13.3
Impact Factor
Theranostics 2022; 12(12):5551-5563. doi:10.7150/thno.74154 This issue Cite
Research Paper
HER2-targeted dual radiotracer approach with clinical potential for noninvasive imaging of trastuzumab-resistance caused by epitope masking
1. Medical Isotopes Research Center and Department of Radiation Medicine, School of Basic Medical Sciences, Peking University, Beijing, 100191, China.
2. Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Nuclear Medicine, Peking University Cancer Hospital & Institute, Beijing 100142, China.
3. Department of Integration of Chinese and Western Medicine, School of Basic Medical Sciences, Peking University, Beijing, 100191, China.
4. Key Laboratory of Protein and Peptide Pharmaceuticals, CAS Center for Excellence in Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, China.
5. Bioland Laboratory (Guangzhou Regenerative Medicine and Health Guangdong Laboratory), Guangzhou 510005, China.
Abstract
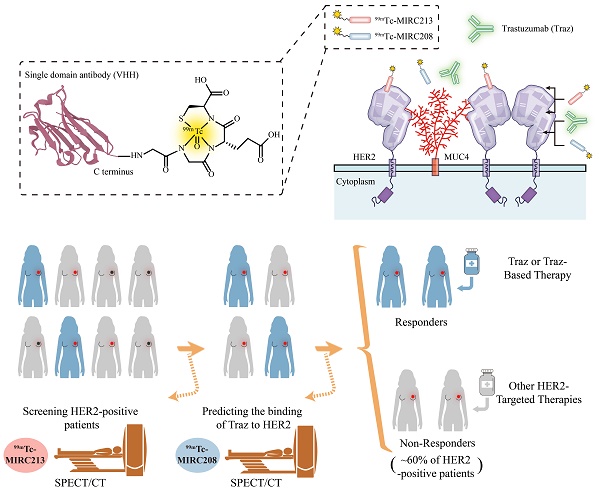
Rationale: The decreased HER2-accessibility by epitope masking is a primary trastuzumab-resistance mechanism. In this study, we developed a HER2-targeted dual radiotracer approach to predict the HER2-trastuzumab engagement noninvasively.
Methods: Two novel HER2-specific VHHs, MIRC208 and MIRC213, were acquired by immunizing alpaca with human HER2 protein, and were site-specifically labeled with 99mTc. Biodistribution and SPECT/CT imaging studies were performed in mice bearing HER2-positive and HER2-negative tumors. The HER2 binding sites of 99mTc-MIRC208 and 99mTc-MIRC213 were investigated by cell binding and SPECT/CT imaging studies. We evaluated the therapeutic predictive ability of our dual-radiotracer imaging approach for trastuzumab treatment in mice bearing MUC4-positive tumors (trastuzumab-resistant JIMT-1 and 87MUC4) and MUC4-negative tumors (trastuzumab-sensitive 7HER2 and NCI-N87). The preliminary clinical studies of 99mTc-MIRC208 were performed in two patients with HER2-positive breast tumors.
Results: 99mTc-MIRC208 and 99mTc-MIRC213 clearly visualized HER2-positive tumors, but not HER2-negative tumors. 99mTc-MIRC208 competes with trastuzumab for HER2-binding while 99mTc-MIRC213 recognizes HER2 on an epitope that is not masked by MUC4. The SPECT/CT studies with 99mTc-MIRC208 and 99mTc-MIRC213 clearly showed that the MUC4-negative and trastuzumab-sensitive 7HER2 and NCI-N87 tumors had very similar tumor uptake with the SUV208/SUV213 (2 h) ratios of 1.11 ± 0.17 in 7HER2 and 1.25 ± 0.22 in NCI-N87. However, the MUC4-positive JIMT-1 tumors showed the decreased SUV208/SUV213 (2 h) ratio (0.63 ± 0.07), which correlated well with the low response rate to trastuzumab therapy. The SUV208/SUV213 (2 h) ratio was reduced to 0.72 ± 0.02 in MUC4-expressing NCI-N87 cells, and resulting in the decreased trastuzumab sensitivity, further supporting the correlation between the SUV208/SUV213 (2 h) ratio and trastuzumab-sensitivity. The primary and metastatic HER2-positive lesions of patients were clearly visualized by 99mTc-MIRC208 SPECT at 2 h post injection.
Conclusion: Overall, we demonstrated that the dual radiotracer imaging strategy is a valid noninvasive approach for the cancer patient selection before trastuzumab therapy. 99mTc-MIRC213 SPECT is utilized to quantify the tumor HER2 expression and screen HER2-positive cancer patients, while 99mTc-MIRC208 SPECT is used to determine the HER2-accessibility of trastuzumab. The SUV208/SUV213 (2 h) ratio is an important biomarker to determine the responsiveness of trastuzumab therapy.
Keywords: SPECT/CT imaging, VHH, Trastuzumab, HER2-targeted therapy
 Global reach, higher impact
Global reach, higher impact