the biofilm matrix, on the cell surface and inside the microbial cells, both planktonic cells and biofilms can be killed and eliminated when exposed to high level of ROS [75-81]. Therefore, it is not easy to induce drug resistance. As known, H2O2 alone at a higher concentration possess good antibacterial performance and serve as a general antibacterial agent. However, it is not safe for healthy tissues in the presence of high concentration of H2O2. Fortunately, POD-like nanozymes can produced highly oxidative •OH by decomposing very low concentration of H2O2 to achieve higher antibacterial efficacy without toxicity to healthy tissues [63-65,81,82]. By virtue of this ability, ferromagnetic nanoparticles were used as a POD-like for oxidative biofilm elimination in the presence of H2O2 [63]. Later, graphene quantum dots (GQDs) were also put forward as safe POD-like mimetics for actual wound disinfection with H2O2 at low dose [81]. Apart from the aforementioned POD-mimetic, some nanomaterials exhibit OXD-mimetic activity that can directly activate oxygen to generate ROS like H2O2, superoxide anions and single oxygen spontaneously which can kill bacteria and eradicate their biofilms [83-91]. By virtue of this ability, many OXD nano-mimetics such as Tb4O7 nanoparticles [88], silica-supported AuNPs [65], Pd nanoparticles [53], Pt/Ag nanoalloys [89] exhibit bactericidal activities in the absence of H2O2. Furthermore, many nanomaterials exhibit multi enzyme-like activities [29,53,65,89-91]. For example, Cu2WS4 nanocrystals exhibit excellent OXD-like and POD-like activities to generate ROS and act as efficient antibacterial agents for healing mouse skin infections [91].
2.2 Combating bacterial infections by DNase-mimetic artificial enzyme
Extracellular DNA (eDNA) is one of important extracellular polymeric substance (EPS) constituents for many bacterial species and has a very significant role in maintaining the biofilm integrity. DNase mimetics can cleave eDNA to inhibit the formation of biofilms and disperse the established biofilms [69,70]. Recently, Qu et al. designed and synthesized bifunctional nanozyme-based synergistic systems by integrating DNase-like and POD-like mimetics for combating biofilms [69]. The bifunctional MOF/Ce-based nanozymes are capable of hydrolyzing eDNA and disrupting established biofilms by the cerium (IV) complexes (DNase mimetics), and kill bacteria exposed in dispersed biofilms by POD-like MOF in the presence of H2O2 [69].
2.3 Combating bacterial infections by HPO-like mimetics
HPO mimetics can prevent biofilm growth and disrupt biofilms by blocking quorum sensing through quenching auto-inducers (signal molecules related to quorum sensing) [71-74]. For example, vanadium pentoxide (V2O5) nanomaterials showing HPO-mimetic properties have been shown to function as biocatalytic bactericidal nano-agents with the assistance of bromide ions (Br-) and H2O2 via the bromination process [73]. As a result, compared to the absence of additives, V2O5 nanowires could inhibit the bacterial growth more efficiently with the assistance of Br- and H2O2. Significant decreases in bacterial growth were observed (the decrease is 78% for E. coli and 96% for S. aureus). Typically, this approach is efficient for bacterial disinfection in mild alkaline environments rather than in acidic environments, thus can be used to marine antifouling. In another work, it was found that CeO2-x nanorods could inhibit the bacterial growth after only 5s of coincubation because of their morphology-dependent HPO-mimetic activity. More importantly, cerium-based nanocatalysts are more biocompatible than the vanadium-based ones, thus have more potentials in clinical antibacterial treatment [74]. In order to overcome the instability of natural enzymes and supply adequate H2O2, Luo et al., presented a semiconductor-based bifunctional nanozyme for antibiofouling that can sustainably self-supply H2O2 from water and O2 by photosynthesis for HPO-mimicking reaction in a sequential manner [29].
2.4 Combating bacterial infections by near infrared (NIR) light laser-induced hyperthermia
Antibacterial photothermal therapy (PTT) has attracted great attentions for bacterial infection treatment as a non-invasive therapeutic technique because of its high efficiency, broad-spectrum antibacterial activities and negligible bacterial resistance [92-97]. Generally, the photothermal agents with high light-to-heat conversion ability can adsorb NIR energy and induce localized hyperthermia, which is able to rupture membranes, damage protein and other inherent bioactive matrix, thus destruct bacteria irreversibly.
3. Nanozyme-based multimodal synergistic therapies in combating bacterial infections
Up to now, a variety of nanozyme-based catalytic platforms have been developed for bacterial disinfection and their biofilm eradiation [13, 18, 47]. Although most of them exhibited acceptable disinfection outcomes, the clinical applications of single modal therapies are still limited because of their low therapeutic efficacy, intolerable cellular toxicity, arrow-spectrum antibacterial activities, non-targeting and short-term physiological stability. Recently, nanozyme-based synergetic systems have been commonly used for increasing the antibacterial efficiency, biosafety and selectivity. Furthermore, in comparison to single bactericidal modality, multimodal synergetic systems minimize the drug-resistance and could achieve site-specific and controlled therapies [47].
In this section, we summarized the multimodal synergistic therapies classified by mechanism and purpose: 3.1 Tuning the surface adhesive property of nanozymes to enhanced bacterial attachment; 3.2 Reducing the usage dose of H2O2 or directly activating O2 to produce ROS; 3.3 Combination of more than one antibacterial mechanism. 3.4 Selective or targeted disinfections; 3.5 Nanozymes for suppressing intracellular bacteria; 3.6 Multifunctional platforms for detection and inhibition of bacteria.
3.1 Tuning the surface adhesive property of nanozymes to enhanced bacterial attachment
By increasing the catalytic activities of nanozymes, more ROS species with high toxicity are produced, thus improving the antibacterial activity. However, ROS species are instable and their diffusion distance is very limited in the practical environment, thus most generated ROS species do not play effective roles in combating bacteria, resulting in low antibacterial efficiency and biosafety [98-104]. Therefore, both enhancement of ROS-generation ability and binding capacity to bacteria are crucial to the realization of efficient antibacterial activity of nanozyme-based antibacterial agents.
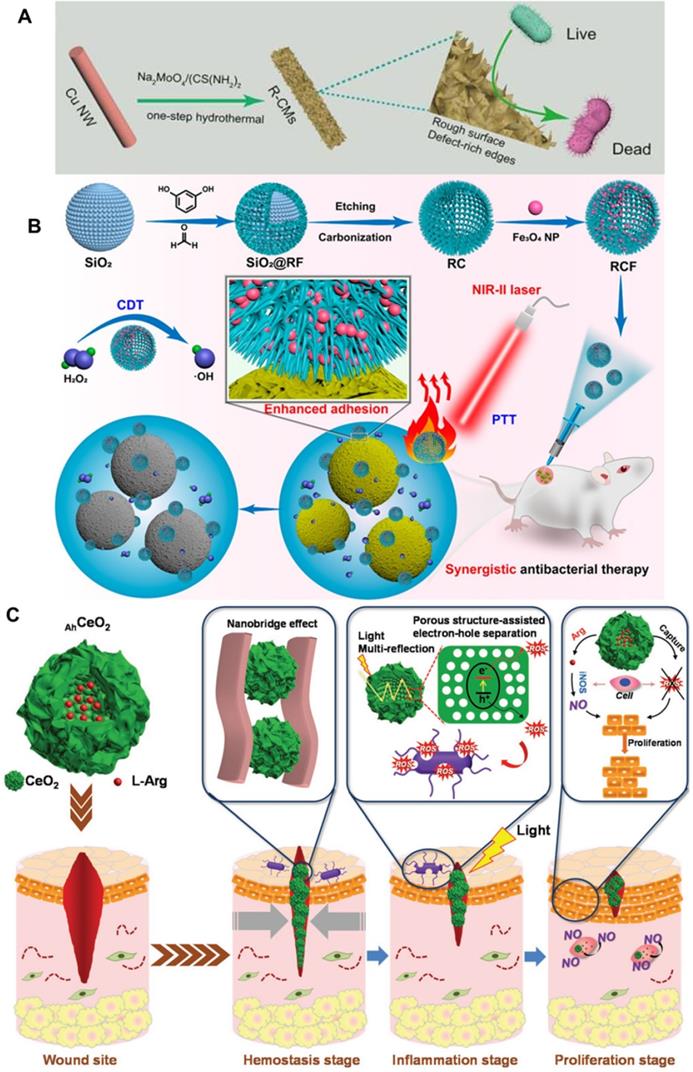
In this regard, Qu et al. constructed a Cu nanowires supported-MoS2 nanozyme with defect-rich active edges and rough surface for enhanced bacterial inhibition (Figure 2A) [39]. The rough surface of the MoS2 endow the nanozyme with enhanced bacteria-capturing ability compared to non-adhesive MoS2. Furthermore, experimental and theoretical studies show that the nanozyme with defect-rich active edges has lower H2O2 adsorption energy, OH* (OH* is the adsorbed intermediates of the H2O2-decomposition process) desorption energy and greater exothermic reaction energy, which endows it with enhanced POD-like activity. In summary, the designed nanozyme has “roughness-enhanced adhesion” and “defect-improved catalytic activity”, and thus has good antibacterial effect on both two typical types of model bacteria in vitro and in vivo. Given the same nature-inspired design philosophy, Shan et al., also reported cuboid-like Cu2WS4 nanocrystals with small size for killing two model bacteria at low concentration in the absence or presence of ambient light [91]. The Cu2WS4 nanocrystals exhibit OXD-like and POD-like catalytic activity thus can produce toxic ROS to kill bacteria. Impressively, the nanoparticles can selectively adsorb on the surface of bacteria because of the strong interaction between the Cu atoms on the nanozyme and amino groups from peptidoglycan in the bacteria cell wall. Thus, the cuboid-like Cu2WS4 nanocrystal with both high enzyme-like activity and selective bacterial adhesive ability endow the nanozymes with excellent antibacterial performance at very low concentration. The simultaneously generated ROS in situ by enzyme-like Cu2WS4 attached on the bacteria surface can efficiently kill bacteria. Furthermore, the as-prepared nanozymes have good biocompatibility and negligible toxicity, further demonstrating their promising potentials in combatting bacterial infections. For the same purpose, Yan et al. proved that Au@AgAu alloy nanoflowers with rough surface is efficient to inhibit bacteria growth with high biocompatibility [104]. Very recently, Liu et al. also proved the importance of the rough surface in a carbon-iron oxide nanoparticles (Figure 2B) related synergistic antibacterial nanoplatform for effective antibacterial therapy [30]. The carbon-iron oxide nanoparticles exhibit excellent POD-like activity and convert NIR light with low power density to hyperthermia efficiently, thus realize synergistic antibacterial system by combining photothermal therapy (PTT) and chemodynamic therapy (CDT). Typically, the carbon-iron oxide nanoparticles show increased bacterial adhesion due to its rough surfaces and benefit both CDT and PTT performance for broad-spectrum synergistic antibacterial effect on Gram-negative Escherichia coli (E. coli), Gram-positive Staphylococcus aureus (S. aureus), and methicillin-resistant Staphylococcus aureus (MRSA). Furthermore, the carbon-iron oxide nanoparticles show satisfactory biocompatibility and can be used as a synergistic antibacterial platform in vivo for healing the rat wound model infected with MRSA.
Hierarchical stimulation is a promising method for the fast wound closure by accelerating the complex and sequential biological process involved in the wound healing process. Ma et al. developed a compact and programmable nano-system for hierarchical stimulation to sequentially promote the hemostasis, inflammation, and proliferation stages (Figure 2C) [105]. The hybrid was prepared by encapsulating L-arginine inside hollow ceria nanoparticles with rough surface. Specially, the surface roughness of the nano-system benefits the hemostasis stage by eliminating wound infection and promoting wound healing. Finally, taking advantage of enzyme-like property of the hollow ceria nanoparticles and the released L-arginine, the nano-system promote the proliferation stage. In summary, this kind of rough surface synergetic modality is simple and efficient for enhanced nanozymes induced bacterial disinfections and would healing.
3.2 Reducing the usage dose of H2O2 or directly activating O2 to produce ROS
3.2.1 Cascade reactions in bacterial infection control
POD or mimetics could convert H2O2 into ROS to kill bacteria or cancer cells. However, their applications in living systems are limited because most of them exhibit optimized activity in acidic environments with a pH range of 3-4. Moreover, the introduction of high concentration of toxic H2O2 would damage normal tissues [88, 106]. Recently, cascade reactions, commonly found in living organisms, are proved to be a novel powerful bacterial infection-control strategy for killing bacteria [107-120]. For example, natural substrates such as glucose and oxygen in human body can be converted directly into ROS by infection-control cascade reactions to kill tumor cells and bacteria without using external toxic H2O2 [108-115, 117-120].
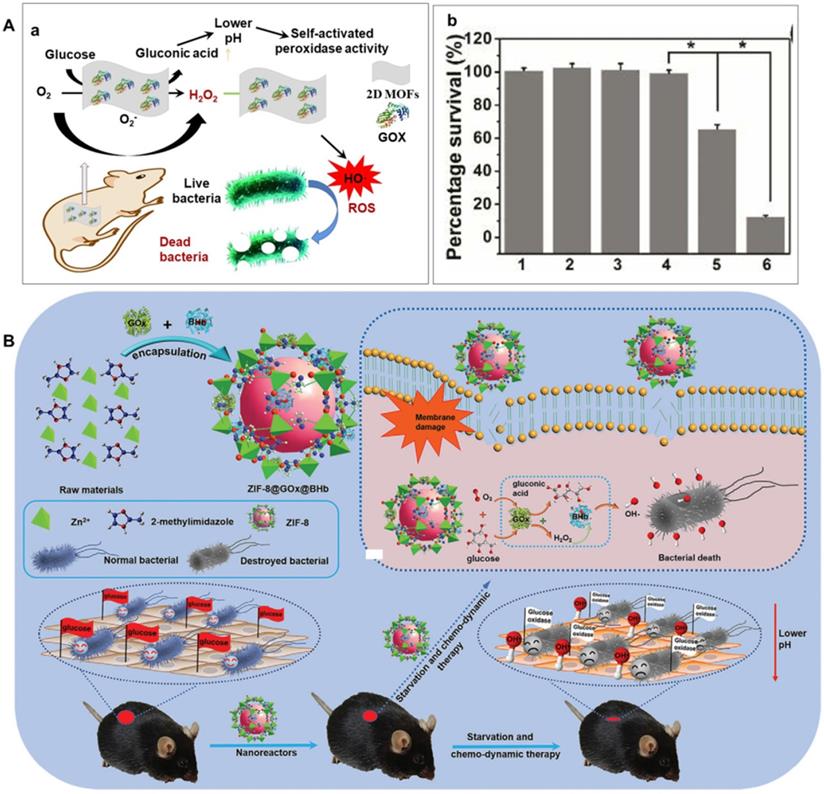
Glucose, one of the most important carbohydrates in the human body, can be oxidized by O2 to produce gluconic acid and H2O2 catalyzed by GOX or mimetics through a reductive and oxidative half-reaction [108-110]. Furthermore, H2O2 can be transformed into ROS by various peroxidase or mimic, such as metal organic frameworks, Fe3O4, Ir nanoplate, Au NPs, Pt and carbon nitride [28, 63-65, 106, 111]. Based on these considerations, as demonstrated in Figure 3A(a), Qu et al. selected ultra-thin two-dimensional MOF nanosheet as a POD-like mimic and simultaneously physically adsorb GOX on it to manufacture a hybrid nano catalyst as a safe, self-activated cascade platform for killing bacteria and healing wound in vivo [31]. In this system, GOX can continuously convert non-toxic glucose into gluconic acid and H2O2. Therefore, the system can act efficiently without introducing external toxic H2O2 with minimal harmful side effects. Meanwhile, the resulting gluconic acid can induce the environment from neutral to medium acidic, which can significantly enhance the POD-like activity of the two-dimensional MOF nanosheet and generate sufficient toxic hydroxyl radials to effectively eliminate bacteria. As shown in Figure 3A(b), compared the other 5 groups, the group incubated with glucose+2D MOF/GOX exhibited the highest killing efficiency against E. coli and S. aureus. Furthermore, the cascade reaction-based platform could effectively eliminate bacteria in vitro and in vivo with negligible biological toxicity [31].
Summary of synergistic antibacterial systems based on nanozymes with rough surface for antibacterial disinfections
| Strategies | Nanomaterials | Enzyme-like activities | Antibacterial mechanisms | Bacteria | Applications | Ref. |
|---|---|---|---|---|---|---|
| Rough surface +Nanozyme+NIR | Defect-rich MoS2 | POD | ROS+PTT | E. coli, S. aureus | Wound healing | [39] |
| Rough surface +Nanozyme+light | Cu2WS4 | POD +OXD | ROS | E. coli, S. aureus | MRSA-Infected wound healing | [91] |
| Rough surface +Nanozyme | Au@AgAu alloy | ~ | ~ | E. coli, S. aureus | Wound healing | [104] |
| Rough surface +Nanozyme | Carbon-iron oxide | POD | ·OH | E. coli, S. aureus | Wound healing | [30] |
| Rough surface +Nanozyme | Hollow ceria nanoparticles | POD | ROS | E. coli, S. aureus | Wound healing | [105] |
A) Defect-rich adhesive nanozymes with rough surface and defect-improved catalytic activity exhibited increased antibacterial activity, adapted permission from ref. 39, copyright 2019 the WILEY-VCH. B) Carbon-iron oxide nanohybrids with rough surface for NIR light-responsive synergistic antibacterial therapy, adapted permission from ref. 30, copyright 2021 the American Chemical Society. C) Hollow cerium oxide nanoparticles with rough surface promote multiple stages of wound healing, adapted permission from ref. 105, copyright 2019 the WILEY-VCH.
A) Schematic presentation of GOX/MOF hybrid nanozyme as a benign and self-activated cascade reaction platform for efficient bacteria killing and in vivo wound healing wound healing (a) and viability analyses of E. coli incubated with different systems (b). 1) PBS, 2) glucose, 3) glucose+2D MOF nanosheet, 4) 2D MOF/GOX, 5) glucose+GOX, 6) glucose+2D MOF/GOX, respectively. Adapted permission from ref. 31, copyright 2019 the American Chemical Society. B) Schematic preparation, mechanism and application of ZIF-8@GOX@BHb for wound infection in diabetic mice through a cascaded catalytic reaction, adapted permission from ref. 120, Copyright 2021, the WILEY-VCH.
Summary of cascade reactions in bacterial infection control
| Strategies | Nanomaterials | Enzyme-like activities | Antibacterial mechanisms | Bacteria | Applications | Ref. |
|---|---|---|---|---|---|---|
| Cascade reaction | MOF | GOX peroxidase | ROS | E. coli, S. aureus | Wound healing | [31] |
| Cascade reaction | V2O5 | haloperoxidases | HOBr+1O2 | E. coli, S. aureus | Marine antifouling | [73] |
| Cascade reaction | GOX-Hb MRs | GOX + peroxidase | OH | MRSA | Antibacterial Agents | [115] |
| Cascade reaction | CaO2/H-G@alginate | Peroxidase | ROS | E. coli, S. aureus | Wound healing | [116] |
| Cascade reaction | GOX-on-Fe-iCOF | GOX + peroxidase | OH | E. coli, S. aureus | Wound healing | [38] |
| Cascade reaction | Au-Au/IrO2@Cu(PABA) | GOX + peroxidase | ROS | E. coli, S. aureus | Antibacterial Agents | [117] |
| Cascade reaction | L-Arg/GOX@CuBDC | GOX + peroxidase + Nitric oxide synthetase | ROS+RNS+NO | E. coli, S. aureus | Antibacterial Agents | [118] |
| Cascade reaction | ZIF-8@Au-GOX(ZAG) | GOX peroxidase | OH | MRSA, S. aureus E. coli | Wound healing | [119] |
| Cascade reaction | ZIF-8@GOX@BHb | GOX peroxidase | OH | MRSA, ESBL E. coli | Wound healing | [120] |
To overcome limitation of the extra usage of H2O2, Tong's group reports an microreactors including both GOX and hemoglobin (Hb) inside a multilayer film as an enzymatic cascade reaction-based antibacterial system for inhibiting bacterial growth and biofilm formation [115]. For the same purpose, another cascade reaction-based antibacterial system was fabricated by introducing CaO2 and hemin-containing graphene into alginate nanocarriers [116]. During the reaction, ROS were generated by local converse of CaO2 and H2O through a localized cascade reaction. This H2O2-free depots has great potential in eradicating biofilm in vivo. Li et al. also developed a glucose-triggered cascade strategy for sterilization and healing wound infection. The loaded GOX in the ionic covalent-organic framework (COF) could convert glucose to H2O2, which could be decomposed directly to be more toxic hydroxyl radicals catalyzed by the pH-dependent POD-like activity of ionic covalent-organic framework-based nanozyme (GFeF) enhanced by the produced gluconic acid. Furthermore, the positively charged GFeF shows stronger adhesion ability to negatively charged bacterial membrane and thus greatly enhanced wound healing effects [38]. This strategy has great potential in the wound healing of diabetic patients without harmful side effects. Similarly, Zhao's group innovatively prepared an Au-Au/IrO2@Cu(PABA) nanocomposite with tandem enzyme-mimicking catalytic activity simultaneously under neutral conditions and utilized in a glucose-triggered cascade strategy for efficient antibacterial application [117]. Inspired by the natural multi-enzyme system, Kang group designed a well-designed L-Arg/GOX@CuBDC biomimetic composite for achieving the synergistic antibacterial effect with good biocompatibility through a double cascade reaction [118]. To further increase the antibacterial efficiency, Wang et al., developed a triple-synergistic strategy for efficient bacterial eradication. In the triple-synergistic antibacterial system, ZIF-8 was used as carrier to integrate gold nanoparticles (Au NPs) and GOX into a cascade reaction to convert glucose into ROS. Typically, the acidic environment generated by the production of gluconic acid facilitate both the ROS production and leaching of Zn2+, thus achieved enhanced overall bacterial eradication at a low dosage for both S. aureus and E. coli. This triple-synergistic strategy exhibits great potential in bacterial eradication and biomedical applications [119].
It is imminent and challenging to develop novel antibacterial agent for killing multidrug-resistant (MDR) bacteria and healing of the diabetic wound where contain more glucose. Thus, diabetic wound healing is still a big challenge. To overcome this problem, as shown in Figure 3B, Niu's group constructed a cascaded nanoreactors (ZIF-8@GOX@BHb) by encapsulating both GOX and (bovine hemoglobin) BHb into porous ZIF-8 by a spatial confinement effect. Benefiting from the two types of enhanced enzyme-like activities and effective cascaded catalytic antibacterial performance of the obtained ZIF-8@GOX@BHb, the cascaded nanoreactors could starve and kill MDR bacteria efficiently. Impressively, the constructed nanoreactors is degradable and biocompatible for combating multidrug-resistant bacteria in vitro and diabetic wound healing in vivo [120].
Recently, to avoid the direct use of extra H2O2 and low pH medium, glucose dependent cascade reactions were used to eradicate bacteria by converting glucose into H2O2 which further decomposed into toxic ROS catalyzed by the acidic environment promoted POD-like nanozyme [118,119]. However, low oxygen content in the anoxic microenvironment of the diabetic wound limit glucose-dependent cascade reaction. To address this, Li et al., designed a defect-rich MoS2@Au@BSA nanozyme attached injectable hydrogels with high multi-enzyme catalytic activities for diabetic wound healing through an O2 self-supplying cascade reaction. The nano-system can catalyze glucose into H2O2 and gluconic acid, which can be converted into ROS by the acid-enhanced POD-like activity of MoS2@Au@BSA for bacteria eradication. Specially, MoS2@Au@BSA exhibit superoxide dismutase-like activity at an alkalescent condition to transform superoxide anions into dioxygen and H2O2, and catalase-like mimetics to decomposes endogenous and exogenous H2O2 into O2, thus facilitate glucose oxidation and accelerates diabetic wound healing [121]. For the same purpose, Li et al. also designed a GOX and hemin co-loaded G4-hydrogel as a cascade reaction container consuming endogenous glucose for wound treatment on diabetic mice [122].
Beside the glucose and O2 were used as substrates in infection-control cascade reactions, Br- and H2O2 has also been used as substrates in the infection-control cascade reactions for preventing marine biofouling [73,74]. For instance, V2O5 nanowires can transform Br- and H2O2 into HOBr and 1O2 species due to its HPO-like activity. The formed 1O2 species exert strong antibacterial activity and thus prevents marine biofouling without toxicity to marine biota [73].
3.2.2 Photo-enhanced nanozyme-based antibacterial therapy
As discussed above, enhancement of catalytic activity and binding capacity to bacteria are both crucial to realization of efficient antibacterial activity of nanozyme-based antibacterial agents. As known, nanozyme's activity can be improved by regulation of size, morphology, and compositions, surface modification, microenvironment adjustment, and application of external power [4, 8, 29, 41, 53, 55]. In recent year, light regulation has proved to be a new and promising method to modulate the activity of nanozymes [40, 123-127]. In the case of nanozymes, light regulation is one of the most attractive methods for tailoring the enzymatic activity owing to its excellent temporal and spatial precision compared to the existing approaches.
A) Schematic illustration of antibacterial mechanism of the photo-modulated nanozymes, adapted permission from ref. 40, Copyright 2019 the Royal Society of Chemistry. B) The catalytic disinfection mechanism proposed for the 2D bimetallic quasi-MOFCe0.5 nanozyme, adapted permission from ref. 134, Copyright 2022 the WILEY-VCH. C) Titanium incorporated Zr-porphyrinic metal-organic frameworks with enhanced antibacterial performance against multidrug-resistant pathogens, adapted permission from ref. 137, Copyright 2020 the WILEY-VCH. D) Silver-infused porphyrinic metal-organic framework as a surface-adaptive, on-demand nanoplatform for synergistic bacteria killing and wound disinfection, adapted permission from ref. 33, Copyright 2019 the WILEY-VCH.
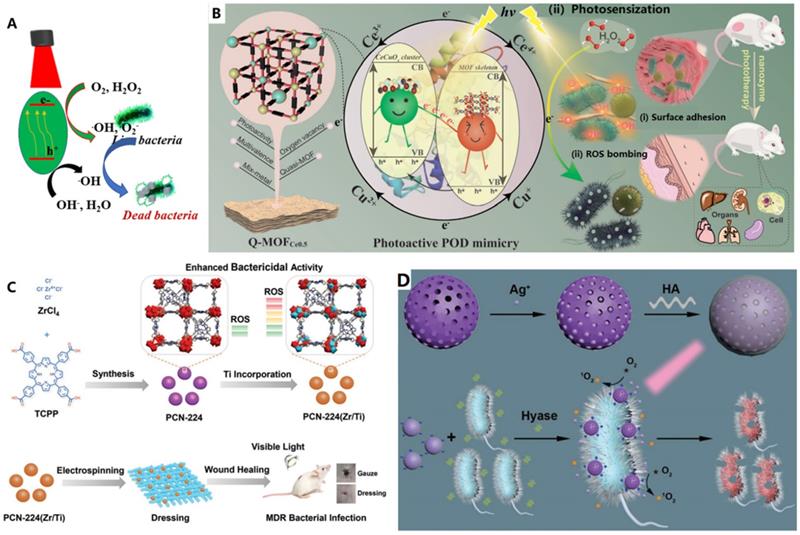
Upon light excitation, some semiconductor-based nanomaterials can be initialized with light and generate ROS which can subsequently oxidize enzymatic substrates. Thus, inorganic nanoparticles used for photocatalysis and photosensitization were typically referred as light-driven oxidase mimetics or light-driven OXD-like nanozymes. In this regard, their introduction into the nanozyme community broadens the research range of the nanozymes [40]. As shown in Figure 4A, under proper light irradiation, the semiconductor adsorbs light and initialized, the electrons in the valence band (VB) can be promoted to the conduction band (CB), leaving holes (h+) in VB. Consequently, O2 and H2O can be oxidized by the photogenerated electrons (e-) or holes to generate ROS such as hydroxyl (.OH) and superoxide (.O2-), which can be harvested for the oxidation of substrates, damage bacterial membrane and intracellular proteins and DNA, etc., thus photo-modulated nanozymes can kill bacterial efficiently [40]. In the case of photosensitization process, the photosensitizer is firstly prompted to the excited singlet state and then activate the triplet state of oxygen to generate reactive oxygen species, which can be used for many photodynamic applications like antibacterial photodynamic therapies. Thus, photosensitizers were also regarded as photo-oxidative nanozymes [40].
Many semiconductor materials have been found to have photo-responsive enzyme-like activity [40,123-125]. Moreover, the catalyst can be activated by a controllable light source, and free radicals can be produced to kill bacteria (Figure 4A) [129-138]. Typically, many reports demonstrated that the enzyme-like activities of some nanozymes can be enhanced by light [55,91,135-138]. For example, Huang et al., reported a bimetallic quasi-MOF (Q-MOFCe0.5) as a POD-like nano-mimetics for photodynamic antibacterial application both in vitro and in vivo under visible light irradiation (Figure 4B) [134]. The representative Q-MOFCe0.5 was prepared by the MOF crystal engineering and controlled thermal transformation of corresponding pristine bimetallic MOF nanosheets. Furthermore, the Q-MOFCe0.5 is a hierarchical heterojunction-like 0D/2D composite in which the partial metal node-derived CeCuOx nanoclusters are uniformly distributed on the decarboxylated MOF skeleton. The heterojunction-like interface endowes the 2D decarboxylated MOF scaffold has isolated nodes-derived Ce-O-Cu sites with oxygen vacancy-coupled multivalent redox cycles and enhanced visible-light photosensitive energy band configuration. Therefore, the biomimetic quasi-MOFCe0.5 nanosheets exhibit enhanced photosensitive POD-like activity to generate sustained reactive oxygen species under visible light for efficient eradicating the surface-adhered bacteria in vitro. Furthermore, the biomimetic quasi-MOFCe0.5 nanosheets can also be used as a safe and on-demand POD-like antibacterial agent for healing potent disinfection of skin wounds in vivo [134]. Liu et al. also reported a dumbbell-shaped Au@CeO2 hybrid nanozyme showed significantly enhanced POD-like activity upon NIR irradiation [135]. By virtue of light-enhanced POD-like activity, Au@CeO2 can generate a large amount of ROS and kill efficiently a broad-spectral bacteria under 808 nm irradiation. Xi et al. reported that the POD-like activity of CuS NPs to generate ROS was enhanced under visible light irradiation [136]. Compared to single bactericidal strategy, these kinds of synergetic modalities make the nanozymes excellent antibacterial candidates for accurate, on-demand and safer bactericidal therapies.
Up to now, most light-activated nanozymes were focused on the production of ROS. Except for the enhanced POD-like capability, some of the photo-trigged nanozymes can activate molecular oxygen directly to generate ROS for killing bacteria without usage of external toxic H2O2. A typical example is antibacterial photodynamic chemotherapy or named photocatalysis (Figure 4A). Among the various semiconductors, organic semiconductor photocatalytic materials have many advantages such as good biocompatibility, low cost, suitable band gap and diverse structural flexibility. Wang et al., constructed an all-organic photocatalytic heterostructure by combing C3N4 and perylene-3,4,9,10-tetracarboxylic diimide (PDINH) which exhibits enhanced photocatalytic efficiency because of the formation of a basal heterostructure. Thus, the nanocomposite could produce more reactive oxygen species to inactivate both Gram-negative and positive bacteria without obvious toxicity to normal tissue cells under simulated solar light (Figure 4B). Furthermore, this all-organic heterostructure show effective therapeutic effect for promoting wound healing in vivo [32].
For instance, as shown in Figure 4C, a bimetallic PCN-224(Zr/Ti) alone was used for photodynamic therapy (PDT) to heal MDR bacteria infected wounds. Under visible light, the obtained bimetallic PCN-224(Zr/Ti) was activated and generate high level of reactive oxygen species to effectively eliminate bacteria. Furthermore, a wound dressing with high biocompatibility and minimal cytotoxicity was prepared by loading the PCN-224(Zr/Ti) NPs onto lactic-co-glycolic acid nanofibers [137]. Similarly, Qu constructed synergistic antimicrobial platform for the treatment of multi-drug resistant bacteria infected wound on mice model. Typically, the platform is powerful surface adaptive and on-demand. The platform was prepared by firstly loading Ag ions onto the photosensitive nMOFs and then coated with hyaluronic acid (HA) (Figure 4D) [33]. Under light irradiation, the nanoparticles showed APDT ability for producing ROS to kill bacteria. Furthermore, in the presence of targeted bacteria, the Ag ions in PCN-224-Ag+ could be released quickly after the HA was degraded by the secreted hyaluronidase. Moreover, the produced positively charged nanoparticles have increased affinity to bacteria. Subsequently, the powerful surface adaptive and on-demand platform showed strong synergistic antibacterial effect on multi-drug resistant bacteria infected wound due to the synergistic performance of the released Ag ions and generated ROS. Wang et al. designed and synthesized a water stable V4+ based MOF material with regular mesoporous structure for the first time, and studied systematically the performance of the material in indoor humidity control and photocatalytic bacteriostasis [130]. Results show that the nanomaterial has dual properties of water adsorption-desorption and photocatalysis bacteriostasis, and can be used for indoor humidity control, especially in the space shuttle cabin and submarine. The killing efficiency of BIT-66 to E. coli was 96% after exposure to visible light (400 nm < λ < 780 nm), which was much higher than that of MCM-41 (55%). ICP-OES and photocatalytic sterilization experiments verified that the excellent antibacterial performance of bit-66 mainly comes from the active oxygen radicals produced by photocatalysis, rather than the release of V4+.
Although antimicrobial photodynamic therapy (aPDT) or photocatalysis has shown great potentials in planktonic bacteria ablation treatment, it is difficult for the photosensitizers (PSs) to penetrate and diffuse inside the biofilm thus high concentration of PSs and dosage of light are desirable which are harmful for normal tissues. Moreover, therapeutic effects were limited because the microenvironment inside biofilm is hypoxic and oxygen rapidly deplete during PDT. To overcome this challenge, Qu et al., fabricated function-adaptive nanoplatform for efficient biofilms eradication [138]. The pH/H2O2-responsive nanoplatform was prepared by encapsulating p-MOF dots (about 5 nm in-size) into human serum albumin-coated MnO2. It was noteworthy that the degradation of MnO2 triggered by the endogenous acidic microenvironment and over-pressed H2O2 could promote the control release of the p-MOF dots. The positively charged p-MOF dots could penetrate into biofilm effectively and attached tightly onto the negatively charged bacterial cell. Importantly, p-MOF dots in the biofilm could not only generate large amount of high ROS, but also can simultaneously generates O2 in situ and lessen hypoxia for biofilms. Thus, benefiting from above synergistic functions, the function-adaptive nanoplatform can effectively ablate bacterial biofilms in vivo without side effect to healthy tissues [138].
3.2.3 Sonodynamic eradication of deep-seated bacterial infection
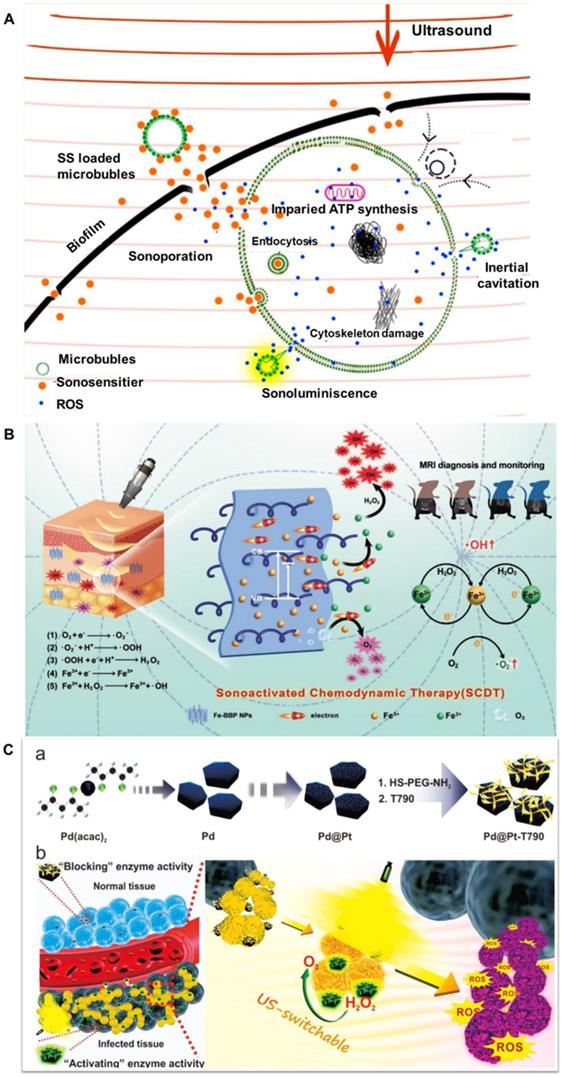
Light or ultrasound induced antibacterial approaches have been proved to be important alternatives against infectious microorganisms. Although antibacterial PDT for superficial tissues have achieved exciting developments, it is inefficient for deep-sited antibacterial infections because it is difficult for the light to penetrate inside the body. Alternatively, antibacterial sonodynamic therapy (SDT) is more attractive alternative than PDT for both superficial and deep-seated microbial infections (Figure 5A). These SDT methods have excellent tissue-penetrating capability and are highly biosafe because ultrasound is nonradioactive and has low tissue attenuation coefficient [139]. However, their further clinical applications are severely limited due to the following disadvantages, such as the therapeutic efficacy of bacteria eradication is still low, and it is difficult to be localized in the infection site, therefore lead to undesired damage to surrounding normal tissues.
Up to now, great attention has been devoted to improve the efficiency of antibacterial SDT by developing new sonosensitizers with good bioavailability and high sonodynamic activity. For instance, Song et al. synthesized Fe-BBP NPs by grafting Fe3+ and branched PEI molecules on the surface of 2D BiOBr NPs via electrostatic interaction that was used as a theranostic platform for sonoactivated chemodynamic therapy (SCDT) against MDR bacterial infection (Figure 5B). Under low-frequency ultrasound, O2 would easily capture excited electrons to produce superoxide anions, while Fe3+ traps an interfacial charge to generate Fe2+, which can react with high concentrations of H2O2 in an inflammation microenvironment to form highly toxic ·OH. The proposed SCDT of Fe-BBP NPs can facilitate an accurate diagnosis and potent therapeutic effect for deep-seated MDR bacterial infection. Furthermore, MRI technique was used to in situ monitor the strong bactericidal effect of Fe-BBP NPs on mice with bacterial myositis [34].
As known, the microenvironment at the inflammation site is hypoxic, and the exposure of sonosensitizers to desired disease site is limited. Furthermore, the oxygen which is essential to the ROS generation depleted rapidly during SDT. Therefore, their practical applications are limited. Very recently, Sun et al. designed an US-switchable nanozyme (Pd@Pt-T790) to enhance the SDT efficacy by alleviating the hypoxia-associated barrier (Figure 5C). The catalase-like activity of Pd@Pt could be significantly blocked by the modification of T790 onto Pd@Pt. However, its activity could be effectively recovered upon US irradiation, which can catalyze the generation of O2 from endogenous H2O2. Due to such “blocking and activating” enzymatic activity, the US-switchable nanozyme could kill bacteria with minimal biotoxicity and side effects on normal tissues. As expected, the Pd@Pt-T790-based SDT nano-system can effectively eradicate MRSA-induced myositis because it could accumulated in infection sites without toxic side effect. In conclusion, the well-designed Pd@Pt-T790 with controllable activity could be used as efficient and precise US-switchable nanozyme to augment sonodynamic eradication of deep-seated bacterial infection [140].
Recently, effective combined diagnosis and therapy in the antibacterial field are high desirable. Thus, Wang et al., prepared multifunctional a Fe@UCNP-HMME nanoplatform for combined therapy and diagnosis. In this system, Fe3O4 cores and UCNPs shell can be used as MRI/UCL dual-modality imaging probes. Moreover, the HMME act as photosensitizer which can be irradiated by the NIR and visible light converted from NIR by the UCNPs for depth PDT and SDT, leading to the temporal induction of cytotoxicity and efficient killing of the deep-seated bacteria through damage of the cell wall structure [141].
A) Proposed mechanisms of antimicrobial sonodynamic therapy, adapted permission from ref. 139, copyright@2021, the American Chemical Society. B) Schematic presentation of sonoactivated chemodynamic therapy (SCDT) against deep MRSA infection and the schedule of MRSA infection, systemic administration of Fe-BBP NPs, SCDT and monitoring in vivo adapted permission from ref.34, copyright@2020, the WILEY-VCH. C) Preparation of Pd@Pt-T790 nanoplatform and its US-switchable nanozyme catalytic oxygen generation-enhanced SDT of bacterial infection, adapted permission from ref. 140, copyright@2020, the American Chemical Society.
Summary of synergetic effects of metal ions and nanozymes for bacterial infection control
| Strategies | Nanomaterials | Enzyme-like activities | Antibacterial mechanisms | Bacteria | Applications | Ref. |
|---|---|---|---|---|---|---|
| Ag++Nanozyme+ light | Ag/AgBr/g-C3N4 | OXD | ROS+Ag | E. coli | Water disinfection | [132] |
| Ag++Nanozyme+ light | ZnO/Ag/RGO | OXD | ROS+Ag+ | E. coli, S. aureus | Bacteria killing | [142] |
| Ag++Nanozyme | Ag/Fe3O4 | POD | .OH+Ag+ | E. coli, S. aureus | Wound healing | [143] |
| Ag++Nanozyme+ light | AgNPs/N-CD@ZnO | OXD | ROS+Ag+ | E. coli, S. aureus | Wound healing | [144] |
| Ag++Nanozyme | NH2-MIL-88B(Fe)-Ag | POD | .OH+Ag+ | E. coli, S. aureus | Wound healing | [28] |
| Ag++Nanozyme+ light | PCN-224-Ag-HA | OXD | ROS+Ag+ | E. coli, S. aureus | Wound disinfection | [33] |
| Ag++Nanozyme | MSN-Ag | OXD | ROS+Ag+ | E. coli, S. aureus | Biofilm disinfection | [35] |
| Ag++Nanozyme | GO/AgNPs/collagen | OXD | ROS+Ag+ | E. coli,S. aureus | Bacteria killing | [145] |
| Zn2++Nanozyme | ZIF-8@Au-GOX(ZAG) | POD+ OXD | Zn2+ | E. coli,S. aureus | Wound disinfection | [119] |
3.3 Combination of more than one antibacterial mechanism
3.3.1 Combination of metal ions and redoxase-like mimetics
Although silver nanoparticles (Ag NPs) are effective agents for killing drug-resistant bacteria, the reduction of the potential toxicity of Ag NPs is highly desirable but challenging. Therefore, the design of novel adaptive agents with lasting emission of Ag+ ions is necessary. Moreover, photocatalytic sterilization is more applicable. Under light irradiation, the photo-catalysts promoted the generation of sufficient ROS and kill the bacteria [32,129-131,142]. More importantly, this green strategy does not induce antibiotic resistance and biotoxicity. Based on these advantages, Yang et al. designed a silver-based ternary composite ZnO/Ag/RGO as an efficient and green antibacterial agent for disinfection treatment. Under light irradiation, the silver nanoparticles in the composite could continuously release silver ions and the ZnO part could effectively generate ROS, respectively, resulting in synergistic antibacterial effect on E. coli and S. aureus [142]. The continuously released silver ions from the silver nanoparticles can firmly adsorbed on the negatively charged bacterial cell membrane, thus interfering with bacterial DNA synthesis and making bacteria lose their ability of division and reproduction; ROS generated by photocatalysis such as ·O2- and ·OH can decompose bacterial cell membrane and cause bacteria to rupture and die. Furthermore, the surface plasmon resonance effect of silver nanoparticles can not only broaden the absorption range of ZnO, but also accelerate the charge separation and effectively inhibit the recombination of photogenerated carriers. Later, Yu et al., prepared a novel antibacterial material by loading Ag NPs and Fe3O4 nanoparticles into chitin nano-fibrous microspheres [143]. Ag-Fe3O4-NMs exhibit excellent antibacterial activity and enhanced wound healing ability attributing to synergistic effect of the released Ag+ and POD-like activity. Very recently, Huang et al. reported that a novel xerogel N-CD@ZnO could capture bacteria rapidly through electrostatic interaction and kill more than 99% of two types of bacteria under NIR light irradiation [144]. Its high antibacterial efficiency is attributed to synergistic effect of continuous release of Ag+ and efficient generation of ROS by N-CD@ZnO. Furthermore, this xerogel could enhance the repair of bacteria-infected wound within 10 days.
In order to resolve the limitation of nanozymes in clinical medicine, Zhang et al., construct a multifunctional “antibiotic” (NH2-MIL-88B(Fe)-Ag). It not only can convert biological level H2O2 into highly active hydroxyl radicals but also can release Ag+ simultaneously, thus eliminate pathogenic bacteria completely [28]. Furthermore, it could enhance the healing of the bacteria-infected wounds with negligible biotoxicity. In order to eliminate the biotoxicity and enhance efficiency of Ag-based antibacterial agent, Qu et al., fabricated an on-demand antimicrobial system with powerful surface-adaptive properties exhibiting a strong synergistic antimicrobial effect [33]. In order to eliminate the toxic effect of Ag NPS, the photoactive MOFs were firstly loaded with Ag ions followed by coating negatively charged HA hyaluronic acid (HA) to prevent the leaking of Ag ions. The nanoplatform shows a strong synergistic antibacterial effect with good biocompatibility with non-targeted cells. However, the degradation of the HA on PCN-224-Ag-HA by the secreted HAase in the targeted bacteria leads to positively charged PCN-224-Ag+ with increased bacterial binding affinity. Benefiting from the synergistic effects of the released Ag+ and generated ROS by photocatalysis, the platform exhibits outstanding efficiency on killing MDR bacteria and accelerates the healing of the bacteria-infected wounds.
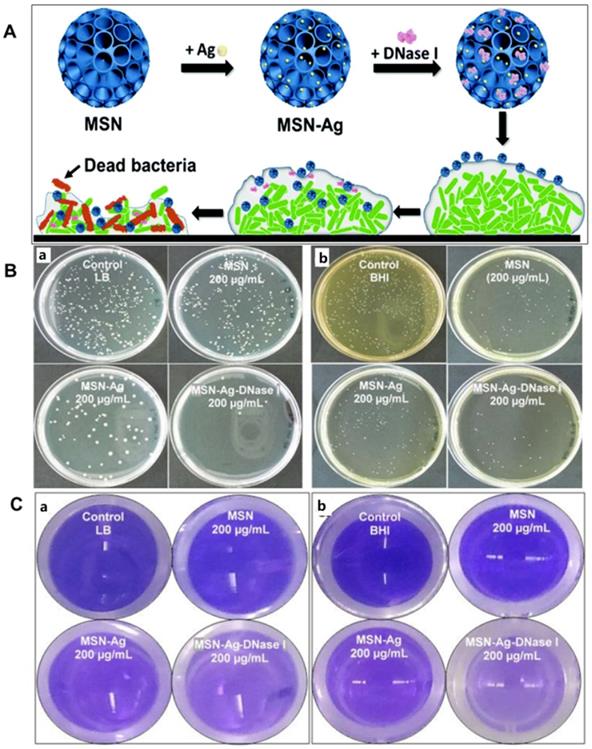
Beside the “intrinsic antimicrobial-resistance”, “infectious biofilm” is another problem that hampers infection control. Most infections are caused by bacteria in their adhering, biofilm-mode of growth [3]. The matrix of EPS act as barriers to protect the bacteria. Therefore, bacteria in the film are usually difficult to kill. It has been proved that deoxyribonuclease I (DNase I) can damage the biofilm matrix by breaking down the extracellular DNA of EPS. Furthermore, Ag nanoparticles could kill the bacteria though ROS generation and other binding with biomolecules inside the cell. In order to use both functions of Ag and DNase I, Xu et al. load DNase I into MSNs with large cone-shaped pores doped with silver nanoparticle for the biofilm treatment (Figure 6A). Due to the high dispersion of Ag NPs and large amount of DNase I in the mesoporous silica, the nano-formulation showed enhanced antibacterial effects for both Gram-negative and Gram-positive biofilms (Figure 6B and 6C) [35]. Beside Ag+, Zn2+ also has good antibacterial effect. Thus, Wang et al. developed a triple-synergistic strategy by combing the glucose-induced cascade reaction and leaching of Zn2+for efficient bacterial eradication. Due to the ROS production and released Zn2+, the triple-synergistic strategy achieved enhanced overall bacterial eradication at a low dosage for both S. aureus and E. coli [119].
A) Schematic preparation of DNase I-loaded MSN-Ag nanocomposite for biofilm treatment. B) Antibacterial effects of nanoparticles towards bacterial biofilms of E. coli (a) and S. mutans (b). C) photograph of dispersal effect of DNase I loaded nanoparticles on bacterial biofilms of E. coli (a) and S. mutans (b) visualized with crystal violet staining, adapted permission from ref. 35, copyright@2020 the Royal Society of Chemistry.
3.3.2 Dual enzyme-like nanozymes as synergetic antibacterial systems
More than 80% of the human bacterial infections are induced by bacterial biofilms, which are hard to eliminate because of its EPS connected by eDNA. Therefore, eDNA plays vital function in maintenance of biofilm structure. DNase can hydrolyze eDNA and thus disassemble biofilms. However, DNase is expensive and instable. To circumvent these issues, Qu et al. designed a series of dual enzyme-mimetic nanozymes to combat biofilms. The dual enzyme-mimetic nanozymes used grafted Ce(IV) complexes as deoxyribonuclease-like mimic to hydrolyze eDNA and MOF doped with Au as POD-like mimetic to generate ROS. Benefiting from the synergetic performance of the above dual enzyme-like activities, the nanozymes could disrupt biofilms and combat bacteria inside [69].
3.3.3 Synergy antibacterial system combining photothermal effect with redoxase-like mimetics
Recently, NIR laser-induced hyperthermia or photothermal therapy (PTT) based on such light absorbing nanomaterials has become a promising and non-invasive strategy to eradicate pathogenic bacteria [147-154]. It can kill microorganisms through a physical sterilization method, in which way the proteins such as enzymes, cell membrane can be effectively destroyed by the hyperthermia effects induced by converting light into local heat. Unfortunately, their clinical application is still a challenge because it is not biosafe with long-term exposure to NIR laser. The construction of synergistic dual phototherapy nanoplatform by combing POD-like nanozymes with photothermal function is expected to facilitate more satisfactory therapeutic effects than single antibacterial mode. Typically, the produced ·OH by nanozymes can damage the cell membrane and endow it with improved permeability and sensitivity to heat, thus hasten bacterial treatment and eliminate the side effects of PTT [147-155]. Moreover, the nanozyme could eliminate inflammatory and promote wound healing without obvious cytotoxicity to healthy mammalian cells. For the same purpose, some nanozyme can generate hyperthermia under light irradiation which can enhance both their POD-like catalytic activity and the membrane permeability, thus significantly improving their antibacterial disinfection ability [147-155].
In this regard, recently, Yin et al. prepared a MoS2 nanoflowers-based synergistic antibacterial system for effective bacteria elimination in vitro and wound disinfection in vivo by integrating its peroxidase-like activity and NIR induced hyperthermia [147]. The PEG-functionalized nanostructured MoS2 could efficiently promote the conversion of H2O2 to more toxic hydroxyl radicals (·OH) for killing bacteria. Furthermore, NIR induced hyperthermia further accelerate the death of E. coli and B. subtilis. Therefore, this synergetic antibacterial system could kill bacteria rapidly with negligible toxicity using low concentrated H2O2. Pan et al. also prepared a photothermal and nanozyme integrated synergetic nanoplatform by combing reduced graphene oxide(r-GO) and iron oxide nanoparticles (IONP) for combating MRSA. The nanocomposite can not only kill bacteria physically by inducing hyperthermia under exposure to near infrared (NIR) energy, but also chemically damage MRSA by degrading H2O2 into highly reactive •OH radicals. Furthermore, the synergetic nanoplatform could effectively kill the bacteria in subcutaneous abscesses and promote wound healing [148]. Recently, Liu et al. used a dumbbell-shaped Au@CeO2 nanozyme as a broad-spectrum antibacterial agent for efficient bacteria disinfection under NIR irradiation. Its high antibacterial efficiency was attributed to the synergetic effects of plasmonic-promoted POD-like activity and NIR induced hyperthermia [135].
In order to enhance the antibacterial properties and reduce biological toxicity of N-doped porous carbon, Wang et al., doped copper single-atoms into N-doped porous carbon (Cu SASs/NPC) by a simple strategy [149]. The Cu-doped NPC exhibits improved catalytic performance of nanozymes and hyperthermia under NIR light, depleting GSH in bacteria and generating plenty of hydroxyl radicals (•OH) to kill bacteria. Benefiting from synergistic antibacterial effects, the Cu-doped NPC can disinfect E. coli and MRSA with almost 100% efficiency and promote the MRSA-infected mouse wounds healing.
Summary of synergetic effects of nanozymes and photothermal for bacterial infection control
| Strategies | Nanomaterials | Enzyme-like activities | Antibacterial mechanisms | Bacteria | Applications | Ref. |
|---|---|---|---|---|---|---|
| Nanozyme+ NIR | PEG-MoS2 | POD | ROS+PTT | E. coli, S. aureus | Wound healing | [147] |
| Nanozyme+ NIR | rGO-IONP | POD | ·OH+ PTT | MRSA | Wound healing | [148] |
| Nanozyme+ NIR | Au@CeO2 | POD | ROS | E. coli, S. aureus | Antibacterial Agents | [135] |
| Nanozyme+ NIR | Cu SASs/NPC | POD | ·OH+ PTT | E. coli, MRSA | Wound healing | [149] |
| Nanozyme+ NIR | AuPtNDs | POD | ROS+PTT | E. coli, S. aureus | Antibacterial therapy | [150] |
| Nanozyme+ NIR | WS2QDs-Van@lipo | POD+OXD | ROS+ PTT | Mu50, E. coli | Anti-biofilm therapies | [36] |
| Nanozyme+ NIR | Au@Cu2-xS | POD | ROS+PTT | E. coli, Faecalis | Root canal therapy | [154] |
| Nanozyme+ NIR | RCF | POD | ·OH+ PTT | E. coli, S. aureus | Wound healing | [30] |
| Nanozyme+ NIR | MoO3-x NDs | POD | ·OH+ PTT | E. coli, MRSA | Wound healing | [155] |
| Nanozyme+ NIR | FePN SAzyme | POD | ·OH+ ROS+PTT | E. coli, S. aureus | Wound healing | [156] |
Zhang et al. also reported a gold-platinum nanodots-based combined therapeutic strategies for bacterial infection and bacterial-infected would healing [150]. Xu reported a WS2QDs-Van@liposome-based photo-controlled antibacterial nanoplatform with both photothermal and nanozyme properties. In this platform, POD-like WS2 QDs and vancomycin were loaded in thermal-sensitive liposome. Under NIR irradiation, the thermal-sensitive liposomes are disrupted due to the photothermal property of WS2QDs. Thus, the antibiotics are released precisely and the antibiotic dosage are greatly reduced. Importantly, a synergistic antibacterial effect could be achieved by combing the antibiotic vancomycin with the hyperthermia effect and POD-like and OXD-like activities of WS2QDs [36].
Biofilm is a three-dimensional structure formed by microorganisms attached to biological or non-biological surfaces. Compared with planktonic-bacteria, it is more challenge to eliminate biofilm-related infections. To overcome the major challenge for removal and sterilization of bacterial biofilms in root canals, Cao et al., proposed an efficient strategy for disinfecting biofilm-protecting bacteria by integrating photothermal and POD-like catalytic activity of nanocomposites (Au@Cu2-xS) [154]. The nanocomposite could convert H2O2 at physiological relevant concentrations to a large amount of •OH to decompose proteins and polysaccharides-the main components of biofilm. Furthermore, the nanocomposite could generate imperative hyperthermia to denaturalize the heat shock proteins. Benefiting from synergistic effect of POD-like activity and photothermal properties, the Au@Cu2-xS nanocomposite-based synergistic antibacterial system achieve high sterilization efficiency against two types of bacteria and is a promising approach to combat bacteria and biofilm in root canals [154]. Zhang et al. reported that an oxygen-vacancy molybdenum trioxide nanodots (MoO3-x NDs) was used as an efficient and safe bacteriostatic combining its photodynamic, photothermal, and peroxidase-like enzymatic activities under NIR irradiation (808 nm) [155]. Furthermore, it can effectively enhance the healing of MRSA-infected wound in living systems. Very recently, Liu et al. also reported that the surface roughness of carbon-iron oxide nanoparticles plays key roles in efficient bacteria disinfections. Benefiting from synergistic effects of rough surface and the photothermal property of carbon nano-shells as well as the POD-like activity of iron oxide nanoparticles, it could realize more effective antibacterial therapy under low-energy NIR-II light irradiation with excellent biocompatibility [30].
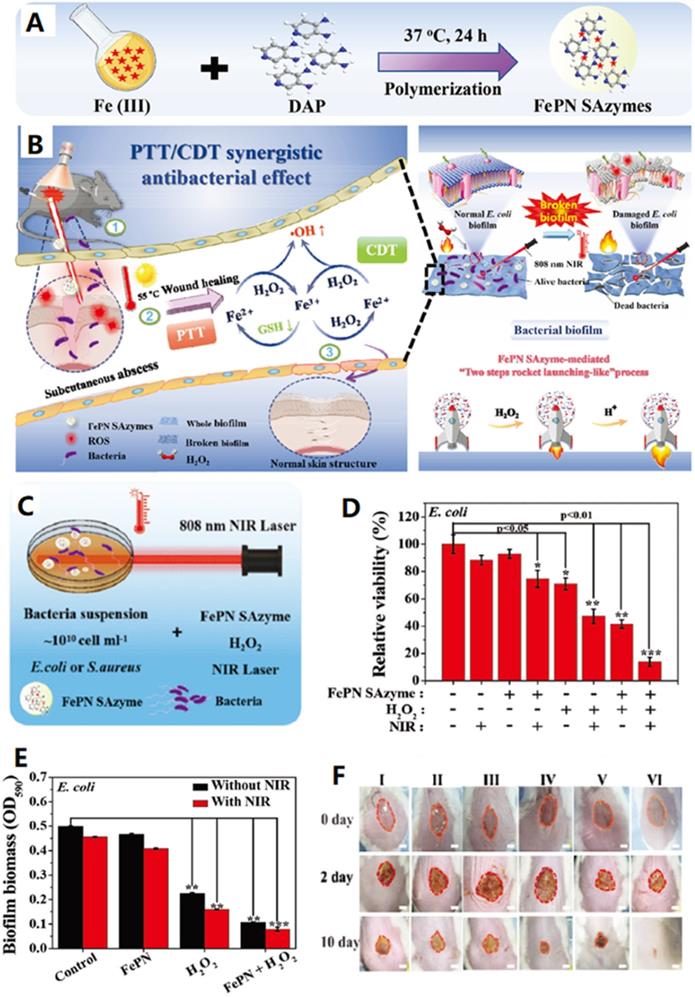
Compared to healthy tissues, biofilm microenvironment is hypoxia, acidic, and is rich of H2O2 and glutathione. Thus, it is more challenge to eliminate biofilm-related infections. In this regard, the exploration of biofilm microenvironment-activated reactions for killing bacteria is an artful way to eradicate bacterial biofilm infections [156,157]. Very recently, Yong's group prepared a Fe-doped polydiaminopyridine nanofusiform-mediated single-atom nanozyme (FePN SAzyme) through a facile one-pot, surfactant-free aqueous polymerization strategy (Figure 7A) and used as a biofilm microenvironment-activated nanozyme-based activatable nanoplatform for healing bacteria-infected wound (Figure 7B) [156]. As shown in Figure 7C and D, FePN SAzyme/H2O2/808 nm laser system show the highest antibacterial activity for inhibiting the growth of E. coli bacteria in the presence of H2O2 under NIR light irradiation, revealing its dominant POD-like antibacterial performance effectively enhanced through combining the photothermal effect. Furthermore, the group of FePN SAzyme/H2O2/808 nm laser system gives the best antibacterial activity for inhibiting formation of the E. coli bacteria-induced biofilm (Figure 7E), because the FePN SAzyme with single Fe atom active center is positively charged and could penetrate and accumulate in biofilms due to electrostatic interactions. Moreover, the high concentration of H2O2 in the biofilm microenvironment promotes its PTT effect which was further accelerated by the mild acidic inflammatory environment. Meanwhile, the nanozyme has enhanced chemo-dynamic effect by promoting the oxidation of biofilm-overexpressed GSH with H2O2 to produce hydroxyl radicals. Additionally, the biofilm-overexpressed H2O2 can be catalytically converted into O2 to eliminate the hypoxia of biofilm. In these regards, the nanoplatform provides significantly synergistic therapy for biofilm-related infections. Encouraged by the above results, the group further confirmed the feasibility of FePN SAzyme for proliferation inhibition of bacteria and potential wound healing efficacy in vivo (Figure 7F). More importantly, the nanoplatform is safe for the healthy tissue because it is simultaneously switchable by the internal and external stimuli. In summary, this nanoplatform has great potential in biofilm-specific anti-infection therapy.
3.4 Selective or targeted disinfections
Nanozyme can kill broad-spectrum of Gram-negative and Gram-positive bacteria. However, it is more significant to selectively discriminate and precisely eliminate pathogenic bacteria. Normally, nanozymes can selectively discriminate and eliminate bacteria by taking advantage of electrostatic and hydrophobic interactions with the target bacteria, molecular recognition, microenvironment response and others.
A) Schematic procedure of the synthesis of FePN SAzyme. B) Schematic illustration of the mechanism of the synergetic PTT and CDT bacteria-infected wound therapy using FePN SAzyme as a biofilm-microenvironment-activated single-atom nanozyme. C) Schematic illustrates NIR irradiation-enhanced ROS generation by peroxidase-like activity of FePN SAzyme for antibacterial treatment. D) Relative viability of E. coli incubated with FePN SAzyme with or without H2O2 under 808 nm laser irradiation. E) Biofilm biomass of E. coli based on the OD590 value for assessing changes in after different treatments. F) Photographs of wound on the mice at different time points after different treatments. I) PBS, II) FePN SAzyme (100 µg/mL), III), H2O2 (100×10-6 M, 5 µL). IV) FePN SAzyme+808 nm (1 W/cm2, 10 min). V) FePN SAzyme+H2O2. VI) FePN SAzyme+808 nm+H2O2. Adapted permission from ref. 156, copyright@2021 the WILEY-VCH.
3.4.1 Selective discrimination of bacteria based on electrostatic and hydrophobic interactions
As known, the surface charge and hydrophobicity of the cell wall components for Gram-positive and Gram-negative bacteria are significant different. Normally, Gram-positive bacteria has negatively charged surface containing teichoic acids and lipoteichoic acids with a thicker peptidoglycan layer. However, Gram-negative bacteria is composed of a bilayer membrane with abundant negatively charged lipopolysaccharides in the outer membrane. Furthermore, there are cross-linked lipopolysaccharides by divalent cations in Gram-negative bacteria, thus inhibiting the interaction of hydrophobic molecules with phospholipid [158]. In this regard, Zhao et al. prepared hydrophobic CNDs for selectively discrimination and elimination for obligate anaerobes. The hydrophobic CNDs can selectively insert into the cell membrane of Gram-positive bacteria through hydrophobic interaction, and adsorb NIR to generate powerful ROS on the targeted bacteria surface under light excitation, inactivating the Gram-positive bacteria by damaging the cell membranes. It is worth to note that CNDs could kill 99.99% of MRSA under 660 nm light irradiation for 5 min, but is not active for killing E. coli. Moreover, CNDs could heal the wounds infected by MRSA faster than the control group without CNDs [159].
Chen et al. reported that antibacterial activities of noble metal-based NPs could be regulated by simply tuning their exposed facets. By investigating the enzyme-like activities and antibacterial performance of Pd NPS with different exposed facets, they found that Pd nanozymes with different crystal surfaces can selectively eliminate Gram-positive or Gram-negative bacteria [53]. There is an important correlation between the surface morphology of Pd nanocrystals and facet-dependent oxidase and POD-like activities. Furthermore, the antibacterial efficiency against Gram-positive bacteria of Pd nanocrystals shows the same trend with their enzyme-like activity for generating ROS. However, in the case of Gram-negative bacteria, a reverse trend is observed. This is because the ROS is not sensitive to Gram-negative bacteria.
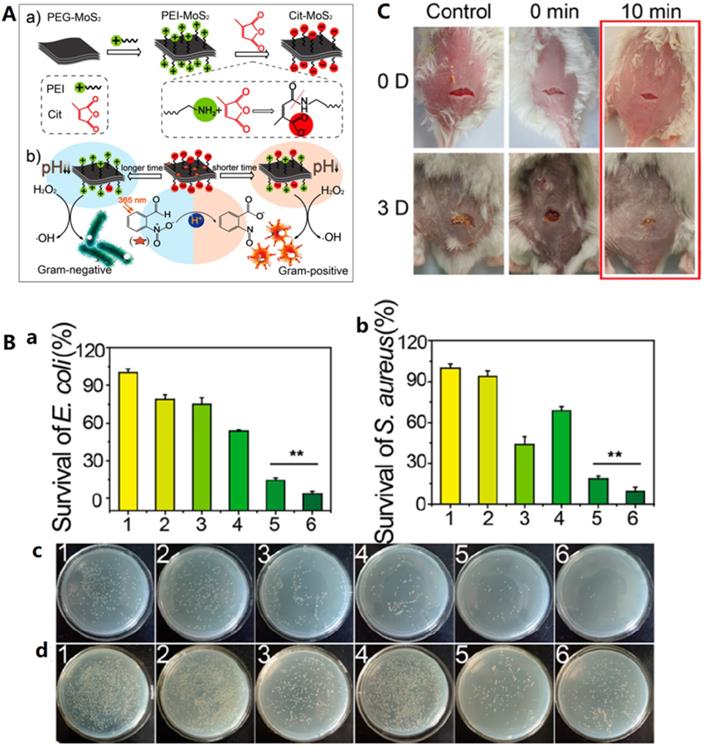
In addition to the different selectivity of the material itself to bacteria, the selective antibacterial activity of nanozyme can also be realized through combination strategy. Qu groups also developed an intelligent strain-selective antimicrobial system using photoacid modified charge-tunable MoS2 [37]. The surface-charge can be modulated from negative to positive by simply changing the light irradiation time. Because of the difference of cell structures of bacterial strains, charge-selective antibacterial activity is achievable by the photo-regulated charge-tunable nanozyme. As shown in Figure 8A, under light, the photoacid molecule 2-nitrobenzaldehyde is converted into 2-nitrobenzoic acid, which has two effects: first, the surface charge of polymer molecule modified molybdenum sulfide in pH response is converted from negative charge to positive charge, which is conducive to the selective combination of materials with bacteria; At the same time, the solution pH changes from neutral to acid, which activates the peroxidase activity of molybdenum sulfide. The above two effects have achieved highly efficient and selective antibacterial performance of light regulation [37]. The antibacterial ability of the system was evaluated by a growth-inhibition assay in liquid LB medium and a plate counting method, the results are summarized in Figure 8B. Compared to other five groups, the group treated with 2-NBA mixed Cit-MoS2 (III) in the presence of H2O2 (irradiation with 365 nm for 40 min) exhibited the best killing ability toward E. coli, confirming the synergistic antibacterial performance between the increased positive charge and enhanced peroxidase-like activity. In addition, similar trend was obtained for S. aureus. The group further investigate the pH environments and the irradiation time effect on the antibacterial selectivity. At weakly acidic conditions, Cit-MoS2 (III) selectively inhibited the growth of E. coli. However, under near neutral pH environment, the system selectively suppressed the growth of S. aureus. Moreover, after long-time irradiation, the surface of Cit-MoS2 (III) is positive, thus is more effective in inhibiting Gram-negative E. coli. Finally, bacteria infected wound on mice model was used to evaluate the Gram-selective antibacterial activity of the designed light-activated antimicrobial system in vivo. As shown in Figure 8C, excellent therapy for S. aureus infected wound on mice was achieved with 10 min of irradiation. Recently, an in vivo activatable pH-responsive PtCo@Graphene nanozyme was used in strain-selective antimicrobial system for selective treatment of Helicobacter pylori (H. pylori) infection. The nanozyme is stable and active in acidic gastric milieu and exhibit targeting bacterial killing ability toward H. pylori without side effect on normal tissues [160].
3.4.2 Selective discrimination of bacteria based on molecular recognition
Many reports proved that Gram-positive or Gram-negative bacteria can be selectively identified and eliminated by conjugating targeting ligands such as antibiotics with phototherapeutic agents [23]. Vancomycin (van) is a narrow-spectrum antibiotic that has been widely used to combat infection arising from gram-positive bacteria such as S. aureus. It is a glycopeptide antibiotic which can bind to a specific C-terminal sequence in the peptidoglycan pentapeptide in the bacterial cell wall. Therefore, Van molecule has the bacteria-targeting ability for the gram-positive bacteria [161-163]. Based on its targeting ability, many vancomycin-functionalized nanomaterials were used for targeted antibacterial applications. For example, Azzam et al., fabricated a novel furry amino magnetic poly-L-ornithine (PLO)/amine-poly(ethylene glycol) (PEG)-COOH/vancomycin (VCM) (AM-PPV) nanospheres with high-loading VCM to track and capture pathogens. The nanospheres could efficiently capture and detect B. cereus with high accuracy and selectivity at low levels due to their relatively rough surface and the bacteria-targeting ability of Van molecule [161]. Furthermore, a vancomycin-functionalized porphyrinic MOFs (PCN-224) was used for targeted antibacterial application. Due to the integrated merits of the PDT feature of PCN-224 and the bacteria-targeting ability of Van molecule, the multifunctional antibacterial agent not only selectively targeted the Gram-positive bacteria but also exhibited enhanced ability for combating bacteria [162].
A) Schematic illustration of gram-selective antimicrobial performance of Cit-MoS2 (III) based-nanozyme under light-modulation. B) Antibacterial activity of the systems by a growth-inhibition assay in liquid LB medium and a plate counting method. Numbers of the surviving bacteria for E. coli (a) and S. aureus (b) by plate counting method, respectively. (c, d) Represent plate counting images of (c) E. coli and (d) S. aureus after different treatments. 1) PBS; 2) 2-NBA (40 min, irradiation time); 3) Cit-MoS2 (III); 4) H2O2; 5) 2-NBA + Cit-MoS2 (III) (40 min, irradiation time); 6) H2O2 + 2-NBA + Cit-MoS2 (III) (40 min, irradiation time), respectively. [Cit-MoS2 (III)] = 500 µg/mL, [H2O2] = 10 mM, [2-NBA] = 600 µM, λ = 365 nm. C) Photographs of S. aureus infected wounds on the mice after treatment with Cit-MoS2 (III) based-nanozyme under different irradiation time, respectively; Adapted permission from ref. 37, copyright@2018, the American Chemical Society.
3.5 Nanozymes for suppressing intracellular bacteria
Although antibiotics are most commonly used agents for antibacterial therapy, the elimination of intracellular bacteria is still challengeable because the intracellular bacteria can be protected by the infected cells by deactivating antibiotics and blocking their antibacterial action. Therefore, it is highly desirable to develop novel and efficient antibiotic-alternatives to eliminate the intracellular intracellular antibiotic-resistant bacteria thus prevent the recurrence of infection and chronic inflammation [164-168].
Serotype Salmonella Enteritidis (S. Enteritidis) is a facultative intracellular pathogen and can cause intracellular infections [169]. Furthermore, it is highly resistant to multiple antibiotics. Thus, the development of novel and environmentally-benign antibiotic-alternatives to eliminate intracellular infectious caused by intracellular S. Enteritidis is important for protecting both animal and human health. In this regard, Shi et al. developed an IONzymes for enhanced autophagic elimination of intracellular S. Enteritidis in LMH cells. Results demonstrated that the IONzymes co-localized with S. Enteritidis in autophagosomes could promote the autophagic activities and generate ROS in the acidic microenvironment, thus lead to enhanced antibacterial performance. Typically, the bacteria in the livers of the S. Enteritidis-infected chickens could be reduced greatly by oral administration of IONzymes. Moreover, by using “non-target high-throughput omics” technologies, the authors confirmed the hepatic oxidation-reduction and autophagy in S. Enteritidis infected chickens are ascribed to the effects of IONzymes on genes. In summary, IONzymes are effective, safe and cost-effective antibiotic alternative for the clinical therapy of the S. Enteritidis associated intracellular infections [170].
Antibacterial photodynamic therapy (aPDT) has proved to be promising method for the elimination of intracellular bacteria by converting nontoxic oxygen to toxic ROS under light irradiation. In this regard, Liu et al. prepared a ZnPc@ZIF-90-M system that can effectively eliminate both planktonic bacteria and macrophage-entrapped antibiotic-resistant bacteria. In this system, photosensitizers of molecular ZnPc loaded in the nanocage of ZIF-90 are monodispersed and effective for ROS generation and antibiotic-resistant bacteria elimination. Furthermore, the coating of a mannosylated shell endow the ZnPc@ZIF-90-M with targeting and internalizing ability with macrophages. In summary, the MOF-based nanoplatform provide a novel direction for designing targeted system for the elimination of stealthy antibiotic-resistant bacteria [171].
3.6 Multifunctional platforms for “detection and inhibition” of bacteria
Developing a dual- or multi-functional platform for “detection and inhibition” of bacteria is in demand but challenging, because bacterial infection has become a major threat to global public health and the use of targeted antibiotics for treatment is often used clinically. Traditional detection methods, such as standard plate colony count, immunoassay and PCR, are complex and time-consuming, most of which require expensive special equipment. Surface enhanced Raman scattering (SERS) may realize the quick, sensitive and effective detection of bacteria. This method can provide sharp, specific fingerprint spectra of the bacteria, thus different kinds of bacteria can be discriminated among from a mixed sample matrix. Furthermore, antimicrobial peptides are attractive bacteria capture due to its high stability and low cost. In view of the above problems, Yuan et al. developed a new biosensor which can simultaneously isolate and detect a variety of bacterial pathogens [24]. As shown in Figure 9A, in this biosensor, the Fe3O4 magnetic nanoparticles modified with antibacterial peptide are used as “capture” probes to selectively separate and magnetically enrich bacteria. 4-MPBA modified gold- silver-graphene oxide (Au@Ag-GO) nanocomposites are used as SERS tags. 4-MPBA can be used as both biomacromolecule recognition and Raman signal molecule for internal standard correction of SERS intensity. Three model pathogens were successfully isolated and detected by this new biosensor. The minimum detection limit of each strain was low to 10 CFU/mL. Moreover, Fe3O4 magnetic nanoparticles modified by antimicrobial peptides have high antibacterial activity and low cellular cytotoxicity, which can be used as antimicrobial agents in long-term blood storage. Finally, the new method is applied to the detection of blood samples from clinical patients. Thirty-nine patients' real blood samples were detected and more than 97% of which were effectively isolated and detected. This new ternary biosensor with the three in one function of “isolation, discrimination and killing” of pathogenic bacteria is expected to have great application potential in clinical diagnosis, blood transfusion safety assurance and other fields.
A) A multifunctional nanoplatform for isolation, discrimination and killing of multiple bacteria in whole blood, adapted permission from ref. 24, copyright@2018, the Royal Society of Chemistry. B) Schematic diagrams of a dual-functional silver platform for detection and inhibition of bacteria (a) and SEM images of bacteria treated for 6 h and 48 h with dual-functional silver platform. Adapted permission from ref. 25, copyright@2019, the WILEY-VCH.
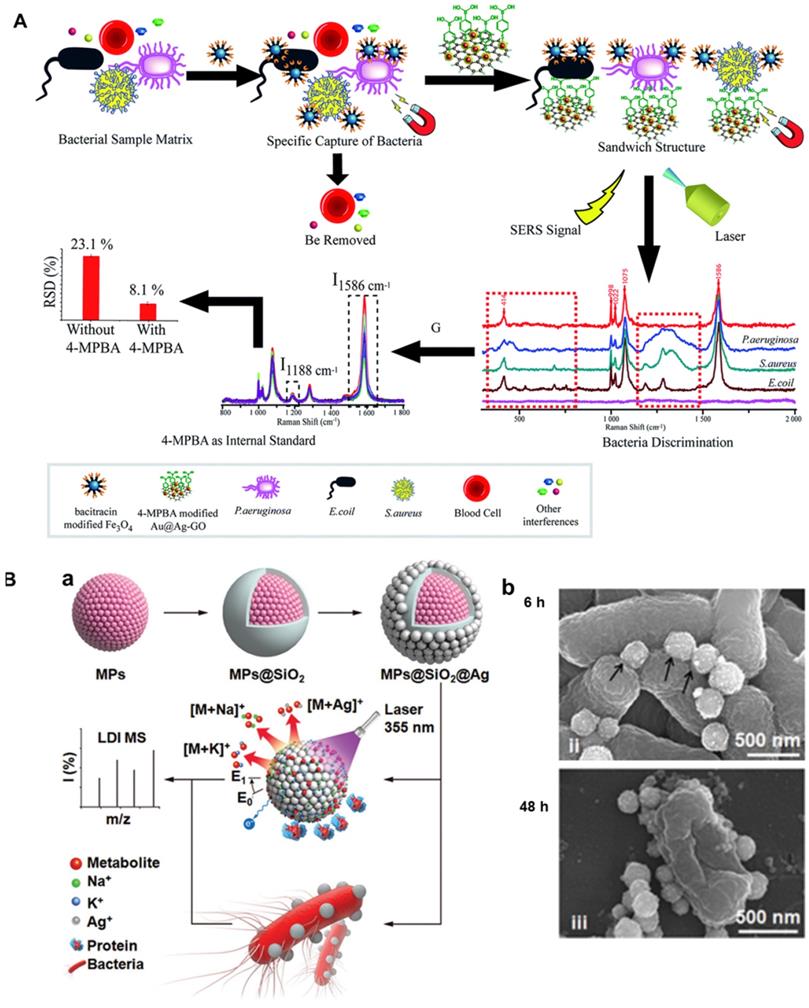
Metabolic analysis is more distal than conventional proteomic and genomic approaches for detection of bacteria in real biological samples. But it is difficult to be applied in bacterial diagnostics because of high complexity of biosystems and low abundance of metabolites. Recently, Vedarethinam et al. also built a multi-functional platform for both detection and kill bacteria [25]. As shown in Figure 9B(a), bacterial metabolites in complex biological liquids can be analyzed by laser desorption ionization mass spectrometry to achieve clinical grade monitoring of serum bacterial infection; Moreover, the surface of magnetic silver with rough surface is easy to be attached on the bacterial surface, which is favorable for the release of silver ions and killing bacteria. Under the cooperation actions of nanoparticles and silver ions, a long-term bacteriostatic effect can be obtained by destroying the bacterial membrane and leading to the disorder of protein synthesis 9B(b). This work provides a novel strategy for the design of multi-functional platforms for “detection and kill bacteria” of pathogenic bacteria. With such multi-functional platform, accurate, rapid and high-throughput detections of bacterial metabolic markers can be realized. Meanwhile, bacteriostatic mechanism can be better understood and more efficient antibacterial effects can be achieved through monitoring metabolic changes.
Electrochemical methods have proven to be one of the most promising strategies for rapid bacteria detection method because of their advantages such as real-time detection, high sensitivity and rapid conversion of chemical signals into electrical signals. Therefore, Wu et al. demonstrated a three-in-one impedance-based electrode sensor which could efficiently capture, rapidly kill and ultra-sensitively detect bacteria [26]. The impedance-based sensor was constructed by decorating Zn-CuO nanoparticles with rough surface and graphene oxide nanosheets on a Ni porous electrode. The integrated platform with burr-like surface could capture 70-80% of bacteria at a concentration of 50 CFU/mL in 20 min, and completely kill bacteria in 30 min. Furthermore, its quantitative detection sensitivity for bacteria is very high and is also effective in a biological sample. Similarly, Wang et al. also constructed an “on-demand” multifunctional magnetic electrode for accurate diagnosis and selectively combating S. aureus. The antibody modified on the magnetic glassy carbon electrode can specifically capture S. aureus in solution. Therefore, the concentration of S. aureus solution can be detected with high detection sensitivity due to the good relevance between the concentration of S. aureus and peak current. Furthermore, benefiting from the controlled released van and good biocompatibility, it could combat S. aureus effectively in the whole blood [21].
4. Summary and outlook
In this review, we summarized the recent progress of nanomaterials-based multimodal synergistic therapies in combating bacterial infections, including the combination of Ag ions and ROS, photothermal and ROS production, and cascade reactions et al. The current state of the art nanozyme-based multimodal synergistic therapies exhibits great potential in treating localized skin infections including wound biofilms in the coming future. But there are still several problems that need to be addressed.
- At present, the antibacterial spectrum for most nanozymes-based antibacterial systems are mainly investigated using E. coli, S. aureus and Pseudomonas aeruginosa (P. aeruginosa) as model bacteria, their bactericidal effects on other pathogenic bacteria are not clear. Furthermore, whether the nanomaterials-based multimodal synergistic antibacterial systems could induce drug resistance should be more concerned. The nanozyme mediated ROS attack is random and less selective. Therefore, the development of new strategies to realize the antibacterial specificity of nanozyme is high desirable. Although nanozyme has good bactericidal activity, its current investigations are mostly limited to in-vitro or skin surface, and little research is on in-vivo application. It is highly desirable to evaluate their bactericidal efficiency and biosafety of nanozyme in vivo.
- Although many efforts have been devoted to the antimicrobial SDT, there are still challenges to be overcome for clinical purposes. Such as the hypersensitivity to light post-therapy because most of the sonosensitizers are photosensitive. Therefore, the development of novel alternatives that are less photosensitive under the exposure of light is one of the attractive strategies to improve the biosafety of the antibacterial SDT.
- Recent studies confirmed that there are bacteria in the tumor and the immune cells. The intracellular bacteria could produce some toxin to make the cells are in an inflammatory state and promote cancer. However, the elimination of intracellular bacteria is still challengeable because the intracellular bacteria can be protected by the infected cells by deactivating antibiotics and blocking their antibacterial action. Therefore, the development of smart and targeting nanozyme-based platforms that could kill both bacteria and cancer simultaneously is highly desirable in the clinical treatment.
- The construction of multi-functional platform of “isolation, detection and killing” for collecting pathogens is one of the most important directions for future development. Furthermore, nanozymes not only could be used for bacterial sterilization by regulating ROS, but also for many other diseases which related to ROS level, such as tumor occurrence, cardiovascular disease and neural aging.
- Recently, nanozymes have been considered to be a new but rapidly developing cross-fields of nanobiology, and their antibacterial applications is just in beginning stage. We believe that with the deepening of research, the applications of nanozymes could be transferred from basic research to clinical transformation as new antibacterial agents. They can be used to prevent and control infection of various diseases, to solve the problem of antibiotic resistance and abuse, and improve the quality of human health life.
Acknowledgements
The authors acknowledge the National Natural Science Foundation of China (NSFC 22072012), Open Fund of Key Laboratory of Biotechnology and Bioresources Utilization (Dalian Minzu University), Ministry of Education (No. KF2020005), China”; Foundation of Liaoning Educational Committee (General Program, LJKZ0032).
Author contributions
All authors listed have made a substantial and intellectual contribution to the work, and all approved it for publication.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Levy SB, Marshall B, Antibacterial resistance worldwide. causes, challenges and responses. Nat Med. 2004;10:S122
2. Nikaido H. Multidrug resistance in bacteria. Annu Rev Biochem. 2009;78:119-46
3. Liu Y, Shi LQ, Su LZ, van der Mei HC, Jutte PC, Ren YJ. et al. Nanotechnology-based antimicrobials and delivery systems for biofilm-infection control. Chem Soc Rev. 2019;48(2):428-46
4. Wei H, Wang EK. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem Soc Rev. 2013;42(14):6060-93
5. Huang YY, Ren JS, Qu XG. Nanozymes: classification, catalytic mechanisms, activity regulation, and applications. Chem Rev. 2019;119(6):4357-412
6. Liang MM, Yan XY. Nanozymes: from new concepts, mechanisms, and standards to applications. Acc Chem Res. 2019;52(8):2190-200
7. Wang H, Wan KW, Shi XH. Recent advances in nanozyme research. Adv Mater. 2019;31(45):1805368
8. Zhang RF, Fan KL, Yan XY. Nanozymes: created by learning from nature. Sci China Life Sci. 2020;63(8):1183-200
9. Tang GH, He JY, Liu JW, Yan XY, Fan KL. Nanozyme for tumor therapy: surface modification matters. Exploration. 2021;1(1):75-89
10. Yang WP, Yang X, Zhu LJ, Chu HS, Li XY, Xu WT. Nanozymes: activity origin, catalytic mechanism, and biological application. Coord Chem Rev. 2021;448:214170
11. Naha PC, Liu Y, Hwang G, Huang Y, Gubara S, Jonnakuti V. et al. Dextran-coated iraon oxide nanoparticles as biomimetic catalysts for localized and pH-activated biofilm disruption. ACS Nano. 2019;13(5):4960-71
12. Ren XY, Chen DX, Wang Y, Li HF, Zhang YB, Chen HY. et al. Nanozymes-recent development and biomedical applications. J Nanobiotechnology. 2022;20(1):92
13. Zhou CY, Wang Q, Jiang J, Gao LZ. Nanozybiotics: Nanozyme-based antibacterials against bacterial resistance. Antibiotics (Basel). 2022;11(3):390
14. Wang Y, Yang YN, Shi YR, Song H, Yu CZ. Antibiotic-free antibacterial strategiesenabled by nanomaterials: progress and perspectives. Adv Mater. 2020;32(18):1904160
15. Duran N, Duran M, de Jesus MB, Seabra AB, Favaro WJ, Nakazato G. Silver nanoparticles: a new view on mechanistic aspects on antimicrobial activity. Nanomedicine: NBM. 2016;12(3):789-99
16. Sanchez-Lopez E, Gomes D, Esteruelas G, Bonilla L, Lopez-Machado AL, Galindo R. et al. Metal-based nanoparticles as antimicrobial agents: an overview. Nanomaterials. 2020;10(2):292
17. Shen MF, Forghani F, Kong XQ, Liu DH, Ye XQ, Chen SG. et al. Antibacterial applications of metal-organic frameworks and their composites. Compr Rev Food Sci Food Saf. 2020;19(4):1397-419
18. Tang Y, Qiu ZY, Xu ZB, Gao LZ. Antibacterial mechanism and applications of nanozymes. Prog Biochem Biophys. 2018;45(2):118-28
19. Huh AJ, Kwon YJ. “Nanoantibiotics”: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J Control Release. 2011;156(2):128-45
20. Hajipour MJ, Fromm KM, Ashkarran AA, de Aberasturi DJ, de Larramendi IR, Rojo T. et al. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012;30(10):499-511
21. Xu TT, Li JM, Zhang SY, Jin YC, Wang R. Integration of diagnosis and treatment in the detection and kill of S. aureus in the whole blood. Biosens Bioelectron. 2019;142:111507
22. Wang ZZ, Dong K, Liu Z, Zhang Y, Chen ZW, Sun HJ. et al. Activation of biologically relevant levels of reactive oxygen species by Au/g-C3N4 hybrid nanozyme for bacteria killing and wound disinfection. Biomaterials. 2017;113:145-57
23. Zhang YQ, Xu WJ, Gan SY, Shan JY, Pen SK, Yuwen LH. et al. Antibody-functionalized MoS2 nanosheets for targeted photothermal therapy of staphylococcus aureus focal infection. Front Bioeng Biotechnol. 2019;7:218
24. Yuan KS, Mei QS, Guo XJ, Xu YW, Yang DT, Sanchez BJ. et al. Antimicrobial peptide based magnetic recognition elements and Au@Ag-GO sers tags with stable internal standards: a three in one biosensor for isolation, discrimination and killing of multiple bacteria in whole blood. Chem Sci. 2018;9(47):8781-95
25. Vedarethinam V, Huang L, Xu W, Zhang R, Gurav DD, Sun XM. et al. Detection and inhibition of bacteria on a dual-functional silver platform. Small. 2019;15(3):1803051
26. Wu RR, Ma Y, Pan JM, Lee SH, Liu JX, Zhu HJ. et al. Efficient capture, rapid killing and ultrasensitive detection of bacteria by a nano-decorated multi-functional electrode sensor. Biosens Bioelectron. 2018;101:52-9
27. Khorsandi K, Keyvani-Ghamsari S, Khatibi Shahidi F, Hosseinzadeh R, Kanwal S. A mechanistic perspective on targeting bacterial drug resistance with nanoparticles. J Drug Deliv. 2021;29(9):941-59
28. Zhang WT, Ren XY, Shi S, Li M, Liu LZ, Han XM. et al. Ionic silver-infused peroxidase-like metal-organic frameworks as versatile “antibiotic” for enhanced bacterial elimination. Nanoscale. 2020;12(30):16330-8
29. Luo Q, Li YL, Huo XB, Li QL, Song YQ, Chen SP. et al. Atomic chromium coordinated graphitic carbon nitride for bioinspired antibiofouling in seawater. Adv Sci. 2022;9(8):2105346
30. Liu ZW, Zhao XY, Yu BR, Zhao NN, Zhang C, Xu FJ. Rough carbon-iron oxide nanohybrids for near-infrared-II light-responsive synergistic antibacterial therapy. ACS Nano. 2021;15(4):7482-90
31. Liu XP, Yan ZQ, Zhang Y, Liu ZW, Sun YH, Ren JS. et al. Two-dimensional metal-organic framework/enzyme hybrid nanocatalyst as a benign and self-activated cascade reagent for in vivo wound healing. ACS Nano. 2019;13(5):5222-30
32. Wang LW, Zhang X, Yu X, Gao EN, Shen ZY. Zhang XY, et al. An all-organic semiconductor C3N4/PDINH heterostructure with advanced antibacterial photocatalytic therapy activity, Adv Materials. 2019;31(33):1901965
33. Zhang Y, Sun PP, Zhang L, Wang ZZ, Wang FM, Dong K. et al. Silver-infused porphyrinic metal-organic framework: surface-adaptive, on-demand nanoplatform for synergistic bacteria killing and wound disinfection. Adv Funct Mater. 2019;29(11):1808594
34. Song ML, Cheng Y, Tian Y, Chu CC, Zhang C, Lu ZX. et al. Sonoactivated chemodynamic therapy: a robust ROS generation nanotheranostic eradicates multidrug-resistant bacterial infection. Adv Funct Mater. 2020;30(43):2003587
35. Tasia WD, Lei C, Cao YX, Ye QS, He Y, Xu C. Enhanced eradication of bacterial biofilms with DNase I-loaded silver-doped mesoporous silica nanoparticles. Nanoscale. 2020;12(4):2328-32
36. Xu MR, Hu Y, Xiao Y, Zhang YY, Sun KL, Wu T. et al. Near-infrared-controlled nanoplatform exploiting photothermal promotion of peroxidase-like and OXD-like activities for potent antibacterial and anti-biofilm therapies. ACS Appl Mater Interfaces. 2020;12(45):50260-74
37. Niu JS, Sun YH, Wang FM, Zhao CQ, Ren JS, Qu XG. Photomodulated nanozyme used for a gram-selective antimicrobial. Chem Mater. 2018;30(20):7027-33
38. Li YT, Wang L, Liu H, Pan Y, Li CN, Xie ZG. et al. Ionic covalent-organic framework nanozyme as effective cascade catalyst against bacterial wound infection. Small. 2021;17(32):2100756
39. Cao FF, Zhang L, Wang H, You YW, Wang Y, Gao N. et al. Defect-rich adhesive nanozymes as efficient antibiotics for enhanced bacterial inhibition. Angew Chem Int Ed. 2019;58(45):16236-42
40. Wu SH, Zhang JY, Wu P. Photo-modulated nanozymes for biosensing and biomedical applications. Anal Methods. 2019;11(40):5081-8
41. Gao LZ, Zhuang J, Nie L, Zhang JB, Zhang Y, Gu N. et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol. 2007;2(9):577-83
42. Zhang D, Zhao YX, Gao YJ, Gao FP, Fan YS, Li XJ. et al. Anti-bacterial and in vivo tumor treatment by reactive oxygen species generated by magnetic nanoparticles. J Mater Chem B. 2013;1(38):5100-7
43. Wu MC, Deokar AR, Liao JH, Shih PY, Ling YC. Graphene-based photothermal agent for rapid and effective killing of bacteria. Acs Nano. 2013;7(2):1281-90
44. Hu G, Song B, Jiang A, Chu B, Shen X, Tang J. et al. Multifunctional silicon-carbon nanohybrids simultaneously featuring bright fluorescence, high antibacterial and wound healing activity. Small. 2019;15(9):1803200
45. Shang YX, Liu FS, Wang YN, Li N, Ding BQ. Enzyme mimic nanomaterials and their biomedical applications. Chembiochem. 2020;21(17):2408-18
46. Ali A, Ovais M, Zhou HG, Rui YK, Chen CY. Tailoring metal-organic frameworks-based nanozymes for bacterial theranostics. Biomaterials. 2021;275:120951
47. Fan X, Yang F, Nie CX, Ma L, Cheng C, Haag R. Biocatalytic nanomaterials: a new pathway for bacterial disinfection. Adv Mater. 2021;33(33):2100637
48. Fu JJ, Shen T, Wu J, Wang C. Nanozyme: a new strategy combating bacterial. J Inorg Mater. 2021;36(3):257-68
49. Li YY, Zhu WX, Li JS, Chu HT. Research progress in nanozyme-based composite materials for fighting against bacteria and biofilms. Colloids Surf B Biointerfaces. 2021;198:111465
50. Li L, Cao LJ, Xiang X, Wu XZ, Ma L, Chen F. et al. ROS-catalytic transition-metal-based enzymatic nanoagents for tumor and bacterial eradication. Adv Funct Mater. 2022;32(1):2107530
51. Xi JQ, Wei G, An LF, Xu ZB, Xu ZL, Fan L. et al. Copper/carbon hybrid nanozyme: tuning catalytic activity by the copper state for antibacterial therapy. Nano Lett. 2019;19(11):7645-54
52. Xi JQ, An LF, Wei G, Huang YL, Li DD, Fan L. et al. Photolysis of methicillin-resistant Staphylococcus aureus using Cu-doped carbon spheres. Biomater Sci. 2020;8(22):6225-34
53. Fang G, Li WF, Shen XM, Perez-Aguilar JM, Chong Y, Gao XF. et al. Differential Pd-nanocrystal facets demonstrate distinct antibacterial activity against gram-positive and gram-negative bacteria. Nat Commun. 2018;9:129
54. Fan H, Li Y, Liu J, Cai R, Gao X, Zhang H. et al. Plasmon-enhanced oxidase-like activity and cellular effect of Pd-coated gold nanorods. ACS Appl Mater Interfaces. 2019;11(49):45416-26
55. Bhattacharyya S, Ali SR, Venkateswarulu M, Howlader P, Zangrando E, De M. et al. Self-assembled Pd-12 coordination cage as photoregulated oxidase-like nanozyme. J Am Chem Soc. 2020;142(44):18981-89
56. Hou C, Chen WQ, Fu LH, Zhang SF, Liang C. Covalent organic frameworks(COFs) materials in enzyme immobilization and mimic enzymes. Prog Chem. 2020;32(7):895-905
57. Zhang L, Yang GP, Xiao SJ, Tan QG, Zheng QQ, Liang RP. et al. Facile construction of covalent organic framework nanozyme for colorimetric detection of uranium. Small. 2021;17(44):2102944
58. Gao LZ, Fan KL, Yan XY. Iron oxide nanozyme: a multifunctional enzyme mimetic for biomedical applications. Theranostics. 2017;7(13):3207-27
59. Kim HY, Park KS, Park HG. Glucose oxidase-like activity of cerium oxide nanoparticles: use for personal glucose meter-based label-free target DNA detection. Theranostics. 2020;10(10):4507-14
60. Qin J, Feng Y, Cheng D, Liu B, Wang Z, Zhao Y. et al. Construction of a mesoporous ceria hollow sphere/enzyme nanoreactor for enhanced cascade catalytic antibacterial therapy. ACS Appl Mater Interfaces. 2021;13(34):40302-14
61. Zhu WS, Wang LY, Li QS, Jiao LZ, Yu XK, Gao XF. et al. Will the bacteria survive in the CeO2 nanozyme-H2O2 system? Molecules. 2021;26(12):3747
62. Bi XL, Bai Q, Wang LN, Du FL, Liu MH, Yu WW. et al. Boron doped graphdiyne: a metal-free peroxidase mimetic nanozyme for antibacterial application. Nano Res. 2022;15(2):1446-54
63. Gao LZ, Giglio KM, Nelson JL, Sondermann H, Travis AJ. Ferromagnetic nanoparticles with peroxidase-like activity enhance the cleavage of biological macromolecules for biofilm elimination. Nanoscale. 2014;6(5):2588-93
64. Yim G, Kim CY, Kang S, Min DH, Kang K, Jang H. Intrinsic peroxidase-mimicking Ir nanoplates for nanozymatic anticancer and antibacterial treatment. ACS Appl Mater Interfaces. 2020;12(37):41062-70
65. Tao Y, Ju EG, Ren JS, Qu XG. Bifunctionalized mesoporous silica-supported gold nanoparticles: intrinsic oxidase and peroxidase catalytic activities for antibacterial applications. Adv Mater. 2015;27(6):1097-104
66. Cao GX, Wu XM, Dong YM, Li ZJ, Wang GL. In situ enzymatically generated photoswitchable oxidase mimetics and their application for colorimetric detection of glucose oxidase. Molecules. 2016;21(7):902
67. Li D, Liu BW, Huang PJJ, Zhang ZJ, Liu JW. Highly active fluorogenic oxidase-mimicking NiO nanozymes. Chem Commun. 2018;54(88):12519-22
68. Li MH, Chen JX, Wu WW, Fang YX, Dong SJ. Oxidase-like MOF-818 annozyme with high specificity for catalysis of catechol oxidation. J Am Chem Socy. 2020;142(36):15569-74
69. Liu ZW, Wang FM, Ren JS, Qu XG. A series of MOF/Ce-based nanozymes with dual enzyme-like activity disrupting biofilms and hindering recolonization of bacteria. Biomaterials. 2019;208:21-31
70. Xu F, Lu QW, Huang PJJ, Liu JW. Nanoceria as a DNase I mimicking nanozyme. Chem Commun. 2019;55(88):13215-8
71. Cheng YY, Liang L, Ye FG, Zhao SL. Ce-MOF with intrinsic haloperoxidase-like activity for ratiometric colorimetric detection of hydrogen peroxide. Biosensors (Basel). 2021;11(7):204
72. Feng W, Han XG, Hu H, Chang MQ, Ding L, Xiang HJ. et al. 2D vanadium carbide MXenzyme to alleviate ROS-mediated inflammatory and neurodegenerative diseases. Nat Commun. 2021;12(1):2203
73. Natalio F, Andre R, Hartog AF, Stoll B, Jochum KP, Wever R. et al. Vanadium pentoxide nanoparticles mimic vanadium haloperoxidases and thwart biofilm formation. Nat Nanotechnol. 2012;7(8):530-5
74. He XY, Tian F, Chang JF, Bai XQ, Yuan CQ, Wang C. et al. Haloperoxidase mimicry by CeO2-x nanorods of different aspect ratios for antibacterial performance. ACS Sustain Chem Eng. 2020;8(17):6744-52
75. Dryden MS, Cooke J, Salib RJ, Holding RE, Biggs T, Salamat AA. et al. Reactive oxygen: a novel antimicrobial mechanism for targeting biofilm-associated infection. J Glob Antimicrob Resist. 2017;8:186-91
76. Hu XQ, Huang YY, Wang YG, Wang XY, Hamblin MR. Antimicrobial photodynamic therapy to control clinically relevant biofilm infections. Front Microbiol. 2018;9:1299
77. Delattin N, Cammue BPA, Thevissen K. Reactive oxygen species-inducing antifungal agents and their activity against fungal biofilms. Future Med Chem. 2014;6(1):77-90
78. Dryden M. Reactive oxygen therapy: a novel therapy in soft tissue infection. Curr Opin Infect Dis. 2017;30(2):143-9
79. Dryden M, Cooke J, Salib R, Holding R, Pender SLF, Brooks J. Hot topics in reactive oxygen therapy: antimicrobial and immunological mechanisms, safety and clinical applications. J Glob Antimicrob Resist. 2017;8:194-8
80. Gambino M, Cappitelli F. Mini-review: biofilm responses to oxidative stress. Biofouling. 2016;32(2):167-78
81. Sun HJ, Gao N, Dong K, Ren JS, Qu XG. Graphene quantum dots-band-aids used for wound disinfection. ACS Nano. 2014;8(6):6202-10
82. Tonoyan L, Montagner D, Friel R, O'Flaherty V. Antimicrobials offered from nature: Peroxidase-catalyzed systems and their mimetics. Biochem Pharmacol. 2020;182:114281
83. Li YF, Yang G, Ren YJ, Shi LQ, Ma RJ, van der Mei HC. et al. Applications and perspectives of cascade reactions in bacterial infection control. Front Chem. 2020;7:861
84. Wang D, Kyere E, Ahmed Sadiq FA. New trends in photodynamic inactivation (PDI) combating biofilms in the food industry-a review. Foods. 2021;10(11):2587
85. Liu QW, Zhang A, Wang RH, Zhang Q, Cui DX. A review on metal- and metal oxide-based nanozymes: properties, mechanisms, and applications. Nanomicro Lett. 2021;13(1):154
86. Li SJ, Pang EN, Gao C, Chang Q, Hu SL, Li N. Cerium-mediated photooxidation for tuning pH-dependent oxidase-like activity. Chem Eng J. 2020;397:125471
87. Zhu DQ, Zhang ML, Pu L, Gai PP, Li F. Nitrogen-enriched conjugated polymer enabled metal-free carbon nanozymes with efficient oxidase-like activity. Small. 2022;18(3):2104993
88. Li C, Sun YR, Li XP, Fan SH, Liu YM, Jiang XM. et al. Bactericidal effects and accelerated wound healing using Tb4O7 nanoparticles with intrinsic oxidase-like activity. J Nanobiotechnology. 2019;17:54
89. Cai SF, Jia XH, Han QS, Yan XY, Yang R, Wang C. Porous Pt/Ag nanoparticles with excellent multifunctional enzyme mimic activities and antibacterial effects. Nano Res. 2017;10(6):2056-69
90. Mu QS, Sun YF, Guo AY, Xu XY, Qin BP, Cai AJ. A bifunctionalized NiCo2O4-Au composite: Intrinsic peroxidase and oxidase catalytic activities for killing bacteria and disinfecting wound. J Hazard Mater. 2021;402:123939
91. Shan JY, Li X, Yang KL, Xiu WJ, Wen QR, Zhang YQ. et al. Efficient bacteria killing by Cu2WS4 nanocrystals with enzyme-like properties and bacteria-binding ability. Acs Nano. 2019;13(12):13797-808
92. Chen Y, Gao YJ, Chen Y, Liu L, Mo A, Peng Q. Nanomaterials-based photothermal therapy and its potentials in antibacterial treatment. J Control Release. 2020;328:251-62
93. Huo JJ, Jia QY, Huang H, Zhang J, Li P, Dong XC. et al. Emerging photothermal-derived multimodal synergistic therapy in combating bacterial infections. Chem Soc Rev. 2021;50(15):8762-89
94. Pallavicini P, Chirico G, Taglietti A. Harvesting light to produce heat: photothermal nanoparticles for technological applications and biomedical devices. Chem Eur J. 2021;27(62):15361-74
95. Xu JW, Yao K, Xu ZK. Nanomaterials with a photothermal effect for antibacterial activities: an overview. Nanoscale. 2019;11(18):8680-91
96. Yougbare S, Mutalik C, Krisnawati DI, Kristanto H, Jazidie A, Nuh M. et al. Nanomaterials for the photothermal killing of bacteria. Nanomaterials. 2020;10(6):1123
97. Zou Y, Zhang YX, Yu Q, Chen H. Photothermal bactericidal surfaces: killing bacteria using light instead of biocides. Biomater Sci. 2021;9(1):10-22
98. Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol. 2008;4(5):278-86
99. Lee KM, Shin SW, Lee WJ, Choi DJ, Ahn Y, Park M. et al. Sunlight-activatable ROS generator for cell death using TiO2/c-Si microwires. Nano Lett. 2021;21(16):6998-7004
100. Roots R. Okada, S. Estimation of life times and diffusion distances of radicals involved in x-ray-induced DNA strand breaks of killing of mammalian cells. Radiat Res. 1975;64(2):306-20
101. Dunnill C, Patton T, Brennan J, Barrett J, Dryden M, Cooke J. et al. Reactive oxygen species (ROS) and wound healing: the functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int Wound J. 2017 14, (1): 89-96
102. Dickinson BC, Chang CJ. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat Chem Biol. 2011;7(8):504-11
103. Chao DY, Dong Q, Chen JX, Yu ZX, Wu WW, Fang YX. et al. Highly efficient disinfection based on multiple enzyme-like activities of Cu3P nanoparticles: a catalytic approach to impede antibiotic resistance. Appl Catal B. 2022;304:121017
104. Yan WJ, Yang LP, Wang HX, Zhang JH, Shen WB. Atomic-engineered gold@silvergold alloy nanoflowers for in vivo inhibition of bacteria. Nanoscale. 2018;10(33):15661-8
105. Ma XM, Cheng Y, Jian H, Feng YL, Chang Y, Zheng RX. et al. Hollow, rough, and nitric oxide-releasing cerium oxide nanoparticles for promoting multiple stages of wound healing. Adv Healthc Mater. 2019: 1900256.
106. Wu RF, Chong Y, Fang G, Jiang XM, Pan Y, Chen CY. et al. Synthesis of Pt hollow nanodendrites with enhanced peroxidase-like activity against bacterial infections: implication for wound healing. Adv Funct Mater. 2018;28(28):1801484
107. Ricca E, Brucher B, Schrittwieser JH. Multi-enzymatic cascade reactions: overview and perspectives. Adv Synth Catal. 2011;353(13):2239-62
108. Fan WP, Lu N, Huang P, Liu Y, Yang Z, Wang S. et al. Glucose-responsive sequential generation of hydrogen peroxide and nitric oxide for synergistic cancer starving-like/gas therapy. Angew Chem Int Ed. 2017;56(5):1229-33
109. Zhang HJ, Toshima N. Glucose oxidation using Au-containing bimetallic and trimetallic nanoparticles. Catal Sci Technol. 2013;3(2):268-78
110. Cao GX, Wu XM, Dong YM. In situ enzymatically generated photo-switchable oxidase mimetics and their application for colorimetric detection of glucose oxidase. Molecules. 2016;21:902
111. Zhang P, Sun D, Cho A, Weon S, Lee S, Lee J. et al. Modified carbon nitride nanozyme as bifunctional glucose oxidase-peroxidase for metal-free bioinspired cascade photocatalysis. Nat Commun. 2019;10:940
112. Huang Y, Zhao MT, Han SK, Lai ZC, Yang J, Tan CL. et al. Growth of Au nanoparticles on 2D metalloporphyrinic metal-organic framework nanosheets used as biomimetic catalysts for cascade reactions. Adv Mater. 2017;29(32):1700102
113. Weng YH, Chen XQ, Chen HH, Yang HL, Gong ZG, Tan HL. Hydrogel microreactor integrated double cascade reactions for synergistic bacterial inactivation and wound disinfection. Chem Eng J. 2022;442(1):136153
114. Zhao JH, Bao XF, Meng T, Wang S, Lu SS, Liu GY. et al. Fe(II)-driven self-assembly of enzyme-like coordination polymer nanoparticles for cascade catalysis and wound disinfection applications. Chem Eng J. 2021;420(1):129674
115. Li T, Li JW, Pang Q, Ma L, Tong WJ, Gao CY. Construction of microreactors for cascade reaction and their potential applications as antibacterial agents. ACS Appl Mater Interfaces. 2019;11(7):6789-95
116. Yan ZQ, Bing W, Ding C, Dong K, Ren JS, Qu XG. A H2O2-free depot for treating bacterial infection: localized cascade reactions to eradicate biofilms in vivo. Nanoscale. 2018;10(37):17656-62
117. Zhong YY, Wang TT, Lao ZT, Lu ML, Liang S, Cui XP. et al. Au-Au/IrO2@Cu(PABA) reactor with tandem enzyme-mimicking catalytic activity for organic dye degradation and antibacterial application. ACS Appl Mater Interfaces. 2021;13(18):21680-92
118. Cheng XQ, Zhang S, Liu HH, Chen HM, Zhou JH, Chen ZW. et al. Biomimetic metal-organic framework composite-mediated cascade catalysis for synergistic bacteria killing. ACS Appl Mater Interfaces. 2020;12(33):36996-7005
119. Wang MX, Zhou X, Li YH, Dong YQ, Meng JS, Zhang S. et al. Triple-synergistic MOF-nanozyme for efficient antibacterial treatment. Bioact Mater. 2022;17:289-99
120. Li DX, Chen T, Zhang YF, Xu YH, Niu HT. Synergistical Starvation and chemo-dynamic therapy for combating multidrug-resistant bacteria and accelerating diabetic wound healing. Adv Healthc Mater. 2021;10(18):2100716
121. Li Y, Fu RZ, Duan ZG, Zhu CH, Fan DD. Injectable hydrogel based on defect-rich multi-nanozymes for diabetic wound healing via an oxygen self-supplying cascade reaction. Small. 2022;18(18):2200165
122. Li YF, Su LZ, Zhang YX, Liu Y, Huang F, Ren YJ. et al. A guanosine-quadruplex hydrogel as cascade reaction container consuming endogenous glucose for infected wound treatment-a study in diabetic mice. Adv Sci. 2022;9(7):2103485
123. Liu YF, Zhou M, Cao W, Wang XY, Wang Q, Li SR. et al. Light-responsive metal-organic framework as an oxidase mimic for cellular glutathione detection. Anal Methods. 2019;91(13):8170-5
124. Liu Q, Cao SF, Sun QQ, Xing CW, Gao W, Lu XQ. et al. A perylenediimide modified SiO2@TiO2 yolk-shell light-responsive nanozyme: Improved peroxidase-like activity for H2O2 and sarcosine sensing. J Hazard Mater. 2022;436:129321
125. Li P, Cao GX, Liu QY, Guo YY, Dong YM, Li ZJ. et al. A novel strategy for amplified probing versatile biomolecules through a photoswitchable biocatalytic cascade. Sens Actuators B Chem. 2018;262:110-7
126. Wang GL, Li XQ, Gao GX, Yuan F, Dong YM, Li ZJ. A novel photoswitchable enzyme cascade for powerful signal amplification in versatile bioassays. Chem Commun. 2017;53(81):11165-8
127. Wang GL, Xu XF, Qiu L, Dong YM, Li ZJ, Zhang C. Dual responsive enzyme mimicking activity of AgX (X = Cl, Br, I) nanoparticles and its application for cancer cell detection. ACS Appl Mater Interfaces. 2014;6(9):6434-42
128. Hu ZM, Jiang X, Xu FJ, Jia J, Long Z, Hou XD. Colorimetric sensing of bithiols using photocatalytic UiO-66(NH2) as H2O2-free peroxidase mimetics. Talanta. 2016;158:276-82
129. Vatansever F, de Melo WCMA, Avci P, Vecchio D, Sadasivam M, Gupta A. et al. Antimicrobial strategies centered around reactive oxygen species-bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol Rev. 2013;37(6):955-9
130. Ma D, Li P, Duan XY, Li ZJ, Shao PP, Lang ZL. et al. A Hydrolytically Stable Vanadium(IV) Metal-Organic Framework with Photocatalytic Bacteriostatic Activity for Autonomous Indoor Humidity Control. Angew Chem Int Ed. 2020;59(10):3905-9
131. Li B, Tan L, Liu XM, Li ZY, Cui ZD, Liang YQ. et al. Superimposed surface plasma resonance effect enhanced the near-infrared photocatalytic activity of Au@Bi2WO6 coating for rapid bacterial killing. J Hazard Mater. 2019;380:120818
132. Yan YC, Zhou XQ, Yu P, Li ZF, Zheng TL. Characteristics, mechanisms and bacteria behavior of photocatalysis with a solid Z-scheme Ag/AgBr/g-C3N4 nanosheet in water disinfection. Appl Catal A Gen. 2020;590:117282
133. Hu YQ, Wang WB, Huang SY, Li J, Zhang YF, Gao YC. et al. A targeted nanozyme based on multiple porphyrins for enhanced photodynamic antibacterial application. Chem Eng J. 2022;431:133704
134. Huang LJ, Sun DW, Pu HB. Photosensitized peroxidase mimicry at the hierarchical 0D/2D heterojunction-like qquasi metal-organic framework interface for boosting biocatalytic disinfection. Small. 2022;18(20):2200178
135. Liu C, Zhang M, Geng HQ, Zhang P, Zheng Z, Zhou YL. et al. NIR enhanced peroxidase-like activity of Au@CeO2 hybrid nanozyme by plasmon-induced hot electrons and photothermal effect for bacteria killing. Appl Catal B. 2021;295:120317
136. Xie YX, Qian Y, Li ZX, Liang ZC, Liu WF, Yang DJ. et al. Near-infrared-activated efficient bacteria-killing by lignin-based copper sulfide nanocomposites with an enhanced photothermal effect and peroxidase-like activity. ACS Sustain Chem Eng. 2021;9(18):6479-88
137. Chen M, Long Z, Dong RH, Wang L, Zhang JJ, Li SX. et al. Titanium incorporation into Zr-porphyrinic metal-organic frameworks with enhanced antibacterial activity against multidrug-resistant pathogens. Small. 2020;16(7):1906240
138. Deng QQ, Sun PP, Zhang L, Liu ZW, Wang H, Ren JS. et al. Porphyrin MOF dots-based, function-adaptive nanoplatform for enhanced penetration and photodynamic eradication of bacterial biofilms. Adv Funct Mater. 2019;29(30):1903018
139. Roy J, Pandey V, Gupta I, Shekhar H. Antibacterial sonodynamic therapy: current status and future perspectives. ACS Biomater Sci Eng. 2021;7(12):5326-38
140. Sun D, Pang X, Cheng Y, Ming J, Xiang SJ, Zhang C. et al. Ultrasound-switchable nanozyme augments sonodynamic therapy against multidrug-resistant bacterial infection. ACS Nano. 2020;14(2):2063-76
141. Wang ZF, Liu CC, Zhao YM, Hu M, Ma DD, Zhang P. et al. Photomagnetic nanoparticles in dual-modality imaging and photo-sonodynamic activity against bacteria. Chem Eng J. 2019;356:811-8
142. Wu YY, Zhang LL, Zhou YZ, Zhang LL, Li Y, Liu QQ. et al. Light-induced ZnO/Ag/rGO bactericidal photocatalyst with synergistic effect of sustained release of silver ions and enhanced reactive oxygen species. Chin J Catal. 2019;40(5):691-702
143. Yu NX, Cai TM, Sun Y, Jiang CJ, Xiong H, Li YB. et al. A novel antibacterial agent based on AgNPs and Fe3O4 loaded chitin microspheres with peroxidase-like activity for synergistic antibacterial activity and wound-healing. Int J Pharm. 2018;552(1-2):277-87
144. Huang B, Liu XM, Li ZY, Zheng YF, Yeung KWK, Cui ZD. et al. Rapid bacteria capturing and killing by AgNPs/N-CD@ZnO hybrids strengthened photo-responsive xerogel for rapid healing of bacteria-infected wounds. Chem Eng J. 2021;414:128805
145. Xie XZ, Mao CY, Liu XM, Zhang YZ, Cui ZD, Yang XJ. et al. Synergistic bacteria killing through photodynamic and physical actions of graphene oxide/Ag/collagen coating. ACS Appl Mater Interfaces. 2017;9(31):26417-28
146. Yin ML, Li ZH, Ju EG, Wang ZZ, Dong K, Ren JS. et al. Multifunctional upconverting nanoparticles for near-infrared triggered and synergistic antibacterial resistance therapy. Chem Commun. 2014;50(72):10488-90
147. Yin WY, Yu J, Lv FT, Yan L, Zheng LR, Gu ZJ. et al. Functionalized nano-MoS2 with peroxidase catalytic and near-infrared photothermal activities for safe and synergetic wound antibacterial applications. ACS Nano. 2016;10(12):11000-11
148. Pan WY, Huang CC, Lin TT, Hu HY, Lin WC, Li MJ. et al. Synergistic antibacterial effects of localized heat and oxidative stress caused by hydroxyl radicals mediated by graphene/iron oxide-based nanocomposites. Nanomed Nanotech Bio Med. 2016;12(2):431-8
149. Wang XW, Shi QQ, Zha ZB, Zhu DD, Zheng LR, Shi LX. et al. Copper single-atom catalysts with photothermal performance and enhanced nanozyme activity for bacteria-infected wound therapy. Bioact Mater. 2021;6(12):4389-401
150. Zhang SN, Lu QJ, Wang FY, Xiao ZY, He LD, He DG. et al. Gold-platinum nanodots with high-peroxidase-like activity and photothermal conversion efficiency for antibacterial therapy. ACS Appl Mater Interfaces. 2021;13(31):37535-44
151. He SY, Feng Y, Sun Q, Xu ZA, Zhang W. Charge-switchable CuxO nanozyme with peroxidase and near-infrared light enhanced photothermal activity for wound antibacterial application. ACS Appl Mater Interfaces. 2022;14(22):25042-9
152. Zhang YF, Ma J, Wang DW, Xu CN, Sheng S, Cheng JF. et al. Fe-TCPP@CS nanoparticles as photodynamic and photothermal agents for efficient antimicrobial therapy. Biomater Sci. 2020;8(23):6526-32
153. Fang QL, Xu KZ, Xiong QS, Xu YQ, Hui AL, Xuan SH. Fe3O4-Au-polydopamine hybrid microcapsules with photothermal-photodynamic synergistic anti-bacterial performance. CrystEngComm. 2021;23(37):6610-9
154. Cao J, Sun Q, Shen AG, Fan B, Hu JM. Nano Au@Cu2-xS with near-infrared photothermal and peroxidase catalytic activities redefines efficient antibiofilm-oriented root canal therapy. Chem Eng J. 2021;422:130090
155. Zhang Y, Li DX, Tan JS, Chang ZS, Liu XY, Ma WS. et al. Near-infrared regulated nanozymatic/photothermal/photodynamic triple-therapy for combating multidrug-resistant bacterial infections via oxygen-vacancy molybdenum trioxide nanodots. Small. 2021;17(1):2005739
156. Xu QQ, Hua YS, Zhang YT, Lv MZ, Wang H, Pi Y. et al. A biofilm microenvironment-activated single-atom iron nanozyme with nir-controllable nanocatalytic activities for synergetic bacteria-infected wound therapy. Adv Healthc Mater. 2021;10(22):2101374
157. Zhang YT, Pi Y, Hua YS, Xie JN, Wang CY, Guo K. et al. Bacteria responsive polyoxometalates nanocluster strategy to regulate biofilm microenvironments for enhanced synergetic antibiofilm activity and wound healing. Theranostics. 2020;10(22):10031-45
158. Ye XT, Feng T, Li L, Wang TJ, Li P, Huang W. Theranostic platforms for specific discrimination and selective killing of bacteria. Acta Biomaterialia. 2021;125:29-40
159. Zhao WB, Wang RT, Liu KK, Du MR, Wang Y, Wang YQ. et al. Near-infrared carbon nanodots for effective identification and inactivation of Gram-positive bacteria. Nano Res. 2022;15(3):1699-708
160. Zhang LF, Zhang L, Deng H, Li H, Tang WT, Guan LY. et al. In vivo activation of pH-responsive oxidase-like graphitic nanozymes for selective killing of helicobacter pylori. Nat Commun. 2021;12(1):2001
161. Azzam AM, Shenashen MA, Selim MS, Mostafa B, Tawfik A, El-Safty SA. Vancomycin-loaded furriness amino magnetic nanospheres for rapid detection of gram-positive water bacterial contamination. Nanomaterials (Basel). 2022;12(3):510
162. Chen LJ, Liu YY, Zhao X, Yan XP. Vancomycin-functionalized porphyrinic metal-organic framework pcn-224 with enhanced antibacterial activity against staphylococcus aureus. Chem Asian J. 2021;16(15):2022-6
163. Chowdhuri AR, Das B, Kumar A, Tripathy S, Roy S, Sahu SK. One-pot synthesis of multifunctional nanoscale metal-organic frameworks as an effective antibacterial agent against multidrug-resistant Staphylococcus aureus. Nanotechnology. 2017;28(9):095102
164. Qi HZ, Shan PP, Wang Y, Li PF, Wang K, Yang LJ. Nanomedicines for the efficient treatment of intracellular bacteria: The "art" principle. Front Chem. 2021;9:775682
165. Cai Q, Fei Y, An HW, Zhao XX, Ma Y, Cong Y. et al. Macrophage-instructed intracellular staphylococcus aureus killing by targeting photodynamic dimers. ACS Appl Mater Interfaces. 2018;10(11):9197-202
166. Elnaggar MG, Jiang KY, Eldesouky HE, Pei YH, Park J, Yuk SA. et al. Antibacterial nanotruffles for treatment of intracellular bacterial infection. Biomaterials. 2020;262:120344
167. Ellis MJ, Tsai CN, Johnson JW, French S, Elhenawy W, Porwollik S. et al. A macrophage-based screen identifies antibacterial compounds selective for intracellular Salmonella Typhimurium. Nat Commun. 2019;10:197
168. Lehar SM, Pillow T, Xu M, Staben L, Kajihara KK, Andlen RV. et al. Novel antibody-antibiotic conjugate eliminates intracellular S. aureus. Nature. 2015;527(7578):323
169. Haraga A, Ohlson MB, Miller SI. Salmonellae interplay with host cells. Nat Rev Microbiol. 2008;6(1):53-66
170. Shi SR, Wu S, Shen YR, Zhang S, Xiao YQ, He X. et al. Iron oxide nanozyme suppresses intracellular Salmonella Enteritidis growth and alleviates infection in vivo. Theranostics. 2018;8(22):6149-62
171. Liu XM, Deng QQ, Zhang L, Sang YJ, Dong K, Ren JS. et al. Elimination of macrophage-entrapped antibiotic-resistant bacteria by a targeted metal-organic framework-based nanoplatform. Chem Commun. 2021;57(23):2903-6
Author contact
![]() Corresponding authors: E-mail: ymzhangedu.cn (Y. Zhang), mikyekenedu.cn (C. Quan); jliac.cn (J. Li).
Corresponding authors: E-mail: ymzhangedu.cn (Y. Zhang), mikyekenedu.cn (C. Quan); jliac.cn (J. Li).
 Global reach, higher impact
Global reach, higher impact