13.3
Impact Factor
Theranostics 2022; 12(13):5615-5630. doi:10.7150/thno.56736 This issue Cite
Research Paper
Translational immunoPET imaging using a radiolabeled GD2-specific antibody in neuroblastoma
1. Werner Siemens Imaging Center, Department of Preclinical Imaging and Radiopharmacy, Eberhard Karls University Tuebingen, Germany
2. Department of Nuclear Medicine and Clinical Molecular Imaging, Eberhard Karls University Tuebingen, Germany
3. Department of Internal Medicine VIII, Eberhard Karls University Tuebingen, Germany
4. Center for Radiopharmaceutical Sciences ETH-PSI-USZ, Paul Scherrer Institute, Villigen, Switzerland
5. Institute of Pathology and Neuropathology, University Hospital Tuebingen, Eberhard Karls University Tuebingen, Germany
6. Provenance Biopharmaceuticals Corp., Carlisle, MA, USA
7. Department of Diagnostic and Interventional Radiology, Eberhard Karls University Tuebingen, Germany
8. Childrens Hospital, Department of Hematology and Oncology, Eberhard Karls University Tuebingen, Germany
9. German Cancer Consortium (DKTK) and German Cancer Research Center (DKFZ), 69120, Heidelberg, Germany
10. Cluster of Excellence iFIT (EXC 2180) "Image Guided and Functionally Instructed Tumor Therapies", University of Tuebingen, Germany
Abstract
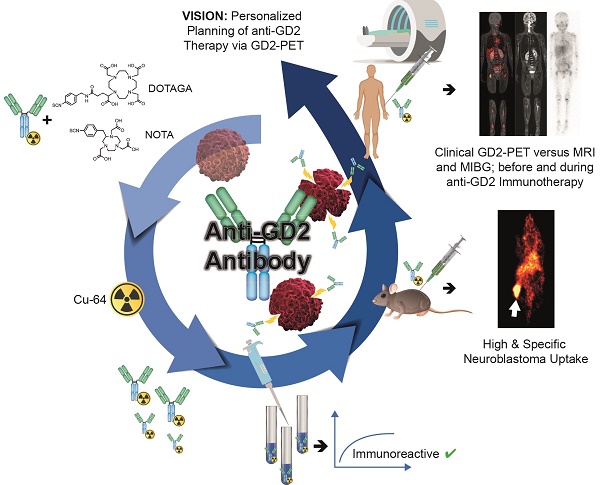
Background: Antibodies targeting surface expressed disialoganglioside GD2 are increasingly used in neuroblastoma immunotherapy and might also have potential for use in radioimmunotherapy. As such targeted treatments might benefit from a dedicated theranostic approach, we studied the influence of radiolabeling on the binding characteristics of ch14.18 antibodies produced by Chinese hamster ovary (CHO) cells and evaluated the benefit of GD2-ImmunoPET as a potential tool for therapy planning.
Methods: 64Cu was used to reduce radiation burden, which is of high importance especially in a pediatric patient population. 64Cu-labeling was accomplished using the chelators NOTA- or DOTAGA-NCS. Radiolabeled antibodies were characterized in vitro. [64Cu]Cu-DOTAGA-ch14.18/CHO was studied in a neuroblastoma mouse model (subcutaneous CHP-134 xenografts). In vivo PET and MR images were acquired at 3 h, 24 h, and 48 h p.i. The specificity of binding was verified using GD2-negative tumors (HEK-293 xenografts), a control antibody and in vivo blocking. A first translational application was performed by PET/MRI in a patient with metastasized neuroblastoma.
Results: Radiolabeling at an antibody-to-chelator ratio ≥1:10 yielded a product with a radiochemical purity of ≥90% and a specific activity of 0.2-1.0 MBq/µg. Radiochelation was stable over 48 h in PBS, mouse serum or EDTA, and 50.8 ± 3.5% and 50.8 ± 2.0% of the radiolabeled conjugates, prepared at antibody-to-chelator ratios of 1:10 or 1:15, were immunoreactive. In vivo, highly specific accumulation (31.6 ± 5.8% ID/g) in neuroblastoma was shown preclinically. Clinical PET/MR scans using [64Cu]Cu-NOTA-ch14.18/CHO (NOTA used for safety reasons) could visualize neuroblastoma metastases.
Conclusions: In vivo, 64Cu-labeled ch14.18/CHO is suitable for specific identification of neuroblastoma in PET. A first patient PET indicated the feasibility of the method for clinical translation and the potential utility in image-guided therapy.
Keywords: neuroblastoma, GD2, PET, ImmuneImaging, theranostic
 Global reach, higher impact
Global reach, higher impact