13.3
Impact Factor
Theranostics 2022; 12(13):5836-5855. doi:10.7150/thno.73400 This issue Cite
Review
Extracellular Vesicles and Circulating Tumour Cells - complementary liquid biopsies or standalone concepts?
1. Department of Pathophysiology, Nicolaus Copernicus University in Toruń, Ludwik Rydygier Collegium Medicum in Bydgoszcz, 85-067 Bydgoszcz, Poland.
2. Department of Internal Medicine I, University Hospital Bonn of the Rheinische Friedrich-Wilhelms-University, 53127 Bonn, Germany.
3. Institute of Molecular Medicine & Experimental Immunology, University Hospital Bonn of the Rheinische Friedrich-Wilhelms-University, 53127 Bonn, Germany.
4. Octavian Fodor Institute for Gastroenterology and Hepatology, Iuliu Haţieganu, University of Medicine and Pharmacy, 400162 Cluj-Napoca, Romania.
5. Department of General, Visceral and Vascular Surgery, German Armed Forces Hospital Hamburg, 22049 Hamburg, Germany.
6. Department of Integrated Oncology, Center for Integrated Oncology (CIO), University Hospital Bonn of the Rheinische Friedrich-Wilhelms-University, 53127 Bonn, Germany.
* Shared first author
Received 2022-3-29; Accepted 2022-7-6; Published 2022-8-1
Abstract
Liquid biopsies do promise a lot, but are they keeping it? In the past decade, additional novel biomarkers qualified to be called like that, of which, some took necessary hurdles resulting in FDA approval and clinical use. Some others are since a while around, well known and were once regarded to be a game changer in cancer diagnosis or cancer screening. But, during their clinical use limitations were observed from statistical significance and questions raised regarding their robustness, that eventually led to be dropped from associated clinical guidelines for certain applications including cancer diagnosis. The purpose of this review isn't to give a broad overview of all current liquid biopsy as biomarkers, weight them and promise a brighter future in cancer prevention, but rather to take a deeper look on two of those who do qualify to be called liquid biopsies now or then. These two are probably of greatest interest conceptually and methodically, and likely have the highest chances to be in clinical use soon, with a portfolio extension over their original conceptual usage. We aim to dig deeper beyond cancer diagnosis or cancer screening. Actually, we aim to review in depth extracellular vesicles (EVs) and compare with circulating tumour cells (CTCs). The latter methodology is partially FDA approved and in clinical use. We will lay out similarities as taking advantage of surface antigens on EVs and CTCs in case of characterization and quantification. But drawing readers' attention to downstream application based on capture/isolation methodology and simply on their overall nature, here apparently being living material eventually recoverable as CTCs are vs. dead material with transient effects on recipient cell as in case of EVs. All this we try to bring in perspective, compare and conclude towards which future direction we are aiming for, or should aim for. Do we announce a winner between CTCs vs EVs? No, but we provide good reasons to intensify research on them.
Keywords: CTC, extracellular vesicles, exosomes, microvesicles, ectosomes, biomarker, liquid biopsy, personalized medicine
Introduction
The history of liquid biopsies in cancer is funded on a historical misunderstanding through many decades how the term liquid biopsies is actually defined vs. how it has been used nowadays as outlined by Todd M. Morgan in his review article from 2018 and by others. Dr. Morgan made his point that defining “what constitutes a liquid biopsy is important here.” [1]. Furthermore, he wrote, that “[t]he term biopsy implies direct measurement of a tumo[u]r, so the liquid biopsy marker should be restricted to tests with specificity approaching that of a tissue biopsy”. In the light of this definition, it's practically impossible to use the term liquid biopsies in association with extracellular vesicles (EVs) if going in line with Dr. Morgan. Probably, it should be substituted with the term personalized cancer diagnostics, or as Dr. Morgan suggested, “…the term liquid biopsy is becoming as commonplace as precision medicine, ”that's because it probably is.”[1].
Despite this interesting opinion, majority of experts and their countless expert reviews including our own, original research titles and other peer-reviewed publications are still using and accepting the term liquid biopsy to define different kind of biomarkers that are obtained from patients applying non-invasive (urine sample, smear) or minimal-invasive (puncture to draw blood) procedures and which are associated with patients' health condition, present, past or prognostic [2].
One of the oldest reports on liquid biopsies was published 1966 by Wichelhausen RN et al., which is matching the widely used definition and isn't a biopsy in the light that patient's cell tissue was obtained and then eventually further cultured and finally analysed accordingly [3]. From far bigger ramification was a publication from 1990 in which Partin A.W. and colleagues reported the quantitative assessment of prostate specific antigen (PSA) in serum samples in association with prostate cancer tumour volume and differentiation and as benign prostatic hyperplasia volume [4]. Interestingly, at that time the authors reported discrepancies between pathological stage and serum PSA that might be explained by a decrease in production of PSA with increasing histological grade according to authors of this study. Few decades later, 1991, the study results were repeated on a broader scale and published in New England Journal of Medicine, a highly respected and very influential clinical peer-review journal, hence, eventually becoming the gold standard in prostate cancer screening via a liquid biopsy [5]. Going in line, 1994, US Food and Drug Administration (FDA) approved PSA in conjunction with a digital rectal exam (DRE) to test asymptomatic men for prostate cancer [6]. PSA is potentially one of the first liquid biopsy marker as approved by the FDA. And probably a story of success saving countless of men lives.
Another liquid biopsy cancer biomarker that was once regarded to be the one that might bring screening and diagnosis in case of hepatocellular carcinoma (HCC) to another level was Alpha-fetoprotein (AFP). This biomarker was even recommended to be included to several international and national guidelines as provided by various organizations as the American Association for the Study of Liver Disease (AASLD) and the European Association for the Study of the Liver (EASL) besides others [7-9] at that time. Due to the promising results, the 2003 HCC guidelines from the British Society of Gastroenterology recommended both this biomarker and abdominal ultrasound for HCC diagnosis [10]. During the course of using this biomarker, several concerns regarding sensitivity and specificity and usefulness of cut-off values appeared, so that finally this marker was soon questioned and eventually dropped for HCC screening and wasn't recommended anymore until today by AASLD or EASL [11-13]. Very unfortunately, AFP had been shown to be relatively insensitive as it is only elevated in 40-60% of HCC cases, thus HCC patients exhibit normal AFP serum levels, particularly during early stage disease [14, 15]. Additionally, AFP levels can also be found elevated in non-HCC patients, including non-cancerous chronic liver diseases, intrahepatic cholangiocarcinoma and metastatic colon cancer [16]. In sum clinical practice guidelines do not recommend AFP (or any other biomarker for that matter) for the diagnosis of HCC [12].
Besides these two proteins based liquid biopsy cancer biomarkers, some other methodical interesting liquid biopsy biomarker in cancer were actually studied, being somehow non-invasively taken as cervical smear that is routinely assessed and used to explore feasibility of experimental use of those samples for DNA testing using both Digene Hybrid Capture assay (DHCA) and polymerase chain reaction (PCR) techniques at that time, 2001, to detect high-risk human papillomaviruses (HPVs) DNA [17]. From nowadays perspective probably to be regarded as another milestone in liquid biopsy in cancer.
Especially in the last 1 ½ decades, liquid biopsy biomarker in cancer took another spin, another boost by taking advantage of novel tracers of various tumours that might be potentially successfully utilized. The “tumour circulome” comprises circulating tumour cells (CTCs), extracellular vesicles (EVs), cell-free tumor DNA (ctDNA), cell-free DNA (cfDNA), circulating tumour RNA (ctRNA), and tumour-educated platelets (TEPs) [18]. All of those biomarkers are in agreement kind of liquid biopsies and as Dr. Morgan would say, precision medicine. However, in this review we would like to discuss differences and similarities between EVs and CTCs. We note comprehensively the limitations and advantages of two upcoming liquid biopsies in cancer screening and diagnosis. Both are somehow ancestors of the tumour mass itself, directly, being a tumour cell as CTCs are or indirectly, as being derived from a tumour cell as EVs are. One of the main differences between both markers is from numeric nature. In the following paragraphs two of those, EVs and CTCs, will be introduced in depth since both are sharing some interesting common features regarding their analysis.
EVs - a star is born
54 years ago, 1967, Wolf P described something like as 'platelet dust' as a trivial by-product of cell degradation in his preparations [19]. His electron microscopic data revealed lipid‐rich particles that may originate from the osmophilic granules of platelets and interestingly the “liberation of this material” as he called, is the result of 'activation' [19]. Nowadays, we call them extracellular vesicles (EVs) and typically activation is one of the major mechanisms in vivo and in vitro besides others to induce the release of small EVs (sEVs) or large EVs (lEVs) [20]. 2011 we published that the induction of lEVs-release by various mechanisms as donor cell activation, as induction of apoptosis in donor cells might eventually result in lEVs differing in the capability to induce fibrolysis in recipient cells in vitro [21]. At that time, we called those EVs 'microparticles' and others 'microvesicles' or 'ectosomes'. Today's International Society For Extracellular Vesicles (ISEV) guidelines, published by ISEV members as us, with the purpose to frame an urgent needed standardisation, actually agreed on to call them correctly as lEVs [22].
During the last decade EV research intensified by a lot, EV research caught finally attention of many research communities including liver research communities and others [23]. Thus, advanced methodologies enabled to categorize EVs into sEVs, typically consistent of exosomes, and smallest microvesicles (MV). On the contrary, lEVs are consistent of microvesicles aka microparticles (MPs)/ectosomes [20, 24, 25]. This careful differentiation implies already that distinction between sEVs and lEVs, isn't razor sharp and exactly defined by a size number. Some might say 100nm might be the border, other 150nm [22, 26]. However, exosomes and MPs/MVs can be distinguished clearly by their biogenesis. MPs/MVs could range in sizes from around 100 to 1000nm and are shed directly from the cell membrane by a “budding process” during cellular activation or early apoptosis [20]. Exosomes are the smallest vesicles, usually below 100 nm, and formed in endosomes and are stored within multi-vesicular bodies (MVB) that release their contents into intracellular space upon fusion with the cell membrane [27]. Several markers for exosomes have been described including head shock protein HSP70 and the integrins as CD63, CD9, CD8 [28-30]. However, some of these might be expressed on MVs/MPs as CD9 and CD81 [31].
Apart from EV size and EV marker issues that are in detail unresolved, lEVs are somewhat representing its donor cell with its membrane-associated proteins on a smaller scale, making lEVs into an appealing field of research. sEVs and lEVs contain lipids, cytosolic proteins, some messenger RNAs and microRNAs [20, 22, 32]. Once EVs were considered to be a kind of a cellular waste system, removing unneeded cytosolic proteins, some messenger RNAs and microRNAs. This idea is still under discussion [33]. Apparently, EVs are more than that. EVs are orchestrating many physiological or pathological effects by their cargo and membrane composition and are a novel horizontal cell to cell communication route, including for the communication between tumour cell and tumour niche, inducing tumour tolerance. Apart from that, eventually its worth to highlight their potential for early cancer screening, cancer diagnosis, especially before metastasis takes place [34]. Until now, not a single human body cell was reported to be incapacitated of the ability to release EVs including tumour cells, “underlining the attractiveness of these vesicles to use as novel minimal invasive biomarkers not only in liver tumours but also in non-malignant liver diseases” [25]. In other words, EVs might be an outstanding tool as an integral part of a new generation of liquid biopsy cancer biomarkers. Moreover, newest work done by von Felden et al. on exosomal unannotated small RNA clusters associated with circulating extracellular vesicles detect early stage liver cancer has given us a preview how beautifully future biomarker studies will take advantage of artificial intelligence (AI) to find novel profiles that helps to manage cancer patients [35]. It is foreseeable that AI might be a game changer [36]. However, validation cohorts/studies are still needed to verify AI driven data bank analyses. Additionally, it is noteworthy to hint that some evidence are given that exosomal proteo-transcriptome may vary compared to their donor cell as shown for 12 cancer cell lines and in human prostate cancer, which is unexpected. How much relevance it could have, e.g implicate a given limitation to which extend EVs are mirroring their donor cells, remains open [37].
The pathophysiological role of EVs is probably manifold and in depth discussed elsewhere. Many in vitro and in vivo animal experiments were done demonstrating a possible role of EVs in extracellular communication between cells proximal or distal, downstream pathway activation in EV recipient cells or even contributing to tumour tolerance [30, 34]. But, those reports could only give a glimpse that EVs, lEVs or sEVs, have actually a more pronounce pathophysiological role then thought and from far bigger clinical interest beyond cancer screening. One of the most prominent examples of EVs capability acting as a novel treatment was shown exemplarily 2017. Kordelas L. et al. performed a first human interventional study where mesenchymal stem cell derived EVs (MSC-EVs) were administered in case of steroid refractory graft-versus-host disease (GvHD) [38]. How precisely MSC EVs did act was debatable, some thought that MSC EVs might be rather the source of a substance that was released by MSC. However, MSCs were isolated from bone marrow donors, expanded and conditioned supernatants were collected being potentially MSC-EV rich. A dose, 1 unit of MSC EVs was defined as the amount of MSC EVs released by 4 × 107 for patient's body weight. This highly cited publication demonstrated a successful experimental clinical application of MSC-EVs where MSC-EVs decreased probably indirectly numbers of patient-derived peripheral blood cells, which secreted pro-inflammatory cytokines IL-1β, TNFα and IFNγ, likely contributing in modulating patient's immune status that led to significant improvement of clinical GvHD symptoms [38]. Following this report, it is fair to conclude that EVs, here MSC derived EVs might play an important future role as a novel therapy option, e.g. in personalized medicine.
CTCs, established and accepted concept
Circulating tumour cells (CTCs), commonly a subset of malignant cells, that are typically disconnected from their primary tumour and circulating in the peripheral blood, can be phenotyped according their surface antigen status or analyzed at their expression-, protein- and genomic level allowing their assessment of their dynamic status of the respective patients at different cancer stages and cancer heterogeneity [39, 40]. Noteworthy, CTCs were first time described by Ashworth T.J. in 1869 in blood of a metastatic cancer patient indicating their role in cancer spread and by doing so paving the ground for modern CTC based diagnostics [41]. Thus, rarely, CTCs may also appear in premalignant conditions, under conditions where “potential” CTCs were detected without any molecular proof of malignancy at this stage [42]. Nowadays, CTCs are regarded to be one of the most prominent kinds of liquid biopsy and their enumeration offers a simple, highly standardized and vigorous way to do precise medical disease pre-diagnosis, prognosis, and therapy response assessment [39, 43, 44]. Several applications in various tumour entities had been published so far. Some of those are very compelling and some are showing interesting conceptual extensions of the common CTC diagnostic routine. The authors covering certain aspects of how CTCs could be potentially applied in pre-diagnosis, prognosis, and therapy response assessment have summarized those.
Considering the blood micro-environment in which CTC circulating, it is quite challenging to find a viable CTC maker to eliminate interference of billions of white blood cells, non-malignant endothelial cells, stem cells, or other blood cells as erythrocytes, thrombocytes, leucocytes etc. These CTC markers should be at best located on the CTC cell membrane, therefore easily accessible and robust enough expressed on those CTCs without pathological inhibition. In other words, its shouldn't be present on non-malignant cells, for example, epithelial markers which could be absent on mesenchymal leukocytes but presented on cancer cells or vice versa, depending if CTC will be positive or negative selected [45]. Newer CTC capturing strategies are either based on their physical properties as size (ISET: isolation by size of epithelial tumour cells) [46], as elasticity and deformability [47] and others that aren't depending on their surface tumour specific antigen profile. Therefore CTC isolation can be generally divided into label and label-free detection as reviewed in detail by Habli Z. et al. [47]. Independent of such methodical advantages, the most established and most robust CTC capture methodology is probably the immunobead assay using epithelial cell adhesion molecules (EpCAM) to select EpCAM+ CTCs (positive selection) and simultaneously to exclude CD45+ white blood cells (negative selection) [48]. Keeping in mind that 1995 Gross H.J. et al. demonstrated that utilizing flow cytometry could in fact discriminate rare cancer cells from other cells in blood and bone marrow introducing the usage of CD45 and “multiple markers, each identified by a separate color of immunofluorescence (yellow and two shades of red) tri-fluorochromes” [49]; at that time a remarkable achievement in flow cytometry. Approximately one decade later, finally, in 2004 the CellSearchTM system was introduced and first data in Clinical Cancer Research published [48]. The CellSearchTM system, as an only Food and Drug Administration (FDA)-cleared immunobead assay detecting EpCAM+CK+CD45- CTCs has been designed to enumerate the number of CTCs in 7.5ml of whole blood under the assumption that under non-pathological conditions EpCAM+CK+CD45- cells will be not present in the peripheral blood. The sensitivity exceeds 90% in metastatic breast, in metastatic prostate, and in metastatic colorectal carcinoma studies [48]. Currently, the CellSearchTM is still regarded as the gold standard by many experts in the field. Typically, in brief, CTCs will be captured, enriched, and fluorescently stained by the automated autoprep system, and last enumerated by the semi-automatic CellSpotter Analyzer [48, 50].
To overcome shortcomings of EpCAM as used by CellSearchTM system and others, the detection error of isolation by size of epithelial tumour cells (ISET) due to size overlapping between CTCs (12 -35µm) and some monocytes /eosinophils (12-20µm) [46], a lot of attention was shifted to the modern nanoelectromechanical CTC chip (NELMEC) and believed that NELMEC can solve aforementioned problems because compare with tumour cells, lower membrane capacitance and higher cytoplasmic conductivity in leukocytes would give less observable movement in the impedance of the SiNG electrode [51]. Another chip based solution, CTC iChip, actually combined size-based enrichment with either EpCAM positive enrichment or CD45 negative depletion with a reported yield of rare cells of 97% and processing rate of 8ml of whole blood/h [52].
To date, the inclusion of CTCs in the clinical assessments for the management of colorectal and breast cancers has not been accepted by the American Society of Clinical Oncology (ASCO) Tumor Marker Guidelines Committee. In 2007, CTCs and disseminated tumour cells (DTCs) were just cited in ASCOS's recommendations on cancer markers [53]. On the contrary, lately, the American Joint Committee of Cancer (AJCC) proposed a new category for TNM staging in breast cancer (M0(i+) as defined by CTCs or DTCs, if evidences of distant metastases are missing [54]. Likewise, no other committees recommended CTC to diagnose cancer or make therapy decisions, but still possible all phases of CTC cancer guidelines will be formulated following the advantage of mature CTC techniques in the near future [55]. The reasons might be manifold, the limited possibility to detect cancer at an early timepoint under the caveat of cancer screening? Some speculated that the heterogeneity of the clinical studies regarding patients/CTC donor selection might play a role asking for CTC guideline (CTC Guide) on study design and study report [55].
Some authors try to extend the common CTC diagnostic routine to additional cancer entities as non-small cell lung cancer (NSCLC) [56, 57], hepatocellular carcinoma (HCC) [58, 59], pancreatic ductal adenocarcinoma (PDAC) [60, 61] besides other cancer entities. Most of these studies were carried out using peripheral blood as a CTC source, in NSCLC pulmonary venous PV-CTC counts were assessed being not significantly related to the personal age, gender, smoking status, even pathological stage, an increased PV-CTC count would be closely associated with the higher risk of cancer recurrence [62]. NSCLC patients with a PV-CTC count higher than 7/7.5ml blood volume post R0-resection are designated to be more frequent postoperative medical examination, due to a higher risk for NSCLC recurrence [62]. Furthermore, few studies could demonstrate the benefit of CTC in pre-screening efforts providing evidence that the existence of CTC was actually detected by an average of 3.2 years earlier than lung nodules discovered in the subsequent CT scan in COPD patients. The authors named this CTC as sentinel CTCs [63].
CTCs vs EVs, a direct comparism
Advantages, limitations pros and cons
Though CTCs and EVs are very different in many ways, living material, ongoing metabolism and reproducible, proliferative vs. non-living material, kind of smallest traces of cells, besides obviously being different in size, macro vs nano-sized. Nevertheless both, CTCs and EVs, share some methodical similarities how to be identified, some minor conceptional and mythological differences how to be phenotyped. Incommon, both are taking advantage of utilizing surface antigens as available on their membrane surface [64]. The widely used CellSearchTM is designed using surface antigens such as EpCAM or CK and others as discussed as a kind of positive selection and CD45 for negative selection of leucocytes, besides DAPI, an intracellular nucleus staining as discussed before [48]. This nucleus staining capability of CTCs is in EVs obviously not given, since EVs are lacking a nucleus and larger genomic material except minor genomic fragments [65, 66]. Additionally, and probably one of the major differences is that an intracellular staining in case of EVs, likely to be called an intravesicular staining, wasn't reported so far to our knowledge, since an intracellular staining relies generally speaking “to poke holes” into the cell membrane using mild membrane solubilizers as Tween 20, as Saponin, as Digitonin and as Leucoperm (0.5% v/v in PBS) [67]. However, a very recent publication demonstrated that EVs bound to a coverslip overnight at 4 degrees Celsius were fixed with paraformaldehyde and were permeabilized using 0.1% Saponin for downstream imaging purposes [68]. Without fixation, such detergence will eventually destroy as reported for sodium dodecyl sulfate (SDS), an anionic detergent, the structural integrity of nano-sized EVs and random those to be useless for quantification efforts [69].
Typically, both type of liquid biomarker, CTCs and EVs, had been phenotyped by flow cytometry by several researchers around the globe using various available models of flow cytometers including advanced imaging flow cytometry as with various resolutions regarding lower detectable size boarder [70-73]. A publication in Nature 2015 on GP1+ (Glypican) sEVs, exosomes, was eventually the most recognized publication and a kind of kick off in EV based cancer diagnosis [74]. On the contrary, 2004 can be regarded as the year when CTCs had their breakthrough by recognition and approval by the FDA. Nevertheless, the usage of sEVs in prostate cancer diagnosis received FDA acknowledged by granting Breakthrough Device Designation to Bio-Techne ExoDx Prostate IntelliScore (EPI) test, [48, 75]. Nevertheless, CTCs had been so far researched for a longer time period than EVs as a kind of cancer liquid biopsy biomarker in patients' blood. Hence it is not surprising that the methodology of CTC isolation and phenotyping is more advanced and to a higher degree standardized. Including FDA approved for the detection of several metastatic cancer entities in the USA. In contrast, the EV research field is somehow still exploring and elaborating the usefulness of EVs in cancer diagnosis and especially with a strong emphasis on prognosis [44]. EVs are still somewhat experimental and guidelines were given to the EV field by the International Society for Extracellular Vesicles (ISEV) 2014 and 2018 [76, 77]. Of note CTC methodology is not final set, neither in case of EVs. New combinations of CTC antigens have been investigated allowing diagnosis and prognosis in case of additional cancer entities, yet not FDA approved [72].
Hypothetically, there is actually an overlap given of used antigens on CTCs and EVs and being specific for cancer entities. The classical CellSearchTM pan cancer marker is EpCAM, reliably used for the detection of CTCs in co-junction with CD45 and DAPI and others (experimental) [44, 48, 58]. Interestingly, EpCAM on lEVs was insufficient in discrimination between investigated cancer entities as colorectal-, as non-small cell lung-, as pancreas carcinoma and thyroid nodules, kind of abnormal growth of thyroid cells forming a lump within the thyroid gland, typically non-malignant [78]. In fact this was foreseeable and the need of combinations of antigens was discussed 2014 already [79]. Only combination of antigens being simultaneously present on the same large EV, here AnnexinV, CD147 (Emmprin) and EpCAM were sufficient to separate patients suffering from thyroid gland and cancers [78]. Additionally, other combinations were reported being specific to a certain extend for biliary malignancies, including the EV based differentiation of HCC from cholangiocarcinoma (CCA) including intrahepatic CCA [73, 80]. Applying cancer entity specific antigens on CTCs was meanwhile achieved by taking advantage of hepatocyte markers as Glypican3 and ASGPR1, in combination with EpCAM utilizing flow cytometry for experimental purpose [72, 81]. ASGPR1 in combination with EpCAM was also successfully applied to detect HCC derived lEVs [80].
Some researchers didn't rely on initial EpCAM-based capture of CTCs allowing them to identify additional CTC populations being associated with cancer progression. Armstrong A.J. et al. explored not only CTCs in patients with metastatic CRPC co-expressing EpCAM or CK, but rather E-cadherin, mesenchymal proteins as Vimentin, as N-cadherin and O-cadherin, thus CD133 as well [82]. All of them eventually missed by the FDA- approved CellSearchTM methodology and other on CTC isolation and numeration depending methods heavily relying on EpCAM. Amstrong's et al. data suggests that CTCs from common epithelial malignancies co-express epithelial and mesenchymal markers, suggesting that EMT/MET transition is likely contributing to metastatic progression. From importance is, that EpCAM is lost earlier than cytokeratin during the epithelial-to-mesenchymal transition resulting in escape from EpCAM-based CTC capture [83]. To our best knowledge EMT/MET transitions surface antigens on EVs were not explored yet.
Another flow cytometric advantage and applicable on CTCs, eventually isn't yet reproduced on EVs, the so-called discrimination of high expressing and low expressing CTCs [84], up to date not achieved on EVs. Scientists around the world can easily providing ample of examples where it does matter if a cell's antigen is high or low/dim expressing [85, 86]. No reports are available if CellSearchTM is capable to do so would be off-label usage. An experimental setting in which the blood waste was enriched with cancer cell line cells (blood waste discarded by CellSearchTM) was collected and passed through a micro-sieves and accounted. In fact, the CellSearchTM cartridge effectively recovered EpCAMhigh tumour cells, whereas the EpCAMlow cells are mainly recovered by micro-sieve [87]. This might be from relevance, since EU-FP7 CTC-Trap program suggested that CTCs expressing high levels of epithelial cell adhesion molecule, EpCAMhigh compared to EpCAMlow CTCs were associated with a different clinical outcome as given in median survival in metastatic prostate and breast cancer patients. In favor of EpCAMhigh CTCs, those were strongly related to shorter survival [84].
As shown, EpCAM does play a prominent role in the FDA approved CellSeachTM system. The hypothetical question that must appear, is obviously, if the CellSearchTM System and other immunoaffinity - positive selection/enrichment based systems will evolve further into a system that is more capable to detect specific cancer entities by utilizing another selection of antigens present on cancer derived CTCs, for example Glypican-3, ASGPR1 and HepPar1 as for liver cancer derived CTCs [88-90]. Of note such move has successfully to pass clinical phases prior FDA approval and clinical use. Would such evaluation of the CellSearchTM system give physicians an advantage in liver cancer diagnosis and prognosis? It is debatable and financial interest might play a role.
However, as discussed above both, CTCs and EVs, can be used for cancer diagnostic applications and of note cancer prognosis [44-46, 62, 74, 80]. Furthermore, at this juncture, we have a real field to maneuver, as many studies have indicated that both structures with high sensitivity and specificity can be used as a liquid biopsy in the diagnosis and prognosis of patients' outcomes and responses to treatment [36, 44, 91]. Nevertheless, many meta-analyses have confirmed the unique diagnostic and prognostic value of CTCs [92-95]. The same properties are attributed by meta-analyses to circulating EVs [96-99]. We conjecture that both of these laboratory markers will soon be introduced into run-of-the-mill clinical diagnostics and that the interpretation of the results may be helped by artificial intelligence and machine learning [30]. In case of cancer prognosis, there might be another prognostic advantage in case of CTCs. CTC clustering. Interestingly, many recent studies have shown that at the beginning of metastasis, CTC clusters are more dangerous than single cells [100]. Recently, it has been identified that Na+/K+ ATPase inhibitors can dissociate CTC clusters into individual cells, thereby inhibiting the spread of cancer, as this might affect DNA hypermethylation at the binding sites of OCT4, NANOG, SOX2, SIN3A and, so on [101]. There's lots of evidence that the formation of tight clusters means shorter remission time, which can use for treatment efficacy monitoring. For example, a patient with lung cancer produced tighter CTC clusters in culture after accepting several cycles of nivolumab, and later PET and CT examinations also further confirmed that the patient had multiple metastases [102]. Obviously, those discoveries provide direction for different therapeutic areas. Again, CTC analysis permits cluster analysis if cell clusters weren't excluded by the used flow cytometry gating strategy. On EV level, nano-size level, we cannot exclude or include reliably EV clusters, typically EVs are ranging a size from 100-1000nm in case of large EVs, giving ample or room for EV duplets or triples. An optical EV flow cytometer as provided by AMNISTM and optimized for the detection of EVs [70], could potentially allow the identification of EV doublets or EV clusters, still doubting on their naturally existence or rather being an artefact due to the isolation process? No experimental data is available on that matter.
Another interesting aspect is, that the source of CTCs and EVs, not on a cellular level, rather, if isolated from peripheral blood (PB), from cancer draining venous blood (DVB) or other organ specific fluids (bile, cyst liquid) might make a difference regarding their diagnostic performance. It was concluded that CTCs numbers may differ after transition through organs, especially high vascularized organs as liver and lung as shown for human colon carcinoma cell line, KM12-HX s, after intraportal vein (i.p.v.) or intravenous (i.v.) injection into rats [103]. Some researchers might consider that peripheral blood might be easier assessable, but numbers of CTCs in cancer draining venous blood (DVB) could evolve to a more robust diagnostic and prognostic performance. Hence, Tsutsuyama M. et al. compared CRC derived CTCs isolated either from DVB or from PB, and demonstrated higher CTC numbers in DCB. Furthermore CTCs numbers in DVB the level of CTCs continued to rise with the cancer deterioration [104]. Noteworthy and somehow a limitation is the fact, that in this experimental setting, DCB was obtained from the mesenteric vein immediately after tumour resection [104]. Going in line in it was reported that higher CTC counts in portal venous blood collected during pancreaticoduodenectomy in periampullary or pancreatic adenocarcinoma without metastases as could predict liver metastases post-surgery [105]. Similar results were reported a year earlier in patients resectable pancreatic cancer [106]. The question that arises now is obviously if EV numbers will be higher in DVB compared to PB as reported for CTCs? Hypothetically the answer should be probably. Surprisingly, in resectable non-small cell lung cancer (NSCLC) difference in size between sEVs isolated from tumour-draining pulmonary vein blood compared to PB were observed. Actually tumour-draining pulmonary vein blood was enriched with sEVs (30-50nm) and smaller EV size was linked to tumour relapsed and shorter overall survival [107]. Unfortunately, the authors didn't discuss the cause of such size differences. 2017 another interesting observation was reported and highly published despite its methodical simplicity, which might let to reasonable faster transfer from an experimental stage towards clinical use. sEV concentrations were compared as detectable in bile and PB in malignant biliary stenosis as pancreatic cancer as cholangiocarcinoma. As expected, sEV concentration in bile was significantly higher and could distinguish malignant vs. non-malignant CBD stenosis with 100% accuracy [108].
The above-discussed differences between CTCs and EVs might be considered minor to a certain degree. Other differences as described next, might be considered major, since CTCs and EVs might obviously not being capable to be used as the other one and vice versa. One of the major differences is, that EVs might be used as a drug delivery system. On the contrary CTCs might be not a desired candidate for drug delivery after any thinkable ex vivo manipulation for obvious reasons. Paclitaxel-mediated mesenchymal stromal cells possible exert an excellent anti-tumour effect by obtaining drugs and subsequently packaging them in EVs [109]. Interestingly, it was suggested in order to overcome possible cross-species adverse EV tolerance effect the usage of bovine milk-derived sEVs for drug delivery [110]. This might be an alternative to artificial produced EVs consuming needed resources as lipids which are in need for mRNA based vaccines [111].
Additionally, EVs might own certain capabilities to support organ/tissue damage repair due to the used EV origin in case of mesenchymal stromal/stem cell (MSC)-derived sEVs as reviewed by Varderidou-Minasian S and Lorenowicz M.J. [112]. Just to highlight the capabilities of MSC-EVs the group around Giebel B. successfully administered MSC-EVs in case of steroid refractory graft-versus-host disease (GvHD) [38]. Until this it was believed that MSC might be a therapy option in severe therapy-refractory acute GvHD. However, how precisely MSC did act was debatable, some thought that MSC might be rather the source of a substance that was released by MSC. However, MSCs were isolated from bone marrow donors, expanded and conditioned supernatants were collected being potentially MSC-EV rich. A dose, 1 unit of MSC-EVs was defined as the amount of MSC-EVs released by 4×107 for patient's body weight. This highly cited publication demonstrated a successful experimental clinical application of MSC-EVs where MSC-EVs decreased numbers of patient-derived peripheral blood cells, which secreted the pro-inflammatory cytokines IL-1β, TNFα and IFNγ, likely contributing modulating patient's immune status and significant improvement of clinical GvHD symptoms [38].
Obviously, CTCs cannot be used to be ex vivo manipulated and then administered back to the donor patient hoping for a kind of cancer cure. But circulating MSC if robust phenotyped, could be likely isolated with a CellSearchTM like methodology too, thus recovered from the CellSearchTM cartridge and ex vivo manipulated or simply serving as MSC-EVs donors and stored for later, similar to the collection of umbilical cord haematopoietic stem cells (HSCs). Many research groups around the globe are working downstream solution on various recovery strategies of CTCs from the cartridge or utilizing other devices, commonly aiming for a possible expansion of CTCs in vitro due to the low numbers of rare CTCs in cancer patient's blood as discussed by Sharma S. et al. [113]. Typically followed by several applications as drug testing, as expression arrays, as genomic analysis for prognosis or as estimating possible drug effectives on designated cancer entities [114-117]. Of note expression arrays and genomic analysis can be done with single cells technology since CTCs are rare and low in numbers. How rare? Recently, in a German study metastatic prostate cancer patients were associated with a median CTC count of 4 (range, 0-820), and a mean CTC count of 27 as baseline before treatment [118]. Similarly, low numbers were associated with prostate cancer [119], However, the initial CellSeachTM publication from 2004 reported for metastatic carcinoma entities as prostate, breast, lung, colorectal, ovarian and others a mean of 60 ± 693 CTCs per 7.5 mL and a used cut-off of 2 CTCs per 7.5mL [48].
Of note, expression analysis on mRNA and protein level, miRNA arrays including small noncoding RNA [32, 120, 121] can be obviously applied to EVs too as shown by many scientists. Aiming for the detection of cancers [74], cancer entity discrimination [73, 80] or for prognostic proposes [98, 122, 123]. Of note, the question is, how feasible EV expression or genomic arrays are in term of a future clinical application? Simplicity might be desired here, as the detection of biliary cancer on the base of total EVs in bile or overall costs per sample [108].
All these possible applications pointing towards personalized medicine and will be discussed in depth later. However, drug testing can be done only on living material as CTCs, since reverse engineer of a living cell by adding up EVs should be regarded as impossible. Therefore, drug testing ex vivo/in vitro, to choose the most effective anti-cancer drug or it's concentration (e.g. for individualized drug susceptibility test) will be only achieved with CTCs [114, 124-126]. In vivo, the effectives of given treatment/drugs, in terms of therapy monitoring can be achieved with CTCs [127] and EVs [128].
Besides additional surface antigens that are actively explored being suitable for CTC enrichment, certain soluble proteins, as cytokines in conjunction with CTCs have been investigated as well, potentially associated with cancer progression and metastasis. 2016 the SUCCESS Study group reported that in pre-therapy primary breast cancer soluble IL-1α was associated with CTC presence in peripheral blood but not within the lymphatic-system indicating IL-1α might suit as a marker for lymphatic cancer invasion. CTC-positive patient no lymph node involvement were reported with high levels of IL-1α, whereas those with lymph node involvement expressed low levels of IL-1α [129]. The SUCCES study group members concluded that reduced levels of IL-1α might be a crucial factor to generate a tissue microenvironment that stimulates cancer expansion [129]. Besides IL-1α, IL-17A was linked with disease-free survival in patients with colorectal carcinoma (CRC), potentially serving as a surrogated marker in combination with CTC in CRC. Same group reported that ablation of IL-17A combined with rGM-CSF effectively suppressed CTCs and prevented organ metastasis in a CRC mice model indicating an novel CRC treatment option too [130].
On the contrary, in PDAC Glyp+ sEVs were correlating with CA19-9: However, Glyp+ sEVs alone were associated with a sensitivity and specificity of 100% [74]. Another synergistic example between a liquid biopsy marker, here lEVs and a soluble antigen was reported recently. Soluble AFP could enhance the discrimination between HCC and CCA in case of AnnV+CD44v6+ lEVs and AnnV+CD44v6+CD133+ lEVs, thus no correlation between AFP values and the investigated EV subpopulation were observed [73]. For a diagnostic application in real life it's doubtful if such synergistic effects are desired, since the initial liquid biomarker as CTCs, as EVs, have to be measured plus an additional liquid biomarker as soluble a protein, as a cytokine. A perfect, a robust liquid biomarker alone should provide the needed information if the investigated specimen is harboring a tumour, underlying tumour entity and mirror tumour dynamics, growth/shrink.
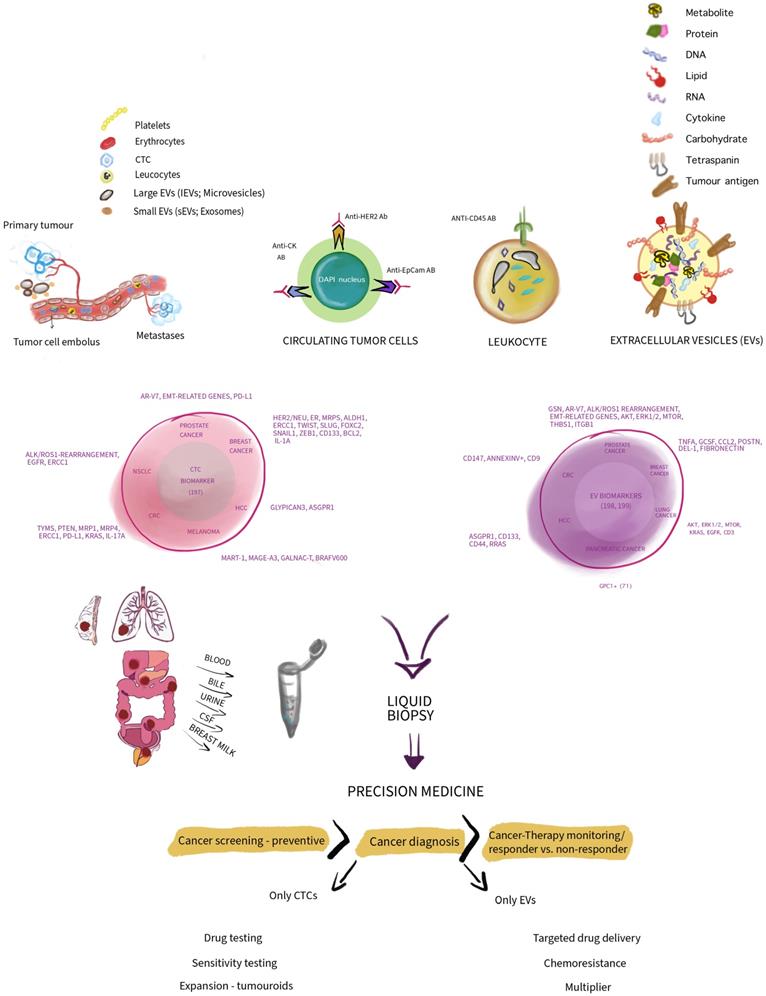
Several thoughts are summarized and depicted in Figure 1.
Aiming for realizing personalized medicine with CTCs
CTCs are not only restricted to cancer screening and cancer diagnosis/prognosis based on phenotyping surface antigens on those CTCs which various methods and devices as discussed above in detail. Moreover, CTCs are harboring genomic DNA, miRNA, mRNA, various kind of proteins and lipids [39]. At the end they are human living material with an ongoing metabolism. Whereas in EVs genomic DNA is generally absent as cell organelles as mitochondria too, except for DNA fragments due to volume restriction [39]. In agreement EVs are death material without an ongoing metabolism. Best case, ATP that is included in EVs might be used up for proteins ensuring temporary an asymmetric membrane composition regarding phosphatidylserine (PS) which is an anionic phospholipid found in cell membranes and EVs [24, 131, 132]. Nevertheless, these characteristics of both biomarkers is allowing several downstream applications after their successful isolation or enrichment. However, we have to keep in mind that CTCs are restricted to an overall cancer phenotype allowing disconnection, dislocation and migration though surrounding tissue infiltrating blood vessels [133]. Such restriction wasn't reported for EVs. EVs were released in colorectal carcinoma (CRC) patients suffering from a non-metastatic or metastatic CRC [78]. Such tumour cell behavior is typical for metastasis, hence metastatic cancer entities.
CTC isolation and recovery are currently in focus and ongoing testing is in progress permitting several downstream applications beyond sequencing genomic material as harbored in CTCs or expression arrays on protein or mRNA level including miRNA profiling. At best, this kind of downstream investigations can be done with little CTC material with low CTC numbers or even as single cell analysis [134]. Providing information regarding various characteristics that individual CTC might have and limiting therapy success if resistance to a chosen drug might be given. Certain mutations might give input regarding chemoresistance, invasion, EMT, extravasation, evasion and migration for instance as reviewed by others in depth. Such information is truly part of personalized medicine effort [135].
Why are CTC numbers crucial? This is due to the fact that CTCs are very rare. Even if 7.5mL of whole blood is the recommended volume for CTC detection by the FDA approved CellSearchTM system, the reported results with this commercially available system is typically ranging from 1-100 and only few specimens are actually acceding >100 CTCs/7.5mL whole blood [48, 119]. A multi centric study reported astonishing low number of CTCs (<6 CTCs/7.5mL) in the majority of cancer patients (78%) suffering from various cancers as bladder, breast, prostate, pancreatic, adenocarcinoma, colorectal, ovarian, lung, head and neck and others [136]. 2015 Miyamoto D.T. et al. reported a successful approach based on the recovery of 77 CTCs out of 13 prostate patients (6/patient) and subsequently expression analysis on mRNA level may help to predict resistance of anti-androgen therapy in spite of patients' heterogeneity regarding androgen receptor-dependent and -independent alterations [137]. On the contrary EVs are actually a multiplier, any cell, including tumour cell may release several sEVs and lEVs, likely increasing the chance to isolate and detect these nano-sized tracers of their donor cell. Typically, detected EV numbers are in a range of 108 to 1012 per mL serum or plasma [26, 138], of note depending on the investigated EV populations and subpopulations. Additionally, we must account that CTCs half-live is limited, somewhat restricted to 1-2.4 hrs as observed in patients suffering from breast cancer [139]. Whereas tumour derived EVs may be detectable up to several days after a R0 resection suggesting a half live of days [74, 78, 80].
A simplified concept depicting circulating tumour cells (CTCs) and extracellular vesicles (EVs) in a comparative manner highlighting and summarizing their differences in composition. Thus, presenting some markers that are in use to associate CTCs and EVs with various cancer entities. Additionally, indicating how CTCs and EVs as biomarkers may be utilized in the context of precision medicine in cancer and associated downstream application. Note, depicted sizes do not show size and diameter differences in reality in vivo. We thank Wioleta Chomko for transformation our scientific thoughts into art.
In spite of CTC's numbers, CTC are living material that could be expanded in a certain time frame depending on their initial numbers [140]. Apparently, low initial CTCS numbers have to be traded for higher expansion phase and higher CTCs numbers fortunately for a shorter expansion phase of those. Current research is primary focusing on novel clinically suitable methods ensuring higher initial CTC numbers, but secondary and from equal importance to shorten CTC's expansion phase, permitting sufficient CTC numbers to test simultaneously and in a short period of time several drugs, granting that the best drug will be administered to the patient [141]. Probably, such an approach would completely be depending on a successful expansion phase allowing a comprehensive drug sensitivity profile. Such effort could significantly reduce time and rule any non-responders to the selected anti-tumoural therapy [127]. Such decision-making of the probably most effective drug is of course very much desired in case of rapid progressing cancers as small cell lung cancer (SCLC) and others. Of note antigen selection for CTC enrichment is crucial. Recently, Lee H.-L. et al. enriched EpCAM+CD45- SCLC CTCs for a further downstream application as a spheroid proliferation assay. Noteworthy, spheroid proliferation was established within 4 weeks and associated with the expression of TTF-1, synaptophysin and PDL-1, permitting further assessment of drug sensitivity of cisplatin and etoposide [115]. CTCs were enriched using a bead-based CTC enrichment strategy based on the RosetteSepTM CTC Enrichment Cocktail kit. Therapy monitoring identifying drug responders from non-responders is desired and doable and part of a personalized medicine effort. 2014 Barbazán J. et al. published a study for which 50 mCRC patients were recruited and receiving a typical first systemic chemotherapy, typically fluoropyrimidines (fluorouracil or capecitabine) alone or in combination with oxaliplatin/irinotecan and biological targeted therapies (bevacizumab, cetuximab) [127].
But what about the feasibility of large-scale testing of FDA approved drugs on patient's own tumour cells? Is it doable? Probably yes, such libraries are in use [142]. Several CTC derived tumour cell lines were established. Eventually, Cayrefourcq L. et al. reported first establishing a cell line from CTCs of a colon cancer patient [143]. As we know, each tumour is somewhat highly personalized, carrying an individual mutation profile besides needed mutations that are typically for malignant cells. Does mutation profiling of pre-established tumour cell lines be beneficial. Rather not, but probably giving direction. Recently, a new concept emerged, creating tumour mass in vitro faster, due to the fact that the initial tumour cell numbers are much advantageous. Classical needle biopsies or resected tumour tissue may lead to sufficient numbers for seeding and growing of so called organoids [144], here tumouroids, on which drugs may be tested prior use in patient's since patients' own biopsy made it possible [145, 146]. This concept is surely and reasonably an alternative, if the trade of needle biopsy for liquid biopsy will result in a shorter expansion phase. Gaining time for the sake of cancer patients is key. However, that might be a desired goal, proof of concept was done with tumour tissue that was obtained during an R0 tumour resection ensuring high amounts of tumour cells for drug testing. Such an approach resulted in a total of 1500 tumouroids that were used for drug screening demonstrating that in this highly personalized approach a combined treatment with AKT and mTOR inhibitors may be a promising strategy for the treatment of patient's CRC [147].
Different cancer-driving mechanisms impact differently on the genomic rearrangements and the genetic heterogeneities of CTCs, that's the rationale behind aiming to maximize diagnostic precision. The high reliable genome integrity index (point mutations, gene amplifications, and whole genomes of single cells) was defined by different molecular assays to assess the molecular heterogeneity of single CTCs from metastatic cancer patients to suggest the evolution of personalized medication [148]. In line, a recent study showed that the up-regulation of Notch activity and the increase of uPAR+/int-β1+ CTCs are newly discovered CTC molecular signals in breast cancer patients who had brain metastasis, which can be used as a magic tool for early detection of micro-metastases in brain setting, or used to make a reasonable therapeutic decision and supervise the response during drugs management [149].
Taken together expansion of CTCs may permit comprehensive molecular profiling of CTCs. Comprehensive in that way, that not few cells or one CTC cell was analyzed, but a certain number which will result in more robust data. However, we have to keep in mind that expansion of CTCs in vitro as organoids, precisely tumouroids may acquire additional mutations/gene due to the length of expansion phase. Broutier L. et al. reported that in case of primary liver cancer (PLC) on average approx. 92% of variants as seen in patient's tissue were retained in the corresponding early tumouroid cultures (< 2 months), and > 80% after months of additional expansion [150]. However, important PLC antigens as typical HCC markers as AFP and as GPC3 and hepatocyte markers as ALB, as TTR, as APOA1 and as APOE were highly expressed in those HCC tumouroids and ductal markers as expected down regulated. Broutier L. et al. concluded that “PLC-derived organoid culture system faithfully recapitulates and maintains the transcriptomic alterations present in the individual patient's tumour subtype.”[150].
Nevertheless, certain CTCs have a magic that is surrounding them, but CTCs number are low in spite of newest efforts to increase those numbers via sub-sequential expansion of CTCs. But what might bring the future? Could be once that we simply go to dialysis center not because of a kidney problem, but rather to get rid of CTCs? To prevent metastatic seed in patients at risk due to a metastatic cancer phenotype? At the same time those CTCs could be recovered and obviously since we wash the whole blood volume for CTCs, captured CTC numbers could be expected to be much higher likely permitting downstream applications as discussed. A time costly expansion of those CTCs could be avoided. This idea of so-called CTCs hemodialysis isn't new. Wang X. et al. discussed their hypothesis 2013 in greater detail [151]. However, 2021 Jarvas G. et al. published a modification of hemodialysis membranes for such purpose to capture EpCAM+ CTCs in an experimental setting. They used HCT116 cancer cells both into buffer solution and added to whole blood. One endpoint was flow cytometry to assess efficiency. Efficiency was given as 69% and approx. 2.1 × 106 cells/m2 as absolute cell capture capacity potential that would result in, if assuming an average patient with 80 kg body and approx. 5 L of blood and a threshold of 5 CTCs/7.5 mL blood, nearly in approx. 3000 CTCs [152], which is a clear improvement. But is it superior than organoids/tumouroids or rather a convenient starting point increasing the chances for a successful organoids/tumouroids culture? Of note if we nail it down to patients' view on choosing between a convenient CTCs hemodialysis or an invasive needle biopsy or tumour resection in order to harvest sufficient primary tumour cells for an organoid/tumouroid culture, we might have a winner here.
In contrast to CTCs, EVs are conveniently present in all body fluids [153] and a multiplier of the donor-cells as discussed above. Hence, numbers are sufficient in contrast to CTCs. However, EVs must be considered to be somewhat experimental and clear guidelines and gold standards must be set including on EV storage. Since thawing and freezing cycles are affecting EV quality, numbers and hence reproducibility as reviewed by others in depth [154, 155]. In the next two paragraphs we would like to discuss potential EV applications as in case of chemoresistance and targeted drug delivery. Two aspects in which CTCs aren't a match, 1st due to low numbers and unresolved expansion limitations and 2nd, obviously, CTCs aren't a vector candidate that could be given back to the CTC donor patients.
EVs and chemoresistance
Contemporary oncology offers a cancer patient several treatment options, of which chemotherapy is stillthe gold standard [156]. Substantive restriction in clinical use of cytostatic drugs is resistance of neoplastic cells to their effects, which is often manifested by resistance to cytostatic drugs with multifarious structures and divergent mechanisms of action [157-159]. This phenomenon is called multiple drug resistance (MDR), and for a long time, researchers have tried to elucidate the complex molecular mechanisms underlying cancer cell resistance to chemotherapeutic agents [158, 159]. Over the last decade, considerable attention has been paid to the role of EVs in MDR [160, 161]. Although EVs are a relatively heterogeneous population, and various EV forms can be investigated in the context of chemoresistance, it is appropriate to clarify that the preponderance of the experimental studies described how sEVsand their associated non-coding RNAs (ncRNAs) regulate chemoresistance. Therefore, in this part of our narrative review, we present succinctly how cancer cells acquire resistance to chemotherapeutic agents through the active transmission of molecular information by sEVs.
Undeniably, sEVs are diligent players in developing chemoresistance in cancer cells [162, 163]. Nonetheless, some conditions must be met for sEVs, here precisely exosomes, to modulate this phenomenon. First, after being formed in the cell within multivesicular bodies (MVBs), the relevant molecule determining chemoresistance must be inserted into the sEV structure, after which exosomes are expelled from the cell into the extracellular environment [164, 165]. Second, sEVs must be absorbed by another cell, where the carried molecules undergo various processes, depending on their nature, that the cell phenotype changes [164, 165]. Typically, sEVs that determine chemoresistance are released predominantly by cancer cells with a natural resistance to specific anti-cancer drugs. Nevertheless, the components of the tumour microenvironment (TME) can also, as shown for sEVs, condition resistance to cytostatics [166-169]. Such properties are attributed to, inter alia, carcinoma-associated fibroblasts (CAFs) [166, 167], tumour-associated macrophages (TAMs) [168], and mesenchymal stem cells (MSCs) [169]. Nevertheless, sEVs released by tumour cells that fulfill a cardinal role in disseminating the chemotherapeutic resistance, and although these changes require sophisticated cytomolecular machinery, the phenomenon appears to be prevalent. As the cutting-edge research shows, the tumours that can zealously generate EVs with such attributes is practically limited to the types of human cells undergoing malignancy [170, 171]. The ability to release in particular sEVs with chemoresistance-modulating properties has been described mostly in the case of gastrointestinal (GI) cancers, including tongue squamous carcinoma (TSC) [172], esophageal cancer [173, 174], gastric cancer (GC) [175, 176], colorectal cancer (CRC) [177, 178], hepatocellular carcinoma (HCC) [179, 180], and pancreatic cancer (PC) [181]. Exosome-mediated chemoresistance has also been reported in cancer cells of ovarian [182] and cervical origin [183], as well as in non-small cell lung cancer (NSCLC) [184, 185] and glioma [186]. This phenomenon can also occur in the course of breast cancer (BC) [187] and acute myeloid leukemia (AML) [188].
However, the rudimentary question is, how do exosomes superintend the chemoresistance? The literature analysis indicates that two main mechanisms are involved; however, the studies discussed the first one more. This cardinal mechanism involves various forms of ncRNAs, which are transported through exosomes to sensitive cells, changing their properties [173, 178]. Therefore, it must be considered here that exosomes constitute a guided missile aimed at chemotherapeutic-sensitive cells. Exosomal microRNAs (miRs) confer chemoresistance via inhibiting cancer suppressor genes in recipient cells [173, 178]. For example, exosomal miR-193 silences transcription factor AP-2 gamma (TFAP2C) in the cisplatin (CDDP)-sensitive esophageal cancer cell line, thus removing CDDP's inhibition of the cell cycle and increasing chemoresistance [173]. In turn, CRC cells acquire resistance to oxaliplatin (OX) by suppressing an apoptosis-related gene, programmed cell death protein 10 (PDCD10) via exosomal miR-46146 [178]. A growing body of evidence reveals that exosomal long ncRNAs (lncRNAs) and circular RNAs (circRNAs) vehemently manipulate the genetic material of cancer cells to achieve chemoresistance [189, 190]. Mechanistically, both types of exosomal ncRNAs, through miR sponging (acting as a competing endogenous RNA; ceRNA), lead to increased expression of oncogenes [183, 184]. An example is the excellent work by Luo X. et al., which suggested that exosomal lncRNA HNF1A antisense RNA 1 (lncRNA HNF1A-AS1) promotes CDDP resistance in cervical cancer (CC) cells by sponging miR-34b to promote the expression of tuftelin1 (TUFT1), which exerts oncogenic roles [183]. Successively, exosomal hsa_circ_0014235 constitutes the ceRNA for miR-520a-5p, thus increasing the expression of cyclin-dependent kinase 4 (CDK4) in NSCL, leading to resistance to CDDP [184]. Phenotypically, such actions of exosomal ncRNAs lead to increased cell proliferation, migration, and invasion, as well as inhibition of cancer cell apoptosis [183, 184]. Other examples of chemotherapeutic agents against which cancer cells become resistant through exosomal ncRNAs are temozolomide (TMZ) [186], 5-fluorouracil (5-FU) [191], and gemcitabine (GEM) [181]. Interestingly, in breast cancer, exosomal lncRNA small nucleolar RNA host gene 14 (SNHG14) mediates resistance to trastuzumab, the monoclonal antibody used in its treatment [192]. The second mechanism is the promotion of chemoresistance through exosomal cargo proteins [171]. Undoubtedly, the mechanisms of action of individual proteins are highly diverse. For example, exosomal fibroblast growth factor 2 (FGF2) from bone marrow stromal cells (BMSCs) is taken up by leukemia cells, which results in their resistance to tyrosine kinase inhibitors (TKIs) [193]. Exosomes can also transport phosphorylated signal transducer and activator of transcription 3 (p-STAT3), increasing the CRC resistance to 5-FU [194].
EV in targeted drug delivery
Advantageously, two can play at that game! If, as proven in the preceding subsection, sEVs may significantly modulate tumour cell response to cytostatics, why not use them as drugs themselves or as drug haulers to cancer cells? Theoretically, the idea is not new as various drugs have long been incorporated into liposomes—a synthetic spherical vesicle [195, 196]. Nevertheless, sEVs and EVs in general might be regarded as universal transmitters of information between cells, and this property should be unquestionably exploited in the study of tumours. In all probability, in cancer therapy, our knowledge that naturally formed sEVs expressing ncRNAs can increase the sensitivity of cancer cells to cytostatics may constitute basis of search for their application in clinical oncology. The second approach is to incorporate cytostatic drug molecules into the structures of EVs. These are multistage processes that require extensive researcher experience and high-class laboratory apparatus. Notwithstanding these constraints, many studies are already registered in ClinicalTrials.gov by researchers who wish to keep abreast of the new developments in this field, although a significant part of these studies still pertains to the role of EVs in the diagnosis and prognosis of neoplastic diseases. In addition, several excellent reviews on the potential use of EVs as cancer treatment options have been published [197-200]. These manuscripts presented advanced data on the engineering of EVs as drug carriers and their potential clinical application in various disorders. In our narrative review, we described, following the structure of the prior subsection, which cytostatic drugs, regardless of the research model, can be delivered to cancer cells by EVs, and the results that are obtained from such transport. The technical details of preparing or modifying EVs are deliberately omitted here as these issues are fully described and seem to be discussed more often than the therapeutic options themselves and the potential effects at the molecular or cellular level.
A review of experimental studies plainly shows that the exosomal transport of two cytotoxic drugs is the most frequently described, namely doxorubicin (DOX) belonging to anthracyclines [201-205] and paclitaxel (PTX) belonging to the taxane family of anti-neoplastic agents [205-209]. Other cytotoxic drugs such as 5-FU [210] or a representative of antimetabolites (e.g., gemcitabine) [211] are, in this context, much less tested. There are two overarching goals behind the research conducted in this area. Firstly, a cytostatic drug attached to exosomes reaches cancer cells more effortlessly, showing greater than standard cytotoxicity and possibly higher clinical effectiveness [201, 203, 205, 210, 211]. Secondly, this form of drug transport may substantially reduce the side effects of cytostatics in cancer patients, especially cardiotoxicity of doxorubicin, likely due to preventing leakage of the drug before it reaches the cancer cell [201, 202, 204, 211].
The exosomal transport of doxorubicin may be used primarily to treat patients diagnosed with breast cancer [202, 203, 212]. This form of drug administration turns out to be of particular importance in human epidermal growth factor receptor 2 (HER2) positive tumours which, compared to HER2 negative cells, preferentially take up exosomes with doxorubicin [203, 212]. Moreover, doxorubicin-carrying exosomes can be potentially used in CRC [204] and the central nervous system neoplasm [205]. This was demonstrated by Yang T. et al., who proved that exosomes with doxorubicin and paclitaxel can cross the blood-brain barrier (BBB) in a zebrafish (danio rerio) model [205].
Exosomes can also become a platform for paclitaxel transport to neoplastic cells, again especially in breast cancer patients [206, 207]. This form of exosomes shows strong anti-tumour properties in vivo [206, 207] even in the case of distant neoplastic metastases, although the level of paclitaxel in exosomes was about 1000 times lower than that in the case of standard drug administration [207]. Paclitaxel-containing exosomes may also show anti-tumour efficacy in glioblastoma [208] and pulmonary metastases [209].
Finally, Liang G. et al. showed that exosomes expressing miR-21 inhibitor oligonucleotide (miR-21i), additionally enriched with 5-FU, exhibit anti-tumour properties, which are manifested by inhibition of the cell cycle and cell proliferation, with the simultaneous intensification of apoptosis [210]. This observation may be of clinical relevance in the treatment of CRC patients [210]. Furthermore, exosomes to which gemcitabine has been attached in treating pancreatic cancer patients may find clinical application [211].
EVs - the dark side
Many interesting findings had been reported on EVs, but being scientist means to be critical too and to address questions. One of the major questions may be if a similar high degree of standardization is achieved on isolation and purification of EVs as in case of the FDA approved CellSeachTM [48]? The answer is that EV isolation and purification is steadily evolving and many various methods are in use as reviewed in depth by others [213-215]. Typically, EVs may be isolated by various methodologies depending on their natural properties as size and affinity [216]. Five of those were recently evaluated and their clinical applicability compared [217]. The latest MISEV guidelines as published in Journal of Extracellular Vesicles (JEV) by the International Society For Extracellular Vesicles (ISEV) which is a professional society of researchers and scientists of EVs, is giving guidance on how to achieve a high degree of purity and standardization and reproducibility of EVs [77]. However, some research indicates that EV content on protein level may be modulated depending on chosen EV purification method, though EV donor cells had been the same [217, 218]. It appears that, for example, precipitation or ultracentrifugation of EVs could result in so-called 'touched EVs' that are either wrapped in polyethylene glycol (PEG, a polymer-based EV purification method) or potentially damaged by physical assault with high g-forces of 100k [219, 220]. Lately a new gold standard might be achieved since steadily more and more researchers are taking advantage of somewhat 'untouched EVs' that were low g isolated followed by size exclusion chromatography (SEC) likely avoiding physical damage or aggregation of those EVs [214, 221, 222]. Overall the EV field is still diverse and fluent on standardization, likely affecting reproducibility of earlier published historical EV data, that might be very well differing from newest EV data on the same topic while now MISEV guidelines were followed or SEC applied. Nevertheless, this is somewhat expected since EV research was 2 decades before a research field reserved and occupied by EV 'nerds'. For example, 2008 approximately 210 publication on exosome(s) were deposed on PubMed, 2021 approximately 5073 (search term exosomes). A similar tendency and total numbers are observable in case of large EVs (search term microvesicles).
On the contrary, CTCs might be a more developed concept and CTC research is highly benefiting due to the fact that CTCs has an historical advantage due to FDA's approval as granted 2004 as discussed above [48]. Thus, Hillig T. et al. compered CTC numbers as accounted by two different methodologies and published in fact that CytoTrackTM and CellSearchTM reviled similar number of CTCs, and similar abilities to identify CTC in vitro [223]. If EVs will ever reach such robustness permitting cross comparism on exact numbers remains open. It might be highly speculative, but the first FDA approval of EVs as a biomarker in screening and diagnosis might bring the needed guidance on standardization in spite of all efforts by ISEV and its members. As it stands for now, FDA issued a public safety notification on exosome products on December the 6th, 2019 (https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/public-safety-notification-exosome-products).
Conclusions
Liquid biopsies do promise a lot and are surely the future as seen during the ongoing SARS-CoV2 pandemic, in which a liquid biopsy made a huge impact on maintaining public health. So, what kind of arrows do we possess in our arrow quiver and which of those might be the sharpest to contest cancer? Assuming that we hit the cancer.
In case of liquid biopsies in cancer we have several, two of them, probably with the biggest perspective and future for a successful application associated with a robust high sensitivity and specificity might be CTCs and EVs. The first one already FDA approved for certain kind of cancer entities, predominantly from epithelial cancer entities with a metastatic nature and the latter one, EVs, that is highly experimental but something new, surely not fully developed and hence associated with big promises.
Both liquid biopsies are contested by its adversary, a highly capable individual beast, the whole cancer with its disconnected CTCs or cancer cells that are shedding EVs constantly for several purposes, mainly to ensure cancer survival and propagation within the host's body. Somehow a one-way direction with an unpleasant ending.
However, we have the tools to make a change. Two of them, CTCs and EVs as discussed in depth and compared. Interestingly, both, CTCs and EVs can be seen as a tool that cancer is using against us, but at the same time it's a tracer for cancer screening, cancer diagnosis, therapy monitoring sorting responders from non-responders as depicted in figure 1. Additionally, individual capabilities of CTCs and EVs are given, that are not shared between both. CTCs may be expended in various means as tumouroids for testing drug sensitivity helping us to tackle cancer. In case of EVs probably their usage as a vector for cancer therapies delivery which may be a promising vision that needs a lot of research to be done. Surely, EVs are a multiplier granting us cancer screening.
Cancer evolution uses both EVs and CTCs for cancer's survival and gaining advantage. We should take advantage of CTCs and EVs too! We should not consider EVs and CTCs just simple biomarkers as discussed above. Both EVs and CTCs orchestrate important processes of carcinogenesis. It seems that EVs are more profound during initiating process that are linked to carcinogenesis while CTCs later on. But, both have the same goal: cancer evolution. We might break cancer's momentum and take advantage. Depending on the tumour stage CTs and EVs could take a prominent future role as a liquid biopsy that fulfill its promise. Hence, the question “Who is better: EVs or CTCs?”, isn't of importance. The one that will help each cancer patient to become tumour free, free of secondary tumour loci is the true winner as a highly individual personalized medicine that includes as a first step cancer screening of note. Arnold Schwarzenegger said once in one of his movies: “If it can bleed, we can kill it.”(McTiernan J. (Director). (1987). PREDATOR [Film]. 20th Century Fox.). We might say once, “if it can release CTCs and EVs, we can heal it!”
Acknowledgements
We are remarkably grateful to Wioleta Chomko for assistance with figures.
Funding
This research was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), DFG Project number 410853455 to M.T.K. and V.L.-K. is funded by the Deutsche Forschungsgemeinschaft under Germany's Excellence Strategy (EXC 2151—390873048) and DFG Project number 411345524. The APC was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Morgan TM. Liquid biopsy: Where did it come from, what is it, and where is it going? Investig Clin Urol. 2019;60:139-41
2. Mocan T, Simao AL, Castro RE, Rodrigues CMP, Slomka A, Wang B. et al. Liquid Biopsies in Hepatocellular Carcinoma: Are We Winning? J Clin Med. 2020 9
3. Wichelhausen RN, McLean RL, Lowrey FB. Reinforcement of diagnostic value of pleural biopsies by culture in liquid medium. Am Rev Respir Dis. 1966;93:288-90
4. Partin AW, Carter HB, Chan DW, Epstein JI, Oesterling JE, Rock RC. et al. Prostate specific antigen in the staging of localized prostate cancer: influence of tumor differentiation, tumor volume and benign hyperplasia. J Urol. 1990;143:747-52
5. Catalona WJ, Smith DS, Ratliff TL, Dodds KM, Coplen DE, Yuan JJ. et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324:1156-61
6. Kouriefs C, Sahoyl M, Grange P, Muir G. Prostate specific antigen through the years. Arch Ital Urol Androl. 2009;81:195-8
7. Bruix J, Sherman M, American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-2
8. Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK. et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-30
9. Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H. et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439-74
10. Ryder SD, British Society of G. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (HCC) in adults. Gut. 2003;52(Suppl 3):iii1-8
11. Giannini EG, Marenco S, Borgonovo G, Savarino V, Farinati F, Del Poggio P. et al. Alpha-fetoprotein has no prognostic role in small hepatocellular carcinoma identified during surveillance in compensated cirrhosis. Hepatology. 2012;56:1371-9
12. European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236
13. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM. et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-50
14. Marrero JA, Su GL, Wei W, Emick D, Conjeevaram HS, Fontana RJ. et al. Des-gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in american patients. Hepatology. 2003;37:1114-21
15. Zhou L, Liu J, Luo F. Serum tumor markers for detection of hepatocellular carcinoma. World J Gastroenterol. 2006;12:1175-81
16. Zamcheck N, Pusztaszeri G. CEA, AFP and other potential tumor markers. CA Cancer J Clin. 1975;25:204-14
17. Brennan MM, Lambkin HA, Sheehan CD, Ryan DD, O'Connor TC, Kealy WF. Detection of high-risk subtypes of human papillomavirus in cervical swabs: routine use of the Digene Hybrid Capture assay and polymerase chain reaction analysis. Br J Biomed Sci. 2001;58:24-9
18. Mader S, Pantel K. Liquid Biopsy: Current Status and Future Perspectives. Oncol Res Treat. 2017;40:404-8
19. Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13:269-88
20. Beyer C, Pisetsky DS. The role of microparticles in the pathogenesis of rheumatic diseases. Nat Rev Rheumatol. 2010;6:21-9
21. Kornek M, Popov Y, Libermann TA, Afdhal NH, Schuppan D. Human T cell microparticles circulate in blood of hepatitis patients and induce fibrolytic activation of hepatic stellate cells. Hepatology. 2011;53:230-42
22. Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750
23. Banales JM, Feldstein AE, Sanger H, Lukacs-Kornek V, Szabo G, Kornek M. Extracellular Vesicles in Liver Diseases: Meeting Report from the International Liver Congress 2018. Hepatol Commun. 2019;3:305-15
24. Kornek M, Schuppan D. Microparticles: Modulators and biomarkers of liver disease. J Hepatol. 2012;57:1144-6
25. Urban SK, Mocan T, Sanger H, Lukacs-Kornek V, Kornek M. Extracellular Vesicles in Liver Diseases: Diagnostic, Prognostic, and Therapeutic Application. Semin Liver Dis. 2019;39:70-7
26. Zhao Z, Wijerathne H, Godwin AK, Soper SA. Isolation and analysis methods of extracellular vesicles (EVs). Extracell Vesicles Circ Nucl Acids. 2021;2:80-103
27. Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nature reviews Immunology. 2002;2:569-79
28. Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B. et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113:E968-77
29. Mathieu M, Nevo N, Jouve M, Valenzuela JI, Maurin M, Verweij FJ. et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat Commun. 2021;12:4389
30. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020 367
31. Crescitelli R, Lasser C, Szabo TG, Kittel A, Eldh M, Dianzani I. et al. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles. 2013 2
32. Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654-9
33. Vidal M. Exosomes: Revisiting their role as "garbage bags". Traffic. 2019;20:815-28
34. Willms E, Cabanas C, Mager I, Wood MJA, Vader P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front Immunol. 2018;9:738
35. von Felden J, Garcia-Lezana T, Dogra N, Gonzalez-Kozlova E, Ahsen ME, Craig A. et al. Unannotated small RNA clusters associated with circulating extracellular vesicles detect early stage liver cancer. Gut. 2021
36. Słomka A, Wang B, Mocan T, González-Carmona M, Strassburg CP, Lukacs-Kornek V. et al. Extracellular vesicles small RNA clusters: hit the nail on the head of liver cancer detection. Hepatobiliary Surg Nutr. 2022;11:100-2
37. Chen TY, Gonzalez-Kozlova E, Soleymani T, La Salvia S, Kyprianou N, Sahoo S. et al. Extracellular vesicles carry distinct proteo-transcriptomic signatures that are different from their cancer cell of origin. iScience. 2022;25:104414
38. Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, Doeppner TR. et al. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28:970-3
39. Pantel K, Speicher MR. The biology of circulating tumor cells. Oncogene. 2016;35:1216-24
40. Tellez-Gabriel M, Heymann MF, Heymann D. Circulating Tumor Cells as a Tool for Assessing Tumor Heterogeneity. Theranostics. 2019;9:4580-94
41. Ashworth TR. A case of cancer in which cells similar to those in the tumours were seen in the blood after death
42. Lopresti A, Malergue F, Bertucci F, Liberatoscioli ML, Garnier S, DaCosta Q. et al. Sensitive and easy screening for circulating tumor cells by flow cytometry. JCI Insight. 2019 5
43. Pantel K, Alix-Panabières C. The clinical significance of circulating tumor cells. Nat Clin Pract Oncol. 2007;4:62-3
44. Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC. et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781-91
45. Alix-Panabières C, Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer. 2014;14:623-31
46. Marquette CH, Boutros J, Benzaquen J, Ferreira M, Pastre J, Pison C. et al. Circulating tumour cells as a potential biomarker for lung cancer screening: a prospective cohort study. Lancet Respir Med. 2020;8:709-16
47. Osmulski P, Mahalingam D, Gaczynska ME, Liu J, Huang S, Horning AM. et al. Nanomechanical biomarkers of single circulating tumor cells for detection of castration resistant prostate cancer. Prostate. 2014;74:1297-307
48. Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C. et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897-904
49. Gross HJ, Verwer B, Houck D, Hoffman RA, Recktenwald D. Model study detecting breast cancer cells in peripheral blood mononuclear cells at frequencies as low as 10(-7). Proc Natl Acad Sci U S A. 1995;92:537-41
50. Riethdorf S, O'Flaherty L, Hille C, Pantel K. Clinical applications of the CellSearch platform in cancer patients. Adv Drug Deliv Rev. 2018;125:102-21
51. Hosseini SA, Abdolahad M, Zanganeh S, Dahmardeh M, Gharooni M, Abiri H. et al. Nanoelectromechanical Chip (NELMEC) Combination of Nanoelectronics and Microfluidics to Diagnose Epithelial and Mesenchymal Circulating Tumor Cells from Leukocytes. Small. 2016;12:883-91
52. Karabacak NM, Spuhler PS, Fachin F, Lim EJ, Pai V, Ozkumur E. et al. Microfluidic, marker-free isolation of circulating tumor cells from blood samples. Nat Protoc. 2014;9:694-710
53. Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S. et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287-312
54. Rossi E, Fabbri F. CTCs 2020: Great Expectations or Unreasonable Dreams. Cells. 2019 8
55. Bünger S, Zimmermann M, Habermann JK. Diversity of assessing circulating tumor cells (CTCs) emphasizes need for standardization: a CTC Guide to design and report trials. Cancer Metastasis Rev. 2015;34:527-45
56. Krebs MG, Hou JM, Sloane R, Lancashire L, Priest L, Nonaka D. et al. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J Thorac Oncol. 2012;7:306-15
57. Naito T, Tanaka F, Ono A, Yoneda K, Takahashi T, Murakami H. et al. Prognostic impact of circulating tumor cells in patients with small cell lung cancer. J Thorac Oncol. 2012;7:512-9
58. Sun YF, Xu Y, Yang XR, Guo W, Zhang X, Qiu SJ. et al. Circulating stem cell-like epithelial cell adhesion molecule-positive tumor cells indicate poor prognosis of hepatocellular carcinoma after curative resection. Hepatology. 2013;57:1458-68
59. Liu Z, Guo W, Zhang D, Pang Y, Shi J, Wan S, et al. Circulating tumor cell detection in hepatocellular carcinoma based on karyoplasmic ratios using imaging flow cytometry
60. Varillas JI, Zhang J, Chen K, Barnes, II, Liu C, George TJ. et al. Microfluidic Isolation of Circulating Tumor Cells and Cancer Stem-Like Cells from Patients with Pancreatic Ductal Adenocarcinoma. Theranostics. 2019;9:1417-25
61. Ankeny JS, Court CM, Hou S, Li Q, Song M, Wu D. et al. Circulating tumour cells as a biomarker for diagnosis and staging in pancreatic cancer. Br J Cancer. 2016;114:1367-75
62. Chemi F, Rothwell DG, McGranahan N, Gulati S, Abbosh C, Pearce SP. et al. Pulmonary venous circulating tumor cell dissemination before tumor resection and disease relapse. Nat Med. 2019;25:1534-9
63. Ilie M, Hofman V, Long-Mira E, Selva E, Vignaud JM, Padovani B. et al. "Sentinel" circulating tumor cells allow early diagnosis of lung cancer in patients with chronic obstructive pulmonary disease. PLoS One. 2014;9:e111597
64. Ferreira MM, Ramani VC, Jeffrey SS. Circulating tumor cell technologies. Mol Oncol. 2016;10:374-94
65. Cai J, Han Y, Ren H, Chen C, He D, Zhou L. et al. Extracellular vesicle-mediated transfer of donor genomic DNA to recipient cells is a novel mechanism for genetic influence between cells. J Mol Cell Biol. 2013;5:227-38
66. Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373-83
67. Jamur MC, Oliver C. Permeabilization of cell membranes. Methods Mol Biol. 2010;588:63-6
68. Maire CL, Fuh MM, Kaulich K, Fita KD, Stevic I, Heiland DH. et al. Genome-wide methylation profiling of glioblastoma cell-derived extracellular vesicle DNA allows tumor classification. Neuro Oncol. 2021
69. Kumeda N, Ogawa Y, Akimoto Y, Kawakami H, Tsujimoto M, Yanoshita R. Characterization of Membrane Integrity and Morphological Stability of Human Salivary Exosomes. Biol Pharm Bull. 2017;40:1183-91
70. Görgens A, Bremer M, Ferrer-Tur R, Murke F, Tertel T, Horn PA. et al. Optimisation of imaging flow cytometry for the analysis of single extracellular vesicles by using fluorescence-tagged vesicles as biological reference material. J Extracell Vesicles. 2019;8:1587567
71. Bhagwat N, Dulmage K, Pletcher CH Jr, Wang L, DeMuth W, Sen M. et al. An integrated flow cytometry-based platform for isolation and molecular characterization of circulating tumor single cells and clusters. Sci Rep. 2018;8:5035
72. Ogle LF, Orr JG, Willoughby CE, Hutton C, McPherson S, Plummer R. et al. Imagestream detection and characterisation of circulating tumour cells - A liquid biopsy for hepatocellular carcinoma? J Hepatol. 2016;65:305-13
73. Urban SK, Sänger H, Krawczyk M, Julich-Haertel H, Willms A, Ligocka J. et al. Synergistic effects of extracellular vesicle phenotyping and AFP in hepatobiliary cancer differentiation. Liver Int. 2020
74. Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J. et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177-82
75. Yekula A, Muralidharan K, Kang KM, Wang L, Balaj L, Carter BS. From laboratory to clinic: Translation of extracellular vesicle based cancer biomarkers. Methods. 2020;177:58-66
76. Lötvall J, Hill AF, Hochberg F, Buzás EI, Di Vizio D, Gardiner C. et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913
77. Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750
78. Willms A, Müller C, Julich H, Klein N, Schwab R, Güsgen C. et al. Tumour-associated circulating microparticles: A novel liquid biopsy tool for screening and therapy monitoring of colorectal carcinoma and other epithelial neoplasia. Oncotarget. 2016;7:30867-75
79. Julich H, Willms A, Lukacs-Kornek V, Kornek M. Extracellular vesicle profiling and their use as potential disease specific biomarker. Front Immunol. 2014;5:413
80. Julich-Haertel H, Urban SK, Krawczyk M, Willms A, Jankowski K, Patkowski W. et al. Cancer-associated circulating large extracellular vesicles in cholangiocarcinoma and hepatocellular carcinoma. J Hepatol. 2017;67:282-92
81. Li J, Chen L, Zhang X, Zhang Y, Liu H, Sun B. et al. Detection of circulating tumor cells in hepatocellular carcinoma using antibodies against asialoglycoprotein receptor, carbamoyl phosphate synthetase 1 and pan-cytokeratin. PLoS One. 2014;9:e96185
82. Armstrong AJ, Marengo MS, Oltean S, Kemeny G, Bitting RL, Turnbull JD. et al. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol Cancer Res. 2011;9:997-1007
83. Gorges TM, Tinhofer I, Drosch M, Röse L, Zollner TM, Krahn T. et al. Circulating tumour cells escape from EpCAM-based detection due to epithelial-to-mesenchymal transition. BMC Cancer. 2012;12:178
84. de Wit S, Manicone M, Rossi E, Lampignano R, Yang L, Zill B. et al. EpCAM(high) and EpCAM(low) circulating tumor cells in metastatic prostate and breast cancer patients. Oncotarget. 2018;9:35705-16
85. Eckert C, Kim YO, Julich H, Heier EC, Klein N, Krause E. et al. Podoplanin discriminates distinct stromal cell populations and a novel progenitor subset in the liver. Am J Physiol Gastrointest Liver Physiol. 2016;310:G1-12
86. Darlington PJ, Stopnicki B, Touil T, Doucet JS, Fawaz L, Roberts ME. et al. Natural Killer Cells Regulate Th17 Cells After Autologous Hematopoietic Stem Cell Transplantation for Relapsing Remitting Multiple Sclerosis. Front Immunol. 2018;9:834
87. de Wit S, van Dalum G, Lenferink AT, Tibbe AG, Hiltermann TJ, Groen HJ. et al. The detection of EpCAM(+) and EpCAM(-) circulating tumor cells. Sci Rep. 2015;5:12270
88. Hamaoka M, Kobayashi T, Tanaka Y, Mashima H, Ohdan H. Clinical significance of glypican-3-positive circulating tumor cells of hepatocellular carcinoma patients: A prospective study. PLoS One. 2019;14:e0217586
89. Zhang Y, Zhang X, Zhang J, Sun B, Zheng L, Li J. et al. Microfluidic chip for isolation of viable circulating tumor cells of hepatocellular carcinoma for their culture and drug sensitivity assay. Cancer Biol Ther. 2016;17:1177-87
90. Xu W, Cao L, Chen L, Li J, Zhang XF, Qian HH. et al. Isolation of circulating tumor cells in patients with hepatocellular carcinoma using a novel cell separation strategy. Clin Cancer Res. 2011;17:3783-93
91. Słomka A, Mocan T, Wang B, Nenu I, Urban SK, Gonzales-Carmona M. et al. EVs as Potential New Therapeutic Tool/Target in Gastrointestinal Cancer and HCC. Cancers (Basel). 2020 12
92. Jiang H, Gu X, Zuo Z, Tian G, Liu J. Prognostic value of circulating tumor cells in patients with bladder cancer: A meta-analysis. PLoS One. 2021;16:e0254433
93. Jiang AM, Zheng HR, Liu N, Zhao R, Ma YY, Bai SH. et al. Assessment of the Clinical Utility of Circulating Tumor Cells at Different Time Points in Predicting Prognosis of Patients With Small Cell Lung Cancer: A Meta-Analysis. Cancer Control. 2021;28:10732748211050581
94. Li Y, Wu G, Yang W, Wang X, Duan L, Niu L. et al. Prognostic value of circulating tumor cells detected with the CellSearch system in esophageal cancer patients: a systematic review and meta-analysis. BMC Cancer. 2020;20:581
95. Bidard FC, Michiels S, Riethdorf S, Mueller V, Esserman LJ, Lucci A. et al. Circulating Tumor Cells in Breast Cancer Patients Treated by Neoadjuvant Chemotherapy: A Meta-analysis. J Natl Cancer Inst. 2018;110:560-7
96. Bunduc S, Gede N, Váncsa S, Lillik V, Kiss S, Juhász MF. et al. Exosomes as prognostic biomarkers in pancreatic ductal adenocarcinoma-a systematic review and meta-analysis. Transl Res. 2022;244:126-36
97. Jafari D, Tiyuri A, Rezaei E, Moradi Y, Jafari R, Jokar Shoorijeh F. et al. Diagnostic accuracy of cerebrospinal fluid and serum-isolated extracellular vesicles for glioblastoma: a systematic review and meta-analysis. Expert Rev Mol Diagn. 2020;20:1075-85
98. Xiang H, Li F, Luo J, Long W, Hong L, Hu Y. et al. A meta-analysis on the relationship of exosomes and the prognosis of lung cancer. Medicine (Baltimore). 2021;100:e25332
99. Guo D, Yuan J, Xie A, Lin Z, Li X, Chen J. Diagnostic performance of circulating exosomes in human cancer: A meta-analysis. J Clin Lab Anal. 2020;34:e23341
100. Yu M. Metastasis Stemming from Circulating Tumor Cell Clusters. Trends Cell Biol. 2019;29:275-6
101. Gkountela S, Castro-Giner F, Szczerba BM, Vetter M, Landin J, Scherrer R. et al. Circulating Tumor Cell Clustering Shapes DNA Methylation to Enable Metastasis Seeding. Cell. 2019;176:98-112 e14
102. Balakrishnan A, Koppaka D, Anand A, Deb B, Grenci G, Viasnoff V. et al. Circulating Tumor Cell cluster phenotype allows monitoring response to treatment and predicts survival. Sci Rep. 2019;9:7933
103. Mizuno N, Kato Y, Shirota K, Izumi Y, Irimura T, Harashima H. et al. Mechanism of initial distribution of blood-borne colon carcinoma cells in the liver. J Hepatol. 1998;28:878-85
104. Tsutsuyama M, Nakanishi H, Yoshimura M, Oshiro T, Kinoshita T, Komori K. et al. Detection of circulating tumor cells in drainage venous blood from colorectal cancer patients using a new filtration and cytology-based automated platform. PLoS One. 2019;14:e0212221
105. Tien YW, Kuo HC, Ho BI, Chang MC, Chang YT, Cheng MF. et al. A High Circulating Tumor Cell Count in Portal Vein Predicts Liver Metastasis From Periampullary or Pancreatic Cancer: A High Portal Venous CTC Count Predicts Liver Metastases. Medicine (Baltimore). 2016;95:e3407
106. Bissolati M, Sandri MT, Burtulo G, Zorzino L, Balzano G, Braga M. Portal vein-circulating tumor cells predict liver metastases in patients with resectable pancreatic cancer. Tumour Biol. 2015;36:991-6
107. Navarro A, Molins L, Marrades RM, Moises J, Viñolas N, Morales S. et al. Exosome Analysis in Tumor-Draining Pulmonary Vein Identifies NSCLC Patients with Higher Risk of Relapse after Curative Surgery. Cancers (Basel). 2019 11
108. Severino V, Dumonceau JM, Delhaye M, Moll S, Annessi-Ramseyer I, Robin X. et al. Extracellular Vesicles in Bile as Markers of Malignant Biliary Stenoses. Gastroenterology. 2017;153:495-504 e8
109. Pascucci L, Coccè V, Bonomi A, Ami D, Ceccarelli P, Ciusani E. et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Control Release. 2014;192:262-70
110. Munagala R, Aqil F, Jeyabalan J, Gupta RC. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016;371:48-61
111. Reichmuth AM, Oberli MA, Jaklenec A, Langer R, Blankschtein D. mRNA vaccine delivery using lipid nanoparticles. Ther Deliv. 2016;7:319-34
112. Varderidou-Minasian S, Lorenowicz MJ. Mesenchymal stromal/stem cell-derived extracellular vesicles in tissue repair: challenges and opportunities. Theranostics. 2020;10:5979-97
113. Sharma S, Zhuang R, Long M, Pavlovic M, Kang Y, Ilyas A. et al. Circulating tumor cell isolation, culture, and downstream molecular analysis. Biotechnol Adv. 2018;36:1063-78
114. Yu M, Bardia A, Aceto N, Bersani F, Madden MW, Donaldson MC. et al. Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science. 2014;345:216-20
115. Lee HL, Chiou JF, Wang PY, Lu LS, Shen CN, Hsu HL. et al. Ex Vivo Expansion and Drug Sensitivity Profiling of Circulating Tumor Cells from Patients with Small Cell Lung Cancer. Cancers (Basel). 2020 12
116. Pearl ML, Dong H, Zhao Q, Tulley S, Dombroff MK, Chen WT. iCTC drug resistance (CDR) Testing ex vivo for evaluation of available therapies to treat patients with epithelial ovarian cancer. Gynecol Oncol. 2017;147:426-32
117. Sinkala E, Sollier-Christen E, Renier C, Rosàs-Canyelles E, Che J, Heirich K. et al. Profiling protein expression in circulating tumour cells using microfluidic western blotting. Nat Commun. 2017;8:14622
118. Bidard FC, Peeters DJ, Fehm T, Nolé F, Gisbert-Criado R, Mavroudis D. et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15:406-14
119. Theil G, Boehm C, Fischer K, Bialek J, Hoda R, Weber E. et al. In vivo isolation of circulating tumor cells in patients with different stages of prostate cancer. Oncol Lett. 2021;21:357
120. Shurtleff MJ, Yao J, Qin Y, Nottingham RM, Temoche-Diaz MM, Schekman R. et al. Broad role for YBX1 in defining the small noncoding RNA composition of exosomes. Proc Natl Acad Sci U S A. 2017;114:E8987-E95
121. Jørgensen M, Bæk R, Pedersen S, Søndergaard EK, Kristensen SR, Varming K. Extracellular Vesicle (EV) Array: microarray capturing of exosomes and other extracellular vesicles for multiplexed phenotyping. J Extracell Vesicles. 2013 2
122. Zhou J, Guo H, Yang Y, Zhang Y, Liu H. A meta-analysis on the prognosis of exosomal miRNAs in all solid tumor patients. Medicine (Baltimore). 2019;98:e15335
123. Shi M, Jiang Y, Yang L, Yan S, Wang YG, Lu XJ. Decreased levels of serum exosomal miR-638 predict poor prognosis in hepatocellular carcinoma. J Cell Biochem. 2018;119:4711-6
124. Khoo BL, Grenci G, Jing T, Lim YB, Lee SC, Thiery JP. et al. Liquid biopsy and therapeutic response: Circulating tumor cell cultures for evaluation of anticancer treatment. Sci Adv. 2016;2:e1600274
125. Smit DJ, Pantel K, Jücker M. Circulating tumor cells as a promising target for individualized drug susceptibility tests in cancer therapy. Biochem Pharmacol. 2021;188:114589
126. Krebs MG, Renehan AG, Backen A, Gollins S, Chau I, Hasan J. et al. Circulating Tumor Cell Enumeration in a Phase II Trial of a Four-Drug Regimen in Advanced Colorectal Cancer. Clin Colorectal Cancer. 2015;14:115-22.e1 -2
127. Barbazán J, Muinelo-Romay L, Vieito M, Candamio S, Díaz-López A, Cano A. et al. A multimarker panel for circulating tumor cells detection predicts patient outcome and therapy response in metastatic colorectal cancer. Int J Cancer. 2014;135:2633-43
128. Pan S, Zhang Y, Natalia A, Lim CZJ, Ho NRY, Chowbay B. et al. Extracellular vesicle drug occupancy enables real-time monitoring of targeted cancer therapy. Nat Nanotechnol. 2021;16:734-42
129. Vilsmaier T, Rack B, König A, Friese K, Janni W, Jeschke U. et al. Influence of Circulating Tumour Cells on Production of IL-1α, IL-1β and IL-12 in Sera of Patients with Primary Diagnosis of Breast Cancer Before Treatment. Anticancer Res. 2016;36:5227-36
130. Tseng JY, Yang CY, Liang SC, Liu RS, Yang SH, Lin JK. et al. Interleukin-17A modulates circulating tumor cells in tumor draining vein of colorectal cancers and affects metastases. Clin Cancer Res. 2014;20:2885-97
131. Skotland T, Hessvik NP, Sandvig K, Llorente A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J Lipid Res. 2019;60:9-18
132. Kay JG, Grinstein S. Sensing phosphatidylserine in cellular membranes. Sensors (Basel). 2011;11:1744-55
133. Krog BL, Henry MD. Biomechanics of the Circulating Tumor Cell Microenvironment. Adv Exp Med Biol. 2018;1092:209-33
134. Keller L, Pantel K. Unravelling tumour heterogeneity by single-cell profiling of circulating tumour cells. Nat Rev Cancer. 2019;19:553-67
135. Barbazán J, Alonso-Alconada L, Muinelo-Romay L, Vieito M, Abalo A, Alonso-Nocelo M. et al. Molecular characterization of circulating tumor cells in human metastatic colorectal cancer. PLoS One. 2012;7:e40476
136. Wang L, Balasubramanian P, Chen AP, Kummar S, Evrard YA, Kinders RJ. Promise and limits of the CellSearch platform for evaluating pharmacodynamics in circulating tumor cells. Semin Oncol. 2016;43:464-75
137. Miyamoto DT, Zheng Y, Wittner BS, Lee RJ, Zhu H, Broderick KT. et al. RNA-Seq of single prostate CTCs implicates noncanonical Wnt signaling in antiandrogen resistance. Science. 2015;349:1351-6
138. Salvi S, Bandini E, Carloni S, Casadio V, Battistelli M, Salucci S. et al. Detection and Investigation of Extracellular Vesicles in Serum and Urine Supernatant of Prostate Cancer Patients. Diagnostics (Basel). 2021 11
139. Meng S, Tripathy D, Frenkel EP, Shete S, Naftalis EZ, Huth JF. et al. Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res. 2004;10:8152-62
140. Eslami SZ, Cortés-Hernández LE, Alix-Panabières C. Epithelial Cell Adhesion Molecule: An Anchor to Isolate Clinically Relevant Circulating Tumor Cells. Cells. 2020 9
141. Maheswaran S, Haber DA. Ex Vivo Culture of CTCs: An Emerging Resource to Guide Cancer Therapy. Cancer Res. 2015;75:2411-5
142. Wang J, Jiang J, Chen H, Wang L, Guo H, Yang L. et al. FDA-approved drug screen identifies proteasome as a synthetic lethal target in MYC-driven neuroblastoma. Oncogene. 2019;38:6737-51
143. Cayrefourcq L, Mazard T, Joosse S, Solassol J, Ramos J, Assenat E. et al. Establishment and characterization of a cell line from human circulating colon cancer cells. Cancer Res. 2015;75:892-901
144. Boj SF, Hwang CI, Baker LA, Chio, II, Engle DD, Corbo V. et al. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160:324-38
145. Shiota J, Samuelson LC, Razumilava N. Hepatobiliary Organoids and Their Applications for Studies of Liver Health and Disease: Are We There Yet? Hepatology. 2021;74:2251-63
146. Tiriac H, Bucobo JC, Tzimas D, Grewel S, Lacomb JF, Rowehl LM. et al. Successful creation of pancreatic cancer organoids by means of EUS-guided fine-needle biopsy sampling for personalized cancer treatment. Gastrointest Endosc. 2018;87:1474-80
147. Nörz D, Mullins CS, Smit DJ, Linnebacher M, Hagel G, Mirdogan A. et al. Combined Targeting of AKT and mTOR Synergistically Inhibits Formation of Primary Colorectal Carcinoma Tumouroids In Vitro: A 3D Tumour Model for Pre-therapeutic Drug Screening. Anticancer Res. 2021;41:2257-75
148. Polzer B, Medoro G, Pasch S, Fontana F, Zorzino L, Pestka A. et al. Molecular profiling of single circulating tumor cells with diagnostic intention. EMBO Mol Med. 2014;6:1371-86
149. Boral D, Vishnoi M, Liu HN, Yin W, Sprouse ML, Scamardo A. et al. Molecular characterization of breast cancer CTCs associated with brain metastasis. Nat Commun. 2017;8:196
150. Broutier L, Mastrogiovanni G, Verstegen MM, Francies HE, Gavarró LM, Bradshaw CR. et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med. 2017;23:1424-35
151. Wang X, Sun Q, Mao F, Shen S, Huang L. CTCs hemodialysis: can it be a new therapy for breast cancer? Med Hypotheses. 2013;80:99-101
152. Jarvas G, Szerenyi D, Tovari J, Takacs L, Guttman A. Modification of Hemodialysis Membranes for Efficient Circulating Tumor Cell Capture for Cancer Therapy. Molecules. 2021 26
153. Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9-17
154. Yuan F, Li YM, Wang Z. Preserving extracellular vesicles for biomedical applications: consideration of storage stability before and after isolation. Drug Deliv. 2021;28:1501-9
155. Gelibter S, Marostica G, Mandelli A, Siciliani S, Podini P, Finardi A. et al. The impact of storage on extracellular vesicles: A systematic study. J Extracell Vesicles. 2022;11:e12162
156. Wu W, Pu Y, Shi J. Nanomedicine-enabled chemotherapy-based synergetic cancer treatments. J Nanobiotechnology. 2022;20:4
157. Ramos A, Sadeghi S, Tabatabaeian H. Battling Chemoresistance in Cancer: Root Causes and Strategies to Uproot Them. Int J Mol Sci. 2021 22
158. Baguley BC. Multiple drug resistance mechanisms in cancer. Mol Biotechnol. 2010;46:308-16
159. Majidinia M, Mirza-Aghazadeh-Attari M, Rahimi M, Mihanfar A, Karimian A, Safa A. et al. Overcoming multidrug resistance in cancer: Recent progress in nanotechnology and new horizons. IUBMB Life. 2020;72:855-71
160. Li J, Gao N, Gao Z, Liu W, Pang B, Dong X. et al. The Emerging Role of Exosomes in Cancer Chemoresistance. Front Cell Dev Biol. 2021;9:737962
161. Tian W, Lei N, Zhou J, Chen M, Guo R, Qin B. et al. Extracellular vesicles in ovarian cancer chemoresistance, metastasis, and immune evasion. Cell Death Dis. 2022;13:64
162. Zhong Y, Li H, Li P, Chen Y, Zhang M, Yuan Z. et al. Exosomes: A New Pathway for Cancer Drug Resistance. Front Oncol. 2021;11:743556
163. Liu Q. The emerging roles of exosomal long non-coding RNAs in bladder cancer. J Cell Mol Med. 2022;26:966-76
164. Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9:19
165. Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal. 2021;19:47
166. Hu Y, Yan C, Mu L, Huang K, Li X, Tao D. et al. Fibroblast-Derived Exosomes Contribute to Chemoresistance through Priming Cancer Stem Cells in Colorectal Cancer. PLoS One. 2015;10:e0125625
167. Deng X, Ruan H, Zhang X, Xu X, Zhu Y, Peng H. et al. Long noncoding RNA CCAL transferred from fibroblasts by exosomes promotes chemoresistance of colorectal cancer cells. Int J Cancer. 2020;146:1700-16
168. Binenbaum Y, Fridman E, Yaari Z, Milman N, Schroeder A, Ben David G. et al. Transfer of miRNA in Macrophage-Derived Exosomes Induces Drug Resistance in Pancreatic Adenocarcinoma. Cancer Res. 2018;78:5287-99
169. Ji R, Zhang B, Zhang X, Xue J, Yuan X, Yan Y. et al. Exosomes derived from human mesenchymal stem cells confer drug resistance in gastric cancer. Cell Cycle. 2015;14:2473-83
170. Mostafazadeh M, Samadi N, Kahroba H, Baradaran B, Haiaty S, Nouri M. Potential roles and prognostic significance of exosomes in cancer drug resistance. Cell Biosci. 2021;11:1
171. Li S, Yi M, Dong B, Jiao Y, Luo S, Wu K. The roles of exosomes in cancer drug resistance and its therapeutic application. Clin Transl Med. 2020;10:e257
172. Wang X, Yu H, Yu Z, Wang D. Exosomal lncRNA HEIH promotes cisplatin resistance in tongue squamous cell carcinoma via targeting miR-3619-5p/HDGF axis. Acta Histochem. 2020;122:151647
173. Shi S, Huang X, Ma X, Zhu X, Zhang Q. Research of the mechanism on miRNA193 in exosomes promotes cisplatin resistance in esophageal cancer cells. PLoS One. 2020;15:e0225290
174. Zang R, Qiu X, Song Y, Wang Y. Exosomes Mediated Transfer of Circ_0000337 Contributes to Cisplatin (CDDP) Resistance of Esophageal Cancer by Regulating JAK2 via miR-377-3p. Front Cell Dev Biol. 2021;9:673237
175. Wang J, Lv B, Su Y, Wang X, Bu J, Yao L. Exosome-Mediated Transfer of lncRNA HOTTIP Promotes Cisplatin Resistance in Gastric Cancer Cells by Regulating HMGA1/miR-218 Axis. Onco Targets Ther. 2019;12:11325-38
176. Zhong Y, Wang D, Ding Y, Tian G, Jiang B. Circular RNA circ_0032821 contributes to oxaliplatin (OXA) resistance of gastric cancer cells by regulating SOX9 via miR-515-5p. Biotechnol Lett. 2021;43:339-51
177. Wang X, Zhang H, Yang H, Bai M, Ning T, Deng T. et al. Exosome-delivered circRNA promotes glycolysis to induce chemoresistance through the miR-122-PKM2 axis in colorectal cancer. Mol Oncol. 2020;14:539-55
178. Xu Y, Zhu M. Novel exosomal miR-46146 transfer oxaliplatin chemoresistance in colorectal cancer. Clin Transl Oncol. 2020;22:1105-16
179. Qu Z, Wu J, Wu J, Luo D, Jiang C, Ding Y. Exosomes derived from HCC cells induce sorafenib resistance in hepatocellular carcinoma both in vivo and in vitro. J Exp Clin Cancer Res. 2016;35:159
180. Liu DX, Li PP, Guo JP, Li LL, Guo B, Jiao HB. et al. Exosomes derived from HBV-associated liver cancer promote chemoresistance by upregulating chaperone-mediated autophagy. Oncol Lett. 2019;17:323-31
181. Zeng Z, Zhao Y, Chen Q, Zhu S, Niu Y, Ye Z. et al. Hypoxic exosomal HIF-1α-stabilizing circZNF91 promotes chemoresistance of normoxic pancreatic cancer cells via enhancing glycolysis. Oncogene. 2021;40:5505-17
182. Li Z, Niu H, Qin Q, Yang S, Wang Q, Yu C. et al. lncRNA UCA1 Mediates Resistance to Cisplatin by Regulating the miR-143/FOSL2-Signaling Pathway in Ovarian Cancer. Mol Ther Nucleic Acids. 2019;17:92-101
183. Luo X, Wei J, Yang FL, Pang XX, Shi F, Wei YX. et al. Exosomal lncRNA HNF1A-AS1 affects cisplatin resistance in cervical cancer cells through regulating microRNA-34b/TUFT1 axis. Cancer Cell Int. 2019;19:323
184. Xu X, Tao R, Sun L, Ji X. Exosome-transferred hsa_circ_0014235 promotes DDP chemoresistance and deteriorates the development of non-small cell lung cancer by mediating the miR-520a-5p/CDK4 pathway. Cancer Cell Int. 2020;20:552
185. Shi Q, Ji T, Ma Z, Tan Q, Liang J. Serum Exosomes-Based Biomarker circ_0008928 Regulates Cisplatin Sensitivity, Tumor Progression, and Glycolysis Metabolism by miR-488/HK2 Axis in Cisplatin-Resistant Nonsmall Cell Lung Carcinoma. Cancer Biother Radiopharm. 2021
186. Wang J, Li T, Wang B. Exosomal transfer of miR-25-3p promotes the proliferation and temozolomide resistance of glioblastoma cells by targeting FBXW7. Int J Oncol. 2021 59
187. Santos JC, Lima NDS, Sarian LO, Matheu A, Ribeiro ML, Derchain SFM. Exosome-mediated breast cancer chemoresistance via miR-155 transfer. Sci Rep. 2018;8:829
188. Bouvy C, Wannez A, Laloy J, Chatelain C, Dogné JM. Transfer of multidrug resistance among acute myeloid leukemia cells via extracellular vesicles and their microRNA cargo. Leuk Res. 2017;62:70-6
189. Yousefi H, Maheronnaghsh M, Molaei F, Mashouri L, Reza Aref A, Momeny M. et al. Long noncoding RNAs and exosomal lncRNAs: classification, and mechanisms in breast cancer metastasis and drug resistance. Oncogene. 2020;39:953-74
190. Wang Y, Liu Q, Wang F. Potential roles of exosome non-coding RNAs in cancer chemoresistance (Review). Oncol Rep. 2021;45:439-47
191. Mao G, Mu Z, Wu DA. Exosomal lncRNA FOXD3-AS1 upregulates ELAVL1 expression and activates PI3K/Akt pathway to enhance lung cancer cell proliferation, invasion, and 5-fluorouracil resistance. Acta Biochim Biophys Sin (Shanghai). 2021;53:1484-94
192. Dong H, Wang W, Chen R, Zhang Y, Zou K, Ye M. et al. Exosome-mediated transfer of lncRNA-SNHG14 promotes trastuzumab chemoresistance in breast cancer. Int J Oncol. 2018;53:1013-26
193. Javidi-Sharifi N, Martinez J, English I, Joshi SK, Scopim-Ribeiro R, Viola SK. et al. FGF2-FGFR1 signaling regulates release of Leukemia-Protective exosomes from bone marrow stromal cells. Elife. 2019 8
194. Zhang Q, Liu RX, Chan KW, Hu J, Zhang J, Wei L. et al. Exosomal transfer of p-STAT3 promotes acquired 5-FU resistance in colorectal cancer cells. J Exp Clin Cancer Res. 2019;38:320
195. Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and Challenges of Liposome Assisted Drug Delivery. Front Pharmacol. 2015;6:286
196. Guimarães D, Cavaco-Paulo A, Nogueira E. Design of liposomes as drug delivery system for therapeutic applications. Int J Pharm. 2021;601:120571
197. Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016;6:287-96
198. Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin. 2017;38:754-63
199. Bunggulawa EJ, Wang W, Yin T, Wang N, Durkan C, Wang Y. et al. Recent advancements in the use of exosomes as drug delivery systems. J Nanobiotechnology. 2018;16:81
200. Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183-95
201. Zou J, Shi M, Liu X, Jin C, Xing X, Qiu L. et al. Aptamer-Functionalized Exosomes: Elucidating the Cellular Uptake Mechanism and the Potential for Cancer-Targeted Chemotherapy. Anal Chem. 2019;91:2425-30
202. Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ. et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383-90
203. Gomari H, Forouzandeh Moghadam M, Soleimani M, Ghavami M, Khodashenas S. Targeted delivery of doxorubicin to HER2 positive tumor models. Int J Nanomedicine. 2019;14:5679-90
204. Li Y, Gao Y, Gong C, Wang Z, Xia Q, Gu F. et al. A33 antibody-functionalized exosomes for targeted delivery of doxorubicin against colorectal cancer. Nanomedicine. 2018;14:1973-85
205. Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R. et al. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm Res. 2015;32:2003-14
206. Wang P, Wang H, Huang Q, Peng C, Yao L, Chen H. et al. Exosomes from M1-Polarized Macrophages Enhance Paclitaxel Antitumor Activity by Activating Macrophages-Mediated Inflammation. Theranostics. 2019;9:1714-27
207. Melzer C, Rehn V, Yang Y, Bähre H, von der Ohe J, Hass R. Taxol-Loaded MSC-Derived Exosomes Provide a Therapeutic Vehicle to Target Metastatic Breast Cancer and Other Carcinoma Cells. Cancers (Basel). 2019 11
208. Zhu Q, Ling X, Yang Y, Zhang J, Li Q, Niu X. et al. Embryonic Stem Cells-Derived Exosomes Endowed with Targeting Properties as Chemotherapeutics Delivery Vehicles for Glioblastoma Therapy. Adv Sci (Weinh). 2019;6:1801899
209. Kim MS, Haney MJ, Zhao Y, Yuan D, Deygen I, Klyachko NL. et al. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: in vitro and in vivo evaluations. Nanomedicine. 2018;14:195-204
210. Liang G, Zhu Y, Ali DJ, Tian T, Xu H, Si K. et al. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J Nanobiotechnology. 2020;18:10
211. Li YJ, Wu JY, Wang JM, Hu XB, Cai JX, Xiang DX. Gemcitabine loaded autologous exosomes for effective and safe chemotherapy of pancreatic cancer. Acta Biomater. 2020;101:519-30
212. Gomari H, Forouzandeh Moghadam M, Soleimani M. Targeted cancer therapy using engineered exosome as a natural drug delivery vehicle. Onco Targets Ther. 2018;11:5753-62
213. Brennan K, Martin K, FitzGerald SP, O'Sullivan J, Wu Y, Blanco A. et al. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci Rep. 2020;10:1039
214. Sidhom K, Obi PO, Saleem A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int J Mol Sci. 2020 21
215. Liangsupree T, Multia E, Riekkola ML. Modern isolation and separation techniques for extracellular vesicles. J Chromatogr A. 2021;1636:461773
216. Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. Biomed Res Int. 2018;2018:8545347
217. Veerman RE, Teeuwen L, Czarnewski P, Güclüler Akpinar G, Sandberg A, Cao X. et al. Molecular evaluation of five different isolation methods for extracellular vesicles reveals different clinical applicability and subcellular origin. J Extracell Vesicles. 2021;10:e12128
218. Jung HH, Kim JY, Lim JE, Im YH. Cytokine profiling in serum-derived exosomes isolated by different methods. Sci Rep. 2020;10:14069
219. Weng Y, Sui Z, Shan Y, Hu Y, Chen Y, Zhang L. et al. Effective isolation of exosomes with polyethylene glycol from cell culture supernatant for in-depth proteome profiling. Analyst. 2016;141:4640-6
220. Cvjetkovic A, Lötvall J, Lässer C. The influence of rotor type and centrifugation time on the yield and purity of extracellular vesicles. J Extracell Vesicles. 2014 3
221. Vanderboom PM, Dasari S, Ruegsegger GN, Pataky MW, Lucien F, Heppelmann CJ. et al. A size-exclusion-based approach for purifying extracellular vesicles from human plasma. Cell Rep Methods. 2021 1
222. Mol EA, Goumans MJ, Doevendans PA, Sluijter JPG, Vader P. Higher functionality of extracellular vesicles isolated using size-exclusion chromatography compared to ultracentrifugation. Nanomedicine. 2017;13:2061-5
223. Hillig T, Horn P, Nygaard AB, Haugaard AS, Nejlund S, Brandslund I. et al. In vitro detection of circulating tumor cells compared by the CytoTrack and CellSearch methods. Tumour Biol. 2015;36:4597-601
Author contact
![]() Corresponding author: miroslawkornekde; Tel.: +49-228-287-15276
Corresponding author: miroslawkornekde; Tel.: +49-228-287-15276
 Global reach, higher impact
Global reach, higher impact