13.3
Impact Factor
Theranostics 2022; 12(12):5488-5503. doi:10.7150/thno.73104 This issue Cite
Research Paper
Co-delivery of phagocytosis checkpoint and STING agonist by a Trojan horse nanocapsule for orthotopic glioma immunotherapy
1. Fujian Provincial Key Laboratory of Brain Aging and Neurodegenerative Diseases, School of Basic Medical Sciences, Fujian Medical University, Fuzhou, Fujian 350122, China.
2. CAS Key Laboratory of Standardization and Measurement for Nanotechnology, CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology, Beijing 100190, China.
3. School of Nanoscience and Technology, Sino-Danish College, University of Chinese Academy of Sciences, Beijing 100049, China.
4. School of Chemical Engineering and Pharmacy, Wuhan Institute of Technology, Wuhan, 430205, China.
*These authors contributed equally to this work.
Abstract
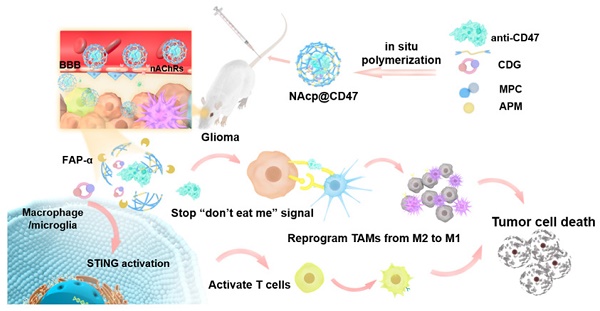
Rationale: Cancer immunotherapy has demonstrated significant antitumor activity in a variety of tumors; however, extensive infiltration of immunosuppressive tumor-associated macrophages (TAMs) in the glioblastoma (GBM) tumor microenvironment (TME) and the existence of the blood-brain barrier (BBB) might lead to failure of the checkpoint blockade therapy.
Methods: Herein, we have developed a smart “Trojan horse” BBB-permeable nanocapsule termed “NAcp@CD47” to deliver anti-CD47 antibodies and stimulator of interferon genes (STING) agonists into GBM tissues in a stealth-like manner to reshaped the immune microenvironment by switching the phenotype of microglia and macrophages.
Results: Both in vitro and in vivo studies demonstrate that NAcp@CD47 could effectively penetrate the BBB, increase the polarization of M1-phenotype TAMs, help reduce tumor immunosuppression, and inhibit the orthotopic GBM growth by phagocytosis of macrophages and microglia.
Conclusions: Our findings indicate that the well-designed NAcp@CD47 not only enhances the phagocytosis of cancer cells but also efficiently enhance antitumor immunogenicity and reverses immune suppression to convert uninflamed “cold” tumors into “hot” tumors.
Keywords: CD47, glioblastoma, immunotherapy, tumor associated macrophages, phagocytosis
 Global reach, higher impact
Global reach, higher impact