13.3
Impact Factor
Theranostics 2022; 12(13):5888-5913. doi:10.7150/thno.75904 This issue Cite
Review
Antigen transfer and its effect on vaccine-induced immune amplification and tolerance
College of Pharmaceutical Sciences, Zhejiang University, 866 Yuhangtang Road, Hangzhou 310058, Zhejiang, China.
#These authors contributed equally to this work.
Received 2022-6-7; Accepted 2022-7-15; Published 2022-8-1
Abstract
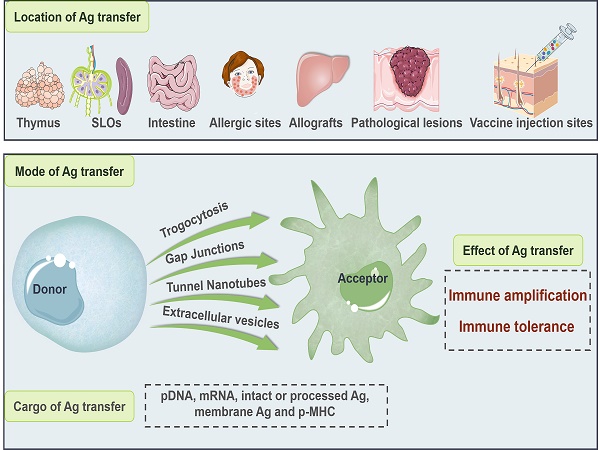
Antigen transfer refers to the process of intercellular information exchange, where antigenic components including nucleic acids, antigen proteins/peptides and peptide-major histocompatibility complexes (p-MHCs) are transmitted from donor cells to recipient cells at the thymus, secondary lymphoid organs (SLOs), intestine, allergic sites, allografts, pathological lesions and vaccine injection sites via trogocytosis, gap junctions, tunnel nanotubes (TNTs), or extracellular vesicles (EVs). In the context of vaccine inoculation, antigen transfer is manipulated by the vaccine type and administration route, which consequently influences, even alters the immunological outcome, i.e., immune amplification and tolerance. Mainly focused on dendritic cells (DCs)-based antigen receptors, this review systematically introduces the biological process, molecular basis and clinical manifestation of antigen transfer.
Keywords: antigen transfer, DCs-based receptor, vaccine, immune amplification, immune tolerance
Introduction
Antigen transfer is an important approach of cell-to-cell communication, where antigenic information is actively transmitted from donor cell to recipient cell in the form of nucleic acid, antigen (Ag) protein/peptide, peptide major histocompatibility complex (p-MHC) and vaccine particle mainly at the thymus, peripheral lymphoid organ, intestine, allergic site, allograft, pathological lesion and vaccine injection site through the contact-dependent pathways including trogocytosis, gap junctions, and tunnel nanotubes (TNTs), and the contact-independent extracellular vesicles (EVs) [1]. In fact, both professional antigen-presenting cells (APCs) and somatic cells (i.e., non-APCs) are potential participants in antigen transfer. Specifically, Ag can be transferred from APCs to APCs, from non-APCs to APCs, from APCs to non-APCs, and even from non-APCs to non-APCs, which is of vital significance for coordinating immune elicitation/amplification and tolerance establishment/maintenance [2].
Encompassing dendritic cells (DCs), B cells and macrophages, APCs are a heterogeneous family with functionally specialized subsets that mediate innate and adaptive immunity upon local microenvironmental cues. Notably, DCs are the most powerful APCs that accommodate a dual regulatory effect in immune activation and tolerance induction. It has been widely recognized that DCs modulate the activation of T cells through both canonical [3] and non-canonical [4-7] Ag presentation pathways, in which the MHC system is flexibly mobilized to elicit potent immune responses against virus infection [8] and tumorigenesis [9, 10]. On the other hand, DCs are paramount in the orchestration of both central and peripheral tolerance. DCs promote central tolerance during the negative selection of autoreactive T cells in the thymus, and induce a tolerogenic or exhausted state of T cells by driving the polarization of regulatory T cells (Tregs) from naïve T cells in the periphery [11]. Such versatile immune competence of DCs is largely attributed to their inherent characteristics, such as: 1) multiple subsets with functionalized phenotypes that constitute a wide-ranging immune surveillance [12, 13], including conventional DC (cDCs) [14-17], Langerhans cells (LCs) [16, 17], plasmacytoid DCs (pDCs) [18], and monocyte-derived DCs (mo-DCs) [19]; 2) rapid sensing and chemotaxis toward sites under the “non-self” invasion [20]; 3) diverse endocytic receptor repertoires and Ag process systems for multi-dimensional activation of T cells [21, 22]; and 4) homing toward the secondary lymphoid organs (SLOs, including lymph nodes (LNs), spleen, Peyer's patches (PPs), adenoids and tonsils) during maturation to provide a timely integration of environmental signals. Moreover, besides direct capture of peripheral Ag [23-26], DCs are capable of collecting antigenic information from Ag-exposed live cells including non-leukocytes and other types or individuals of APCs [14, 23, 27-29], serving as Ag acceptors to ensure an all-round supervision over the body and promote the flexible modulation of immune activation [1] and tolerance [30].
Indeed, the existence of intercellular antigen transfer largely reshapes our understanding about the mode of action of vaccines. For locally administrated vaccines, the accessibility and availability of peripheral Ag by SLOs-resident DCs plays a central role in the in-situ activation of T-/B- lymphocytes and consequently determines the immunological outcomes [31]. However, considering the poor mobilization ability of tissue-resident DCs and the potential cell damage caused by the “non-self” attack, a direct contact with the source Ag may be difficult, risky and not necessary. As a matter of fact, APCs and non-APCs predominate at the vaccine sites can both be positively vaccinated and act as intermediaries that provide antigenic information to DCs, such as keratinocytes (KCs) [32], muscle cells [33], LCs [34, 35], migratory DCs [36], macrophages and B cells. As a matter of fact, antigen transfer from infected, transformed, or vaccinated live cells to DCs prevents the risk of cell damage caused by direct virus/tumor contact, compensates the insufficient availability of certain types of DCs to distal Ag, and enhances specific immune responses against natural infection, tumorigenesis and vaccine inoculation [2, 37-39].
Therefore, rationally utilize and regulate antigen transfer for improved vaccine efficacy might demonstrate some clinical significance. However, current understanding about the biological process and molecular basis of antigen transfer is insufficient [40], which may limit the efficiency and safety of current vaccines. Herein, mainly focused on DCs-based Ag receptors, this review systematically introduces the mode, location and participant of antigen transfer, especially in the context of vaccine inoculation, which may provide guidance for the design and development of vaccines.
Pathological and physiological significance of antigen transfer
Pathological significance of antigen transfer
Antigen transfer refers to the intercellular trafficking (active behavior) of antigenic information from the donor to the acceptor, which effectively promotes the availability of Ag and extends the breadth and duration of immune response. APCs, as represented by DCs, fail to elicit immune responses when directly exposed to viruses that are highly invasive and cell-destructive (e.g., herpes simplex virus (HSV), Epstein-Barr virus (EBV), cytomegalovirus and some influenza viruses) [37]. Likewise, transformed or malignant cells may reshape the microenvironment to inactivate infiltrating immune cells, as both the number and the LNs-migrating ability of tumor-infiltrating DCs drastically decreased with time [41].
Under these circumstances, Ag is transferred from infected or transformed live cells to DCs, which greatly reduces the risk of direct virus/tumor contact and magnifies specific immune response to natural infection and tumorigenesis.
Physiological significance of antigen transfer
It is reported that compared to APCs, most somatic cells (e.g., muscle cells, keratinocytes) abundant at the sites of vaccine administration can be positively vaccinated and even display higher competence in nucleic acid-transfection and protein-uptake [2]. However, these cells are generally low in the expression of co-stimulatory molecules, which deprives their ability of direct T-cell activation upon vaccination. In response to this situation, Ag is transferred from vaccinated somatic cells to the nearby APCs to activate specific immune response.
On the other hand, tissue-resident DCs, important components of the lymphoid organs that far outnumber their circulating counterparts have poor mobilization ability, which greatly limits their accessibility to the peripheral Ag. However, these DCs, LN-resident CD8α+ DCs in particular, have been shown to present Ag from other cells (e.g., circulating DCs), leading to efficient elicitation of the cytotoxic T lymphocyte (CTL) response [38, 39]. Therefore, antigen transfer from other cells to tissue-resident DCs may compensate the low availability of these certain types of APCs to distal Ag and magnifies specific immune response.
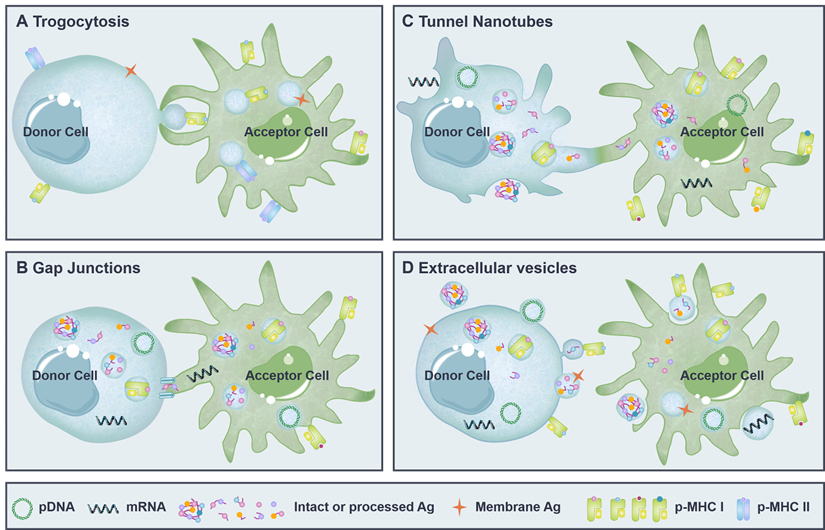
Four modes of antigen transfer. (A) Trogocytosis. Cells in close contact can directly "bite" and internalize membrane-associated Ags and/or p-MHCs from each other. (B) Gap junction. Adjacent cells exchange intracellular antigenic information (pDNA, mRNA, Ag protein/peptide and p-MHCs) via hexamer channels. (C) Tunnel nanotubes (TNTs). Cell-cell connection by actin-based membrane protrusions that establish cytoplasmic continuity between distant cells and enable the exchange of cytoplasmic Ags and cell surface-associated Ags. (D) Extracellular vesicles (EVs). Donor cells bud directly from the plasma membrane to generate microvesicles containing p-MHCs and/or membrane-associated Ags, or secrete exosomes derived from the intracellular Ag-incorporating endosomes. These microvesicles and exosomes diffuse into the extracellular space to be captured by acceptor cells. Ag: antigen; mRNA: messenger RNA; pDNA: plasmid DNA; p-MHC I/II: peptide-major histocompatibility complex class I/II molecules.
Mode of antigen transfer
Intercellular antigen transfer is largely mediated by the contact-dependent pathways including trogocytosis [27], tunnel nanotubes (TNTs) [42] and gap junctions [43], as well as the contact-independent extracellular vesicles [15] (Figure 1). Both microvesicles bud directly from the plasma membrane [1] and trogocytosis [44] are able to transfer membrane-associated Ags and functional p-MHCs presented on the cell surface, whereas exosomes derived from the late endosomes [45, 46], gap junctions [47] and TNTs [48] mainly transfer cytoplasmic Ag in the form of nucleic acid, Ag protein/peptide, and p-MHCs.
In fact, different modes of antigen transfer are involved in various physiological and pathological conditions. Trogocytosis is generally observed between cells with active membrane mobility. DCs trogocytose membrane fragments containing functional p-MHCs from neighboring cells are able to initiate immune response efficiently [49]. Meanwhile, immunosuppressive molecules transferred to DCs via trogocytosis may lead to impaired immunity [50, 51]. Gap junctions are hexamer channels formed within adjacent cells that facilitate the intracellular Ag exchange. For example, pathogenic and harmless antigen captured by gut-resident macrophages can be transferred to migratory DCs through gap junctions to induce protective immunity and establish oral tolerance, respectively [52-54]. TNTs are actin-based membrane protrusions (up to 150 µm in length) that enable cell-to-cell connection over a longer distance. TNTs are the main mediators of lymphatic meshwork that support the quick activation of LN-resident DCs and promote the efficient induction of immune response [55]. Likewise, TNTs formed with malignant cells or virus-infected cells may accelerate the spread of diseases [55, 56]. EVs, on the other hand, enable a contact-independent Ag transfer between the donor and the acceptor. Tumor Ag transferred to DCs via EVs may consequently promote anti-tumor immunity or induce T-cell tolerance, depending mainly on the form of transferred Ag and the maturation state of receptor DCs [57-60].
Trogocytosis
Generally, DCs phagocytize apoptotic and necrotic debris from the extracellular space for canonical Ag presentation and non-canonical Ag cross presentation [61-63]. However, recent studies have demonstrated that DCs can also obtain antigenic information from living cells through a contact-dependent pathway called "trogocytosis" (also known as "nibbling", Figure 1A) [27, 40]. Trogocytosis is an active process whereby acceptor cells conjugate to donor cells for extraction of surface molecules and membrane fragments [64]. In the context of antigen transfer, membrane Ag and p-MHCs displayed on the surface of donor cells are transferred to DCs in close proximity via trogocytosis, which mainly involves close cell-to-cell contact, formation of “immunological synapse”-like structure, cross-cellular transport of plasma membrane-associated cargos, and separation of cells, leading to elicited immune responses or maintained peripheral tolerance [14, 65-67]. Notably, the special biological characteristics of DCs facilitate the development of trogocytosis, including high membrane deformability and elasticity, rapid sensing and chemotaxis in respond to inflammation, and extensive interaction with other cells [10, 68]. On the contrary, lines of evidence indicate that macrophages, which readily phagocytose apoptotic cells, cannot trogocytose membrane from viable cells, possibly due to limited expression of surface scavenger receptors [40, 44], too acidic endosomal/phagosomal environment, or high levels of lysosomal proteases [69].
In tumor-bearing patients, compared to apoptotic or necrotic tumor cells, live tumor cells expressing various tumor-associated antigens (TAAs) and tumor-specific antigens (TSAs) are the most abundant source of Ag with high immunogenicity. Therefore, trogocytosis of viable tumor cells by DCs contributes to an efficient and versatile Ag presentation for the activation of anti-tumor immune response [44]. Meanwhile, during virus infection (e.g., human immunodeficiency virus (HIV) and EBV), DCs are able to preferentially acquire viral Ag from infected cells including lymphocytes, macrophages and non-hemopoietic cells, without risks of self-infection and immune dysfunction. On the other hand, DCs directly infected by virus may serve as Ag donors to provide sustained Ag for epidermal resident LCs or recruited circulating DCs [70-73].
However, attention should be paid to the fact that immunosuppressive molecules may also spread and spoil the immune microenvironment during trogocytosis. For example, human leukocyte antigen-G (HLA-G), a nonclassical HLA-class I molecule usually over-expressed by malignant cells, can directly inhibit the function, chemotaxis and viability of immune cells through receptor binding [74]. Furthermore, the systemic immune environment can be further deteriorated when HLA-G is transferred to DCs via trogocytosis, which limits the activation of effector T cells, promotes the expansion of immunosuppressive cells (such as Tregs and myeloid-derived suppressive cells (MDSCs)), and even induces the apoptosis of immune cells, rendering tumor cells with greater metastatic potential [50, 51]. Similarly, virus with high invasiveness and viability may also accelerate the speed and scale of transmission through antigen transfer. For example, although DCs are largely resistant to productive virus infection, they express high levels of C-type lectins, the main attachment factors of HIV at the surface of dermal and mucosal DCs. As a result, myeloid DCs, pDCs and LCs are all susceptible to infection with HIV, leading to impaired antigen-presenting function. In addition, follicular DCs (FDCs) capture large quantities of HIV as persistent reservoirs of virion to promote viral pathogenesis. Furthermore, HIV-pulsed DCs can transfer virion to T cells through “trans-infection” (across the virological synapse or DC-derived exosomes) and/or “cis-infection” (mediated by the de novo viral production within DCs) for facilitated viral dissemination and escaped antiviral immunity [75-77].
Gap junctions
Gap junctions are clusters of intercellular hemichannels mainly composed of plasma membrane protein Connexin and formed in closely apposed neighboring cells [43] (Figure 1B), especially in DCs, B cells, monocytes and activated lymphocytes that have a high expression of Connexin 43 (Cx43) [78, 79]. In such communication channels, ions and small molecules can be passively diffused [80]. Moreover, gap junctions provide a pathway mediating the direct cell-to-cell transfer of Ag in the form of nucleic acids, proteins (molecular weights below 1 kDa, or amino acid residues less than 11) [81, 82], p-MHCs, and other signaling molecules [83]. Of note, Cx43-based gap junctions are more favorable for the intercellular transfer of MHC I-restricted peptides with molecular weights lower than 1 kDa, instead of the theoretically larger MHC II-restricted peptides [62, 84].
Accumulating evidence suggests that gap junction plays an important role in the initiation and amplification of immune responses. It's reported that infection with bacteria Salmonella up-regulates the expression of Cx43 in both human and murine melanoma cells, which promotes the formation of functional gap junctions between melanoma cells and adjacent DCs to facilitate the intercellular transfer of antigenic peptides. Consequently, DCs present Ag on their surface to initiate specific cytotoxic T cells against tumor growth. Notably, such Cx43-dependent antigen transfer induces cross presentation and CD8+ T cell activation more efficient than that of standard Ag loading in generating anti-tumor responses [85, 86]. Macrophages, although with limited capacity of Ag cross-presentation and CD8+ T cell activation, may serve as transfer stations of Ag to promote immune responses. Specifically, tumor rejection Ags are phagocytosed by macrophages [87] and subsequently transferred to DCs through gap junction-mediated intercellular transmission, which promotes the maturation of DCs and augments antitumor T cell responses [88]. Such antigenic communication between macrophages and DCs can also be observed in the intestine. Mazzini et al. [47, 52] revealed that CX3CR1+ macrophages sampled over the intestine for suspicious “non-self” substances and delivered captured soluble Ags to DCs through gap junction. Subsequently, Ag-exposed DCs migrated toward the draining lymph nodes (dLNs) to prime or tolerize T cells, depending on the microenvironmental signals. FDCs have also been shown to form immune cell clusters with cognate follicular B cells by Cx43-mediated gap junction for direct Ag delivery [89], supporting the development and maturation of B cells in the germinal center [79].
Tunnel nanotubes
Tunnel nanotubes (TNTs) (Figure 1C), also known as “filopodia bridges”, “membrane tubes” and “nanotubules” [90], are non-adherent, filamentous actin (F-actin) -based cytoplasmic protrusions [91] widely found in immune cells, neurons, tumor cells [56] and epithelial cells. TNTs enable cell-to-cell communication over long distance by plasma membrane bridges [92] (e.g., TNTs in macrophages can extend more than 150 μm [93]), which establishes cytoplasm continuity [94] and facilitates intercellular information exchange. Specifically, nucleic acids, proteins, lipid nanoparticles, organelles (such as vesicles, lysosomes, mitochondria and autophagosomes) and even pathogenic particles [95] can be transported from donor cells to acceptor cells via TNTs [42]. To date, “cell dislodgment" and "actin-driven" are the two widely recognized mechanisms accounting for the formation of intercellular TNTs [96]. However, more efforts are needed to fully address the molecular basis and immunological significance of TNTs.
Despite insufficient understanding of TNTs-involved Ag transfer, lines of evidence suggest that such long and thin membrane tubes actively mobilize the immune regulatory networks by connecting multiple cells and promoting the intracellular sharing of antigenic information [97]. It should be mentioned that the unique membrane structures of DCs including elaborate dendrites, sophisticated pseudopodia and delicate ruffles support the deformation and rearrangement of plasma membrane [98, 99], which also consists the structural basis of TNTs. Peripheral Ag-exposed DCs migrate to the dLNs within 48 h in a chemokine receptor 7 (CCR7)-dependent manner [14, 23], during which DCs undergo maturation with extensive dendritic stretching and remarkable morphological change, laying the foundation for immune cell communication and T cell activation [100]. Then, LNs-resident DCs acquire Ag from their migratory counterparts by TNTs, which increases the availability of Ag and consequently magnifies immune response [1, 100]. In addition, p-MHC class II complexes and costimulatory B7 family proteins (e.g., CD86 molecules) are shared within two adjacent B cells [101] or B cells and macrophages [102] through TNTs-mediated interconnection networks, which improves the efficiency of Ag-dependent T cell activation and induces a wide-ranging mobilization of the immune system.
Nevertheless, TNTs formed within tumor cells are reported to accelerate tumor metastasis by propagating metabolic plasticity, angiogenic ability and therapy resistance [56]. Besides, TNTs can be exploited by pathogens such as HIV-1 for direct cell-to-cell spread [55].
Extracellular Vesicles
Extracellular vesicles (EVs) (Figure 1D) are small spherical lipid bilayer particles released into the extracellular environment by almost all types of cells, including APCs, somatic cells and tumor cells. According to different mechanisms of biogenesis, EVs are mainly categorized into microvesicles (also named as microparticles) that bud directly from the plasma membrane [103] and exosomes secreted as a consequence of the fusion of multivesicular endosomes (MVEs) with the plasma membrane [104]. EVs loaded with cargos (e.g., lipids, proteins and nucleic acids) are diffused into the interstitial space or the circulation to be internalized by receptor cells via phagocytosis, endocytosis, macropinocytosis, lipid rafts-mediated internalization, or direct plasma membrane fusion [105, 106]. EVs remain attached to recipient cells can also transfer donor-derived cargos. For example, during allogenic organ transplantation, donor DCs migrate from the graft to lymphoid tissues and transfer MHC molecules to recipient cDCs through EVs. These EVs are internalized or remain attached to the recipient cDCs, instead of fusing with the plasma membrane of the acceptor APCs, which consequently enhanced the activation of alloreactive T cells. In this regard, depletion of recipient DCs after allograft can be used to delay graft rejection [107].
EVs-mediated Ag transfer from tumor cells or virus-infected cells to DCs is of great importance to the initiation and maintenance of specific immune responses [15]. DCs are able to selectively engulf cancer cell-derived EVs incorporating antigenic protein, epitope peptide and/or p-MHCs through extracellular vesicles-internalizing receptors (EVIR) [45, 46], which coordinates antitumor response with quick mobilization and high efficiency [57]. Of note, EVs can be easily isolated from the sera or malignant effusions of patient, representing as rich reservoirs of the whole panel of tumor Ag that may elicit a broad array of T cell clones against multiple Ag epitopes. Indeed, several EVs have been collected, modified and used as the next-generation cell-free cancer vaccines in personalized tumor immunotherapy [108, 109].
However, EVs with insufficient co-stimulatory signals and/or adjuvant-like components may induce immune tolerance when internalized by immature DCs [110]. Moreover, immunosuppressive molecules can also be transferred through tumor cells-derived EVs [111-114] to impair the maturation and immunological function of immune cells.
Location of antigen transfer
A growing number of studies have demonstrated that Ag is transferred at various physiological and pathological compartments that mainly include thymus [115], SLOs [1], intestine [47], allergic sites [116], allografts [117], lesions [34, 35] and vaccine injection sites [2], which largely determines the immunological consequence (i.e., immune activation or tolerance). And more efforts are needed to unveil other potential sites, as well as the associated outcomes, of antigen transfer.
Thymus
Thymus is primarily responsible for the establishment of central tolerance that avoids autoimmune responses [118]. Specifically, autoreactive T cells are negatively selected and eliminated in the thymic medulla before entering the periphery, which blocks the recognition of T cell receptors (TCRs) with tissue-restricted self-Ags and prevents specific cytotoxic killing against normal cells [30]. Firstly, a subpopulation of medullary thymic epithelial cells (mTECs) displays the vast majority of autoantigens by generating corresponding p-MHCs, a process that involves the transcription factor autoimmune regulator (AIRE) [119, 120]. Then, the resultant p-MHCs are subjected to other APCs in the medullary microenvironment such as DCs, B cells and macrophages, especially resident CD8α+ DCs, possibly through trogocytosis, exosomes and uptake of apoptotic bodies that are irrespective of the subcellular localization or expression pattern of Ag [121]. As a result, medullary thymocytes that express TCRs with high affinity for autoantigen-associated p-MHCs presented by these APCs are either deleted through apoptosis or undergo lineage deviation that gives rise to Tregs and other 'unconventional' T cell populations [122]. Notably, during negative selection, CD8+ and CD4+ single positive T cells may travel at a rate of 10 μm per minute in the medullary areas to increase the interaction with these APCs [123]. It should be mentioned that scavenger receptor CD36 is involved in the process of antigen transfer from mTECs to DCs in the form of EVs that contain mTECs cell surface proteins (i.e., intact p-MHC class I and II complexes) [115]. However, more efforts are needed to unveil the mechanistic details of such EVs-engaged antigenic communication and explore the participation of other antigen transfer approaches.
Secondary lymphoid organs
SLOs, especially LNs and spleen, are highly organized structures that filter lymph and blood for suspicious Ags in these fluids, allow the entry of Ag-loaded DCs, and facilitate the antigenic interaction between DCs, B cells and T cells, serving as the “transit hubs” of adaptive immunity. There are a variety of specialized stromal cells, bone marrow cells and lymphocytes constituting the structural organization of SLOs for efficient Ag encounter and intercellular transfer. For example, LNs are anatomically composed of paracortex (T cell zone), cortex (B cell zone with follicles and germinal centers) and medulla (including subcapsular sinus (SCS), medullary sinuses, medullary cords and hilus) [124]. These functionalized compartments are closely connected to orchestrate immune response against foreign substances.
Circulating DCs migrate back to the LNs upon peripheral Ag stimulation through afferent lymphatic vessels and in a chemokine-dependent manner for Ag transfer and lymphocyte activation [125, 126]. Notably, the T cell immunity elicited within SLOs is found to be compartmentalized by route of lymphatic transport. In response to the administration of vaccinia virus (a replication-competent live attenuated vaccine), skin DCs fail to relocate to the dLNs from site of infection, which ablates vaccine efficacy [127]. To delineate the underlying mechanisms, O'Melia et al. [128] designed a suite of nanoscale biomaterial tools to track and quantify the Ag access and presentation within LNs, thereby optimizing antitumor CD8+ T cell responses [121]. They found that in the melanoma context, the extent of Ag presentation by dLNs-resident APCs remained unchanged despite the sustained access of lymph-draining Ag while the presentation of cell-transported Ag was increased, which was partially caused by the phenotypes of DCs accessed via different lymphatic transport mechanisms. Specifically, passively drained Ag was presented mainly by pDCs and cDCs that displayed an immunosuppressive phenotype. In contrast, actively transported Ag was presented by dDCs and LCs that exhibited an immunepotentiating phenotype. However, the complex communication among different cells, especially the intercellular antigen transfer, is still incompletely understood. More detailed discussion of intra-SLOs antigen transfer can be found at the following chapters (i.e., 5.1 From APCs to APCs).
Generally, antigen can be transferred within the SLOs in multiple forms, including Ag protein/peptide, Ag-encoding nucleic acid, functional p-MHC, immune complex, and vaccine particle. More importantly, the form of Ag may affect the mode and even the immunological consequence of Ag transfer. In SLOs, LNs in particular, exogenous Ag or Ag fragments (i.e., antigenic complexes, protein and peptide) transferred to DCs by trogocytosis, EVs, TNTs or gap junctions can be canonically presented on the MHC class II molecules to activate specific CD4+ T cells [3, 62, 129] or cross-presented via the MHC class I molecule-restricted pathway to initiate specific CD8+ T cells [63]. Meanwhile, Ag-encoding nucleic acids (e.g., mRNA, pDNA) transferred to DCs, probably through EVs, TNTs or gap junctions, can be translated into “endogenous Ag” and then preferentially presented on the MHC class I molecules or undergo Ag translocation to the endosomes for MHC class II-favored cross presentation [6, 7, 130, 131]. Besides, functional p-MHC I and/or p-MHC II can be transferred to DCs mainly through trogocytosis and EVs, which facilitates an efficient elicitation and magnification of T-cell responses [49, 132-134].
Intestine
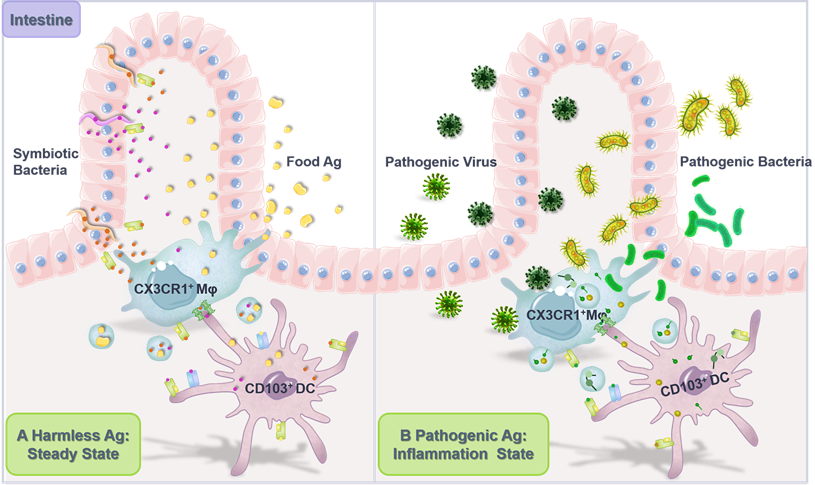
Chronically exposed to both innocuous and pathogenic Ags, intestine constitutes the largest and most complex part of the immune system where acquired oral tolerance to harmless dietary proteins and commensal bacteria is established while specific immune response against pathogenic microbes can be elicited [135]. It is increasingly recognized that in the intestine, antigen transfer among phagocytes with specialized functions [136] plays a vital role in mediating the balance between tolerance (Figure 2A) and protective immunity (Figure 2B).
Intestinal APCs, especially DCs, are in dispensable for triggering peripheral Foxp3+ Tregs polarization from naïve T cells and inducing oral tolerance [137, 138]. And default responses to harmless Ags may otherwise lead to food allergies, inflammatory bowel disease, and even colorectal cancer [139, 140]. Mazzini et al. [52] found that soluble food Ags are internalized by gut-resident CX3CR1+ macrophages and quickly transferred to migratory CD103+ DCs in a Cx43-dependent and plasma membrane-required manner (i.e., through gap junction), which consequently promoted Treg differentiation and induced oral tolerance to these Ags. Meanwhile, McDole et al. [141] suggested that in steady state, goblet cells in the epithelium of small intestine transported low molecular weight soluble Ags from the intestinal lumen to tolerogenic CD103+ DCs in the lamina propria to promote intestinal immune homeostasis. However, the underlying mechanisms accounting for such Ag transfer from goblet cells to DCs remain to be fully elucidated. Segmented filamentous bacteria (SFB) and other intestinal resident commensal bacteria adhere tightly to intestinal epithelial cells (IECs) via hook-like structures, and Ag proteins from these bacteria can be transferred into the cytosol of IECs through adhesion-directed endocytosis to affect host T cell homeostasis [142]. Specifically, at the tip of the SFB-IEC synapse, SFB generates endocytic vesicles containing microbial cell wall-associated proteins, including an Ag that induces mucosal T helper type 17 (Th17) cell differentiation, to be acquired by host IECs for elicitation of specific T cell responses.
On the other hand, intercellular antigen transfer might also occur in the context of gastrointestinal infections that consequently induces protective immune defense against potentially pathogenic Ags [53, 54]. In the rectal mucosal biopsies of patients with acute campylobacter colitis or cholera, mononuclear phagocytic cells (mainly macrophages and DCs) in the superficial rectal mucosa exhibit a higher prevalence of ultrastructural features of activation. Macrophages are found to actively insert pseudopodia through intestinal epithelial cell gaps to capture pathogenic Ag, while DCs that are superior in Ag presentation and T cell activation display active membrane processes, enhanced macropinocytosis and elevated phagosomal/lysosomal activity [143], indicating that macrophages and DCs might share antigenic information within the intestine through multiple pathways to coordinate the anti-infection immune responses.
Antigen transfer in the intestine. (A) Intercellular transfer of harmless Ag establishes intestinal homeostasis. Gut-resident CX3CR1+ macrophages (Mφ) continuously sample the gut lumen for harmless soluble Ag, including Ag from dietary proteins and commensal bacteria. Subsequently, Mφ captured Ag is transferred to intestinal migratory CD103+ DCs, which then migrate back to the dLNs to induce T cell tolerance, establishing intestinal flora homeostasis and preventing food allergy. (B) Intercellular transfer of pathogenic Ag induces pro-inflammatory responses against infection. Upon intestinal invasion of pathogenic bacteria and viruses, CX3CR1+ Mφ collect potentially pathogenic Ag from infected intestinal tissue cells or directly from the pathogen, which was further transferred to CD103+ DCs through gap junctions and EVs for presentation and T cell activation, inducing specific protective immune responses.
Allergic sites
Allergy, also termed as allergic disease or anaphylactic reaction, refers to hypersensitivity of the immune system in response to the exposure of typically harmless Ags. To date, mounting evidences have suggested that intercellular transfer of immunoreactive substance or Ag is closely associated with the development of exaggerated immune response to allergens such as pollens, dust mites, furry animal dander, drugs and foods.
Mast cells (MCs) are well recognized as key effector cells of allergic reactions, which respond to endogenous or exogenous danger signals by secreting a plethora of mediators including histamine, proteases and cytokines in the form of mast cell granules (MCGs) that can be released by degranulation within seconds on activation to initiate immune responses, neutrophil recruitment and allergen clearance. On skin inflammation, MCs-exocytosed intact MCGs are engulfed by and degraded within dermal DCs to promote DC maturation and migration to the dLNs for subsequent T cell priming [144]. In turn, it is reported that CD301b+ perivascular DCs continuously sample the blood and relay Ag to neighboring MCs and other DCs through an active discharge of surface-associated Ags on microvesicles (MVs) generated by vacuolar protein sorting 4 (VPS4) to potentiate inflammation and anaphylaxis against blood-borne Ags [115]. Moreover, in the case of allergic asthma and atopic dermatitis (AD), the interplay between tissue structural cells and DCs is largely responsible for CD4+ T helper type 2 (Th2) cell-induced dysregulated type 2 inflammation (Th2 sensitization) to environmental allergens [145]. For instance, when exposed to house dust mite (HDM), airway epithelial cells generate danger-associated molecular patterns (DAMPs), chemokines and cytokines to recruit, activate and skew DCs toward Th2 phenotype that promotes the pulmonary inflammatory reactions. Whereas skin KCs recognize HDM through Toll-like receptors (TLRs) and produce type 2 immune cytokines to activate cDC2 subsets and induce their migration to the dLNs for elicitation of Th2 response. Moreover, individuals with autoimmune diseases, such as systemic lupus erythematosus (SLE) that produces systemic inflammation in multiple organs, have platelets that continuously recruit and release mitochondrial DNA (mtDNA) as a source of circulating autoantigen to exacerbate the self-attack of immune system [146].
Allografts
Similar to that of allergy, the rapid acquisition of antigenic information from allograft by host APCs induces severe immune rejection and graft organ necrosis. During allogeneic organ transplantation, host DCs rapidly integrate intact donor p-MHC class I complexes through cross dressing or uptake and process donor Ags into allopeptides bound to self-MHC molecules, which induces massive proliferation of reactive T cells and leads to graft rejection [147, 148]. In addition, donor DCs migrated from the graft to the SLOs may release EVs to facilitate an efficient passage of donor MHC molecules to host cDCs, which triggers full activation of alloreactive T cells and impedes graft survival [107]. On the other hand, DCs in successfully transplanted patients undergo continuous transfer of p-MHCs from donor DCs and/or donor somatic cells to DCs, and these MHC-dressed DCs may induce immune tolerance to benefit a long-term graft survival by upregulating their own programmed death-ligand 1 (PD-L1) [149].
In order to minimize the Ag transfer-associated graft rejection, Borges et al. [150] incubated skin grafts with the anti-inflammatory mycobacterial protein DnaK, which promoted a March 1-dependent reduction of MHC class II molecules on donor CD103+ DCs, thereby inhibiting the transfer of p-MHCs to recipient DCs and prolonging the survival of transplanted skin. Meanwhile, Zhang et al. [151] used CRISPR/Cas9 to ablate costimulatory CD40 at the genomic level in DCs dressed with donor p-MHCs to inhibit their maturation and LNs-homing, which not only induced long-term graft tolerance but also prevented severe immunosuppressive side effects.
Pathological lesions
Antigen transfer at the lesions (e.g., sites under physical damage, chemical stimulation, ultraviolet irradiation, pathogen infection and tumorigenesis) may serve as a critical line of immune defense. For example, in human skin models and genital herpes lesion biopsies, HSV is first taken up by LCs that patrol over the epidermis. Subsequently, HSV-loaded LCs migrate to the dermis and transfer HSV Ag to CD103+ cDCs with a superior antigen-presenting ability and more motivated LNs-homing for initiation of immune response (passive Ag transfer, as HSV-infected LCs undergo apoptosis to be further taken up by dermal DCs) [152, 153]. Notably, cDC1s that feature high expression of C-type lectin-like receptor 9A (CLEC9A) are capable of binding dead-cell debris and promoting the cross presentation of corpse-associated Ags, which facilitates their relay of Ag from the donor cells or directly from the pathogens [154, 155]. Moreover, during skin inflammation, an intensive and long-lasting synapse-like contact between migratory DCs and stationary MCs culminates in the functional transfer of DC-restricted proteins to MCs, including MHC class II complexes, which may ensure the host defense during DC migration to the dLNs or critical periods of migration-based DC absence [156]. In the context of tumorigenesis, p-MHC class I complexes and other membrane structures containing the “non-self” Ags that presented on the surface of tumor cells can be directly transferred to DCs via trogocytosis [44], while intracellular Ags can be transmitted to DCs through exosomes [157]. Squadrit et al. [57] reported a lentivirus-encoded chimeric receptor named extracellular vesicle-internalizing receptor (EVIR) to facilitate the specific and efficient uptake of cancer cell-derived EVs by DCs, which exploited the cross dressing of pre-formed p-MHC class I complexes for improved activation of specific T cell responses against tumor.
However, as aforementioned, some immunosuppressive molecules might also be transferred to immune cells through trogocytosis, gap junctions, TNTs and EVs to modulate immune responses and promote disease progression [50, 51, 74]. For example, natural killer (NK) cells acquire carcinoembryonic antigen (CEA) from the surface of CEA-expressing cells via trogocytosis and exhibit inhibited cytolytic activity and dampened degranulation function [158]; T cells exposed to tumor-derived exosomes incorporating PD-L1 display suppressed activation in the dLNs [159]; and TNT-connected astrocytoma cells may promote tumor progression and resistance to therapy [160].
Vaccine injection sites
Prophylactic and therapeutic vaccines are generally administrated into the intramuscular, subcutaneous or intradermal compartments. Different physiological sites differ in the cell type, cell abundance and lymphatic system. Therefore, the site of vaccine inoculation may affect the efficacy of Ag transfer as well as the strength and duration of immune response [2].
For example, upon intramuscularly injection, self-amplifying mRNAs (SAM®)-encoded Ag is expressed by muscle cells and then transferred to nearby APCs, which consequently promotes the activation the CD8+ T-cell responses [33]. In addition, mRNA-based vaccine is taken up by both immune and non-immune cells in the skin upon intradermal administration [32]. Functional Ag may be expressed by these vaccinated non-immune cells and then transferred to APCs to promote the induction of adaptive immunity. Moreover, studies suggest that many keratinocyte-specific molecules can be transferred to epidermal-resident LCs as mRNA and protein probably via TNTs or dendrites [161, 162].
Administrated vaccine antigen internalized by non-APCs at the injection site can be transferred to tissue-resident APCs or migratory DCs. Meanwhile, migratory DCs may migrate towards the draining SLOs and transfer both directly captured and indirectly acquired (transferred from non-APCs) Ag to LN-resident or splenic DCs. Detailed information will be discussed in the following sections.
Participant of antigen transfer
The phenomenon of antigen transfer was initially identified in T cell activation. In fact, within minutes of cognate T cells interacting with APCs, p-MHCs on the surface of APCs form clusters at the site of T cell contact. Subsequently, clusters containing p-MHCs are internalized by T cells via TCR-mediated trogocytosis. As a result, T cells are subjected to the Ag-specific cytolysis by neighboring T cells (termed “fratricide”), which may lead to suppressed T cell immunity [163]. Meanwhile, T cells may also acquire p-MHCs from other target cells through contact-dependent immunological synapses, and Tregs are especially adept at removing MHC class II and costimulatory molecules from APCs via trogocytosis to induce immune tolerance [164]. In addition to T cell-based Ag receptors, NK cells [165, 166] and basophils [167] can also acquire Ag from APCs, thereby impacting the potency, durability, and even consequence of immune responses.
Generally, antigen transfer is a reciprocal interaction that theoretically can occur between any cells with active membrane mobility, including that from APCs to APCs, from non-APCs to APCs, from APCs to non-APCs, and even from non-APCs to non-APCs. In this review, we focus on DCs-based Ag receptors and the associated immunological outcomes (Table 1).
Antigen transfer with APCs-based receptors
| Donor cell | Acceptor cell | Pathway | Ag form | Location | Immunological outcome | Ref. |
|---|---|---|---|---|---|---|
| APCs to APCs | ||||||
| Migratory cDC1 | LNs-resident cDC1/2 | TNTs, EVs, trogocytosis, gap junctions | p-MHC I/II | LNs | Initiate anti-tumor immune response | [1, 48, 55, 134] |
| pDCs | cDC1 | EVs | antigen protein/peptide, or p-MHC I | LNs | Cross prime CD8+ T cells and induce durable immunity | [132, 133] |
| B cells and FDCs, respectively | FDCs and B cells, respectively | EVs | p-MHC II | Follicle | Immunocomplexes deposit on FDCs and cognitive B cells differentiation | [183, 184] |
| LCs | Dermal cDCs | EVs, trogocytosis, gap junctions, TNTs | Processed Ag and intact p-MHCs | Skin | Induce immune defense against HSV | [152] |
| B cells | mo-DCs | Possibly by EVs, trogocytosis, gap junctions, TNTs | Processed Ag and intact p-MHC II | / | Mo-DCs obtain processed Ag to activate T cells | [280] |
| Macrophages | DCs | Gap junctions | Dietary Ag | Intestine | Establish oral tolerance | [52, 206] |
| Macrophages | DCs | Gap junctions, EVs | Ingested or processed Ag | Intestine and skin | Resist the infection by Mycobacterium, Salmonella, Listeria and other pathogens | [176, 178, 211] |
| Macrophages | B cells | Possibly by gap junctions, TNTs | p-MHCs | Lymphoid follicles | Initiate the early activation of cognate B cells | [183] |
| B cells | B220+ Macrophages | EVs | Processed Ag fragments or Ag particles | Peritoneum | Macrophages acquire the ability to activate CD4+ T cells | [187] |
| cDCs | B cells | Possibly by gap junctions, TNTs | Processed Ag fragments, Ag particles and intact p-MHC II | Lymphoid follicles | Activate cognate B cells | [180, 185] |
| Non-APCs to APCs | ||||||
| Gene edited 4T1/B16 tumor cells with high expression of MHC I/II | Tumor infiltrating cDC1 | Possibly by EVs, trogocytosis, gap junctions, TNTs | p-MHC I/II | Tumor site | Activate tumor specific CD4+ T cells | [189] |
| Fibrosarcoma tumor cells | cDC2 | Possibly by EVs, trogocytosis, gap junctions, TNTs | p-MHC I | Tumor site | Promote antitumor CD8+ T cell immunity | [190] |
| Melanoma cells and epithelial cells near the colorectal tumor | pDCs | Possibly by EVs, trogocytosis, gap junctions, TNTs | p-MHC I | Tumor site | Compensate the poor cross presentation and phagocytic ability of pDCs | [191] |
| Tumor cells and commensal bacteria, respectively | Intestinal commensal bacteria and DCs, respectively | Possibly by EVs, TNTs, trogocytosis | p-MHC I | Tumor site, intestine | Upregulate reactive IFN-γ+ T cells and sensitize immune checkpoint blockade efficacy | [202-205] |
| UVB irradiated mutate melanocytes | Skin-resident DCs and tumor infiltrating DCs | Possibly by EVs, trogocytosis, gap junctions, TNTs | Possibly p-MHC I | Tumor site, mutated skin | Promote the cure rate of malignant melanoma | [281] |
| HCV or HCV infected hepatocytes | pDCs | Contact-dependent gap junctions, TNTs, EVs | HCV RNA | HCV infected liver | Triger TLR 7 activation induced type-I IFN release by pDCs to inhibit HCV infection | [192, 193] |
| KCs | Multiple DCs subsets in skin and LNs | Possibly by EVs, trogocytosis, gap junctions, TNTs | Ag-encoding mRNA and protein | Vaccine injection site and draining LNs | Induce an enhanced immune response without immune cell depletion upon repeated inoculation of mRNA vaccine | [32] |
| Muscle cells | Mo-DCs | Possibly by trogocytosis, gap junctions, TNTs | mRNA transfected Ag fragments and/or p-MHC I | Vaccine injection site | Elicit potent Ag-specific CD8+ T cell immune responses | [33] |
| KCs | LCs | TNTs | Ag-encoding mRNA and protein | Vaccine injection site | Promote vaccine effect | [161, 162] |
| Symbiotic bacteria and IECs | IECs, macrophages, and DCs | Gap junctions, EVs | Ag fragments | Intestine | Maintain intestinal homeostasis | [136, 142, 282] |
| mTECs | Thymus-resident CD8α+ DCs | EVs | p-MHC I/II | Thymus | Establish central tolerance | [115, 122] |
| Graft cells | DCs in organ recipients | Trogocytosis, EVs | p-MHC I | Transplanted organ | Induce activation and proliferation of allergen-reactive T cells | [107, 147, 148] |
| Mast cells | DCs | EVs | Possibly ingested and/or processed Ag fragments, Ag particles, and intact p-MHC II | Near the allergic site | Induce acute inflammatory injury, such as severe vascular leakage, at the allergic sites | [144] |
| Epithelial cells | DCs | Possibly by EVs, trogocytosis, gap junctions, TNTs | Possibly ingested and/or processed Ag fragments, Ag particles, and intact p-MHC II | Allergic skin | Cause allergen-associated Th2 immune responses | [145] |
| Platelets | DCs, Macrophages | EVs | Mitochondria DNA and multiple autoantigens | Kidney | Aggravate systemic lupus erythematosus | [146] |
From APCs to APCs
It is widely recognized that DCs, especially cDCs, are indispensable coordinators of the adaptive immunity, yet elicitation of specific immune response may not rely solely on the direct antigenic stimulation on DCs. Accumulating evidence suggests that Ag or Ag complex can be transferred from other types or individuals of APCs to DCs [168] (Figure 3), contributing to an improved availability of Ag that mobilizes the immune system with higher efficiency.
DCs and DCs
S.L. Nutt et al. [79] have summarized that heterogeneous DCs subpopulations are closely associated with each other in the systemic immune network despite distinct developmental, locational, phenotypical and functional hallmarks, which constitutes the immunological basis of T cell activation and tolerance [169, 170].
It is reported that in response to CD40L-expressing Th cells or recombinant CD40L, networks of TNTs are induced by DC1 (i.e., DCs matured in the presence of inflammatory mediators of type-1 immunity) to support the direct intercellular transfer of endosome-associated vesicles and Ag between DCs [55]. Aline et al. [171] demonstrated that DCs-derived exosomes encompassing functional MHC class I/II and costimulatory molecules were capable of inducing protective immunity against toxoplasmosis, serving as a novel cell-free vaccine. Specifically, part of the adoptively transferred Toxoplasma gondii-pulsed DC-derived exosomes accumulated in the spleen and were most likely internalized by spleen-resident CD8α+ DCs, which elicited a strong systemic T helper type 1 (Th1)-biased specific immune response. In addition, protein antigens in DCs-derived exosomes can be transferred to and presented by recipient DCs to induce the activation of allogeneic T cells, which may be used to facilitate cancer immunotherapy [172, 173]. On the other hand, inflammatory signals induce the LNs-homing of migratory cDC1 and its subsequent Ag transfer to LNs-resident DCs through tight synaptic interaction [15], which facilitates the accumulation of Ag in LNs-resident DCs for activation of specific effector CD8+ T cells [1]. pDCs, formerly known as natural interferon producing cells (NIPCs), are the main producers of type I Interferon (IFN) [18] and play a key role in antiviral immunity. Although the capability of pDCs to generate in vivo cross-primed CD8+ T cells remains controversial, they have been shown to transfer antigen (possibly Ag protein, peptide, or p-MHC I [133]) to the bystander cDCs via EVs, which leads to efficient cross-priming of naive CD8+ T cells and induction of durable immunity. Notably, although both cDC1s and cDC2s are capable of acquiring Ag from pDCs, cDC1s, instead of cDC2s, are required for CTL activation upon pDCs-targeted vaccination [132]. Furthermore, monocytes loaded with protein or peptide antigen can transfer Ag to splenic DCs through cell-cell contact and the formation of Cx43-containing gap junctions, which leads to efficient activation of CTLs and potent antitumor responses [174].
Macrophages and DCs
With intricated membrane structures and dynamic membrane activities, macrophages and DCs are closely associated in the context of antigen transfer (Figure 2). Although macrophages prevail in phagocytosis, their ability of Ag cross presentation is far inferior than that of DCs. However, studies suggest that there exists a complicated interplay between macrophages and DCs in the process and presentation of Ag. For instance, upon dead cell accumulation in vivo, macrophages transfer phagocytosed Ag to DCs via exosomes for potent antigen presentation and efficient T‐cell activation [181]. In addition, despite inefficient cellular uptake of Listeria monocytogenes (Lm), DCs are capable of taking up microparticles (MPs) released by Lm-infected macrophages. These MPs transport Lm Ag to DCs for presentation, propagating DC-elicited protective immunity against Lm infection [175]. Similarly, macrophages act as transmitters to convey Ag for presentation by DCs in response to the invasion of other pathogens such as mycobacterium [176, 177] and salmonella [178]. Moreover, it is found that infected macrophages secrete EVs containing Cdc42 (a protein responsible for increased cellular endocytic activity) to enhance the cellular uptake of recipient cells [179], which may further facilitate the antigenic cross-talk between macrophages and DCs.
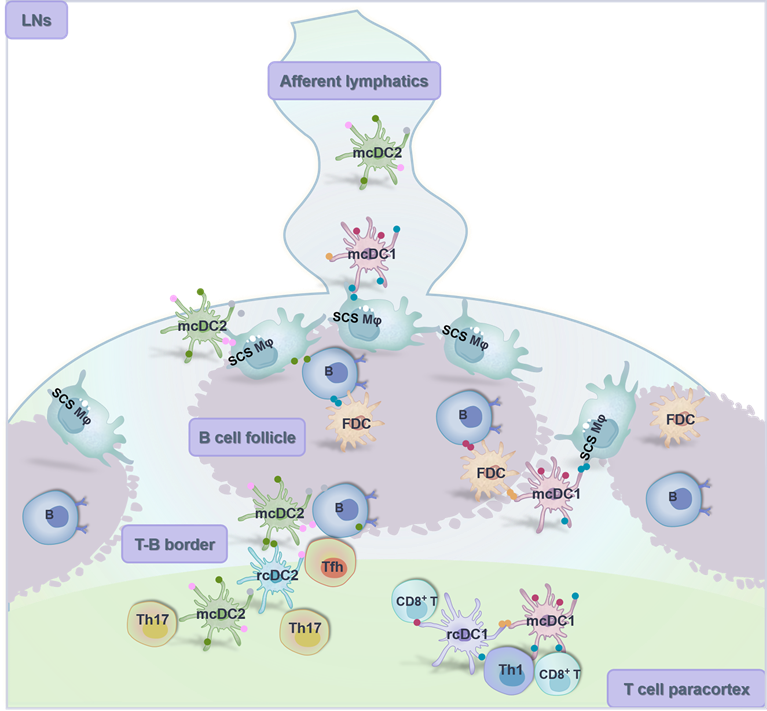
Close intercommunication among B cells, macrophages and DCs in the LNs. Peripheral migratory conventional DC1 and DC2 (i.e., mcDC1 and mcDC2) move back to the draining LNs via afferent lymphatics and transfer Ag (including viruses, particulate Ag and immune complexes) to CD169+ subcapsular sinus (SCS) macrophages that line the follicle-proximal side of the SCS. Then, these SCS macrophages display Ag to follicular B cells via cellular protrusions, and follicular DCs (FDCs) located therein engage in reciprocal Ag sharing with B cells for elicitation of germinal center reactions. Afterwards, mcDC1 and mcDC2 pass through the T-B boundary and enter the T cell-localized medullary zone to share Ag with resident conventional DCs (rcDCs) for efficient activation of immune responses.
B cells, macrophages and DCs
A successful elicitation of the humoral immunity depends primarily on the close antigenic interaction among B cells, macrophages and DCs. In both LNs and spleen, the maturation and native antigen presentation of B cells requires the support from follicular dendritic cells (FDCs) and CD169+ subcapsular sinus (SCS) macrophages [180]. Specifically, at the T-B border, SCS macrophages display Ag including processed viral particles, vaccine particles and immune complexes [181, 182] to both cognate and non-cognate B cells via TNTs-like cellular protrusions that extend into follicles. SCS macrophages-mediated Ag recognition by cognate B cells through B cell antigen receptors (BCRs) initiates the early activation and the subsequent migration to T-B border of B cells [183]. Meanwhile, immune complexes are transferred from SCS macrophages to non-cognate B cells, and then ferried into the follicle for deposition on FDCs [183, 184]. Then, FDCs retain native Ag for prolonged presentation to B cells that evokes the germinal center reactions and promotes the maturation of effector and memory B cells. Notably, evidence suggests that in the lymphoid germinal center, direct intercellular communication through gap junctions is involved in FDCs-FDCs and FDCs-B cells interaction, in which multiple signal molecules and Ag fragments/complexes can be shared [89]. On the other hand, there is mounting evidence that both migratory and resident cDCs may encounter cognate B cells at the T-B border and contribute to their early initiation [180, 185]. Besides, Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) signaling on DCs promotes B cell differentiation into class-switched plasmablasts and facilitates their exit from germinal center and migration towards local mucosa and skin [186]. Furthermore, result illustrates that Ag acquired by B cells through BCRs can be specifically transferred to B220+ macrophages through direct cell-cell contact, which enables the macrophages to activate CD4+ T cells [187].
Antigen transfer from non-APCs to APCs in the context of anti-cancer/-infection immunity. (A) Tumor cells serve as the largest Ag reservoir for migratory conventional DCs (mcDCs), plasmacytoid DCs (pDCs) and macrophages. Ag is transferred from tumor cells to these APCs through both contact dependent (i.e., trogocytosis, gap junctions and TNTs) and independent (i.e., EVs) pathways, leading to activation and expansion of T cells in situ or in the dLNs. (B) Hepatitis C virus (HCV)-infected parenchymal hepatocytes release virus RNA and protein Ag through both contact dependent and independent pathways for activation of Kupffer cells and pDCs against virus invasion. TAAs, tumor associated antigens; TSAs, tumor specific antigens.
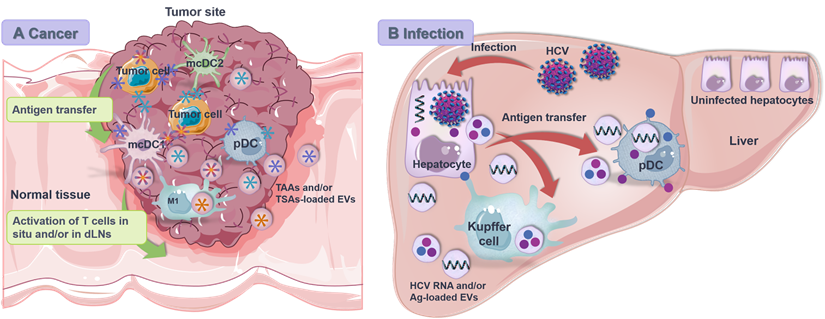
From non-APCs to APCs
Given the homologous expression of MHC class I molecules by all nucleated cells, non-APCs can also serve as Ag donor cells to APCs, especially to DCs, including malignant/transformed cells, vaccinated muscle cells and KCs, and even harmless commensal bacteria. Antigen transfer from non-APCs to APCs may promote the immune response against the “non-self” or facilitate the spread of invasive pathogens.
Tumor cells and DCs
Tumor infiltrating DCs assume different functional states that affect the antigen transfer and overall antitumor immunity (Figure 4A). In the TME, interferon regulatory factor 8 (IRF8)-dependent CD103+ cDC1 (CD141+ cDC1 in human) are the only APCs that can cross present tumor rejection Ags for activation of specific CTLs [23], which are sparsely distributed and frequently threatened by the hostile immunosuppressive environment [188]. In this situation, antigen transfer, especially cross dressing (i.e., p-MHCs transfer), from tumor cells to cDC1s stands as an efficient means of Ag presentation and reactive T cell activation [44, 189]. On the other hand, cDC2 is developmentally driven by interferon regulatory factor 4 (IRF4) and highly specialized in MHC II-restricted presentation [29]. Recently, Duong et al. [190] investigated the transcriptional profiles of intra-tumoral DCs within regressor tumors and identified an activation state of CD11b+ cDC2 with interferon-stimulated gene signatures. Stimulated by exogenous IFN-β, these cDC2 acquired and presented intact tumor-derived p-MHC class I complexes to induce CD8+ T cell-involved antitumor immunity against progressor tumors in mice lacking cDC1 [188, 190]. Moreover, Bonaccorsi et al. [191] identified that pDCs, although inefficient in internalizing cell membrane fragments by phagocytosis, were able to acquire membrane patches and associated molecules from cancer cells of different histotypes in a cell-to-cell contact-dependent manner that closely resembled “trogocytosis”. As a result, tumor cell-derived Ag was displayed by pDCs and recognized by specific CD8+ T cells to promote anti-tumor cellular immune response.
Virus infected cells and DCs
During hepatitis C virus (HCV) infection, exosomes mediate the intercellular transfer of immunostimulatory HCV RNA from infected cells to neighboring non-infectible pDCs to trigger the generation of type I IFN [192] (Figure 4B). Both HCV-infected cells and purified HCV RNA-packaged exosomes are sufficient to activate pDCs without infecting them. Notably, the exosomal viral RNA transfer is dependent on active viral replication, direct cell-cell contact and TLR 7 signaling [193]. Nevertheless, such exosome-mediated transfer of viral RNA may enhance virus clearance by activating Kupffer cells and pDCs or promote virus infection by delivering infectious viral genomes to cells that are permissive for viral replication.
Vaccinated somatic cells and DCs
To improve the efficacy of protein- or nucleic acid- based vaccines, substantial efforts have been paid to promote the site-specific accumulation of vaccine components in SLOs, and even in APCs [194-199], which increases the availability of vaccine Ag by DCs to amplify specific immune response and establish a durable memory. However, a targeted delivery of vaccine preparation proposes great demands for its physiochemical properties (e.g., particle size, potential and surface modification) and route of administration [200, 201]. Moreover, compared to professional APCs that have a limited cell abundance in different vaccination sites, somatic cells with larger quantity and widespread distribution display higher competence in messenger RNA (mRNA)-transfection and protein-uptake, which may impact the magnitude and duration of specific immunoresponse by transferring Ag to nearby APCs (specific mechanisms of Ag transfer need to be further identified) [2]. Indeed, most somatic cells are biologically equipped with abundant cytoplasmic free ribosomes (such as KCs and muscle cells) or rough endoplasmic reticulum-attached ribosomes (such as hepatocytes and fibroblasts) to support their antigenic communication with surrounding DCs [33]. For example, KCs actively transfer Ag, including Ag-encoding mRNA [161] and protein [162], to the skin-resident LCs mainly in a contact-dependent fashion, impacting the efficacy and safety of transdermal- and intramuscular- injected vaccines.
Commensal bacteria and DCs
It is reported that several bacteria participate in tumor immunosurveillance and antitumor immune response. Rong et al. [202] studied the bacteria-reactive CD8+ T cell response in HBV-associated hepatocellular carcinoma patients and found that circulating CD8+ T cells displayed remarkable enhanced immune responses against a series of commensals and bacteria, including Escherichia coli (E. coli), Enterococcus faecium, Bifidobacterium longum, Bacteroides fragilis, and Enterococcus hirae. And the ratio of CD8+ T cell-to-Foxp3+ Treg was positively correlated with the proportion of Bifidobacterium longum-reactive and Enterococcus hirae-specific CD8+ T cells, whereas the frequency of PD-1+ CD8+ T cells was negatively correlated with the frequency of Enterococcus hirae-specific CD8+ T cells. Moreover, these bacteria-reactive responses were MHC class I-restricted and dependent on the presence of APCs, indicating that certain commensal bacteria might act as Ag mediators between cancer cells and APCs to increase the proportion and viability of tumor-reactive IFNγ+ T cells [202], which is also observed in MC38 colon cancer, MCA-205 sarcoma and RET melanoma [203-205].
Vaccine effect of antigen transfer: immune amplification or tolerance
Antigen transfer plays an important role in coordinating immune amplification and tolerance. When the receptor cells are tolerogenic DCs, immature DCs, some pDCs and even certain types of non-APCs [110, 138, 165, 206-208], antigen transfer may promote the expansion of immunosuppressive Tregs/MDSCs and even induce the apoptosis of specific T cells, leading to tolerance [47]. Immune tolerance is fundamental to the maintenance of homeostasis. For example, antigen transfer from mTECs to DCs in the thymus enables the deletion of self-reactive T cells and promotes the establishment of central tolerance [118-120]. In patients with autoimmune diseases, harmless Ag (e.g., autoantigens and dietary proteins) are recognized as pathogenic Ag, which consequently causes local/systemic inflammatory responses that are harmful and even fatal. In this situation, antigen transfer that induces tolerance to specific Ag may limit autoimmune responses and help restore homeostasis [146, 209, 210]. On the other hand, antigen transfer to mature APCs, especially DCs, may facilitate the access and presentation of Ag that contributes to a more efficient and versatile elicitation of the adaptive immunity [1, 48, 55, 134, 190, 211], which is frequently used to enhance the preventive and therapeutic effects of vaccines [26].
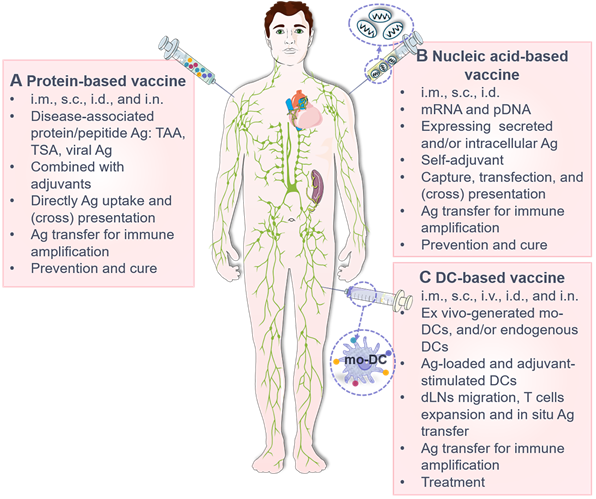
Vaccines are powerful weapons against pathogenic evasion [26] and tumor progression [212-216], which elicit specific T/B lymphocyte-mediated effector and memory immune responses upon single or repeated inoculation. Considering the efficacy and biosafety, most recently licensed vaccines are typically protein/peptide-based subunit vaccines that usually used in combination with adjuvants, nucleic acid-based vaccines (especially mRNA vaccine) [217-219], and DCs-based vaccines [220, 221]. And transcutaneous local injection is the most applied route of administration for these vaccines [222], including:
1) Intradermal injection (i.d.), which is most frequently used for the inoculation of bacillus Calmette-Guérin (BCG), rabies and smallpox vaccines [223] for its little invasiveness, avoided drug degradation in the gastrointestinal tract, and escaped hepatic first-pass effect. Vertebrate skin comprises epidermis and dermis. Epidermis is composed of abundant KCs and few melanocytes and LCs. In contrast, dermis is rich in collagen and elastin fibers but low in cell density. Dermal APCs (such as cDCs, mo-DCs, LCs and macrophages) [224] and lymphatic system facilitate a quick and effective initiation of immune response, conferring dermis a highly immunocompetent site for vaccine delivery [225, 226].
2) Subcutaneous injection (s.c.), that is most suitable for the administration of live-attenuated vaccines against polio, measles, mumps, rubella and yellow fever. Subcutaneous compartments incorporate blood vessels, nerves, loose connective tissue and adipose tissues, where fibroblast, mast cell and macrophage are most abundant. Subcutaneous drainage system is underdeveloped, which prolongs the in-situ Ag dwelling and serves as Ag reservoirs.
3) Intramuscular injection (i.m.). As the most commonly used route of delivery for licensed vaccine, especially inactivated vaccines against hepatitis A/B (HepA/B), HPV, influenza, and the currently prevalent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), i.m. is easy to perform and generally well tolerated, with a low risk for adverse reactions [212-214, 227]. Muscle tissue is composed primarily of myocytes, with few APCs, blood vessels and nerves. Therefore, higher dosage, adjuvant-incorporation, and multiple administration is usually recommended for eliciting an expected immunoprotection [228].
In addition, intravenous (i.v.) and intranodal (i.n.) injection have also been studied [229]. However, their feasibility and safety needs to be further optimized before putting into clinical use [230]. It's worth noting that a long-term persistence of immunogens/immunomodulators or sustained expression of vaccine products was observed at the site of delivery following i.d., s.c., and i.m. (superficial injection). Meanwhile, upon i.v., i.m. (deep injection), and intraperitoneal injection (i.p.), significant antigenic signal was detected in the liver early post administration [231, 232], suggesting that the biodistribution of vaccine is route-dependent, and liver may be an important anatomical compartment for mounting immunoreactions.
Antigen transfer takes place after vaccine inoculation, which is primarily grouped into the following categories according to the type and tissue distribution of vaccine (Table 2): 1) Antigen (such as Ag-encoding nucleic acids, Ag peptides/fragments, intact Ag proteins, particulate Ag, immune complex and functional p-MHCs) transfer from vaccinated APCs and/or non-APCs to neighboring DCs at site of administration in the context of protein/peptide-based vaccines (Figure 5A) and nucleic acid-based vaccines (Figure 5B); 2) Antigen transfer from Ag-pulsed DCs to nearby APCs including LCs, cDCs and macrophages at vaccine inoculation site in terms of DCs-based vaccines (Figure 5C); and 3) Antigen sharing from Ag-laden DCs to SCS macrophages, B cells, FDCs and cDCs at the dLNs to activate germinal center reactions.
Protein-based vaccines
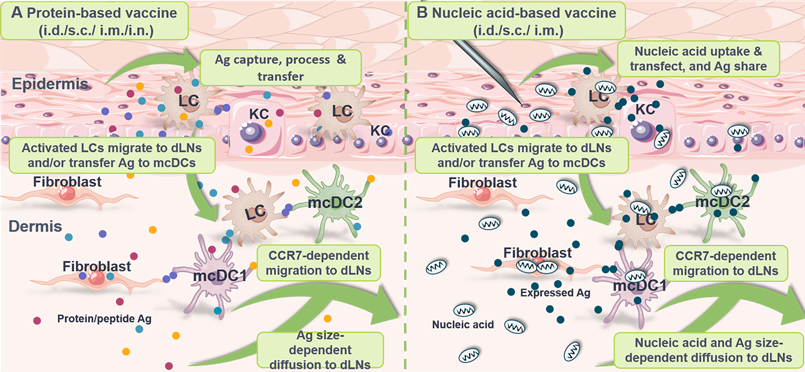
Increasing studies suggest that the efficiency of T cell-mediated adaptive immunity against peripheral infections and particulate vaccine systems (such as nanoparticles, microparticles and adjuvant-formulated proteins) depends heavily on the ability of LNs-homing and Ag presentation by peripheral DCs [233]. In addition, it seems that most soluble Ags cannot penetrate into the paracortex and cortex of LNs, which directly limits the Ag accessibility of LNs-resident DCs [237, 238]. At the same time, anatomic studies indicate that Ag diffused into the LNs in a size-dependent manner seems to accumulate only at the proximal ends near the afferent lymphatic vessels, whereas Ag carried by migratory DCs penetrates deep into the medullary zone [128]. On the other hand, vaccine Ag transferred from vaccinated muscle cells, KCs, fibroblasts and other tissue cells to skin-resident DCs in the epidermis and dermis is reported to facilitate a durable immune response under limited dosage of vaccine inoculation [32, 33] (Figure 6A). Therefore, antigen transfer to DCs is of physiological and clinical significance.
Antigen transfer and its immunological effects by different types of vaccine
| Vaccine type | Vaccine component | Administration route | Major Ag donor cells | Major Ag receptor cells | Immunological outcome | Ref. |
|---|---|---|---|---|---|---|
| Protein-based vaccine | ||||||
| Protein | Multivalent HPV protein Ag and adjuvant AS04 | i.m. | Muscle cells and skin-resident DCs, respectively | Skin-resident DCs and LNs-resident DCs, respectively | Prevent HPV induced infections and cancers | [212-214] |
| Protein | 5-20 recombinant/fusion tumor neoantigens | s.c. | KCs and skin-resident DCs, respectively | Skin-resident DCs and LNs-resident DCs, respectively | 71.4 % of cancer patients are under control with specific CTL response elicited | [236] |
| Protein | TAAs (HER-2) and immunostimulatory molecules modified plasma membrane vesicles (PMVs) | s.c. | Breast cancer cells | DCs in subcutaneous compartment and LNs | Induce both cellular and humoral immunity against HER-2-expressing tumor cells | [241] |
| Protein | M2e-displaying outer membrane vesicles (OMVs) | s.c. | Escherichia coli | Skin somatic cells and DCs | Initiate specific humoral immunity against influenza A (H1N1) | [240] |
| Protein | Oligodendrocyte-derived EVs containing multiple myelin Ags | i.v. | Oligodendrocyte, monocyte, cDCs | mo-DCs | Induce immunosuppressive monocytes and apoptosis of autoreactive CD4+ T cells in several autoimmune encephalomyelitis models | [283] |
| Protein | OVA | s.c. | Skin somatic cells and CCR9+ pDCs, respectively | CCR9+ pDCs and thymus cDCs, respectively | Induce pDCs-mediated thymic central tolerance | [249] |
| Nucleic acid-based vaccine | ||||||
| pDNA | OVA pDNA | i.m. | KCs | CD103+/CD8α+ DCs | Activate OVA-specific CD8+ T cells | [49] |
| pDNA | Bacillus anthracis protective antigen domain 4 (PA-D4) pDNA | i.d. by electroporation | KCs | Skin-resident DCs | Induce potent Anthrax-associated humoral immune response | [284] |
| pDNA | OVA pDNA and GM-CSF -loaded mesoporous silica microrods (MSRs) | s.c. | KCs and migratory DCs, respectively | Skin-resident DCs and LNs-resident DCs, respectively | Elicit OVA-specific CTL response, Th1 humoral response and CD8+ effector and memory T cell responses | [285] |
| mRNA | Influenza A mRNA delivered by Lipofectamine 2000 | i.m. | Muscle cells | mo-DCs | Cross prime CD8+ T cells in vivo | [33] |
| mRNA | Protamine mRNA | i.d. | KCs and migratory DCs, respectively | Migratory DCs and LNs-resident DCs, respectively | Induce functional Ags in the dLNs and massive activation of T cells | [32] |
| DCs-based vaccine | ||||||
| mo-DCs | Mo-DCs loaded with both keyhole limpet hemocyanin (KLH) and TAA | i.d., i.n. | mo-DCs | CD163+ macrophages and LNs-resident DCs | Induce Ag-specific immune response in patients with melanoma | [273] |
| mo-DCs | In vivo activated mo-DCs | s.c. | mo-DCs | LNs-resident CD8α+ DCs | Activate B16-OVA specific CD8+ T cell immune response | [279] |
| mo-DCs | Tumor whole cell lysate-pulsed mo-DCs | i.d. | mo-DCs | Possibly DCs and macrophages in the dLNs and vaccine injection site | Nearly half of the patients generate specific immune responses against glioblastoma, with survival time prolonged | [268, 277] |
| mo-DCs | Tumor whole cell lysate-pulsed mo-DCs | s.c. | mo-DCs | Possibly DCs and macrophages in LNs and vaccine injection site | Induce renal cell cancer-specific Th1 immune response | [286] |
| cDC2 and pDCs | Three TAAs/mRNA-pulsed cDC2 and pDCs | i.d. | cDC2 and pDCs | LNs-resident DCs | Increase metastatic castration-resistant prostate cancer (mCRPC) reactive IFN-γ+ CTLs | [276] |
| cDC2 | TAAs (gp100 and tyrosinase) -pulsed cDC2 | i.d. | cDC2 | LNs-resident DCs | Prolong progression free survival in some melanoma patients | [274] |
| pDCs | TAAs (gp100 and tyrosinase) -pulsed pDCs | intra-LN | pDCs | LNs-resident DCs | Prolong the survival of melanoma patients with 1-2 years | [275] |
| pDCs | Peripheral Ag (OVA) -loaded pDCs | i.v. | CCR9+ pDCs | Thymus-resident cDCs | Induce central tolerance | [249] |
The bivalent (2vHPV, Cervarix), quadrivalent (4vHPV, Gardasil) and nine-valent (9vHPV, Gardasil 9) human papillomavirus (HPV) vaccines are primarily composed of noninfectious virus-like particles (VLP) that display potent protection against cervical infections caused by HPV, condylomas and some HPV-related cancers [212-214, 234, 235]. Recently, accumulating evidence indicates that muscle cells at site of injection may act as Ag reservoirs/donors for DCs during i.m. administration to promote the establishment of a sustained anti-viral effector and memory immune defense [236-239]. Antigen transfer is also involved in other protein-based vaccines and contributes to an efficient disease prevention and control. Rappazzo et al. [240] reported influenza vaccines based on bacteria-derived outer membrane vesicles (OMVs) and ectodomain of the influenza M2 protein (M2e). Briefly, OMVs were engineered to display M2e by transforming E. coli with a plasmid encoding the transmembrane protein ClyA followed by the Ag of interest, which elicited high IgG titers and protects against lethal doses of the mouse-adapted H1N1 influenza strain PR8 in BALB/c mice, probably due to the OMVs-mediated Ag transfer to APCs in vivo.
Characteristics of the currently most licensed three vaccine types. (A) Protein-based vaccines. Widely used in clinical practice, these vaccines incorporate disease-associated Ag protein/peptide (usually insufficient in immunogenicity) and adjuvants (such as alum and Freund's adjuvants) and are mainly administrated via i.m., s.c., i.d., and i.n. (less adopted). After administration, Ag is transferred from vaccinated somatic cells (such as muscle cells, keratinocytes and fibroblasts) and APCs (such as rDCs, LCs and macrophages) to DCs at the injection site to amplify immune responses for disease prevention and treatment. (B) Nucleic acid-based vaccines. These vaccines contain Ag-encoding mRNA or plasmid DNA (pDNA) with self-adjuvant effects and are inoculated mainly by i.d., s.c. and i.m. After injection, vaccine particles/naked nucleic acids are internalized, translated, processed and presented by local somatic cells and APCs, or undergo Ag (the original Ag, translated/process/displayed Ag fragments or Ag complexes) transfer to DCs, serving as prophylactic and therapeutic agents. (C) DC-based vaccines. Primarily administrated through i.d., s.c., i.m., i.v. and i.n. (less adopted), these vaccines are mainly composed of ex vivo-cultured mo-DCs derived from autologous/allogeneic mononuclear progenitor cells or endogenous cDCs/pDCs isolated and enriched from blood to provide an individualized therapeutic effect. These DCs are pulsed with Ag and adjuvant prior to administration, and Ag-laden DCs can also transfer Ag to nearby APCs in vivo. i.d.: intradermal injection; i.m.: intramuscular injection; i.n.: intranodal injection; i.v.: intravenous injection; s.c.: subcutaneous injection; TAA: tumor associated antigen; TSA: tumor specific antigen.
Antigen transfer in protein-based vaccines and nucleic acid-based vaccines. (A) After local administration of protein-based vaccines, Ag is captured, processed, presented and/or intercellularly shared by epidermal LCs and keratinocytes (KCs). Subsequently, activated Langerhans cells (LCs) migrate to the dermis for activation of migratory conventional DCs (mcDC1 and mcDC2) via antigen transfer. Meanwhile, fibroblasts in the dermis may also internalized and transfer Ag to DCs. Activated dermal cDCs and LCs then homing to the dLNs in a CCR7-dependent manner to induce immune response. Meanwhile, free Ag particles may also diffuse into the dLNs in a size-dependent manner to directly activate adaptive immunity. (B) Nucleic acid-based vaccines are locally administrated to be internalized and transfected by LCs, KCs and fibroblasts. Subsequently, these vaccinated cells may transfer Ag to skin DCs for dLNs-homing and immune activation. Similarly, nucleic acids and expressed Ag may directly drain toward the dLNs to induce immune response. i.d., intradermal injection; s.c., subcutaneous injection; i.m., intramuscular injection; i.n., intranodal injection.
Patel et al. [241] reported a biocompatible particulate protein delivery system that may exploit the phenomenon of Ag transfer from tumor cells to DCs for improved immunization. Briefly, plasma membrane vesicles (PMVs) are prepared from biological materials (such as cultured cells and isolated tissues) and surface-modified by glycosylphosphatidylinositol (GPI)-anchored TAAs (breast cancer Ag: human epidermal growth factor receptor-2 (HER-2) in this work) and immunostimulatory molecules (such as interleukin (IL)-12 and B7-1), which induced both cellular and humoral immunity against a HER-2-expressing tumor cell challenge along with delayed tumor growth and partial regression of established tumors.
DCs-derived exosomes (Dex) are loaded with costimulatory molecules, functional p-MHCs and other immune cell-interacting elements, and are especially enriched in p-MHC class II complexes, by 10-100-fold that of DCs, which might lead to a more efficient Ag transfer to other DCs and remarkable immunological impacts [242]. It should be noted that the immune effects (i.e., stimulation or inhibition) and biological activity of Dex depend on the activation status of donor DCs and the follow-up artificial manipulation of the isolated endosomes. For instance, compared with that from immature DCs, Dex from mature murine DCs are enriched in MHC class II, costimulatory B7.2, intercellular adhesion molecule 1 (ICAM-1) and depleted in milk fat globule-epidermal growth factor-factor VIII (MFG-E8), which are 50- to 100-fold more potent in functional T-cell activation both in vitro and in vivo [243]. And the involvement of exosomes in the induction of host defense and immune evasion has been reviewed by Schorey et al. [244] in detail. Indeed, with advances in molecular and cellular biology, such cell-free multifunctional protein delivery platform might have widespread applications in mediating antigen transfer for a desired immune regulation [245-248].
As mentioned before, antigen transfer might also induce immune tolerance and dampen the protective effect of protein-based vaccines. Hadeiba et al. [249] found that peripheral pDCs engulfed subcutaneously injected exogenous Ag in the absence of TLR signals, and subsequently migrated to the thymus in a CCR9-dependent manner to delete Ag-reactive thymocytes and induce immune tolerance. Specifically, pDCs themselves fail to make physical contacts with CD4+ T cells, and are incapable of directly inducing T cell proliferation. Nonetheless, pDCs transport and transfer Ag to thymic APCs to abort the activation and clonal expansion of cognate CD4+ T cells, inducing Ag-specific systemic tolerance [208]. However, the mechanistic details of such Ag transfer in tolerogenic T cell induction needs further exploration. In addition, given that co-stimulatory membrane molecules and immunostimulatory soluble molecules are low-expressed in most non-APCs, the direct transfer of functional p-MHC class I complexes from Ag-pulsed non-APCs to immature DCs may sometimes induce T-cell tolerance and/or exhaustion due to insufficient costimulatory signals [2, 59, 60, 110].
Nucleic acid-based vaccines
Compared to protein/peptide-based subunit vaccines that are generally inadequate in immunogenicity, nucleic acid-based vaccines have self-adjuvant effect and can act as pathogen-associated molecular patterns (PAMPs) to stimulate pattern-recognition receptors (PRRs, such as TLR-3/-7/-8/-9) for amplified immune responses [32, 250], which have emerged as promising vaccine platforms in anti-cancer and anti-viral immunotherapy [251, 252]. And antigen transfer during the inoculation of nucleic acid-based vaccines is increasingly gaining attention for its potential clinical benefits (Figure 6B).
Considering the great discrepancy in lymphatic draining system and cell abundance of different vaccination sites, the administration route and delivery vehicle of the nucleic acid of interest significantly shapes the efficiency and duration of vaccine responses. DNA- and mRNA-based vaccines are commonly delivered via i.d. [253], i.m. [254] and s.c. [255], or through the less adopted i.n. [256], i.v. [257], intra-tumoral injection [258], intra-splenic injection [259], and intranasal administration [260].
After administration, Ag-encoding nucleic acids are directly captured, processed and presented by DCs through MHC class I-biased pathway, or indirectly transferred to DCs from transfected somatic cells in the form of exogenous protein/peptide to induce MHC class II-preferred Ag presentation. In addition, it is reported that pDNA-vaccinated somatic cells may 1) present associated p-MHC class I complexes on the cell surface to be recognized and even cytolyzed by cognate CD8+ T cells [261]; or 2) be phagocytized by DCs for further process and presentation [262]. For example, Li et al. [49] found that following vaccination with ovalbumin-encoding pDNA (OVA-pDNA, i.m.), CD103+/CD8α+ DCs obtain antigenic information from transfected KCs via cross dressing to efficiently activate both naïve and memory CD8+ T cells. Similarly, after administration, mRNA vaccines are extensively internalized and expressed by muscle cells and KCs, and the resultant Ag protein/peptide can be transferred to nearby APCs for CD8+ T cell activation and immune amplification [32, 33].
DCs-based vaccines
The adoptive transfer of ex vivo-activated DCs has been widely recognized with good biosafety and sufficient efficacy in clinical anti-tumor therapy [263]. Briefly, CD34+ bone marrow progenitors or CD14+ peripheral blood monocytes are isolated and stimulated with granulocyte-macrophage colony stimulating factor (GM-CSF) and IL-4 in vitro to generate mo-DCs [264, 265]. Then, the resultant mo-DCs are pulsed with 1) TAAs and/or TSAs [266, 267]; 2) tumor whole cell lysates [268]; or 3) tumor-derived EVs [269, 270], and concurrently stimulated with adjuvants for maturation [271]. However, the clinical efficacy of mo-DCs-based vaccine is greatly limited by its inferior ability of CCR7-dependent LNs homing and Ag cross presentation [272]. As a result, a large proportion of mo-DCs remained at the site of administration, lost viability and are eliminated by phagocytes [273]. In this consideration, some current clinical trials have used enriched cDCs and pDCs that directly collected from the peripheral blood and activated in vitro before administration, which might have broader immunotherapeutic applications as these DCs subsets display superior LNs homing and T-cell cross priming [274-276].
Nevertheless, multiple clinical phase II and phase III studies have shown that the less mobilized mo-DCs are sufficient in eliciting potent immune responses in cancer therapy [268, 276-278], which may attribute to the antigen transfer from mo-DCs to other APCs both at the site of injection and the dLNs [29, 273, 279] (Figure 7). For example, Huang et al. [174] found that even undifferentiated monocytes loaded with Ag protein or peptide induced robust CD8+ T cell responses by Ag transfer to endogenous splenic CD8+ DCs in a cell-to-cell contact-dependent fashion and through Cx43-mediated intercellular gap junctions. On the other hand, DCs retain at the injection site may transfer Ag to tissue-resident LCs or circulating cDCs through multiple contact- and non-contact- dependent pathways including EVs and trogocytosis to sensitize the immune system for specific activation [72]. Meanwhile, dead cells, cell derbies and apoptotic bodies of those DCs can be phagocytized by infiltrating CD163+ macrophages as an approach of passive Ag transfer. Consequently, Ag-laden macrophages migrate to the liver for further Ag sharing and T-/B-cell activation [273]. In short, intercellular antigen transfer during the administration of DCs-based vaccines may serve as an efficacious strategy to amplify immune response.
Antigen transfer in DC-based vaccines. After local injection, adoptively transferred DCs (as represented by monocyte-derived DCs (mo-DCs)) transfer Ag to Langerhans cells (LCs) in the epidermis (1) and migratory conventional DCs (mcDCs) in the dermis (2). Then, part of Ag-loaded mo-DCs, LCs and mcDCs migrate to the dLNs in a CCR7-dependent manner to activate adaptive immune responses (3-1). In addition, mo-DCs that undergo apoptosis in situ and their apoptotic bodies are mainly phagocytosed by macrophages (3-2) and transported to the liver for immune activation (4).
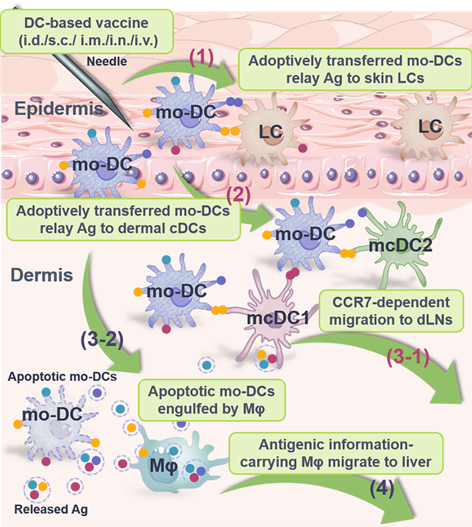
Conclusions and Discussion
In this review, mainly focused on DCs-based antigen receptors, we summarize the recent understanding of antigen transfer and its impact on immune amplification and tolerance. We conclude that antigen transfer plays an important role in coordinating immune responses against the invasion of the “non-self”. Therefore, appropriately manipulating antigen transfer may promote the preventive and/or therapeutic effects of vaccines, which depends heavily on a rational design of the vaccine component and administration route. Meanwhile, undesired antigen transfer may induce tolerance or cause allergy, graft rejection and autoimmune diseases. In these regards, reasonable intervention that blocks or disturbs antigen transfer is needed.
Of note, antigen transfer-associated immune amplification and tolerance can sometimes be interconvertible. In fact, persistent and/or excessively/insufficiently-dosed Ag stimulation may induce a tolerogenic phenotype of DCs that leads to vaccine failure. To avoid the induction of unwanted tolerance to an antigen of interest, 1) adjuvants that facilitate the recruitment, mobilization, and maturation of DCs can be supplemented; 2) the route of vaccine delivery that determines the participants of antigen transfer needs further consideration; 3) the dose and type (i.e., protein-, nucleic acid-, or cells- based vaccines) of vaccine should be carefully selected as different mechanisms of antigen transfer may be involved.
Up to now, the key steps and mediators directing the intercellular antigen transfer remain obscure, and the immunological and pathological consequences of antigen transfer in different biological processes require further exploration. Therefore, more efforts are needed for proper regulation over the mode, site and participant of antigen transfer that might contribute to a more satisfactory immune outcome.
Abbreviations
Ag: antigen; p-MHCs: peptide-MHC complexes; SLOs: secondary lymphoid organs; TNTs: tunnel nanotubes; EVs: extracellular vesicles; DCs: dendritic cells; APCs: antigen-presenting cells; Tregs: regulatory T cells; cDCs: conventional DCs; LCs: Langerhans cells; pDCs: plasmacytoid DCs; mo-DCs: monocyte-derived DCs; LNs: lymph nodes; PPs: Peyer's patches; KCs: keratinocytes; HSV: herpes simplex virus; EBV: Epstein-Barr virus; CTL: cytotoxic T lymphocyte; TAAs: tumor-associated antigens; TSAs: tumor-specific antigens; HIV: human immunodeficiency virus; HLA-G: human leukocyte antigen-G; MDSCs: myeloid-derived suppressive cells; FDCs: follicular DCs; Cx43: Connexin 43; dLNs: draining lymph nodes; F-actin: filamentous actin; CCR7: chemokine receptor 7; MVEs: multivesicular endosomes; EVIR: extracellular vesicles-internalizing receptors; mTECs: medullary thymic epithelial cells; AIRE: autoimmune regulator; SCS: subcapsular sinus; SFB: Segmented filamentous bacteria; IECs: intestinal epithelial cells; Th17: T helper type 17; Mφ: macrophages; MCs: Mast cells; MCGs: mast cell granules; VPS4: vacuolar protein sorting 4; AD: atopic dermatitis; Th2: T helper type 2; HDM: house dust mite; DAMPs: danger-associated molecular patterns; TLRs: Toll-like receptors; SLE: systemic lupus erythematosus; mtDNA: mitochondrial DNA; PD-L1: programmed death-ligand 1; CLEC9A: C-type lectin-like receptor 9A; EVIR: extracellular vesicle-internalizing receptor; NK: natural killer; CEA: carcinoembryonic antigen; Th1: T helper type 1; NIPCs: natural interferon producing cells; IFN: type I Interferon; Lm: Listeria monocytogenes; MPs: microparticles; BCRs: B cell antigen receptors; LOX-1: Lectin-like oxidized low-density lipoprotein receptor-1; mcDCs: migratory conventional DCs; rcDCs: resident conventional DCs; IRF8: interferon regulatory factor 8; IRF4: interferon regulatory factor 4; HCV: hepatitis C virus; mRNA: messenger RNA; E. coli: Escherichia coli; HepA/B: hepatitis A/B; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; pDNA: plasmid DNA; HPV: human papillomavirus; VLP: virus-like particles; OMVs: outer membrane vesicles; M2e: influenza M2 protein; PMVs: plasma membrane vesicles; GPI: glycosylphosphatidylinositol; HER-2: human epidermal growth factor receptor-2; IL: interleukin; Dex: DCs-derived exosomes; ICAM-1: intercellular adhesion molecule 1; PAMPs: pathogen-associated molecular patterns; PRRs: pattern-recognition receptors; GM-CSF: granulocyte-macrophage colony stimulating factor.
Acknowledgements
This work was supported by the National Nature Science Foundation of China (81573365, 82003667).
Author Contributions
Y. Shi & Y. Lu: Conceptualization, Visualization, Writing-original draft, and Writing-review & editing. J. You: Supervision, Funding acquisition and Writing-review & editing.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ruhland MK, Roberts EW, Cai E, Mujal AM, Marchuk K, Beppler C. et al. Visualizing synaptic transfer of tumor antigens among dendritic cells. Cancer Cell. 2020;37:786-99
2. Shi Y, Lu Y, Qin B, Jiang M, Guo X, Li X. et al. Antigen transfer from non-APCs to APCs impacts the efficacy and safety of protein- and mRNA- based vaccines. Nano Today. 2021;41:101326
3. Hilligan KL, Ronchese F. Antigen presentation by dendritic cells and their instruction of CD4+ T helper cell responses. Cell Mol Immunol. 2020;17:587-99
4. Li W, Yang J, Luo L, Jiang M, Qin B, Yin H. et al. Targeting photodynamic and photothermal therapy to the endoplasmic reticulum enhances immunogenic cancer cell death. Nat Commun. 2019;10:3349
5. Shi Y, Zhu C, Liu Y, Lu Y, Li X, Qin B. et al. A vaccination with boosted cross presentation by ER-targeted antigen delivery for anti-tumor immunotherapy. Adv Healthc Mater. 2021: e2001934.
6. Crotzer VL, Blum JS. Autophagy and its role in MHC-mediated antigen presentation. J Immunol. 2009;182:3335-41
7. Mintern JD, Macri C, Chin WJ, Panozza SE, Segura E, Patterson NL. et al. Differential use of autophagy by primary dendritic cells specialized in cross-presentation. Autophagy. 2015;11:906-17
8. Eickhoff S, Brewitz A, Gerner MY, Klauschen F, Komander K, Hemmi H. et al. Robust anti-viral immunity requires multiple distinct T cell-dendritic cell interactions. Cell. 2015;162:1322-37
9. Weinstock M, Rosenblatt J, Avigan D. Dendritic cell therapies for hematologic malignancies. Mol Ther Methods Clin Dev. 2017;5:66-75
10. Garris CS, Arlauckas SP, Kohler RH, Trefny MP, Garren S, Piot C. et al. Successful anti-PD-1 cancer immunotherapy requires T cell-dendritic cell crosstalk involving the cytokines IFN-gamma and IL-12. Immunity. 2018;49:1148-61
11. Hasegawa H, Matsumoto T. Mechanisms of tolerance induction by dendritic cells in vivo. Front Immunol. 2018;9:350
12. Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419-26
13. Nutt SL, Chopin M. Transcriptional networks driving dendritic cell differentiation and function. Immunity. 2020;52:942-56
14. Roberts EW, Broz ML, Binnewies M, Headley MB, Nelson AE, Wolf DM. et al. Critical role for CD103(+)/CD141(+) dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in melanoma. Cancer Cell. 2016;30:324-36
15. Balan S, Bhardwaj N. Cross-presentation of tumor antigens is ruled by synaptic transfer of vesicles among dendritic cell subsets. Cancer Cell. 2020;37:751-3
16. Papaioannou NE, Salei N, Rambichler S, Ravi K, Popovic J, Kuentzel V. et al. Environmental signals rather than layered ontogeny imprint the function of type 2 conventional dendritic cells in young and adult mice. Nat Commun. 2021;12:464
17. Sterrett S, Peng BJ, Burton RL, LaFon DC, Westfall AO, Singh S. et al. Peripheral CD4 T follicular cells induced by a conjugated pneumococcal vaccine correlate with enhanced opsonophagocytic antibody responses in younger individuals. Vaccine. 2020;38:1778-86
18. Berod L, Sparwasser T. pDCs take a deep breath to fight viruses. Immunity. 2016;44:1246-8
19. Dutertre C-A, Becht E, Irac SE, Khalilnezhad A, Narang V, Khalilnezhad S. et al. Single-cell analysis of human mononuclear phagocytes reveals subset-defining markers and identifies circulating inflammatory dendritic cells. Immunity. 2019;51:573-89
20. Worbs T, Hammerschmidt SI, Foerster R. Dendritic cell migration in health and disease. Nat Rev Immunol. 2017;17:30-48
21. Pelgrom LR, Patente TA, Sergushichev A, Esaulova E, Otto F, Ozir-Fazalalikhan A. et al. LKB1 expressed in dendritic cells governs the development and expansion of thymus-derived regulatory T cells. Cell Res. 2019;29:406-19
22. Obermajer N, Urban J, Wieckowski E, Muthuswamy R, Ravindranathan R, Bartlett DL. et al. Promoting the accumulation of tumor-specific T cells in tumor tissues by dendritic cell vaccines and chemokine-modulating agents. Nat Protoc. 2018;13:335-57
23. Salmon H, Idoyaga J, Rahman A, Leboeuf M, Remark R, Jordan S. et al. Expansion and activation of CD103(+) dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity. 2016;44:924-38
24. Barry KC, Hsu J, Broz ML, Cueto FJ, Binnewies M, Combes AJ. et al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat Med. 2018;24:1178-91
25. Garg AD, More S, Rufo N, Mece O, Sassano ML, Agostinis P. et al. Trial watch: Immunogenic cell death induction by anticancer chemotherapeutics. Oncoimmunology. 2017;6:e1386829
26. Liang JG, Su D, Song T-Z, Zeng Y, Huang W, Wu J. et al. S-Trimer, a COVID-19 subunit vaccine candidate, induces protective immunity in nonhuman primates. Nat Commun. 2021;12:1346
27. Harshyne LA, Watkins SC, Gambotto A, Barratt-Boyes SM. Dendritic cells acquire antigens from live cells for cross-presentation to CTL. J Immunol. 2001;166:3717-23
28. Shin J-Y, Wang C-Y, Lin C-C, Chu C-L. A recently described type 2 conventional dendritic cell (cDC2) subset mediates inflammation. Cell Mol Immunol. 2020;17:1215-7
29. Krishnaswamy JK, Gowthaman U, Zhang B, Mattsson J, Szeponik L, Liu D. et al. Migratory CD11b(+) conventional dendritic cells induce T follicular helper cell-dependent antibody responses. Sci Immunol. 2017;2:eaam9169
30. Perry JSA, Russler-Germain EV, Zhou YW, Purtha W, Cooper ML, Choi J. et al. CD36 mediates cell-surface antigens to promote thymic development of the regulatory T cell receptor repertoire and allo-tolerance. Immunity. 2018;48:923-36.e4
31. Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity. 2013;39:38-48
32. Kowalczyk A, Doener F, Zanzinger K, Noth J, Baumhof P, Fotin-Mleczek M. et al. Self-adjuvanted mRNA vaccines induce local innate immune responses that lead to a potent and boostable adaptive immunity. Vaccine. 2016;34:3882-93
33. Lazzaro S, Giovani C, Mangiavacchi S, Magini D, Maione D, Baudner B. et al. CD8 T-cell priming upon mRNA vaccination is restricted to bone-marrow-derived antigen-presenting cells and may involve antigen transfer from myocytes. Immunology. 2015;146:312-26
34. Botting RA, Rana H, Bertram KM, Rhodes JW, Baharlou H, Nasr N. et al. Langerhans cells and sexual transmission of HIV and HSV. Rev Med Virol. 2017 27
35. Preza GC, Tanner K, Elliott J, Yang OO, Anton PA, Ochoa M-T. Antigen-presenting cell candidates for HIV-1 transmission in human distal colonic mucosa defined by CD207 dendritic cells and CD209 macrophages. AIDS Res Hum Retroviruses. 2014;30:241-9
36. Levin C, Bonduelle O, Nuttens C, Primard C, Verrier B, Boissonnas A. et al. Critical role for skin-derived migratory DCs and Langerhans cells in T-FH and GC responses after intradermal immunization. J Invest Dermatol. 2017;137:1905-13
37. Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol. 2007;7:543-55
38. Lee HK, Zamora M, Linehan MM, Iijima N, Gonzalez D, Haberman A. et al. Differential roles of migratory and resident DCs in T cell priming after mucosal or skin HSV-1 infection. J Exp Med. 2009;206:359-70
39. Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y. et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153-62
40. Harshyne LA, Zimmer MI, Watkins SC, Barratt-Boyes SM. A role for class A scavenger receptor in dendritic cell nibbling from live cells. J Immunol. 2003;170:2302-9
41. Santana-Magal N, Farhat-Younis L, Gutwillig A, Gleiberman A, Rasoulouniriana D, Tal L. et al. Melanoma-secreted lysosomes trigger monocyte-derived dendritic cell apoptosis and limit cancer immunotherapy. Cancer Res. 2020;80:1942-56
42. Zhu C, Shi Y, You J. Immune cell connection by tunneling nanotubes: The impact of intercellular cross-talk on the immune response and its therapeutic applications. Mol Pharmaceut. 2021;18:772-86
43. Neijssen J, Herberts C, Drijfhout JW, Reits E, Janssen L, Neefjes J. Cross-presentation by intercellular peptide transfer through gap junctions. Nature. 2005;434:83-8
44. Das Mohapatra A, Tirrell I, Benechet AP, Pattnayak S, Khanna KM, Srivastava PK. Cross-dressing of CD8 alpha(+) dendritic cells with antigens from live mouse tumor cells is a major mechanism of cross-priming. Cancer Immunol Res. 2020;8:1287-99
45. Wakim LM, Bevan MJ. Cross-dressed dendritic cells drive memory CD8+ T-cell activation after viral infection. Nature. 2011;471:629-32
46. Andre F, Scharz NEC, Chaput N, Flament C, Raposo G, Amigorena S. et al. Tumor-derived exosomes: A new source of tumor rejection antigens. Vaccine. 2002;20:A28-A31
47. Shakhar G, Kolesnikov M. Intestinal macrophages and DCs close the gap on tolerance. Immunity. 2014;40:171-3
48. Schiller C, Huber JE, Diakopoulos KN, Weiss EH. Tunneling nanotubes enable intercellular transfer of MHC class I molecules. Hum Immunol. 2013;74:412-6
49. Li L, Kim S, Herndon JM, Goedegebuure P, Belt BA, Satpathy AT. et al. Cross-dressed CD8 alpha(+)/CD103(+) dendritic cells prime CD8(+) T cells following vaccination. Proc Natl Acad Sci U S A. 2012;109:12716-21
50. Lin A, Yan W-H. Intercellular transfer of HLA-G: its potential in cancer immunology. Clin Transl Immunol. 2019;8:e1077
51. LeMaoult J, Caumartin J, Daouya M, Switala M, Rebmann V, Arnulf B. et al. Trogocytic intercellular membrane exchanges among hematological tumors. J Hematol Oncol. 2015;8:24
52. Mazzini E, Massimiliano L, Penna G, Rescigno M. Oral tolerance can be established via gap junction transfer of fed antigens from CX3CR1+ macrophages to CD103+ dendritic cells. Immunity. 2014;40:248-61
53. Satpathy AT, Briseño CG, Lee JS, Ng D, Manieri NA, Kc W. et al. Notch2-dependent classical dendritic cells orchestrate intestinal immunity to attaching-and-effacing bacterial pathogens. Nat Immunol. 2013;14:937-48
54. Arnold IC, Zhang X, Urban S, Artola-Borán M, Manz MG, Ottemann KM. et al. NLRP3 controls the development of gastrointestinal CD11b+ dendritic cells in the steady state and during chronic bacterial infection. Cell Rep. 2017;21:3860-72
55. Zaccard CR, Watkins SC, Kalinski P, Fecek RJ, Yates AL, Salter RD. et al. CD40L induces functional tunneling nanotube networks exclusively in dendritic cells programmed by mediators of type 1 immunity. J Immunol. 2015;194:1047-56
56. Pinto G, Brou C, Zurzolo C. Tunneling nanotubes: The fuel of tumor progression? Trends Cancer. 2020;6:874-88
57. Squadrito ML, Cianciaruso C, Hansen SK, De Palma M. EVIR: chimeric receptors that enhance dendritic cell cross-dressing with tumor antigens. Nat Methods. 2018;15:183-6
58. Leone DA, Peschel A, Brown M, Schachner H, Ball MJ, Gyuraszova M. et al. Surface LAMP-2 is an endocytic receptor that diverts antigen internalized by human dendritic cells into highly immunogenic exosomes. J Immunol. 2017;199:531-46
59. Yang X, Meng S, Jiang H, Zhu C, Wu W. Exosomes derived from immature bone marrow dendritic cells induce tolerogenicity of intestinal transplantation in rats. J Surg Res. 2011;171:826-32
60. Peche H, Heslan M, Usal C, Amigorena S, Cuturi MC. Presentation of donor major histocompatibility complex antigens by bone marrow dendritic cell-derived exosomes modulates allograft rejection. Transplantation. 2003;76:1503-10
61. Purcell AW, McCluskey J, Rossjohn J. More than one reason to rethink the use of peptides in vaccine design. Nat Rev Drug Discov. 2007;6:404-14
62. Afridi S, Hoessli DC, Hameed MW. Mechanistic understanding and significance of small peptides interaction with MHC class II molecules for therapeutic applications. Immunol Rev. 2016;272:151-68
63. Embgenbroich M, Burgdorf S. Current concepts of antigen cross-presentation. Front Immunol. 2018;9:1643
64. Ahmed KA, Munegowda MA, Xie Y, Xiang J. Intercellular trogocytosis plays an important role in modulation of immune responses. Cell Mol Immunol. 2008;5:261-9
65. Wetzel SA, McKeithan TW, Parker DC. Peptide-specific intercellular transfer of MHC class II to CD4(+) T cells directly from the immunological synapse upon cellular dissociation. J Immunol. 2005;174:80-9
66. Osborne DG, Wetzel SA. Trogocytosis results in sustained intracellular signaling in CD4(+) T cells. J Immunol. 2012;189:4728-39
67. Kim H-R, Mun Y, Lee K-S, Park Y-J, Park J-S, Park J-H. et al. T cell microvilli constitute immunological synaptosomes that carry messages to antigen-presenting cells. Nat Commun. 2018;9:3630
68. Boettcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S. et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell. 2018;172:1022-37
69. Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES. Differential lysosomal proteolysis in antigen-presenting CeRs determines antigen fate. Science. 2005;307:1630-4
70. Nasr N, Lai J, Botting RA, Mercier SK, Harman AN, Kim M. et al. Inhibition of two temporal phases of HIV-1 transfer from primary Langerhans cells to T Cells: The role of Langerin. J Immunol. 2014;193:2554-64
71. Sarrami-Forooshani R, Mesman AW, van Teijlingen NH, Sprokholt JK, van der Vlist M, Ribeiro CMS. et al. Human immature Langerhans cells restrict CXCR4-using HIV-1 transmission. Retrovirology. 2014;11:52
72. Mayr L, Su B, Moog C. Langerhans cells: The 'Yin and Yang' of HIV restriction and transmission. Trends Microbiol. 2017;25:170-2
73. de Witte L, Nabatov A, Pion M, Fluitsma D, de Jong MAWP, de Gruijl T. et al. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat Med. 2007;13:367-71
74. Lin A, Yan W-H. Heterogeneity of HLA-G expression in cancers: Facing the challenges. Front Immunol. 2018;9:2164
75. Donahue DA, Schwartz O. Actin' on HIV: How dendritic cells spread infection. Cell Host Microbe. 2016;19:267-9
76. Menager MM, Littman DR. Actin dynamics regulates dendritic cell-mediated transfer of HIV-1 to T cells. Cell. 2016;164:695-709
77. Kongsomros S, Manopwisedjaroen S, Chaopreecha J, Wang S-F, Borwornpinyo S, Thitithanyanont A. Rapid and efficient cell-to-cell transmission of avian influenza H5N1 virus in MDCK cells is achieved by trogocytosis. Pathogens. 2021;10:483
78. Segretain D, Falk MA. Regulation of connexin biosynthesis, assembly, gap junction formation, and removal. Biochim Biophys Acta Biomembr. 2004;1662:3-21
79. Rajnai H, Teleki I, Kiszner G, Meggyeshazi N, Balla P, Vancsik T. et al. Connexin 43 communication channels in follicular dendritic cell development and in follicular lymphomas. J Immunol Res. 2015;2015:528098
80. Tittarelli A, Mendoza-Naranjo A, Farias M, Guerrero I, Ihara F, Wennerberg E. et al. Gap junction intercellular communications regulate NK cell activation and modulate NK cytotoxic capacity. J Immunol. 2014;192:1313-9
81. Weber PA, Chang H-C, Spaeth KE, Nitsche JM, Nicholson BJ. The permeability of gap junction channels to probes of different size is dependent on connexin composition and permeant-pore affinities. Biophys J. 2004;87:958-73
82. Oviedo-Orta E, Howard Evans W. Gap junctions and connexin-mediated communication in the immune system. Biochim Biophys Acta Biomembr. 2004;1662:102-12
83. Handel A, Yates A, Pilyugin SS, Antia R. Gap junction-mediated antigen transport in immune responses. Trends Immunol. 2007;28:463-6
84. Rock KL, York IA, Goldberg AL. Post-proteasomal antigen processing for major histocompatibility complex class I presentation. Nat Immunol. 2004;5:670-7
85. Mendoza-Naranjo A, Saez PJ, Johansson CC, Ramirez M, Mandakovic D, Pereda C. et al. Functional gap junctions facilitate melanoma antigen transfer and cross-presentation between human dendritic cells. J Immunol. 2007;178:6949-57
86. Saccheri F, Pozzi C, Avogadri F, Barozzi S, Faretta M, Fusi P. et al. Bacteria-induced gap junctions in tumors favor antigen cross-presentation and antitumor immunity. Sci Transl Med. 2010;2:44ra57
87. Farache J, Koren I, Milo I, Gurevich I, Kim K-W, Zigmond E. et al. Luminal bacteria recruit CD103(+) dendritic cells into the intestinal epithelium to sample bacterial antigens for presentation. Immunity. 2013;38:581-95
88. Guo L, Wei R-X, Sun R, Yang Q, Li G-J, Wang L-Y. et al. "Cytokine-microfactories" recruit DCs and deliver tumor antigens via gap junctions for immunotherapy. J Control Release. 2021;337:417-30
89. Krenacs T, vanDartel M, Lindhout E, Rosendaal M. Direct cell/cell communication in the lymphoid germinal center: Connexin43 gap junctions functionally couple follicular dendritic cells to each other and to B lymphocytes. Eur J Immunol. 1997;27:1489-97
90. Dupont M, Souriant S, Lugo-Villarino G, Maridonneau-Parini I, Verollet C. Tunneling nanotubes: Intimate communication between myeloid cells. Front Immunol. 2018;9:43
91. Ljubojevic N, Henderson JM, Zurzolo C. The ways of actin: Why tunneling nanotubes are unique cell protrusions. Trends Cell Biol. 2021;31:130-42
92. Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007-10
93. Eugenin EA, Gaskill PJ, Berman JW. Tunneling nanotubes (TNT) are induced by HIV-infection of macrophages: A potential mechanism for intercellular HIV trafficking. Cell Immunol. 2009;254:142-8
94. Gerdes H-H, Carvalho RN. Intercellular transfer mediated by tunneling nanotubes. Curr Opin Cell Biol. 2008;20:470-5
95. Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Koehler K. et al. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat Cell Biol. 2008;10:211-9
96. Chauveau A, Aucher A, Eissmann P, Vivier E, Davis DM. Membrane nanotubes facilitate long-distance interactions between natural killer cells and target cells. Proc Natl Acad Sci U S A. 2010;107:5545-50
97. Watkins SC, Salter RD. Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity. 2005;23:309-18
98. Mylvaganam S, Freeman SA, Grinstein S. The cytoskeleton in phagocytosis and macropinocytosis. Curr Biol. 2021;31:R619-R32
99. Salter RD, Tuma-Warrino RJ, Hu PQ, Watkins SC. Rapid and extensive membrane reorganization by dendritic cells following exposure to bacteria revealed by high-resolution imaging. J Leukoc Biol. 2004;75:240-3
100. Bousso P. T-cell activation by dendritic cells in the lymph node: lessons from the movies. Nat Rev Immunol. 2008;8:675-84
101. Osteikoetxea-Molnar A, Szabo-Meleg E, Toth EA, Oszvald A, Izsepi E, Kremlitzka M. et al. The growth determinants and transport properties of tunneling nanotube networks between B lymphocytes. Cell Mol Life Sci. 2016;73:4531-45
102. Halasz H, Ghadaksaz AR, Madarasz T, Huber K, Harami G, Toth EA. et al. Live cell superresolution-structured illumination microscopy imaging analysis of the intercellular transport of microvesicles and costimulatory proteins via nanotubes between immune cells. Methods Appl Fluoresc. 2018;6:045005
103. Wang Q, Lu Q. Plasma membrane-derived extracellular microvesicles mediate non-canonical intercellular NOTCH signaling. Nat Commun. 2017;8:709
104. Leone DA, Rees AJ, Kain R. Dendritic cells and routing cargo into exosomes. Immunol Cell Biol. 2018;96:683-93
105. Mulcahy LA, Pink RC, Carter DRF. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014 3
106. Mashouri L, Yousefi H, Aref AR, Ahadi Am, Molaei F, Alahari SK. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18:75
107. Liu Q, Rojas-Canales DM, Divito SJ, Shufesky WJ, Stolz DB, Erdos G. et al. Donor dendritic cell-derived exosomes promote allograft-targeting immune response. J Clin Investig. 2016;126:2805-20
108. Naseri M, Bozorgmehr M, Zoeller M, Ranaei Pirmardan E, Madjd Z. Tumor-derived exosomes: The next generation of promising cell-free vaccines in cancer immunotherapy. Oncoimmunology. 2020;9:1779991
109. Alexander M, Hu R, Runtsch MC, Kagele DA, Mosbruger TL, Tolmachova T. et al. Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat Commun. 2015;6:7321
110. Coutant F, Miossec P. Altered dendritic cell functions in autoimmune diseases: distinct and overlapping profiles. Nat Rev Rheumatol. 2016;12:703-15
111. Ning Y, Shen K, Wu Q, Sun X, Bai Y, Xie Y. et al. Tumor exosomes block dendritic cells maturation to decrease the T cell immune response. Immunol Lett. 2018;199:36-43
112. Maus RLG, Jakub JW, Nevala WK, Christensen TA, Noble-Orcutt K, Sachs Z. et al. Human melanoma-derived extracellular vesicles regulate dendritic cell maturation. Front Immunol. 2017;8:358
113. Maus RLG, Jakub JW, Hieken TJ, Nevala WK, Christensen TA, Sutor SL. et al. Identification of novel, immune-mediating extracellular vesicles in human lymphatic effluent draining primary cutaneous melanoma. Oncoimmunology. 2019;8:e1667742
114. Hosseini R, Asef-Kabiri L, Yousefi H, Sarvnaz H, Salehi M, Akbari ME. et al. The roles of tumor-derived exosomes in altered differentiation, maturation and function of dendritic cells. Mol Cancer. 2021;20:83
115. Perry JSA, Russler-Germain EV, Zhou YW, Purtha W, Cooper ML, Choi J. et al. Transfer of cell-surface antigens by scavenger receptor CD36 promotes thymic regulatory T cell receptor repertoire development and allo-tolerance. Immunity. 2018;48:923-36
116. Choi HW, Suwanpradid J, Kim IH, Staats HF, Haniffa M, MacLeod AS. et al. Perivascular dendritic cells elicit anaphylaxis by relaying allergens to mast cells via microvesicles. Science. 2018;362:eaao0666
117. Mastoridis S, Londono M-C, Kurt A, Kodela E, Crespo E, Mason J. et al. Impact of donor extracellular vesicle release on recipient cell "cross-dressing" following clinical liver and kidney transplantation. Am J Transplant. 2021;21:2387-98
118. Vrisekoop N, Monteiro JP, Mandl JN, Germain RN. Revisiting thymic positive selection and the mature T cell repertoire for antigen. Immunity. 2014;41:181-90
119. Perry JSA, Lio C-WJ, Kau AL, Nutsch K, Yang Z, Gordon JI. et al. Distinct contributions of aire and antigen-presenting-cell subsets to the generation of self-tolerance in the thymus. Immunity. 2014;41:414-26
120. Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don't see). Nat Rev Immunol. 2014;14:377-91
121. Koble C, Kyewski B. The thymic medulla: a unique microenvironment for intercellular self-antigen transfer. J Exp Med. 2009;206:1505-13
122. Perry JSA, Hsieh C-S. Development of T-cell tolerance utilizes both cell-autonomous and cooperative presentation of self-antigen. Immunol Rev. 2016;271:141-55
123. Ueda Y, Katagiri K, Tomiyama T, Yasuda K, Habiro K, Katakai T. et al. Mst1 regulates integrin-dependent thymocyte trafficking and antigen recognition in the thymus. Nat Commun. 2012;3:1098
124. Gasteiger G, Ataide M, Kastenmüller W. Lymph node - an organ for T-cell activation and pathogen defense. Immunol Rev. 2016;271:200-20
125. León B, Lund FE. Compartmentalization of dendritic cell and T-cell interactions in the lymph node: Anatomy of T-cell fate decisions. Immunol Rev. 2019;289:84-100
126. Ufer F, Vargas P, Engler Jan B, Tintelnot J, Schattling B, Winkler H. et al. Arc/Arg3.1 governs inflammatory dendritic cell migration from the skin and thereby controls T cell activation. Sci Immunol. 2016;1:eaaf8665
127. Aggio JB, Krmeska V, Ferguson BJ, Wowk PF, Rothfuchs AG. Vaccinia virus infection inhibits skin dendritic cell migration to the draining lymph node. J Immunol. 2021;206:776-84
128. O'Melia MJ, Rohner NA, Manspeaker MP, Francis DM, Kissick HT, Thomas SN. Quality of CD8+ T cell immunity evoked in lymph nodes is compartmentalized by route of antigen transport and functional in tumor context. Sci Adv. 2020;6:eabd7134
129. Neefjes J, Jongsma MLM, Paul P, Bakke O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol. 2011;11:823-36
130. Keller SA, Bauer M, Manolova V, Muntwiler S, Saudan P, Bachmann MF. Cutting Edge: Limited specialization of dendritic cell subsets for MHC class II-associated presentation of viral particles. J Immunol. 2010;184:26-9
131. Unanue ER, Turk V, Neefjes J. Variations in MHC class II antigen processing and presentation in health and disease. Annu Rev Immunol. 2016;34:265-97
132. Fu C, Peng P, Loschko J, Feng L, Phuong P, Cui W. et al. Plasmacytoid dendritic cells cross-prime naive CD8 T cells by transferring antigen to conventional dendritic cells through exosomes. Proc Natl Acad Sci U S A. 2020;117:23730-41
133. Yao Y, Fu C, Zhou L, Mi QS, Jiang A. DC-derived exosomes for cancer immunotherapy. Cancers (Basel). 2021 13
134. Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. Indirect activation of naive CD4(+) T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3:1156-62
135. Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331-41
136. Cerovic V, Bain CC, Mowat AM, Milling SWF. Intestinal macrophages and dendritic cells: what's the difference? Trends Immunol. 2014;35:270-7
137. Esterházy D, Loschko J, London M, Jove V, Oliveira TY, Mucida D. Classical dendritic cells are required for dietary antigen-mediated induction of peripheral Treg cells and tolerance. Nat Immunol. 2016;17:545-55
138. Joeris T, Gomez-Casado C, Holmkvist P, Tavernier Simon J, Silva-Sanchez A, Klotz L. et al. Intestinal cDC1 drive cross-tolerance to epithelial-derived antigen via induction of FoxP3+CD8+ Tregs. Sci Immunol. 2021;6:eabd3774
139. Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D. et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254-8
140. Longman RS, Diehl GE, Victorio DA, Huh JR, Galan C, Miraldi ER. et al. CX(3)CR1(+) mononuclear phagocytes support colitis-associated innate lymphoid cell production of IL-22. J Exp Med. 2014;211:1571-83
141. McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, Knoop KA. et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483:345-9
142. Ladinsky Mark S, Araujo Leandro P, Zhang X, Veltri J, Galan-Diez M, Soualhi S. et al. Endocytosis of commensal antigens by intestinal epithelial cells regulates mucosal T cell homeostasis. Science. 2019;363:eaat4042
143. Pulimood AB, Ramakrishna BS, Rita AB, Srinivasan P, Mohan V, Gupta S. et al. Early activation of mucosal dendritic cells and macrophages in acute Campylobacter colitis and cholera: An in vivo study. J Gastroenterol Hepatol. 2008;23:752-8
144. Dudeck J, Froebel J, Kotrba J, Lehmann CHK, Dudziak D, Speier S. et al. Engulfment of mast cell secretory granules on skin inflammation boosts dendritic cell migration and priming efficiency. J Allergy Clin Immunol. 2019;143:1849-64.e4
145. Deckers J, De Bosscher K, Lambrecht BN, Hammad H. Interplay between barrier epithelial cells and dendritic cells in allergic sensitization through the lung and the skin. Immunol Rev. 2017;278:131-44
146. Melki I, Allaeys I, Tessandier N, Lévesque T, Cloutier N, Laroche A. et al. Platelets release mitochondrial antigens in systemic lupus erythematosus. Sci Transl Med. 2021;13:eaav5928
147. Hughes AD, Zhao D, Dai H, Abou-Daya KI, Tieu R, Rammal R. et al. Cross-dressed dendritic cells sustain effector T cell responses in islet and kidney allografts. J Clin Investig. 2020;130:287-94
148. Smyth LA, Lechler RI, Lombardi G. Continuous acquisition of MHC: peptide complexes by recipient cells contributes to the generation of anti-graft CD8(+) T cell immunity. Am J Transplant. 2017;17:60-8
149. Ono Y, Perez-Gutierrez A, Nakao T, Dai H, Camirand G, Yoshida O. et al. Graft-infiltrating PD-L1hi cross-dressed dendritic cells regulate antidonor T cell responses in mouse liver transplant tolerance. Hepatology. 2018;67:1499-515
150. Borges TJ, Murakami N, Machado FD, Murshid A, Lang BJ, Lopes RL. et al. March1-dependent modulation of donor MHC II on CD103(+) dendritic cells mitigates alloimmunity. Nat Commun. 2018;9:3482
151. Zhang Y, Shen S, Zhao G, Xu C-F, Zhang H-B, Luo Y-L. et al. In situ repurposing of dendritic cells with CRISPR/Cas9-based nanomedicine to induce transplant tolerance. Biomaterials. 2019;217:119302
152. Kim M, Truong NR, James V, Bosnjak L, Sandgren KJ, Harman AN. et al. Relay of herpes simplex virus between Langerhans cells and dermal dendritic cells in human skin. PLOS Pathog. 2015;11:e1004812
153. Ronchese F, Hilligan KL, Mayer JU. Dendritic cells and the skin environment. Curr Opin Immunol. 2020;64:56-62
154. Canton J, Blees H, Henry CM, Buck MD, Schulz O, Rogers NC. et al. The receptor DNGR-1 signals for phagosomal rupture to promote cross-presentation of dead-cell-associated antigens. Nat Immunol. 2021;22:140-53
155. Zeng B, Middelberg APJ, Gemiarto A, MacDonald K, Baxter AG, Talekar M. et al. Self-adjuvanting nanoemulsion targeting dendritic cell receptor Clec9A enables antigen-specific immunotherapy. J Clin Investig. 2018;128:1971-84
156. Dudeck J, Medyukhina A, Fröbel J, Svensson C-M, Kotrba J, Gerlach M. et al. Mast cells acquire MHCII from dendritic cells during skin inflammation. J Exp Med. 2017;214:3791-811
157. Zhang B, Yin Y, Lai RC, Lim SK. Immunotherapeutic potential of extracellular vesicles. Front Immunol. 2014;5:518
158. Stern-Ginossar N, Nedvetzki S, Markel G, Gazit R, Betser-Cohen G, Achdout H. et al. Intercellular transfer of carcinoembryonic antigen from tumor cells to NK cells. J Immunol. 2007;179:4424
159. Poggio M, Hu T, Pai C-C, Chu B, Belair CD, Chang A. et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell. 2019;177:414-27.e13
160. Osswald M, Jung E, Sahm F, Solecki G, Venkataramani V, Blaes J. et al. Brain tumour cells interconnect to a functional and resistant network. Nature. 2015;528:93-8
161. Su Q, Igyártó BZ. Keratinocytes share gene expression fingerprint with epidermal Langerhans cells via mRNA transfer. J Invest Dermatol. 2019;139:2313-23.e8
162. De La Cruz Diaz JS, Kaplan DH. Langerhans cells spy on keratinocytes. J Invest Dermatol. 2019;139:2260-2
163. Huang J-F, Yang Y, Sepulveda H, Shi W, Hwang I, Peterson PA. et al. TCR-mediated internalization of peptide-MHC complexes acquired by T cells. Science. 1999;286:952-4
164. Nakayama M, Hori A, Toyoura S, Yamaguchi S-I. Shaping of T cell functions by trogocytosis. Cells. 2021;10:1155
165. Nakayama M, Takeda K, Kawano M, Takai T, Ishii N, Ogasawara K. Natural killer (NK)-dendritic cell interactions generate MHC class II-dressed NK cells that regulate CD4(+) T cells. Proc Natl Acad Sci U S A. 2011;108:18360-5
166. Marcenaro E, Pesce S, Sivori S, Carlomagno S, Moretta L, Moretta A. KIR2DS1-dependent acquisition of CCR7 and migratory properties by human NK cells interacting with allogeneic HLA-C2(+) DCs or T-cell blasts. Blood. 2013;121:3396-401
167. Yamanishi Y, Miyake K, Iki M, Tsutsui H, Karasuyama H. Recent advances in understanding basophil-mediated Th2 immune responses. Immunol Rev. 2017;278:237-45
168. de Heusch M, Blocklet D, Egrise D, Hauquier B, Vermeersch M, Goldman S. et al. Bidirectional MHC molecule exchange between migratory and resident dendritic cells. J Leukoc Biol. 2007;82:861-8
169. Nierkens S, Tel J, Janssen E, Adema GJ. Antigen cross-presentation by dendritic cell subsets: one general or all sergeants? Trends Immunol. 2013;34:361-70
170. Eisenbarth SC. Dendritic cell subsets in T cell programming: location dictates function. Nat Rev Immunol. 2019;19:89-103
171. Aline F, Bout D, Amigorena S, Roingeard P, Dimier-Poisson I. Toxoplasma gondii antigen-pulsed-dendritic cell-derived exosomes induce a protective immune response against T. gondii infection. Infect Immun. 2004;72:4127-37
172. Stefanie Hiltbrunner PL, Maria Eldh, Maria-Jose Martinez-Bravo, Arnika K. Wagner, Mikael C.I. Karlsson, and Susanne Gabrielsson. Exosomal cancer immunotherapy is independent of MHC molecules on exosomes. Oncotarget. 2016;7:38707-17
173. Samuel M, Gabrielsson S. Personalized medicine and back-allogeneic exosomes for cancer immunotherapy. J Intern Med. 2021;289:138-46
174. Huang M-N, Nicholson LT, Batich KA, Swartz AM, Kopin D, Wellford S. et al. Antigen-loaded monocyte administration induces potent therapeutic antitumor T cell responses. J Clin Investig. 2020;130:774-88
175. Zhang Y, Zhang R, Zhang H, Liu J, Yang Z, Xu P. et al. Microparticles released by Listeria monocytogenes-infected macrophages are required for dendritic cell-elicited protective immunity. Cell Mol Immunol. 2012;9:489-96
176. Girvan A, Aldwell FE, Buchan GS, Faulkner L, Baird MA. Transfer of macrophage-derived mycobacterial antigens to dendritic cells can induce naive T-cell activation. Scand J Immunol. 2003;57:107-14
177. Athman JJ, Wang Y, McDonald DJ, Boom WH, Harding CV, Wearsch PA. Bacterial membrane vesicles mediate the release of mycobacterium tuberculosis lipoglycans and lipoproteins from infected macrophages. J Immunol. 2015;195:1044
178. Lindenbergh MFS, Stoorvogel W. Antigen presentation by extracellular vesicles from professional antigen-presenting cells. Annu Rev Immunol. 2018;36:435-59
179. Zhang Y, Jin X, Liang J, Guo Y, Sun G, Zeng X. et al. Extracellular vesicles derived from ODN-stimulated macrophages transfer and activate Cdc42 in recipient cells and thereby increase cellular permissiveness to EV uptake. Sci Adv. 2019;5:eaav1564
180. Heath WR, Kato Y, Steiner TM, Caminschi I. Antigen presentation by dendritic cells for B cell activation. Curr Opin Immunol. 2019;58:44-52
181. Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27:160-71
182. Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M. et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110-4
183. Phan TG, Grigorova I, Okada T, Cyster JG. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat Immunol. 2007;8:992-1000
184. Denzer K, van Eijk M, Kleijmeer MJ, Jakobson E, de Groot C, J. Geuze H. Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface. J Immunol. 2000;165:1259
185. Gonzalez SF, Lukacs-Kornek V, Kuligowski MP, Pitcher LA, Degn SE, Kim Y-A. et al. Capture of influenza by medullary dendritic cells via SIGN-R1 is essential for humoral immunity in draining lymph nodes. Nat Immunol. 2010;11:427-34
186. Joo H, Li D, Dullaers M, Kim T-W, Duluc D, Upchurch K. et al. C-type lectin-like receptor LOX-1 promotes dendritic cell-mediated class-switched B cell responses. Immunity. 2014;41:592-604
187. Harvey BP, Gee RJ, Haberman AM, Shlomchik MJ, Mamula MJ. Antigen presentation and transfer between B cells and macrophages. Eur J Immunol. 2007;37:1739-51
188. Maier B, Leader AM, Chen ST, Tung N, Chang C, LeBerichel J. et al. A conserved dendritic-cell regulatory program limits antitumour immunity. Nature. 2020;580:257-62
189. Dolan BP, Gibbs KD, Ostrand-Rosenberg S. Tumor-specific CD4+T cells are activated by “cross-dressed” dendritic cells presenting peptide-MHC class II complexes acquired from cell-based cancer vaccines. J Immunol. 2006;176:1447
190. Duong E, Fessenden TB, Lutz E, Dinter T, Yim L, Blatt S. et al. Type I interferon activates MHC class I-dressed CD11b+ conventional dendritic cells to promote protective anti-tumor CD8+ T cell immunity. Immunity. 2021;55:308-23
191. Bonaccorsi I, Morandi B, Antsiferova O, Costa G, Oliveri D, Conte R. et al. Membrane transfer from tumor cells overcomes deficient phagocytic ability of plasmacytoid dendritic cells for the acquisition and presentation of tumor antigens. J Immunol. 2014;192:824-32
192. Dreux M, Garaigorta U, Boyd B, Décembre E, Chung J, Whitten-Bauer C. et al. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe. 2012;12:558-70
193. Takahashi K, Asabe S, Wieland S, Garaigorta U, Gastaminza P, Isogawa M. et al. Plasmacytoid dendritic cells sense hepatitis C virus-infected cells, produce interferon, and inhibit infection. Proc Natl Acad Sci U S A. 2010;107:7431
194. Tullett KM, Leal Rojas IM, Minoda Y, Tan PS, Zhang JG, Smith C. et al. Targeting CLEC9A delivers antigen to human CD141+ DC for CD4+ and CD8+T cell recognition. JCI Insight. 2016;1:e87102
195. Radford K, Pearson F, Masterman K-A, Tullett K, Haigh O, Walpole C. et al. Targeting human CD141+DC using CLEC9A antibodies for cancer immunotherapy. Cancer Immunol Res. 2019 7
196. Dey M, Chang AL, Miska J, Wainwright DA, Ahmed AU, Balyasnikova IV. et al. Dendritic cell-based vaccines that utilize myeloid rather than plasmacytoid cells offer a superior survival advantage in malignant glioma. J Immunol. 2015;195:367
197. Le Moignic A, Malard V, Benvegnu T, Lemiègre L, Berchel M, Jaffrès PA. et al. Preclinical evaluation of mRNA trimannosylated lipopolyplexes as therapeutic cancer vaccines targeting dendritic cells. J Control Release. 2018;278:110-21
198. Van der Jeught K, De Koker S, Bialkowski L, Heirman C, Joe PT, Perche F. et al. Dendritic cell targeting mRNA lipopolyplexes combine strong antitumor T-cell immunity with improved inflammatory safety. Acs Nano. 2018;12:9815-29
199. Patel PM, Ottensmeier C, Mulatero C, Lorigan P, Plummer R, Hannaman D. et al. An adjuvant clinical trial of SCIB1, a DNA vaccine that targets dendritic cells in vivo, in fully resected melanoma patients. J Clin Oncol. 2015;33:9035
200. Lu Y, Shi Y, You J. Strategy and clinical application of up-regulating cross presentation by DCs in anti-tumor therapy. J Control Release. 2022;341:184-205
201. Hossain MK, Wall KA. Use of dendritic cell receptors as targets for enhancing anti-cancer immune responses. Cancers. 2019;11:418
202. Rong Y, Dong Z, Hong Z, Jin Y, Zhang W, Zhang B. et al. Reactivity toward Bifidobacterium longum and Enterococcus hirae demonstrate robust CD8+ T cell response and better prognosis in HBV-related hepatocellular carcinoma. Exp Cell Res. 2017;358:352-9
203. Routy B, Le Chatelier E, Derosa L, Duong Connie PM, Alou Maryam T, Daillère R. et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91-7
204. Vétizou M, Pitt Jonathan M, Daillère R, Lepage P, Waldschmitt N, Flament C. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079-84
205. Fluckiger A, Daillère R, Sassi M, Sixt Barbara S, Liu P, Loos F. et al. Cross-reactivity between tumor MHC class I-restricted antigens and an enterococcal bacteriophage. Science. 2020;369:936-42
206. Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW. et al. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206:3101-14
207. Lu L-F, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K. et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997-1002
208. Kohli K, Janssen A, Foerster R. Plasmacytoid dendritic cells induce tolerance predominantly by cargoing antigen to lymph nodes. Eur J Immunol. 2016;46:2659-68
209. Wang F, Zong R, Chen G. Erythrocyte-enabled immunomodulation for vaccine delivery. Journal of Controlled Release. 2022;341:314-28
210. Steptoe RJ, Ritchie JM, Jones LK, Harrison LC. Autoimmune diabetes is suppressed by transfer of proinsulin-encoding Gr-1+ myeloid progenitor cells that differentiate in vivo into resting dendritic cells. Diabetes. 2005;54:434-42
211. Xu Y, Liu Y, Yang C, Kang L, Wang M, Hu J. et al. Macrophages transfer antigens to dendritic cells by releasing exosomes containing dead-cell-associated antigens partially through a ceramide-dependent pathway to enhance CD4(+) T-cell responses. Immunology. 2016;149:157-71
212. Zizza A, Banchelli F, Guido M, Marotta C, Di Gennaro F, Mazzucco W. et al. Efficacy and safety of human papillomavirus vaccination in HIV-infected patients: a systematic review and meta-analysis. Sci Rep. 2021;11:4954
213. Lehtinen M, Dillner J. Clinical trials of human papillomavirus vaccines and beyond Nat Rev Clin Oncol. 2013; 10: 400-10.
214. Drolet M, Benard E, Perez N, Brisson M, Boily M-C, Ali H. et al. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet. 2019;394:497-509
215. Higano CS, Small EJ, Schellhammer P, Yasothan U, Gubernick S, Kirkpatrick P. et al. Sipuleucel-T. Nat Rev Drug Discov. 2010;9:513-4
216. Small EJ, Higano CS, Kantoff PW, Whitmore JB, Frohlich MW, Petrylak DP. Time to disease-related pain and first opioid use in patients with metastatic castration-resistant prostate cancer treated with sipuleucel-T. Prostate Cancer Prostatic Dis. 2014;17:259-64
217. Herishanu Y, Avivi I, Aharon A, Shefer G, Levi S, Bronstein Y. et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137:3165-73
218. Blakney AK, McKay PF. Next-generation COVID-19 vaccines: here come the proteins Comment. Lancet. 2021;397:643-5
219. Lederer K, Castano D, Atria DG, Oguin TH, Wang S, Manzoni TB. et al. SARS-CoV-2 mRNA vaccines foster potent antigen-specific germinal center responses associated with neutralizing antibody generation. Immunity. 2020;53:1281-95.e5
220. Menshenina AP, Kit OI, Zlatnik EY, Bondarenko ES, Novikova IA, Moiseenko TI. et al. Dynamics of the immune status in patients with cervical cancer receiving complex treatment with dendritic cell vaccine. J Clin Oncol. 2019 37
221. Hickerson A, Clifton GT, Brown TA, Campf J, Myers JW, Vreeland TJ. et al. Clinical efficacy of vaccination with the autologous tumor lysate particle loaded dendritic cell (TLPLDC) vaccine in metastatic melanoma. J Clin Oncol. 2019 37
222. Karande P, Mitragotri S. Transcutaneous immunization: An overview of advantages, disease targets, vaccines, and delivery technologies. Annu Rev Chem Biomol Eng. 2010;1:175-201
223. Schnee M, Vogel AB, Voss D, Petsch B, Baumhof P, Kramps T. et al. An mRNA vaccine encoding rabies virus glycoprotein induces protection against lethal infection in mice and correlates of protection in adult and newborn pigs. PLoS Negl Trop Dis. 2016;10:e0004746
224. Clausen BE, Stoitzner P. Functional specialization of skin dendritic cell subsets in regulating T cell responses. Front Immunol. 2015;6:534
225. Weide B, Carralot J-P, Reese A, Scheel B, Eigentler TK, Hoerr I. et al. Results of the first phase I/II clinical vaccination trial with direct injection of mRNA. J Immunother. 2008;31:180-8
226. Weide B, Pascolo S, Scheel B, Derhovanessian E, Pflugfelder A, Eigentler TK. et al. Direct injection of protamine-protected mRNA: Results of a phase 1/2 vaccination trial in metastatic melanoma patients. J Immunother. 2009;32:498-507
227. Nappi F, Iervolino A, Avtaar Singh SS. COVID-19 pathogenesis: From molecular pathway to vaccine administration. Biomedicines. 2021;9:903
228. Johansen P, Storni T, Rettig L, Manolova V, Lang KS, Qiu Z. Antigen kinetics determines immune reactivity. Proc Natl Acad Sci U S A. 2008;105:5189-94
229. Broos K, Van der Jeught K, Puttemans J, Goyvaerts C, Heirman C, Dewitte H. et al. Particle-mediated intravenous delivery of antigen mRNA results in strong antigen-specific T-cell responses despite the induction of type I interferon. Mol Ther Nucleic Acids. 2016;5:e326
230. Lowenfeld L, Mick R, Datta J, Xu S, Fitzpatrick E, Fisher CS. et al. Dendritic cell vaccination enhances immune responses and induces regression of HER2(pos) DCIS independent of route: Results of randomized selection design trial. Clin Cancer Res. 2017;23:2961-71
231. Pardi N, Tuyishime S, Muramatsu H, Kariko K, Mui BL, Tam YK. et al. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J Control Release. 2015;217:345-51
232. Maier MA, Jayaraman M, Matsuda S, Liu J, Barros S, Querbes W. et al. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol Ther. 2013;21:1570-8
233. Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10:787-96
234. de Sanjose S, Delany-Moretlwe S. HPV vaccines can be the hallmark of cancer prevention. Lancet. 2019;394:450-1
235. Hall MT, Simms KT, Lew J-B, Smith MA, Brotherton JML, Saville M. et al. The projected timeframe until cervical cancer elimination in Australia: a modelling study. Lancet Public Health. 2019;4:E19-E27
236. Fang Y, Mo F, Shou J, Wang H, Luo K, Zhang S. et al. A pan-cancer clinical study of personalized neoantigen vaccine monotherapy in treating patients with various types of advanced solid tumors. Clin Cancer Res. 2020;26:4511-20
237. Hilf N, Kuttruff-Coqui S, Frenzel K, Bukur V, Stevanovic S, Gouttefangeas C. et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565:240-5
238. Kyi C, Roudko V, Sabado R, Saenger Y, Loging W, Mandeli J. et al. Therapeutic immune modulation against solid cancers with intratumoral Poly-ICLC: A pilot trial. Clin Cancer Res. 2018;24:4937-48
239. Morse MA, Bradley DA, Keler T, Laliberte RJ, Green JA, Davis TA. et al. CDX-1307: a novel vaccine under study as treatment for muscle-invasive bladder cancer. Expert Rev Vaccines. 2011;10:733-42
240. Rappazzo CG, Watkins HC, Guarino CM, Chau A, Lopez JL, DeLisa MP. et al. Recombinant M2e outer membrane vesicle vaccines protect against lethal influenza A challenge in BALB/c mice. Vaccine. 2016;34:1252-8
241. Patel JM, Vartabedian VF, Bozeman EN, Caoyonan BE, Srivatsan S, Pack CD. et al. Plasma membrane vesicles decorated with glycolipid-anchored antigens and adjuvants via protein transfer as an antigen delivery platform for inhibition of tumor growth. Biomaterials. 2016;74:231-44
242. Pitt JM, André F, Amigorena S, Soria J-C, Eggermont A, Kroemer G. et al. Dendritic cell-derived exosomes for cancer therapy. J Clin Investig. 2016;126:1224-32
243. Segura E, Amigorena S, Théry C. Mature dendritic cells secrete exosomes with strong ability to induce antigen-specific effector immune responses. Blood Cells Mol Dis. 2005;35:89-93
244. Schorey JS, Harding CV. Extracellular vesicles and infectious diseases: new complexity to an old story. J Clin Investig. 2016;126:1181-9
245. Nikfarjam S, Rezaie J, Kashanchi F, Jafari R. Dexosomes as a cell-free vaccine for cancer immunotherapy. J Exp Clin Cancer Res. 2020;39:258
246. Viaud S, Théry C, Ploix S, Tursz T, Lapierre V, Lantz O. et al. Dendritic cell-derived exosomes for cancer immunotherapy: What's next? Cancer Res. 2010;70:1281
247. Dooley K, McConnell RE, Xu K, Lewis ND, Haupt S, Youniss MR. et al. A versatile platform for generating engineered extracellular vesicles with defined therapeutic properties. Mol Ther. 2021;29:1729-43
248. Yang W, Deng H, Zhu S, Lau J, Tian R, Wang S. et al. Size-transformable antigen-presenting cell-mimicking nanovesicles potentiate effective cancer immunotherapy. Sci Adv. 2021;6:eabd1631
249. Hadeiba H, Lahl K, Edalati A, Oderup C, Habtezion A, Pachynski R. et al. Plasmacytoid dendritic cells transport peripheral antigens to the thymus to promote central tolerance. Immunity. 2012;36:438-50
250. Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines — a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261-79
251. Miao L, Zhang Y, Huang L. mRNA vaccine for cancer immunotherapy. Mol Cancer. 2021;20:41
252. Rice J, Ottensmeier CH, Stevenson FK. DNA vaccines: precision tools for activating effective immunity against cancer. Nat Rev Cancer. 2008;8:108-20
253. Fotin-Mleczek M, Duchardt KM, Lorenz C, Pfeiffer R, Ojkić-Zrna S, Probst J. et al. Messenger RNA-based vaccines with dual activity induce balanced TLR-7 dependent adaptive immune responses and provide antitumor activity. J Immunother. 2011;34:1-15
254. Hassett KJ, Benenato KE, Jacquinet E, Lee A, Woods A, Yuzhakov O. et al. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol Ther Nucleic Acids. 2019;15:1-11
255. Oberli MA, Reichmuth AM, Dorkin JR, Mitchell MJ, Fenton OS, Jaklenec A. et al. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano letters. 2017;17:1326-35
256. Van Lint S, Goyvaerts C, Maenhout S, Goethals L, Disy A, Benteyn D. et al. Preclinical evaluation of TriMix and antigen mRNA-based antitumor therapy. Cancer Res. 2012;72:1661-71
257. Wilgenhof S, Van Nuffel AMT, Benteyn D, Corthals J, Aerts C, Heirman C. et al. A phase IB study on intravenous synthetic mRNA electroporated dendritic cell immunotherapy in pretreated advanced melanoma patients. Ann Oncol. 2013;24:2686-93
258. Van Lint S, Renmans D, Broos K, Goethals L, Maenhout S, Benteyn D. et al. Intratumoral delivery of TriMix mRNA results in T-cell activation by cross-presenting dendritic cells. Cancer Immunol Res. 2016;4:146-56
259. Zhou WZ, Hoon DS, Huang SK, Fujii S, Hashimoto K, Morishita R. et al. RNA melanoma vaccine: induction of antitumor immunity by human glycoprotein 100 mRNA immunization. Human gene therapy. 1999;10:2719-24
260. Phua KK, Staats HF, Leong KW, Nair SK. Intranasal mRNA nanoparticle vaccination induces prophylactic and therapeutic anti-tumor immunity. Sci Rep. 2014;4:5128
261. Lin Y-Y, Belle I, Blasi M, Huang M-N, Buckley AF, Rountree W. et al. Skeletal muscle is an antigen reservoir in integrase-defective lentiviral vector-induced long-term immunity. Mol Ther Methods Clin Dev. 2020;17:532-44
262. Shirota H, Petrenko L, Hong C, Klinman DM. Potential of transfected muscle cells to contribute to DNA vaccine immunogenicity. J Immunol. 2007;179:329
263. Kuhn S, Yang J, Ronchese F. Monocyte-derived dendritic cells are essential for cD8(+) T cell activation and antitumor responses after local immunotherapy. Front Immunol. 2015;6:584
264. Thurner B, Haendle I, Roder C, Dieckmann D, Keikavoussi P, Jonuleit H. et al. Vaccination with Mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999;190:1669-78
265. Unal A, Birekul A, Unal MC, Karakus E, Koker Y, Ozkul Y. et al. Dendritic cell production from allogeneic donor Cd34+ stem cells and mononuclear cells; cancer vaccine. Blood. 2016;128:5723
266. Mehrotra S, Britten CD, Chin S, Garrett-Mayer E, Cloud CA, Li M. et al. Vaccination with poly (IC: LC) and peptide-pulsed autologous dendritic cells in patients with pancreatic cancer. J Hematol Oncol. 2017;10:82
267. Ding Z, Li Q, Zhang R, Xie L, Shu Y, Gao S. et al. Personalized neoantigen pulsed dendritic cell vaccine for advanced lung cancer. Signal Transduct Target Ther. 2021;6:26
268. Liau LM, Ashkan K, Tran DD, Campian JL, Trusheim JE, Cobbs CS. et al. First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J Transl Med. 2018;16:142
269. Gu X, Erb U, Buechler MW, Zoeller M. Improved vaccine efficacy of tumor exosome compared to tumor lysate loaded dendritic cells in mice. Int J Cancer. 2015;136:E74-E84
270. Sorrentino D, Chiarle R, Manenti S, Giuriato S. PO-422 development of an ALK lymphoma-derived autophagosomal and dendritic cells vaccine. ESMO Open. 2018;3:A396
271. Liu F, Sun J, Yu W, Jiang Q, Pan M, Xu Z. et al. Quantum dot-pulsed dendritic cell vaccines plus macrophage polarization for amplified cancer immunotherapy. Biomaterials. 2020;242:119928
272. Le Borgne M, Etchart N, Goubier A, Lira SA, Sirard JC, van Rooijen N. et al. Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8+ T cell cross priming in vivo. Immunity. 2006;24:191-201
273. Verdijk P, Aarntzen EHJG, Lesterhuis WJ, Boullart ACI, Kok E, van Rossum MM. et al. Limited amounts of dendritic cells migrate into the T-cell area of lymph nodes but have high immune activating potential in melanoma patients. Clin Cancer Res. 2009;15:2531-40
274. Schreibelt G, Bol KF, Westdorp H, Wimmers F, Aarntzen EHJG, Duiveman-de Boer T. et al. Effective clinical responses in metastatic melanoma patients after vaccination with primary myeloid dendritic cells. Clin Cancer Res. 2016;22:2155-66
275. Tel J, Aarntzen EHJG, Baba T, Schreibelt G, Schulte BM, Benitez-Ribas D. et al. Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res. 2013;73:1063-75
276. Westdorp H, Creemers JHA, van Oort IM, Schreibelt G, Gorris MAJ, Mehra N. et al. Blood-derived dendritic cell vaccinations induce immune responses that correlate with clinical outcome in patients with chemo-naive castration-resistant prostate cancer. J Immunother Cancer. 2019;7:302
277. Inoges S, Tejada S, de Cerio AL-D, Perez-Larraya JG, Espinos J, Idoate MA. et al. A phase II trial of autologous dendritic cell vaccination and radiochemotherapy following fluorescence-guided surgery in newly diagnosed glioblastoma patients. J Transl Med. 2017;15:104
278. Bol KF, Aarntzen EHJG, in 't Hout FEM, Schreibelt G, Creemers JHA, Lesterhuis WJ. et al. Favorable overall survival in stage III melanoma patients after adjuvant dendritic cell vaccination. Oncoimmunology. 2016;5:e1057673
279. Qu C, Nguyen VA, Merad M, Randolph GJ. MHC class I/peptide transfer between dendritic cells overcomes poor cross-presentation by monocyte-derived APCs that engulf dying cells. J Immunol. 2009;182:3650-9
280. Harvey BP, Raycroft MT, Quan TE, Rudenga BJ, Roman RM, Craft J. et al. Transfer of antigen from human B cells to dendritic cells. Mol Immunol. 2014;58:56-65
281. Lo Jennifer A, Kawakubo M, Juneja Vikram R, Su Mack Y, Erlich Tal H, LaFleur Martin W. et al. Epitope spreading toward wild-type melanocyte-lineage antigens rescues suboptimal immune checkpoint blockade responses. Sci Transl Med. 2021;13:eabd8636
282. Knoop Kathryn A, Gustafsson Jenny K, McDonald Keely G, Kulkarni Devesha H, Coughlin Paige E, McCrate S. et al. Microbial antigen encounter during a preweaning interval is critical for tolerance to gut bacteria. Sci Immunol. 2017;2:eaao1314
283. Casella G, Rasouli J, Boehm A, Zhang W, Xiao D, Ishikawa Larissa Lumi W. et al. Oligodendrocyte-derived extracellular vesicles as antigen-specific therapy for autoimmune neuroinflammation in mice. Sci Transl Med. 2020;12:eaba0599
284. Kim NY, Son WR, Choi JY, Yu CH, Hur GH, Jeong ST. et al. Immunogenicity and biodistribution of Anthrax DNA vaccine delivered by intradermal electroporation. Curr Drug Deliv. 2020;17:414-21
285. Thanh Loc N, Yin Y, Choi Y, Jeong JH, Kim J. Enhanced cancer DNA vaccine via direct transfection to host dendritic cells recruited in injectable scaffolds. Acs Nano. 2020;14:11623-36
286. Floercken A, Kopp J, van Lessen A, Movassaghi K, Takvorian A, Joehrens K. et al. Allogeneic partially HLA-matched dendritic cells pulsed with autologous tumor cell lysate as a vaccine in metastatic renal cell cancer A clinical phase I/II study. Hum Vaccin Immunother. 2013;9:1217-27
Author contact
![]() Corresponding author: Jian You, College of Pharmaceutical Sciences, Zhejiang University, 866 Yuhangtang Road, Hangzhou 310058, Zhejiang, China. Office: 086-571-88981651, Email: youjiandocedu.cn.
Corresponding author: Jian You, College of Pharmaceutical Sciences, Zhejiang University, 866 Yuhangtang Road, Hangzhou 310058, Zhejiang, China. Office: 086-571-88981651, Email: youjiandocedu.cn.
 Global reach, higher impact
Global reach, higher impact