13.3
Impact Factor
Theranostics 2022; 12(13):5727-5743. doi:10.7150/thno.71872 This issue Cite
Research Paper
RNA N6-methyladenosine reader YTHDC1 is essential for TGF-beta-mediated metastasis of triple negative breast cancer
1. Department of Systems Biology, Beckman Research Institute of City of Hope, Monrovia, CA 91016, USA
2. Department of Cancer Genetics and Epigenetics, Beckman Research Institute of City of Hope, Duarte, CA91010, USA
3. Gehr Family Center for Leukemia Research, City of Hope, Duarte, CA 91010, USA
4. Department of Immunology, School of Basic Medicine, Hebei Medical University, 361 Zhongshan East Road, Shijiazhuang, Hebei 050017, China
5. Department of Radiation Oncology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong 510080, China
6. Department of Pharmacology, School of Pharmacy, China Medical University, Shenyang 110122, China
7. Department of Liver Surgery, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200127, China
*These authors contributed to this work equally.
Abstract
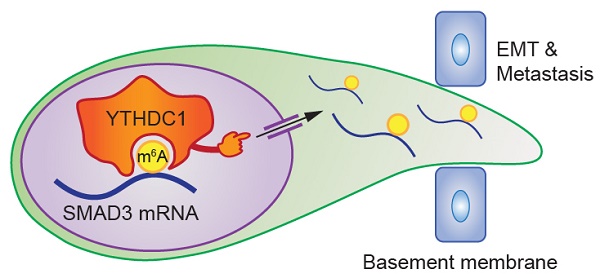
RNA N6-methyladenosine (m6A) modification and its regulators fine tune gene expression and contribute to tumorigenesis. This study aims to uncover the essential role and the underlying molecular mechanism(s) of the m6A reader YTHDC1 in promoting triple negative breast cancer (TNBC) metastasis.
Methods: In vitro and in vivo models were employed to determine the pathological function of YTHDC1 in TNBC metastasis. To identify bona fide YTHDC1 target RNAs, we conducted RNA-seq, m6A-seq, and RIP-seq, followed by integrative data analysis and validation assays.
Results: By analyzing The Cancer Genome Atlas (TCGA) dataset, we found that elevated expression of YTHDC1 is positively correlated with poor prognosis in breast cancer patients. Using a mammary fat pad mouse model of TNBC, YTHDC1 significantly promoted lung metastasis of TNBC cells. Through multiple transcriptome-wide sequencing and integrative data analysis, we revealed dysregulation of metastasis-related pathways following YTHDC1 depletion and identified SMAD3 as a bona fide YTHDC1 target RNA. Depletion of YTHDC1 caused nuclear retention of SMAD3 mRNA, leading to lower SMAD3 protein levels. Loss of YTHDC1 led to impaired TGF-β-induced gene expression, leading to inhibition of epithelial-mesenchymal transition (EMT) and suppressed TNBC cell migration and invasion. SMAD3 overexpression was able to restore the response to TGF-β in YTHDC1 depleted TNBC cells. Furthermore, we demonstrated that the oncogenic role of YTHDC1 is mediated through its recognition of m6A as m6A-binding defective mutants of YTHDC1 were unable to rescue the impaired cell migration and invasion of YTHDC1 knockout TNBC cells.
Conclusions: We show that YTHDC1 plays a critical oncogenic role in TNBC metastasis through promoting the nuclear export and expression of SMAD3 to augment the TGF-β signaling cascade. Overall, our study demonstrates that YTHDC1 is vital for TNBC progression by enhancing TNBC cell survival and TGF-β-mediated EMT via SMAD3 to enable the formation of distant metastasis and highlights the therapeutic potential of targeting the YTHDC1/m6A/SMAD3 axis for TNBC treatment.
Keywords: N6-methyladenosine, YTHDC1, TGF-β, SMAD3, metastasis
 Global reach, higher impact
Global reach, higher impact