13.3
Impact Factor
Theranostics 2022; 12(12):5272-5298. doi:10.7150/thno.73566 This issue Cite
Review
Progress in advanced nanotherapeutics for enhanced photodynamic immunotherapy of tumor
1. School of Preclinical Medicine, Chengdu University, Chengdu 610106, P. R. China.
2. School of Food and Bioengineering, Chengdu University, Chengdu 610106, P. R. China.
3. Key Disciplines of Clinical Pharmacy, Affiliated Hospital and Clinical Medical College of Chengdu University, Chengdu 610081, P. R. China.
4. Department of Thoracic and Cardiac Surgery, Affiliated Hospital of Chengdu University, Chengdu 610081, P. R. China.
#These authors contributed equally to this work.
Received 2022-4-3; Accepted 2022-6-25; Published 2022-7-4
Abstract
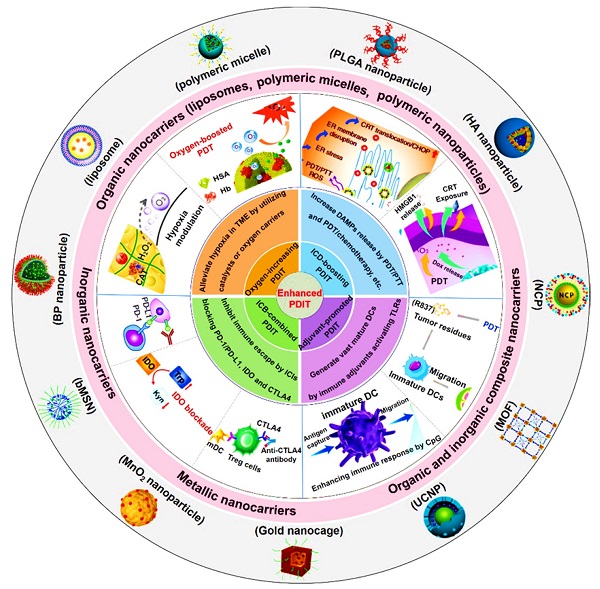
Clinically, the conventional treatments of cancer are still often accompanied by tumor recurrence, metastasis and other poor prognosis. Nowadays, more attention has been paid to photodynamic therapy (PDT), which is regarded as an adjuvant antineoplastic strategy with superiorities in great spatiotemporal selectivity and minimal invasiveness. In addition to eliminating tumor cells via reactive oxygen species (ROS), more meaningfully, this phototherapy can trigger immunogenic cell death (ICD) that plays a vital role in photodynamic immunotherapy (PDIT). ICD-based PDIT holds some immunotherapeutic potential due to further enhanced antitumor efficacy by utilizing various combined therapies to increase ICD levels. To help the PDIT-related drugs improve pharmacokinetic properties, bioavailability and system toxicity, multifunctional nanocarriers can be reasonably designed for enhanced PDIT. In further consideration of severe hypoxia, low immunity and immune checkpoints in tumor microenvironment (TME), advanced nanotherapeutics-mediated PDIT has been extensively studied for boosting antitumor immunity by oxygen-augment, ICD-boosting, adjuvant stimulation and combined checkpoints blockade. Herein, this review will summarize different categories of nanocarriers consisting of their material type, targeting and stimuli-responsiveness. Moreover, we will focus on the latest progress of various strategies to enhance the antitumor immune effect for PDIT and elucidate their corresponding immune-activation mechanisms. Nevertheless, there are several thorny challenges in PDIT, including limited light penetration, tumor hypoxia, immune escape and the development of novel small-molecule compounds that replace immune checkpoint inhibitors (ICIs) for easy integration into nanosystems. It is hoped that these issues raised will be helpful to the preclinical study of nanotherapeutics-based PDIT, thus accelerating the transformation of PDIT to clinical practice.
Keywords: photodynamic therapy, immunogenic cell death, nanotherapeutics, tumor immunotherapy
Introduction
As a global malignant disease, cancer usually shows a very high mortality rate. The main clinical treatments for cancer are still surgery, chemotherapy and radiotherapy nowadays [1]. Unfortunately, high recurrence, high metastasis and other poor prognosis in cancer patients can't be effectively avoided only by employing the conventional clinical medication. To address these problems, some antitumor adjuvant therapies like photodynamic therapy (PDT) have been gradually developed, which refers to the conversion of oxygen (O2) to cytotoxic ROS via photosensitizers (PSs) under light, and directly triggering the intrinsic mitochondrial oxidative damage-related apoptotic pathways, thereby achieving the goal of killing tumor cells [2, 3]. The dominant features of PDT present great spatiotemporal selectivity and minimal invasiveness [4]. In addition to destroying tumor cells by ROS, PDT can even induce immunogenic cell death (ICD), which is accompanied by the generation and release of damage-associated molecular patterns (DAMPs) including calreticulin (CRT), high mobility group box 1 (HMGB1) and adenosine triphosphate (ATP), which can be recognized by a variety of immune cells, and thus provoking the specific antitumor immune response [5, 6]. Numerous studies have demonstrated that ICD-based immunotherapeutic strategies hold great potential in cancer therapy [7, 8]. Thus more and more attention has also been paid to ICD-focused PDIT that owns an enhanced suppressive effect on certain tumors.
Visible light can be applied to penetrate tissues and trigger conventional PSs to generate ROS for PDT. Whereas, owing to the insufficient penetration of visible light, deep tumors become difficult to eradicate effectively [9]. Moreover, most of the PSs such as chlorin e6 (Ce6) and protoporphyrin IX (PpIX) in the free state usually show some disadvantageous properties, including low stability, suboptimal selectivity, poor cell absorption and poor tumor retention [10, 11]. Besides, the common immunomodulators like imiquimod (R837) are easy to be eliminated quickly while transported in the body. Meanwhile, as a result of the limited ability of tumor targeting and the complexity and heterogeneity of tumor microenvironment (TME), it's hard to develop the precise targeted therapeutic reagents [12]. To overcome the above defects, hydrophilic and surface-modifiable nanomaterials, which can be passively accumulated in tumor sites via the enhanced permeability and retention (EPR) effect, are widely applied in tumor PDIT [13, 14]. As a thriving therapeutic modality, the nanomaterials-based advanced nanotherapeutics can effectively assist drug delivery, thus improving the therapeutic effect of drugs. Specifically, the nanocarriers like liposomes, polymeric micelles or other nanomaterials can easily carry PSs (e.g., Ce6, PpIX), immune adjuvants (e.g., R837, cytosine-phosphate-guanine (CpG)), immune checkpoint inhibitors (ICIs) (e.g., small interfering RNA (siRNA), antibody), chemotherapy drugs (e.g., doxorubicin (DOX), paclitaxel), etc. to directly target tumor sites, further enhancing the stability of the delivery drugs and reducing systemic toxicity via the encapsulation and release of drugs [15, 16].
Tumor cells can be effectively eliminated by advanced nanotherapeutics-mediated PDIT. To be specific, tumor ICD can be induced after realizing PDT effect, to further promote the maturation of antigen-presenting cells (APCs) like dendritic cells (DCs) that can activate effector T cells, and thus advancing the immunosuppressive effect on tumor cells. However, there are some constraints such as severe hypoxia, poor ICD and immune escape that greatly suppress the antitumor efficacy during ICD-based PDIT. Among them, high hypoxia in TME results in limited efficacy of O2-dependent PDT [17]. Increasing evidence has indicated that, catalase (CAT) and manganese dioxide (MnO2) can catalyze endogenous hydrogen peroxide (H2O2) to produce O2 [18, 19]. So it is usually considered to alleviate tumor hypoxia by using nanocarriers loading CAT, MnO2 or other O2 catalysts in PDIT. Additionally, direct delivery of O2 through oxygen carriers such as perfluorocarbons (PFCs) and hemoglobin (Hb) is also a feasible way to remodel the tumor hypoxic microenvironment. Furthermore, the low-level ICD induced by common PDT strategies has become a critical concern in PDIT [20]. So far, the combination strategies like PDT synergized with chemotherapy or photothermal therapy (PTT) have been developed for increased tumor ICD [21], whose characteristics are to expedite antigen presentation via CRT exposure, HMGB1 release and ATP secretion. Similarly, some adjuvants like CpG can be easily integrated into nanoplatforms, which accelerate antigen presentation by stimulating toll-like receptors (TLRs) and further provoke effector T cells, thus enhancing specific antitumor immunity. Besides, immune escape of tumor cells becomes the one of essential features of tumor progression [22]. On account of the lack of costimulatory molecules that are able to activate T cells in immunosuppressive TME, fewer effector T cells can be generated and infiltrated into the tumor region, implying that malignant tumor cells can easily elude immune surveillance [23, 24]. As a dominant driver of tumor immune escape, immune checkpoints, such as programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1), cytotoxic T-lymphocyte-associated protein 4 (CTLA4) and indoleamine 2,3-dioxygenase (IDO), have long become a topic of great interest in biomedical field [25-27]. Generally, activated T cells can be notably inhibited by those immune checkpoints after antigen recognition [28], which leads to an inadequate antitumor immunity. In the past decade, the clinical application of immune checkpoint inhibitors (ICIs) has greatly promoted the development of cancer immunotherapy [29-31]. On the whole, the combination of ICD-based PDIT with immune checkpoint blockade (ICB) can bring about a superior tumor-suppressive potency by successfully inhibiting tumor immune escape.
In this review, we first introduce the material property of various nanocarriers containing organic nanocarriers, inorganic nanocarriers, metallic nanocarriers, and organic and inorganic composite nanocarriers, and concretely describe their functional diversities that include specific cell targeting and stimuli-responsiveness. Then, we discuss in depth the mechanisms and applications of several strategies associated with nanotherapeutics-mediated PDIT, which contain “Oxygen-increasing PDIT”, “ICD-boosting PDIT”, “Adjuvant-promoted PDIT” and “ICB-combined PDIT” (Scheme 1). Last, we summarize the current challenges and development prospects of PDIT, and put forward possible solutions to its existing defects and exposed problems. We hope to expand the advantages of advanced nanotherapeutics-based PDIT, thus shortening the transformation of PDIT from laboratory research to clinical trials.
Reasonable design of nanocarriers for PDIT
Nanocarriers-based PDIT is increasingly appreciated as an alternative antitumor strategy. Generally, nanometer size is regarded as a typical physical feature in nanosystem. Notably, the size of nanocarriers can usually be controlled to be relatively big to avoid invading capillaries, while also being small enough to avoid phagocytosis by the macrophages of the reticuloendothelial system [51]. Hence, suitable particle size of nanocarriers should be designed for PDIT under certain conditions to achieve prolonged circulation and selective extravasation [52]. Except for the particle size, the shape, charge and composition of functional nanocarriers also play a vital role in helping the payloads improve safety, pharmacokinetic properties and bioavailability, while determining the tissue distribution and cell internalization of drugs [53]. In addition, targeted nanocarriers based on different targeting molecules can achieve accurate localization and accumulation in tumor sites via active targeting or passive targeting like EPR. Particularly, active targeting can enhance therapeutic efficacy by increasing cell specificity and uptake [54]. Also, stimulus-responsive nanocarriers are able to control the cargoes release to specific sites under different external or internal stimuli, thus increasing the concentration of drug in tumor region. Collectively, reasonable design of various targeted and responsive nanocarriers with different sizes, shapes, charges and compositions is essential for antitumor accuracy and effectiveness of PDIT. Next, here we summarize the structural characteristics of various nanoplatforms for delivering cargoes in PDIT, and their corresponding functionalities such as targeting and stimulus response (Table 1).
Different types of nanocarriers for delivering drugs
Multiple types of nanocarriers are capable of being utilized for delivering PDIT-related antitumor drugs. In the section, we mainly discuss four types of nanocarriers, including organic nanocarriers, inorganic nanocarriers, metallic nanocarriers, and organic and inorganic composite nanocarriers.
Organic nanocarriers
Liposomes
Liposomes, the spherical vesicles composed of cholesterol, phosphatidylcholine and phosphatidylethanolamine, have a hydrophilic core and double-shell with good biocompatibility [63, 64]. Although conventional liposomal nanocarriers have some disadvantages such as the poor stability and rapid removal from blood circulation [65], liposomes can still be widely applied to encapsulate and deliver a variety of drug molecules, such as peptides, siRNA, chemotherapy drugs, etc., thus effectively reducing the toxicity of drugs, improving their pharmacokinetic properties, and enhancing the efficacy of tumor therapy [66]. In general, liposomes can integrate therapeutic reagents through active extrusion or passive diffusion. However, since different drugs have diverse pharmacokinetic characteristics, there are some differences in the form of packages between these drugs. Specifically, the hydrophilic drugs can enter the inner core of liposomes by passive diffusion, and the lipophilic drugs can be loaded into lipid bilayer resulted from hydrophobic interactions. Of course, liposomes can also synchronously encapsulate both hydrophilic and hydrophobic drugs to fulfill the co-delivery. Besides, nucleic acid-based drugs with negative charge can be carried and delivered by cationic charge adsorption effect on the surface of liposomes [67, 68]. Ding et al. used a liposome loaded with Ce6 and phosphoinositide 3-kinase gamma (PI3Kγ) inhibitor IPI-549 through hydrophobic interaction, which was capable of targeting myeloid-derived suppressive cells (MDSCs) in tumor immune microenvironment (TIME) and reversing the immunosuppressive state, thus enhancing PDIT efficacy to eradicate colon cancer [55]. Additionally, Kim et al. reported a liposome that encapsulated gemcitabine (GEM) in a hydrophilic core through hydrophilic interaction, which was beneficial to the treatment of biliary duct cancer by employing the therapeutic pattern of PDIT [56]. Similarly, Wang et al. also reported a redox-activatable liposome constructed by phospholipid-porphyrin conjugates, where IDO inhibitor NLG-8189 was embedded via hydrophilic forces, leading to the obvious augment of tumor ICD level, thus boosting the therapeutic effect of systemic anti-tumor immunity [38].
Polymeric micelles
Typically, polymeric micelles with spherical structure can be formed spontaneously while amphiphilic block copolymers are put into an aqueous solution, which consist of a hydrophobic inner core and a hydrophilic outer shell [69]. Unfortunately, polymeric micelles are less stable in biological fluids and have more complex properties [70], but there are still numerous advantages in drug delivery for tumor therapy. Specifically, the core of polymeric micelles can be made up of hydrophobic polymer segments, in which liposoluble drugs such as debydrochlorination DOX and paclitaxel can be effectively wrapped by hydrophobic effect [71]. Additionally, the hydrophilic components of block copolymers constitute the hydrophilic shell of micelles to ensure the high solubility of polymeric micelles in blood, thus facilitating the targeted delivery of drugs [72]. At present, it is common to design different types of polymeric micelles to deliver chemotherapeutic drugs for anti-tumor PDIT. For instance, Wang et al. designed a polymeric micelle based on negatively charged siRNA that could be adsorbed onto the surface of the micelle shell via electrostatic adsorption effect [39]. The polymeric micelle was unassembled under weak acid in TME, and siRNA was subsequently released to achieve PD-L1 gene knockdown (KD), resulting in enhanced immunosuppression against metastatic lung tumors. Cai et al. also used the same micelle embedding siRNA to achieve PD-L1 KD, which increased the number of CD8+ T cells and generated a potent antitumor immunity for PDIT [73]. In addition, Peng et al. designed a micelle modified by triphenylphosphonium (TPP) groups called as TPPM, which encapsulated the Ce6 in the hydrophobic core through physical hydrophobic forces, and was loaded with TPP on the surface of the shell via covalent grafting, thus being conducive to realizing the increased PDIT after targeting the mitochondria of tumor cells [74].
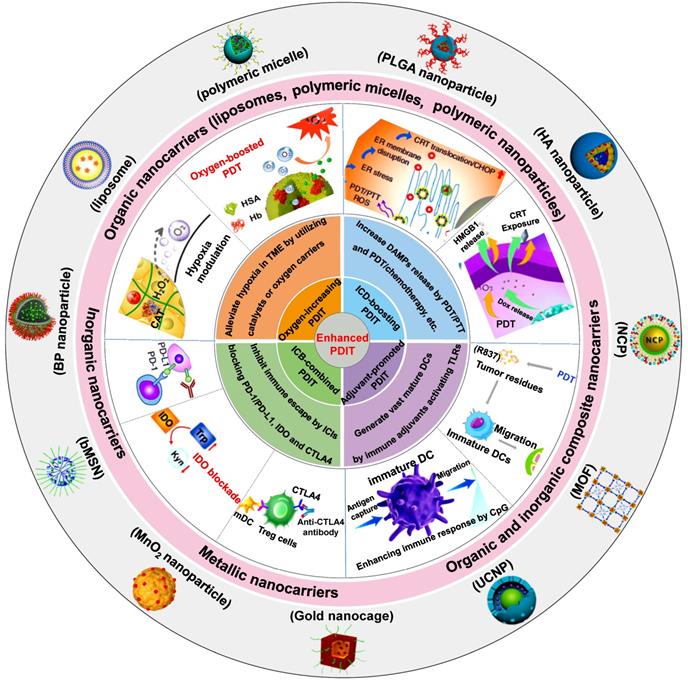
Schematic illustration of various advanced nanotherapeutics for enhanced PDIT. Partial images were adapted with permission from [32] copyright 2019, [33] copyright 2019, [34] copyright 2016, [35] copyright 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim; [36] copyright 2018, [37] copyright 2019, [9] copyright 2017, [38] copyright 2019, [39] copyright 2016, [40] copyright 2015, [41] copyright 2020, [42] copyright 2019 American Chemical Society; [43] copyright 2019, [44] copyright 2016 Springer Nature Limited; [45] copyright 2021, [46] copyright 2021 Wiley‐VCH GmbH; [47] copyright 2019, [48] copyright 2018 Ivyspring International Publisher; [49], copyright 2020 Springer Nature Switzerland AG; [50], copyright 2018 Elsevier Ltd.
Various nanoformulations for delivering therapeutic reagents during PDIT
| Category | Nanoformulation | Reagent | Drug-loading mechanism | Targeting ligand/receptor | Stimulus response | Target | Refs. |
|---|---|---|---|---|---|---|---|
| Organic nanocarrier | Liposome | Ce6/IPI-549 | Hydrophobic forces | IPI-549/PI3Kγ | pH | Colon cancer | [55] |
| Gemcitabine | Hydrophilic interaction | / | Photo | Biliary tract cancer | [56] | ||
| Polymeric micelle | Mitoxantrone | Hydrophobic forces | Anti-EpCAM/EpCAM | Photo | Liver cancer | [57] | |
| PPa/siRNA | Covalent binding/Electrostatic absorption | siRNA/PD-L1 | pH | Melanoma | [39] | ||
| Polymeric nanoparticle | MET/Ce6 | Hydrophobic forces | VRGDK/Integrin αvβ3 | Enzyme | Breast cancer | [58] | |
| IR-780/IMT/GITR | Electrostatic absorption/Hydrophobic forces | GITR antibody/Treg cells | pH | Melanoma/Colon cancer | [48] | ||
| JQ1/PPa/HA | Host-guest interaction | HA/CD44 | Redox | Pancreatic cancer | [46] | ||
| PpIX/PCPK | Hydrophobic forces | PCPK/PM | Enzyme | Breast cancer | [59] | ||
| Inorganic nanocarrier | GQDs | Ce6/HA | Covalent binding | HA/CD44 | Photo | Lung cancer | [60] |
| Graphene | IR820/CpG/TPP | Hydrophobic forces | TPP/Mitochondrion | Photo | Breast cancer | [61] | |
| BP | HA | / | HA/CD44 | Photo | Leukemia | [14] | |
| Metallic nanocarrier | Fe3O4 nanoparticle | Ce6 | Covalent binding | cell membrane/tumor tissue | Redox/pH | Breast cancer | [62] |
| Organic and inorganic composite nanocarrier | NCP | OXA/Pyrolipid | Hydrophobic forces | / | / | Colorectal cancer | [44] |
| UCNP | Ce6/R837 | Hydrophobic forces | / | / | Colorectal cancer | [9] | |
| MOF | H2TCCP/CpG/HA | Coordination effect/Electrostatic absorption | HA/CD44 | Enzyme | Liver cancer | [33] |
Polymeric nanoparticles
Polymeric nanoparticles (NPs), usually possess a size of 10-500 nm and a spherical structure, can be constructed by the self-assembly of natural or synthetic copolymers such as poly(lactic-co-glycolic acid) (PLGA), polysaccharides and natural proteins. Notably, remarkable varies of achievable drug loading in polymeric NPs limit their extensive application [75]. But undoubtedly, owing to favorable physicochemical features (e.g., easily controllable size and surface charge, the availability of various functional groups to conjugate cargoes) [76], polymeric NPs have become the greatly attractive nanocarriers. Generally, polymeric nanospheres or nanocapsules can be formed to wrap and deliver therapeutic drugs for cancer therapy [77]. Namely, the nanosphere is composed of the polymeric matrix, where drugs can be physically retained or adsorbed by electrostatic forces; the nanocapsule is made up of a polymeric shell that wraps around an oily core, in which hydrophobic drugs can be dissolved. It should be noted that the drugs can also be adsorbed on the wall of the polymeric shell [78]. For PLGA NPs, Yang et al. constructed a composite nanostructure containing the PLGA nanocompartment and ferritin (FRT) nanocompartment [79]. Specifically, the IDO inhibitor NLG919 was encapsulated in the PLGA nanocompartment through hydrophobic forces, and the zinc hexadecafuoro-phthalocyanine (ZnF16Pc) as a PS was physically loaded into the FRT nanocompartment, ultimately leading to an optimal tumor suppression by enhanced PDIT. Moreover, Ou et al. reported a PLGA NP modified by GITR antibody that was a kind of tumor necrosis factor (TNF) receptor family-related proteins, which simultaneously encapsulated imatinib (IMT) into the core of the nanocarrier via hydrophobic forces and absorbed the positively charged PS IR-780 on the polymeric shell by electrostatic interaction, thus quickly damaging tumor cell membrane during PDIT [48]. For polysaccharide NPs, hyaluronic acid (HA) NPs are widely applied in the relevant research of tumor PDIT. Sun et al. proposed a HA NP called as HCJSP based on HA-CD, AD-SS-JQ1 and AD-SS-PPa for pancreatic cancer therapy, in which HA-CD consisted of cyclodextrin (CD) grafting HA, and AD-SS-JQ1 and AD-SS-PPa were constituted by adamantine (AD) connecting with bromodomain and extraterminal protein 4 (BRD4) inhibitor JQ1 or pyropheophorbidea (PPa) via disulfide bonds, respectively [46]. Interestingly enough, the drug-loading pattern of HCJSP was different from the conventional modes such as hydrophobic or electrostatic interaction. In this study, both CD and AD were conjugated by molecular docking and host-guest interaction for the introduction of JQ1 and PPa into the HCJSP nanosystem. For natural protein NPs, Hu et al. synthesized a polymeric NP based on polypeptides (MA-pepA-Ce6 NP) that could be cleaved by matrix metalloproteinase-2 (MMP-2), where Ce6 was covalently grafted into the hydrophobic nanocore via amido bond, and metformin (MET) was conjugated into this polypeptides-based nanoplatform via the acid-sensitive bond [58]. As expected, this tactic effectively inhibited the PD-L1 ligand of tumor cells, thus enhancing the anti-breast cancer immune response of PDIT. In addition, dopamine is also applied to synthesize polymeric NPs. Wu et al. fabricated a hybrid polymer NPs consisted of polydopamine (PDA) and upconversion nanoparticle (UCNP), where Ce6 was encapsulated on the surface of mixed NPs for the combination of PDT/PTT. Specifically, the internal PDA was regarded as the photothermal core for PTT and the UCNP shell was used for PDT. Under 980 nm laser irradiation, synergistic photoimmunotherapy could achieve the augmented level of tumor ICD by exposing CRT, and further induced abundant mature DCs to activate cytotoxic T lymphocytes (CTLs), ultimately generating a potent antitumor immunity and effectively inhibiting tumor metastasis [80].
Inorganic nanocarriers
As a suitable inorganic nanocarrier, black phosphorus (BP) has been widely used in cancer treatment due to its advantages of high biocompatibility, good biodegradation, large surface area and negative charge [81]. For example, Li et al. constructed a novel NIR/ROS-sensitive BPQDs nanovesicle (BPNV), which encapsulated CpG into the vesicular cavity for antitumor immune activation in PDIT, leading to extensive damage of tumor cells via increasing TNF-α, IL-6 and IL-12 in serum [35].
As is well-known, the graphene with a two-dimensional (2D) and honeycomb structure possesses a large surface area to easily load more PSs, targeting moieties or chemotherapy drugs [82]. However, as a 0D graphene material, graphene quantum dots (GQDs/GOQDs) are highly desirable for antitumor immunity [60], which exhibit good distribution characteristics within tumor cells and show low toxicity to surrounding tissues. Also, it has been demonstrated that, the single-layer structure of graphene can increase its transparency to facilitate light penetration, and its internal π-π stacking structure can be easier to cooperate with the drug molecules, thus improving its overall solubility and stability [83]. Nafiujjaman et al. employed the GQDs binding with Ce6 and HA via ester bonds for tumor PDIT, which promoted the production of large amounts of ROS, and thus triggered a strong antitumor immune response [60].
Likewise, mesoporous silica (SiO2) NPs, that also own a high specific surface area and pore volume, can enable the delivery of more antitumor cargoes [84]. Additionally, good biocompatibility of SiO2 can ensure the endocytosis of more drugs [62]. Xu et al. designed a biodegradable mesoporous SiO2 NP (bMSN) loading CpG via electrostatic absorption and Ce6 through hydrophobic interaction, which expedited the maturation of DCs in PDIT, ultimately showing strong antitumor effects on both local and distant tumors in C57BL/6 mice [42].
Metallic nanocarriers
As a common metal-O2 catalyst, MnO2 with large surface areas, good absorption and degradation abilities has been extensively applied in antitumor PDIT [85]. Zhou et al. constructed a multifunctional anti-cancer nanoplatform, in which the mesoporous SiO2 shell and MnO2 shell were separately coated on the surface of Cu9S5 nanocrystals, and the adjuvant CpG was also loaded by electrostatic forces, contributing to producing a large number of ROS to destroy tumor cells [86].
Furthermore, although magnetic ferriferrous oxide (Fe3O4) NPs possess low toxicity, they are still greatly valuable for the delivery of anti-cancer drug. Moreover, Fe3O4 can also catalyze the decomposition of endogenous H2O2 to produce O2, thereby significantly improving the internal hypoxia of tumors [87]. For instance, Wang et al. prepared the Janus magnetic mesoporous organosilicon NPs (M-MONs) constituted by Fe3O4 head and SiO2 body to load the Ce6, which showed an obvious inhibition effect on primary and distant tumors [62].
Organic and inorganic composite nanocarriers
As a common organic and inorganic composite nanomaterial, non-toxic and porous MOFs consist of metal ions or clusters and organic ligands, which can reduce toxicity to surrounding tissues and enhance the loading capacity [88]. Lan et al. developed a novel MOFs-based PS (Fe-TBP) that was composed of Fe3O clusters and 5,10,15,20-tetra(p-benzoato)porphyrin (TBP) ligand. After the PDT effect was achieved under light, the anti-PD-L1 antibody was further administrated to realize the combined treatment of PDT and ICB, thus leading to increased CD4+ and CD8+ T cells that can specifically eliminate tumor cells. From in vivo outcomes, tumor elimination rate reached more than 90% in the mouse model of colorectal cancer [89].
For another typical composite material, UCNPs can be more stably transferred to aqueous solutions responsible for their oleic acid stability [90]. Moreover, UCNPs can generate large amounts of oxygen radicals under NIR to disrupt the redox homeostasis within tumor cells [91]. For the research on UCNPs, Xu et al. designed the UCNPs for the treatment of colorectal cancer. Namely, UCNPs were loaded with Ce6 and R837 via hydrophobic forces, which facilitated the maturation of DCs and release of related cytokines, further markedly reinforcing the efficacy of PDIT by combining PDT with CTLA4-blockade therapy [9].
Furthermore, the nanoscale coordination polymers (NCPs), composed of organic bridging linkers and metal ions, possess highly tunable constituents and structures, which can enable the combination of various therapeutic cargoes or modalities [92, 93]. By this token, NCPs may be also commonly applied in the sufficient delivery of multiple antitumor reagents during PDIT. He et al. reported a NCP nanoparticle carrying chemotherapeutic cisplatin and PS pyrolipid for enhanced PDIT, which could exhibit marked therapeutic effect on head and neck cancer via the combination of PDT and chemotherapy [40].
Various functionalities of nanocarriers for delivering drugs
Targeted nanocarriers
As is well-known, nanocarriers can achieve passive targeting of tumor tissues during PDIT via exploiting EPR effect [14, 38]. Furthermore, by functionally modifying the nanocarriers, active targeting of tumor sites, including cell membrane targeting, mitochondrial targeting and TME targeting, can be successfully achieved for more tumor entry of nanomedicines. In this section, we will focus on nanocarriers with active targeting functions.
Cell membrane targeting
Some specific markers on the surface of various cancer cells, such as epithelial cell adhesion molecule (EpCAM) and type I transmembrane glycoprotein CD44, can be used for cell membrane targeting in tumor PDIT. EpCAM is highly expressed on the cell membrane of many types of cancer including hepatocellular carcinoma, colon cancer, lung cancer and gastric cancer [94], which mainly plays a crucial role in the signal transduction, proliferation and differentiation of tumor cells, and has been utilized for the development of PDT based on targeted micelles [95]. Han et al. designed an EpCAM antibody-modified micelle loaded with mitoxantrone (MX) to treat hepatocellular carcinoma. Mice treated with these micelles showed significant fluorescence intensity, indicating that the EpCAM-conjugated micelles owned a good active targeting ability [57]. As another tumor surface antigen, CD44 protein is highly activated during tumor invasion and metastasis [96, 97]. Zhang et al. designed targeted BP NPs modified by PEGylated HA (HA-BP) for tumor PDIT [14], which could selectively accumulate at the tumor site through active targeting mediated by the HA receptor CD44. In vitro and in vivo experiments showed that HA-BP NPs had good biocompatibility, stability and therapeutic effect. Besides, the αvβ3 subtype in the integrin family, related to tumor proliferation, invasion and metastasis, is also highly expressed on the surface of various cancer cells [98]. Hu et al. reported a αvβ3 receptor-targeted NP (MA-pepA-Ce6 NP) for PDIT [58]. Of which, a small molecule PD-L1 inhibitor MET was covalently conjugated to Ce6 via a peptide linker (GPLGVRGDK, pepA). MA-pepA-Ce6 NP could quickly release MET and VRGDK-Ce6 after enzymatic response in TME. Subsequently, VRGDK-Ce6 could bind to the integrin αvβ3 receptor on tumor cell membrane to achieve tumor cell targeting, thus enhancing its tumor penetration and tumor cell internalization.
Mitochondrial targeting
It has been reported that, PSs can be delivered to mitochondria that may be more sensitive to ROS by using nanocarriers, thus resulting in better PDIT efficacy [99]. The reason for this is that damaged mitochondria can increase the production of ROS and further show a greater tendency to trigger apoptosis [100]. Accordingly, mitochondria have been considered as a preferred subcellular target in PDIT [101]. As a representative mitochondrial-inclined lipophilic cation, alkyl TPP has been widely applied to modify some molecules such as PSs for improving the selectivity of mitochondrial absorption, and reducing the toxic effect on normal tissues. Wu et al. developed a mitochondrial-targeted graphene NP encapsulated with IR820 and CpG for tumor PDIT [61]. In this study, TPP-modified graphene nanocarriers could specifically deliver IR820 into mitochondria and realize the photodynamic effect of mitochondrial localization. In another research, Peng et al. also prepared a TPP-based mitochondria-targeting nanocarrier for PDIT, where the positively charged polymeric micelle containing TPP groups was regarded as the core to load Ce6, and the charge transformational layer obtained from anionic 2,3-dimethylmaleic anhydride modified Biotin-PEG4000-NH2 via electrostatic interaction was regarded as the shell [74]. Notably, the nanocarrier could expose TPP in tumor extracellular microenvironment, and the exposed TPP groups had the capacity of targeting the mitochondria of tumor cells, which facilitated Ce6 to generate ROS within mitochondria, thereby presenting an excellent antitumor effect in PDIT. Similarly, Yang et al. also employed a mitochondrial-targeted dual-loading system that covalently bound Ce6 and TPP-modified PEI to the CRISPR-Cas9 system targeting the Ptpn2 gene [10]. As a result, the mitochondrial-targeting of nanocarriers can be expectably obtained by TPP modification during PDIT.
TME targeting
TME, containing the stromal cells, immune cells, vascular system, lymphatic system and extracellular matrix at the tumor site, usually plays a vital role in the occurrence, development and treatment of various cancers [102]. Hence, it will be beneficial to improve PDIT effect by various targeting molecules-based nanocarriers delivering therapeutic cargoes to the components in TME, such as cancer-associated fibroblasts (CAFs), tumor-associated macrophages (TAMs), and tumor vasculature.
As a crucial component of TME, CAFs exist in diverse cancers [103], which affect tumor growth and metastasis. In addition, fibroblast activation protein (FAP), highly expressed on the surface of CAFs cell membrane, can become a useful tumor-targeting antigen for PDIT. For instance, Zhen et al. used FAP-specific single-chain variable fragments-modified FRT ZnF16Pc nanocage to achieve PDIT-based antitumor effect by active targeting of CAFs in TME [104]. Also, TAMs are the most abundant leucocyte subset in many cancers and also play a vital role in boosting cancer progression [105]. Actually, the elimination of TAMs by nanocarriers targeting their highly expressed surface receptors (e.g., mannose receptors) has been considered as a promising antitumor approach [106]. Gao et al. reported a mannose-decorated mesoporous calcium silicate nanocomposite carrying indocyanine green (ICG) as a PS for PDT/PTT, which could obviously target TAMs in TME by the affinity between mannose and its receptor, thus effectively promoting tumor cell apoptosis [107]. In addition, due to the overexpression of neuropilin-1 (NRP-1) on angiogenic endothelial cells, it is usually regarded as a common target of tumor vasculature-targeted therapy [108]. Youssef et al. designed a gold nanorod loaded with PPa and “KDKPPR” peptide moiety for tumor PDT, which could achieve specific TME targeting by “KDKPPR” targeting NRP-1 of tumor vasculature, thus inducing a significant antitumor efficacy [109].
Stimulus-responsive nanocarriers
Generally, antitumor cargoes are first delivered to a specific tumor site by functional nanocarriers, and then quickly released in response to environmental stimuli. Notably, the stimulus-responsive drug delivery system can spatiotemporally control on-demand release of drugs, improve therapeutic efficacy and decline system toxicity [110]. In this section, we will mainly emphasize the drug release of stimulus-responsive nanocarriers under internal stimuli (pH, redox potential gradient, enzymes) or external stimuli (photo response).
Among most stimuli, pH sensitivity is usually applied to trigger drug release [111]. The acid-sensitive nanocarriers can trigger pH response in the TME (pH 6.8~7.2) or lysosome (pH 4.5~5.5) to release related drugs in PDIT [112, 113]. In fact, pH-sensitive nanocarriers like liposomes have been gradually developed since the 1980s, which can be protonated in lysosomes to achieve pH response [114, 115]. Yu et al. used pH-responsive nanoplatforms to deliver siRNA. In the TME with pH 6.8~7.2, acid-responsive micelles could be stimulated by pH to decompose and release siRNA for PD-L1 gene KD of tumor cells, thus enhancing the antitumor immune effect of PDIT [39]. In addition, Yang et al. constructed a self-assembled smart nanovesicle composed of pH-responsive block copolymer (PEG-b-cationic polypeptide), which could realize the pH response under the lysosomal environmental stimulation of pH 4.5~5.5, to release the PS HPPH and IDO inhibitor IND, thus heightening the effect of PDIT [116]. Indeed, this pH-sensitive smart nanovesicle offered great diversity and potential for the construction of nanomaterials for PDIT.
In addition to pH response, the intracellular redox potential gradient can also be served as a stimulus response to control drug release from nanocarriers. As a common reductant that acts on disulfide bonds, GSH is usually used in nano-drug delivery systems, whose concentration in the cytoplasm presents 0.5~10 mM [117]. Once the disulfide bond is destructed by GSH, the delivered drugs can be instantly released to exert their effects. Xu et al. developed bMSN embedding TLR9 agonist CpG and Ce6 for tumor PDIT, which achieved a superior antitumor potency in PDIT by releasing immunostimulant CpG and antigenic peptides after breaking disulfide bonds [42]. Moreover, this strategy was also used in multiple tumor-bearing mice models, demonstrating their potential to treat advanced cancers. Yu et al. used a GSH-responsive nanosystem-based PDIT strategy for the treatment of breast cancer [118]. Specifically, the PPa and IDO-1 inhibitor NLG919 were linked with compounds containing disulfide bonds, and further loaded into OXA prodrugs to form the light-inducible nanocargo (LINC). After the LINC was intravenously injected and delivered into 4T1 tumor cells, disulfide bonds were broken due to redox response, further facilitating the release of PPa and NLG919 from LINC.
Enzymes, such as phospholipase and nitroreductase, can not only be applied as catalysts to participate in the organism's life activities, but also as responsive stimulants to achieve the controlled release of drugs from nanocarriers [119, 120]. In a related research, a MMP-2-responsive multifunctional liposomal nanocarrier was introduced, where the MMP-2-sensitive linker could be disconnected by the catalysis of MMP-2 to release the protective long PEG segments [121]. In addition, the functional application of enzyme response has been widely concerned in the studies of nanocarrier-based PDIT. For instance, Hu et al. prepared an enzymatically cleavable self-delivery NP (MA-pepA-Ce6 NP). Noticeably, pepA could be specifically cleaved by MMP-2 in TME, and then the photodynamic reagent Ce6 and PD-L1 inhibitor MET could be effectively released to eliminate breast cancer [58]. Furthermore, Song et al. synthesized a chimeric peptide NP, which integrated PpIX with the checkpoint blocker IMT via a caspase-responsive peptide sequence Asp-Glu-Val-Asp. Once exposure to caspase stimulation, IMT could be easily released from decomposed nanocarriers to enhance tumor PDIT by activating antitumor immunity, ultimately effectively destroying primary and metastatic lung tumors [122].
Other external physical stimuli such as photo response have also been extensively explored for various nanodrug delivery systems. Usually, the light source can divide into ultraviolet light, visible light or NIR. The photosensitive nanocarriers loaded with therapeutic drugs can be cleaved to release drugs under the stimulation of the above light in PDIT. Sun et al. developed a ROS-sensitive polymeric nanocarrier to achieve light-controlled drug release, which enhanced the antitumor immune response of PDIT under NIR [123]. Furthermore, Hu et al. explored a lipid-polymer hybrid nanocarrier with a ROS-responsive core for on-demand release of DOX under NIR, thus augmenting the efficacy of nanocarrier-based PDIT [124].
Various strategies for enhancing PDIT effectiveness
Different types of nanocarriers (organic, inorganic, metallic, organic and inorganic composite nanocarriers) and their functionalities (targeting and stimulus response) have been summarized above in detail. Overall, the employment of nanocarriers can effectively ensure therapeutic efficacy and reduce off-target adverse reactions in the process of drug delivery. However, in recent years, several factors limiting the effectiveness of antitumor immunity in PDIT have attracted significant interest. As we known, O2-dependent PDT mainly produces inhibitory effects on tumors via cytotoxic ROS. Namely, when the ROS accumulates in large quantities within mitochondria, apoptosis-inducing factors can be further released to cause tumor cell death. Actually, higher O2 content can help the photodynamic nanosystem produce stronger antitumor inhibition effect [125]. Whereas, both natural hypoxic state and PDT-mediated O2 depletion can results in low O2 concentrations of tumor area, subsequently contributing to an attenuated PDIT efficacy [126]. Meanwhile, most conventional PDT strategies inducing tumor ICD cannot normally pose an apparent threat to tumor cells. In addition, although tumor cells can be recognized and killed by intrinsic T cells in the body's immune system, it has been found that tumor cells evolve the ability of immune escape, which can make T cells more difficult to effectively recognize the tumor cells, further resulting in a poor immune response. So, this negative regulation signaling has been often referred to as the immunosuppressive checkpoints [28], including PD-1/PD-L1, CTLA4 and IDO. Accordingly, it is vital to combine different checkpoint-blockade mechanisms to remove the constraints of these negative factors above, thereby reinforcing antitumor immune effect of PDIT.
Next, the underlying mechanisms of different immune-related drugs for enhanced tumor PDIT are detailedly illustrated. There are currently four main therapeutic modalities to enhance antitumor effectiveness of PDIT (Table 2). First, to increase the O2 concentration in TME, delivering various O2 catalysts and oxygen carriers through the functional nanoplatforms can alleviate tumor hypoxia and increase the production of PDT-triggered ROS. Second, to boost tumor ICD effect, it is necessary to raise tumor ICD level and heighten the immunogenicity of tumor cells via combination strategy like PDT plus chemotherapy or PTT. After the significant activation of tumor ICD, the immune signal DAMPs secreted by dying tumor cells can further provoke the release of “eat me” signal of tumor [127], which can trigger the activation of the antitumor immune system for enhancing the antitumor immunity of PDIT. Third, to sufficiently stimulate immune response in PDIT, immunoregulatory adjuvants such as TLR agonists (R837 and CpG) can be integrated into nanocarriers, to promote the maturation of DCs and enhance immune responses that eradicate tumor cells. Last, to block immunosuppressive effect of checkpoints, PDT can be combined with ICB-based immunotherapy, in which ICIs, including anti-PD-1/PD-L1 antibodies, siRNA, anti-CTLA4 antibodies and IDO inhibitors, are usually embedded in NPs to effectively avoid the immune escape of tumor cells, ultimately inducing the death of tumor cells by immune-activated CTLs.
Oxygen-increasing PDIT
The PSs can become excited states under light, which will generate free radicals or transfer energy to the surrounding O2 for producing ROS through a series of reactions, thus destroying tumor cells. Nevertheless, except for greatly restricting the antitumor efficiency of PDT, tumor hypoxia may even cause cancer recurrence [145]. Especially, relevant studies in colorectal cancer cases showed that, there was a higher probability of tumor recurrence in cancer patients with hypoxia-induced fat mass and obesity-associated protein degradation [146]. In recent years, much progress has been made in alleviating hypoxia in TME [147-149]. It has been reported that, high concentrations of H2O2 in TME present a new opportunity for reversing the hypoxia-related drug resistance in tumor therapy [150, 151]. Hence, some catalysts such as MnO2, cerium oxide (CeO2) and CAT, that own the capacity to catalyze the decomposition of endogenous H2O2 to produce O2, are usually introduced into nanosystems and widely applied for enhanced PDIT. In addition, directly delivering oxygen to the tumor site by utilizing PFCs or Hb can also effectively relieve hypoxia in TME, thus boosting the efficacy of PDIT.
(A) Schematic representation of iPS-MnO2@Ce6-mediated antitumor photodynamic immunotherapy. (B) Confocal laser microscopy images of oxygen-sensing probe in iPS cells indicating the intracellular oxygen level incubated with nanoprobes for the certain time. (C) Mature DCs in tumor tissues and TDLN of staining with CD80 and CD86 for flow cytometry assay. (D) Images of H&E and Ki67 stained tumor slides from the mice after various treatments. Adapted with permission from [49], copyright 2020 Springer Nature Switzerland AG. (E) Schematic illustration of the mechanism of photodynamic tumor immunotherapy with MnO2 nanoparticles. Adapted with permission from [128], copyright 2019 Ivyspring International Publisher. Abbreviations: iPSs: induced pluripotent stem cells; ICG: indocyanine green; H&E: hematoxylin and eosin; CTLs: cytotoxic T lymphocytes; TDLN: tumor draining lymph node.
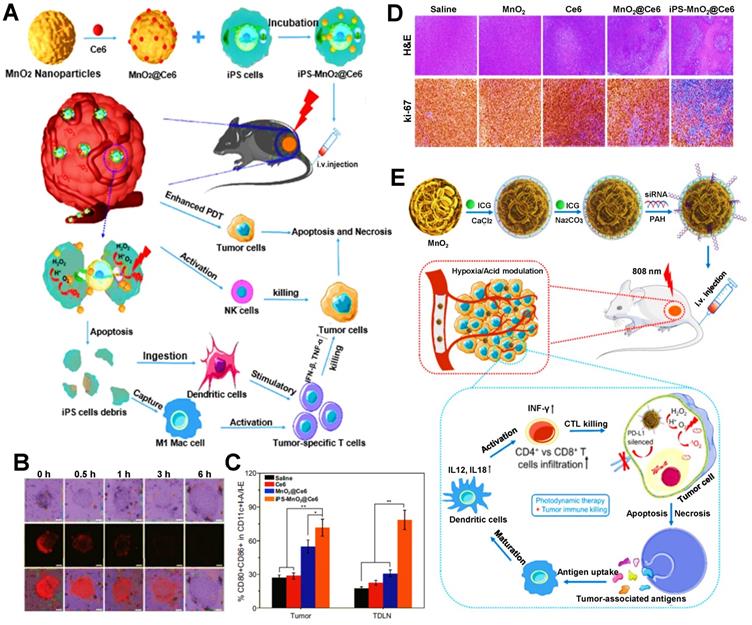
Previous studies have shown that, MnO2 exhibits a high degree of specificity and reactivity to H2O2, which can consume hydrogen ions to produce O2 and manganese ions [150, 152]. Liu et al. reported a MnO2@Ce6 NP encapsulated in induced pluripotent stem cells (iPSs) for enhanced PDIT efficacy (Figure 1A) [49]. The increasing O2 content in iPSs was found after measuring intracellular O2 levels with the oxygen-sensing probe (Figure 1B), which also confirmed the catalytic effect of MnO2 on endogenous H2O2. Under laser irradiation, the photodynamic conversion effect of Ce6 induced a large amount of O2 to be converted into ROS, thus enhancing the suppressive effect of ROS on tumor cells due to the increase in the amount of ROS. In addition, after injecting different nanoformulations into each group of tumor-bearing mice, it was found that the proportion of DCs maturation in mice treated with iPS-MnO2@Ce6 was higher than that in other treatment groups (Figure 1C). To certify the antitumor potency, after the tumor sections of each group were stained, the results showed that the tumor cell density in the iPS-MnO2@Ce6 group was the lowest (Figure 1D). From these data, it could be seen that the generation of sufficient O2 not only remarkably eliminated tumors by promoting high production of ROS, but also exhibited the enhanced effect of anti-tumor immunity in PDIT. Besides, Pan et al. designed a CaCO3/MnO2 nanoplatform loaded with ICG and siRNA to enhance tumor PDIT (Figure 1E) [128]. More specifically, the catalysis of H2O2 triggered by MnO2 augmented the O2 concentrations in TME, which further increased the contents of ICG-induced ROS under light, consequently raising the potency of antitumor immunity in PDIT. Besides, as common catalysts, CeO2 and titania (TiO2) are also widely used in PDIT. Zuo et al. constructed a mesoporous SiO2 NP co-loaded with CeO2, PS IR780 and MET, which generated enough O2 and Ce2+ after the etching of CeO2 in TME to boost IR780-mediated PDT, thus inducing a powerful antitumor PDIT effect [129]. Additionally, for the catalyst TiO2, Zheng et al. prepared the Au@TiO2 core-shell NP carrying DOX, where TiO2 as a new-type PS was able to catalyze endogenous H2O2 to produce more O2, thus effectively inhibiting tumor cells by enhanced PDT [130].
Except for the applications of MnO2, CeO2 and TiO2, CAT is also commonly used as a catalyst to ameliorate tumor hypoxia in PDIT. For instance, Meng et al. developed a PEG double acrylate (PEGDA) hydrogel (Ce6-CAT/PEGDA) combined with Ce6, immune adjuvant R837 and CAT for tumor PDIT [32]. After hydrogel was injected into mice and exposed to 660 nm light, ROS induced by Ce6 could induce PEGDA polymerization (Figure 2A).
Various strategies for enhancing the antitumor efficacy of PDIT
| Strategy | Nanoformulation | Reagent | Mechanism of enhanced PDIT | Target | Refs. |
|---|---|---|---|---|---|
| Oxygen-increasing PDIT | MnO2 | ICG and siRNA | Generate more oxygen by the catalysis of MnO2 and silence; PD-L1 by siRNA | Lung cancer | [128] |
| MSN | CeO2, IR780 and MET | Alleviate hypoxia in tumor by the etching of CeO2 in TME | Melanoma | [129] | |
| Au@TiO2 | DOX | Produce enhanced PDT by TiO2 catalyzing H2O2 to release more oxygen | Cervical cancer | [130] | |
| SiO2 | Ce6 and CAT | Overcome tumor hypoxia by CAT triggering decomposition of tumor endogenous H2O2 | Breast cancer | [131] | |
| Polymeric nanoparticle | Ce6 and NLG919 | Elicit stronger PDT efficacy by fluorinated polymers directly carrying O2 | Breast cancer | [132] | |
| Hybrid protein oxygen nanocarrier | Ce6 | Induce more sufficient PDT by Hb directly delivering enough O2 | Breast cancer | [36] | |
| ICD-boosting PDIT | Polymeric nanoparticle | HPPH and DOX | Enhance the population of TAAs and DCs recruitment by DOX inducing exposure of CRT and release of HMGB1 | Colorectal cancer | [37] |
| UCNP | RB and DOX | Enhance ICD by DOX inducing release of DAMPs | Breast cancer | [133] | |
| Boehmite | Ce6 and MLT | Express more DAMPs by MLT disrupting cell membrane via forming transmembrane pores | Breast cancer | [134] | |
| Liposome | Purpurin 18 | Induce the exposed increase of CRT by the combination of PDT and PTT | Breast cancer | [135] | |
| Hollow gold/liposome | ICG/Hemoglobin | Boost ICD by ICG inducing synergistic PDT/PTT | Melanoma | [43] | |
| Nanoneedle | Aluminum phthalocyanine tetrasulfonate | Generate stronger ICD by hyperthermia and ROS generation | Cervical cancer | [136] | |
| Polymeric nanoparticle | TCPP | Amplify ICD by TCPP inducing endoplasmic reticulum stress | Breast cancer | [137] | |
| ICD-boosting PDIT | Nanoaggregate | ICG | Induce powerful ICD based on enhanced PDT by discrete ICG concurrently alleviating ACQ and photobleaching | Breast cancer | [138] |
| NCP nanoparticle | Pt and ICG | Tailor aggregation of ICG and integrate the complementarity of PDT/PTT/chemotherapy to magnify the ICD effect | Breast cancer | [139] | |
| Adjuvant-promoted PDIT | MOF | H2TCPP, CpG and ACF | Release more cytokines by CpG activating TLR9 on the endosomal membrane | Liver cancer | [33] |
| Polymeric nanoparticle | Ce6 and CpG | Promote DCs maturation by CpG activating TLR9 | Melanoma | [140] | |
| Hydrogel | Ce6 and R837 | Amplify the immunogenicity of TAAs by R837 activating TLR7 on the lysosome membrane | Breast cancer | [45] | |
| ICB-combined PDIT | Nanoprobe | Phthalocyanine dye and anti-PD-1 antibody | Inhibit immune escape of tumors by anti-PD-1 antibody blocking PD-1/PD-L1 pathway | Breast cancer | [141] |
| Polymeric nanoparticle | IR780 and anti-PD-L1 peptide | Enhance tumor infiltration of effector T cells by anti-PD-L1 peptide blocking PD-L1 | Melanoma | [142] | |
| Polymeric micelle | PPa and siRNA | Improve immune response by siRNA inducing PD-L1 KD | Melanoma | [39] | |
| UCNP | Ce6, R837 and anti-CTLA4 antibody | Abrogate the activity of Tregs by anti-CTLA4 antibody blocking CTLA4 | Colorectal cancer | [9] | |
| Polymeric nanoparticle | Zinc phthalocyanine and anti-CTLA4 antibody | Boost the activation of T cells by anti-CTLA4 antibody binding to CTLA4 | Breast cancer | [143] | |
| Liposome | PpIX and NLG919 | Increase Trp to enhance the activity of T cells by NLG919 inhibiting IDO | Breast cancer | [47] | |
| Polymeric nanoparticle | Ce6 and NLG919 | Decrease Kyn to generate more CD8+ T cells by NLG919 interfering the activity of IDO | Colorectal cancer | [144] |
In detail, this light-triggered in situ gel released CAT to break down endogenous H2O2 in tumor cells under light irradiation, thus resulting in increased O2 concentrations in TME. Therefore, these findings showed that, compared with the H2O2 group, both the CAT group and Ce6-CAT group could trigger more generation of O2 in the solution of H2O2 (Figure 2B). When given light in the environment with sufficient O2, more ROS production was induced by Ce6, thus improving the ability to destroy tumor cells. In subsequent in vivo studies, the tumor volume and cell density in the Ce6-CAT/PEGDA group presented the smallest after 7 days of treatment (Figure 2C-E), fully reflecting the augmented antitumor immunotherapeutic efficacy resulted from oxygen-increasing PDIT. Similarly, Yang et al. also prepared a kind of mitochondrial targeted/pH-responsive SiO2 NP integrated with CAT and Ce6 [131]. As expected, the catalytic effect of CAT increased intracellular O2 content of tumor cells, and then a large number of ROS induced by Ce6 was produced under 660 nm NIR, thus effectively enhancing the immunotherapeutic efficacy of PDIT. Furthermore, Shi et al. also reported a CAT-based liposome with PS MBDP and DOX, which could reverse immunosuppressive TME by CAT catalyzing intratumoral H2O2, thus strengthening killing effect on breast cancer during PDIT [153].
(A) The scheme showing the formation of Ce6-CAT/PEGDA hydrogel for applying in enhanced PDIT. (B) The dissolved oxygen generation by various formulations. (C) Individual and (D) average tumor growth curves of 4T1 tumor-bearing mice from various formulations. (E) Images of H&E stained tumor slices from diverse groups of 4T1 tumor-bearing mice. Adapted with permission from [32], copyright 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (F) Schematic illustration of the mechanism of enhanced PDIT via utilizing PFCs. Adapted with permission from [132], copyright 2019 Elsevier Ltd. Abbreviations: CAT: catalase; PEGDA: PEG double acrylate; PFCs: perfluorocarbons; IDO: indoleamine 2,3-dioxygenase.
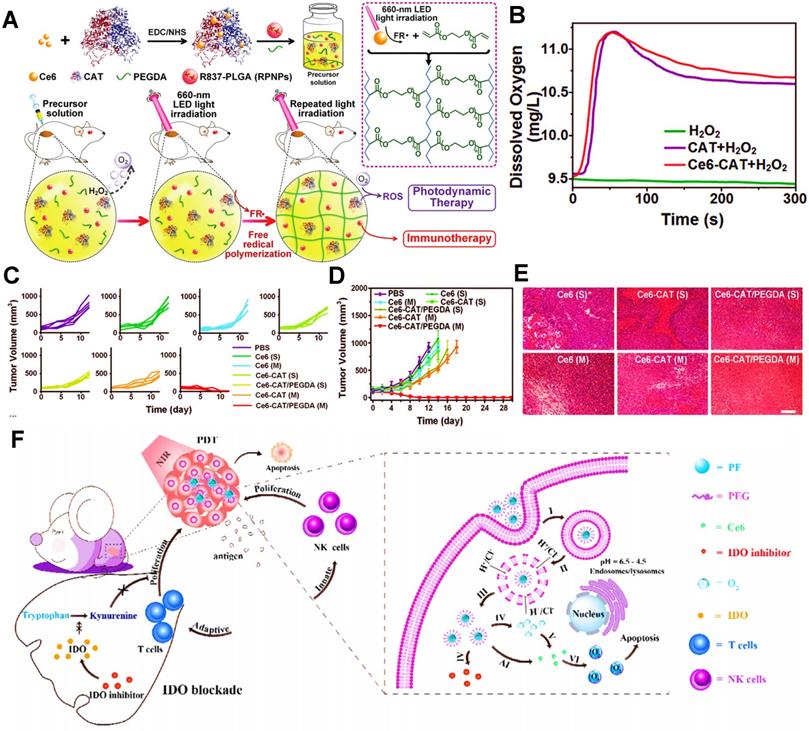
In addition, PFCs, as the inert chemical with extremely high O2 solubility, are capable of effectively storing oxygen molecules [154], which can be applied in PDIT to increase O2 concentration. For instance, Xing et al. constructed a fluorinated polymeric nanoparticle loaded with Ce6 and NLG919 (an IDO inhibitor) for synergistic tumor PDIT, which could induce stronger PDT efficacy by fluorinated polymers directly carrying high concentrations of oxygen and suppress immune escape of tumors by blocking IDO, thereby enhancing suppressive ability against breast cancer cells of PDIT (Figure 2F) [132]. Aside from PFCs, Hb can also have a vital role in improving anoxic environment due to its high oxygen carrying capacity. Luo et al. prepared a tumor-targeted oxygen-carrying hybrid protein nanocarrier composed of Hb and albumin, which encapsulated DOX and Ce6 and dissolved a large number of O2 by Hb so as to produce enhanced PDT via strengthening O2 self-supply and ROS generation, thus leading to the effective elimination of tumors [155]. Similarly, Chen et al. also fabricated a bioinspired hybrid protein oxygen nanocarrier containing Hb for improved PDIT, which could achieve the co-delivery of enough O2 and Ce6 to induce more sufficient PDT, ultimately evoking intense antitumor immunity [36].
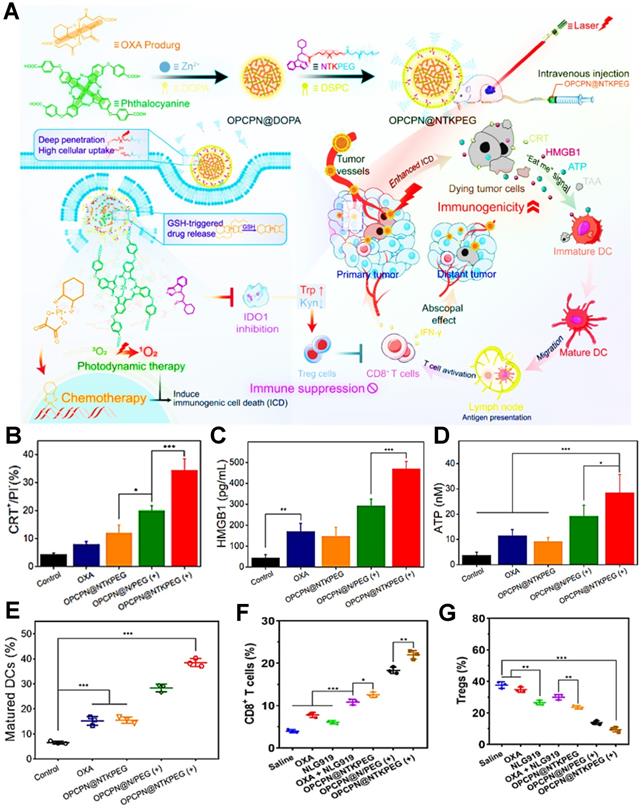
ICD-boosting PDIT
During the tumor PDIT, ICD is characterized by releasing DAMPs-based immune signal from dying tumor cells. These DAMPs can interact with various receptors such as phagocytosis-related receptors, purinergic receptors and pattern-recognition receptors on the surface of innate immune cells to realize ICD-induced antitumor immune responses [5]. However, tumor ICD activation is often limited by the low immunogenicity of tumors. Therefore, a series of ICD-boosting PDIT strategies such as the combination of PDT and chemotherapy have gradually attracted widespread attention. For instance, Huang et al. constructed a laser/GSH-responsive oxaliplatin (OXA)/phthalocyanine-based coordination polymer NP (OPCPN), which could achieve phthalocyanine-triggered PDT under laser irradiation and promote the ICD effect by OXA prodrugs exposing CRT, releasing HMGB1 and secreting ATP, further enhancing the antitumor immunity by PDT/chemotherapy combining with IDO inhibitor prodrug (NTKPEG) (Figure 3A) [156]. To confirm PDT/chemotherapy could cause more powerful ICD, the research results suggested that, compared with the OXA and OPCPN@NTKPEG groups, OPCPN@NTKPEG (+) group-induced CRT exposure nearly increased 4.3-fold and 2.9-fold, respectively. Moreover, both HMGB1 release and ATP secretion from the tumor cells treated with OPCPN@NTKPEG (+) exhibited over 2 times as many as those from the groups of OXA and OPCPN@NTKPEG (Figure 3B-D). In addition, the release of DAMPs obviously caused DCs maturation, subsequent tumor infiltration of CD8+ T cells and decline of regulatory T cells (Tregs), which resulted in the highest content of mature DCs, CD8+ T cells and minimum expression of Tregs in the OPCPN@NTKPEG (+) group compared to other groups (Figure 3E-G), and eventually realized the best antitumor immunotherapeutic effect in PDIT. Likewise, Jin et al. designed a mixed nanocarrier based on UCNP as the core and SPTP micelle as the shell for enhanced PDIT, in which DOX and the PS rose bengal (RB) were integrated into this composite nanocarrier for boosting ICD effect by upregulating the expression of CRT and HMGB1 within tumors, thus dramatically augmenting antitumor immune response of PDIT [133]. Yang et al. also synthesized a photo-responsive MSN co-loaded with chemo-drug DOX and PS methylene blue, which could amplify ICD effect by the synergistic PDT/chemotherapy under red light irradiation, subsequently evoking more powerful antitumor PDIT while combining with a PD-1 checkpoint blockade [157]. Moreover, melittin (MLT) served as a common chemotherapeutic agent can be also employed for enhanced PDIT efficacy. For example, Liu et al. designed a serum albumin-coated boehmite encapsulated with Ce6 and a honey bee venom-based MLT peptide for improving PDT-mediated ICD levels [134]. As a non-selective cytolytic peptide, MLT disrupted tumor cell membranes by forming transmembrane pores, which led to the initial damage of tumor cells. Furthermore, the formation of transmembrane pores by MLT also facilitated the accumulation of drugs in tumor cells and induced the expression and release of more DAMPs, thus markedly reinforcing the immune responses of PDIT.
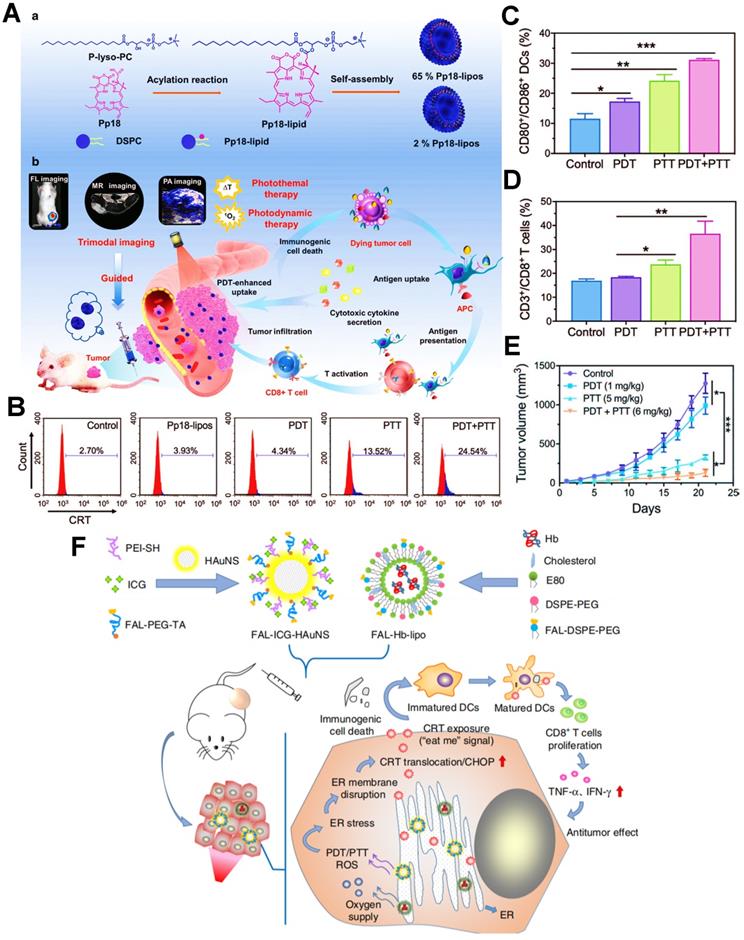
Furthermore, as an excellent antitumor immunotherapeutic strategy, the combination of PDT and PTT (PDT/PTT) based on nanomedicines also presents an apparent performance in exerting a potent ICD effect. In this regard, Sun et al. designed a versatile liposome-like nanoporphyrin carrying purpurin 18 as a PS for the synergistic PDT/PTT on the 4T1 tumor-bearing mice (Figure 4A) [135]. Upon exposure to the 705 nm laser, the PDT effect could be carried out for generating a large amount of ROS, and meanwhile the purpurin 18 owned an excellent capability of photothermal conversion, thereby further achieving the collaborative antitumor efficacy of PDT/PTT. To further demonstrate the ICD-boosting effect, the flow cytometric analysis of CRT revealed that the level of CRT exposure on 4T1 cell membrane during the PDT/PTT had a nearly six-fold increase compared with applying PDT alone (Figure 4B). On account of enhanced tumor ICD, it took the remarkable rise in the activation ratios of mature DCs and effector T cells (Figure 4C, D). Indeed, the in vivo data proved that tumor cells could be noticeably suppressed because of the stronger immune responses provoked by PDT/PTT (Figure 4E). Likewise, Li et al. established an endoplasmic reticulum (ER)-targeted nanosystem incorporating ICG and pardaxin peptides for PDT/PTT-mediated tumor immunotherapy, which further heightened tumor ICD followed by accelerated maturation of DCs and effective release of pro-inflammatory cytokines, thus ultimately exerting an excellent PDIT effectiveness (Figure 4F) [43]. In another study, Liu et al. used a nanoneedle loaded with the PS aluminum phthalocyanine tetrasulfonate for enhanced tumor PDIT, which effectively facilitated the level of ICD via PDT/PTT-based combination therapy, thus resulting in high-efficiency treatment of tumors [136]. As an aside, as distinguished from combination therapy, Deng et al. designed a reduction-sensitive polymeric NP loading ER-targeted PS TCPP-TER for the enhancement of PDIT via amplifying ICD [137]. TCPP-TER could selectively accumulate in the ER of tumor cells upon NIR irradiation and locally induce ROS production that triggered oxidative stress of ER, which resulted in increased expression of CRT and HMGB1, and then elicited a stronger immune response of tumor suppression. Additionally, enhanced ICD can also be achieved by tailoring photophysical properties of PSs. Zhao et al. prepared a discrete ICG-loaded nanoaggregate by sterically hindered aggregation degree editor for improved tumor PDIT, which could concurrently alleviate aggregation-caused-quenching (ACQ) and photobleaching, thus evoking a powerful antitumor immune response by amplifying the ICD level of tumor [138].
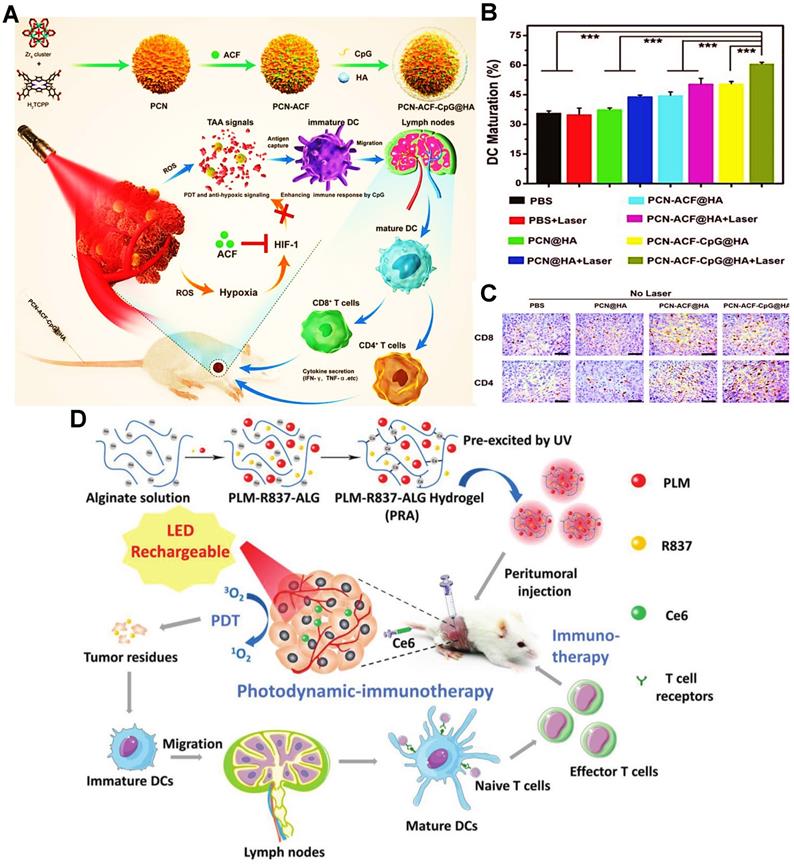
Adjuvant-promoted PDIT
To improve the immunogenicity of antigens, the adjuvants can be supplemented to sub-unit or recombinant vaccines for boosting antitumor immunity [158]. Inspired by this principle, some adjuvants like CpG or R837 can usually be encapsulated into nanocarriers for strengthening the antigen presentation of APCs during PDIT. For instance, Cai et al. reported a MOFs-based NP loading PS H2TCPP, adjuvant CpG and hypoxia-induced factor-1 inhibitor ACF for enhancing the effectiveness of tumor PDIT (Figure 5A) [33]. During the PDIT, tumor cells were effectively eradicated by ROS under 670 nm laser irradiation, followed by the abundant release of tumor associated antigens (TAAs) and DAMPs. In terms of the level of DCs maturation within tumors, compared to other groups without laser irradiation or the treatment group of CpG, it was found that the PCN-ACF-CpG@HA group with laser irradiation exhibited the highest percentage (61.21%) of DCs maturation (Figure 5B). The probable reason was that CpG released from MOF NP could activate TLR9 on the endosomal membrane of DCs, and further released a large number of cytokines to induce DCs maturation. Moreover, the ability of antigen presentation was also improved by the increased mature DCs, thus evoking a mass of activated T cells to eliminate tumor cells. As expected, the more tumor infiltration of CD4+ T cells and CD8+ T cells were distinctly observed in the PCN-ACF-CpG@HA group under laser irradiation (Figure 5C). Similarly, Wen et al. designed thiol-activated bovine serum albumin NPs (TABNs) for tumor PDIT [140]. Specifically, TABNs were first anchored onto the surface of tumor cells, and then the thiol-exposed BSA molecules were introduced to link TABNs to an albumin-based net that spatially caged tumor cells. In addition, Ce6 and CpG could be individually attached onto the tumor cell surface through hydrophobic and electrostatic interactions. The immunoregulatory CpG was further applied to activate TLR9, thus maintaining immunostimulation by increased expression of heat shock protein 70 (HSP70) and continuous exposure of tumor antigens. The final results showed that the strong and persistent immunostimulation promoted the sufficient maturation of activated CTLs, leading to the enhancement of immunotherapeutic effect in PDIT. In another research, Shu et al. proposed an enhanced PDIT strategy, namely that a hydrogel loaded with Ce6 and adjuvant R837 was fabricated for improving adaptive immune responses against tumors (Figure 5D) [45]. R837 regarded as a TLR agonist could specifically activate TLR7 on the lysosome membrane, thereby heightening the immunogenicity of TAAs. As a consequence, it was demonstrated that a big number of mature DCs could be activated to accelerate the antitumor immune response by that strategy above.
ICB-combined PDIT
At present, ICB-based immunotherapy has become a first-line treatment option for most cancers [159, 160]. It is worth noting that PDIT combined with ICB can produce a synergistic effect and enhance the response rate of ICIs [152, 161], thus producing the optimal antitumor potency of PDIT.
(A) Schematic illustration of PDT/chemotherapy combining with IDO inhibitor prodrugs to enhance antitumor efficacy. (B) Flow cytometric examination of CRT exposure. (C) Determination of HMGB1 release and (D) ATP secretion during antitumor PDIT. (E) Mature DCs ratio of tumor-bearing mice with different strategies. (F) Intratumoral infiltration ratio of CD8+ T cells and (G) Tregs in PDIT. Adapted with permission from [156], copyright 2021 American Chemical Society. Abbreviations: OXA: oxaliplatin; DOPA: dihydroxyphenylalanine; NTKPEG: NLG919-thioketal-PEG; Trp: tryptophan; Kyn: kynurenine; GSH: glutathione; OPCPN: oxaliplatin/phthalocyanine-based coordination polymer nanoparticle; CRT: calreticulin; HMGB1: high mobility group box 1; TAA: tumor-associated antigen.
PD-1/PD-L1 blockade
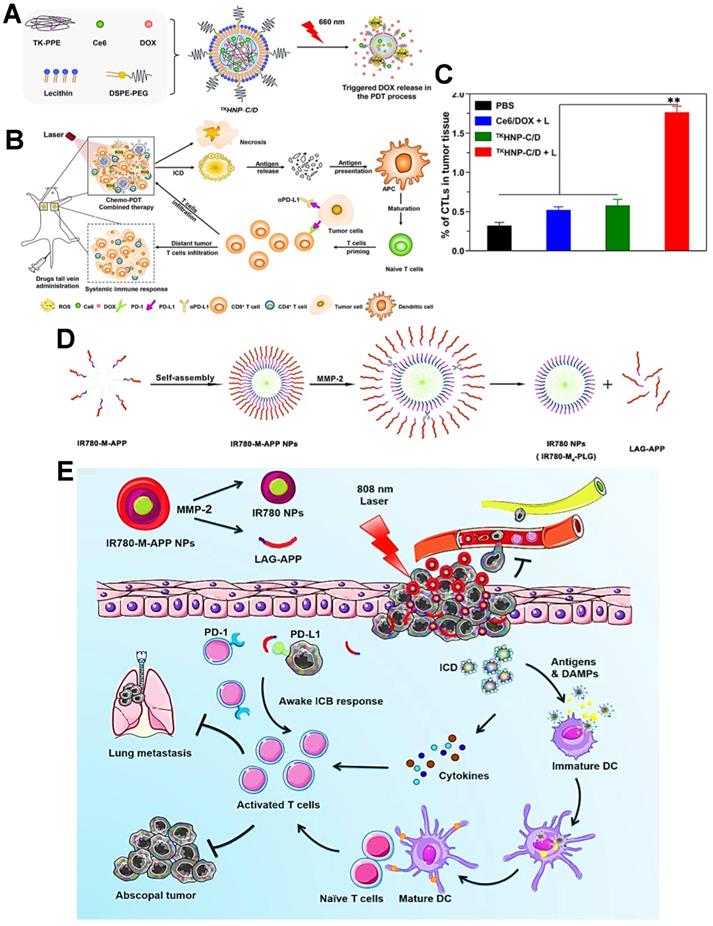
As a common immune checkpoint, the PD-1 receptor on the surface of the T cell membrane can bind to the PD-L1 on the surface of the tumor cell membrane, which directly impedes the activity of T cells and prevent T cells from attacking tumor cells [162]. To avoid this issue, a variety of therapeutic strategies have been well studied for this type of ICB, among which the blocking strategy based on PD-1/PD-L1 immune checkpoint holds great potential [142]. Indeed, the anti-PD-1/PD-L1 antibody has been currently considered as an effective ICI for cancer immunotherapy. For anti-PD-1 antibody, Gao et al. used an integrin αvβ6-targeted phthalocyanine dye-labeled probe combined with anti-PD-1 antibody-based ICB for the synergistic tumor PDIT. During this combination strategy, phthalocyanine dye-labeled probe could evoke powerful antitumor efficacy of PDT by generating sufficient ROS under 690 nm laser, and anti-PD-1 antibody could achieve the specific blockade of checkpoint PD-1 for impeding immune escape of tumors, ultimately effectively suppressing breast cancer growth and lung metastasis [141]. In terms of anti-PD-L1 antibody, Hu et al. reported lipid-polymer hybrid NPs loaded with Ce6 and DOX, which could finally achieve enhanced effectiveness of PDIT under light irradiation (Figure 6A) [124].
(A) Schematic illustration of the preparation of Pp18-lipos and application in enhanced PDIT. (B) Flow cytometry analysis of the level of CRT exposure after different phototherapies. (C) Frequency of tumor-infiltrating mature DCs in tumor-bearing mice with different treatments. (D) Corresponding quantification of CD3+/CD8+ T cells of mice by various treatments. (E) Tumor growth curves by different phototherapies. Adapted with permission from [135], copyright 2020 Wiley-VCH GmbH. (F) Schematic illustration of the formulation of nanocarriers and the mechanism of enhanced ICD via ER-targeting PDT/PTT. Adapted with permission from [43], copyright 2019 Springer Nature Limited. Abbreviations: ER: endoplasmic reticulum; Pp18: purpurin 18; PEI: polyethylenimine; HAuNS: hollow gold nanospheres; Hb: hemoglobin; E80: egg phosphatidyl lipid-80; TA: thioctic acid.
(A) Schematic illustration of the preparation of PCN-ACF-CpG@HA and principle for enhanced tumor PDIT. (B) Frequency of DCs maturation in tumor-bearing mice receiving different treatments by flow cytometry. (C) The CD4 and CD8 immunohistochemical images of tumors by using various formulations. Adapted with permission from [33], copyright 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (D) Schematic illustration of the fabrication procedure and application of PLM-R837-ALG hydrogel during PDIT. Adapted with permission from [45], copyright 2021 Wiley-VCH GmbH. Abbreviations: ACF: acriflavine; H2TCPP: tetrakis (4-carboxyphenyl) porphyrin; TAAs: tumor associated antigens; TLR: toll-like receptor; HIF-1: hypoxia inducible factor-1; HA: hyaluronic acid; ALG: alginate; PLM: persistent luminescence material.
In this system, ROS-based photodynamic immunogenicity induced by Ce6 and tumor immunogenicity elicited by DOX could lead to a certain antitumor immunotherapeutic effect that was still blocked by PD-1/PD-L1 pathway. Accordingly, to strengthen specific T cells-based immune response during PDIT, the anti-PD-L1 antibody was further adopted and injected into tumor-bearing mice. As expected, the anti-PD-L1 antibody could effectively bind to PD-L1 on tumor cell membrane, and then competitively block the interaction between PD-L1 and PD-1 (Figure 6B), thus facilitating the sufficient tumor infiltration of activated CTLs that owned the ability to eliminate tumors (Figure 6C). In this research, PDIT combined with anti-PD-L1 antibodies exhibited a powerful systemic immune response, resulting in obvious elimination of both primary and distant tumors [44]. Also, Wang et al. proposed an ICB-combined PDIT strategy for PD-L1 blockade by using anti-PD-L1 peptide instead of anti-PD-L1 antibody [142]. In detail, a MMP-2-responsive polymeric NP co-loading the PS IR780 and anti-PD-L1 peptide was constructed for improved PDIT (Figure 6D). Under 808 nm NIR irradiation, the photodynamic conversion of IR780 enabled PDT-induced tumor ICD by generating ROS. More importantly, the anti-PD-L1 peptide released from NP specifically blocked checkpoint PD-L1, leading to the improved effect of PDIT on destroying tumor cells and suppressing lung metastasis of tumors (Figure 6E).
(A) Schematic representation of triggered DOX release from TKHNP-C/D under 660 nm laser. (B) The working principle of antitumor immune responses in PDT combined with PD-1/PD-L1 blockade therapy. (C) Flow cytometric analysis of the intratumoral infiltration of CTLs by various treatments. Adapted with permission from [124], copyright 2019 Elsevier Ltd. (D) The preparation and MMP-2 response of IR780-M-APP NPs. (E) Schematic illustration of antitumor immune responses based on combination of PDT and ICB immunotherapy under 808 nm laser. Adapted with permission from [142], copyright 2020 Elsevier B.V. Abbreviations: TK-PPE: thioketal phosphoester; MMP-2: matrix metalloproteinase-2; APP: anti-PD-L1 peptide.
(A) Schematic illustration of preparation of POP micelleplexes loaded with PPa and siRNA and pH response in the acid surrounding. (B) Schematic drawing of POP/PD-L1 micelleplex-mediated tumor PDIT. (C) Western blot assay of PD-L1-KD in B16-F10 cells after receiving different POP micelleplexes loaded with 40, 80, 160 nM siRNA, respectively. (D) Proportion of tumor-infiltrating CD8+ T cells during PDIT. (E) Tumor growth inhibition curves by various strategies. (F) Images of TUNEL and H&E staining of the primary tumors. Adapted with permission from [39], copyright 2016 American Chemical Society. Abbreviations: siRNA: small interfering RNA; KD: knockdown; NF-κB: nuclear factor kappa B; PBS: phosphorus buffer saline; TUNEL: terminal deoxynucleotidyl transferase-mediated dUTP nick- end labeling.
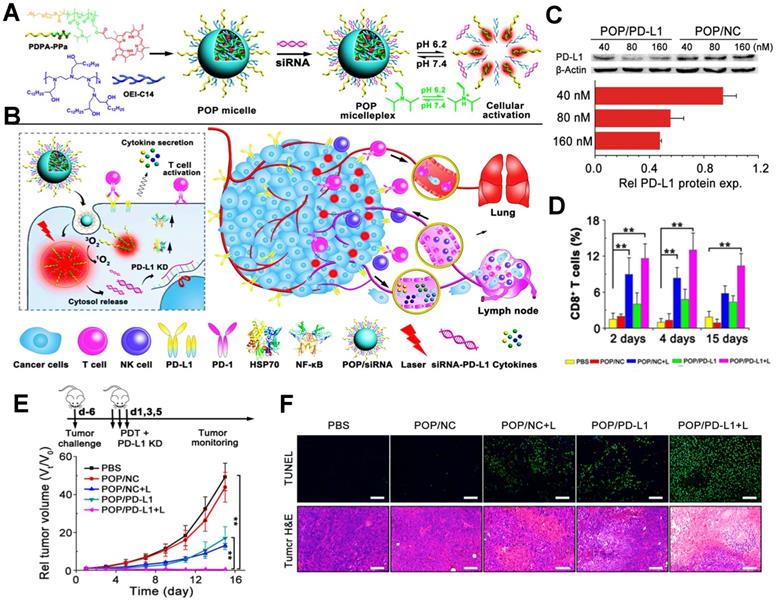
In addition, PDIT strategy can also combine with siRNA to block the immune checkpoint PD-L1 for the elimination of tumor cells. For instance, Wang et al. used an acid-activatable micelleplex nanoplatform (POP micelles) carrying the PS PPa and PD-L1 checkpoint-blocked siRNA, which could further enable the enhanced effect of anti-tumor PDIT (Figure 7A, B) [39]. In a further study, to prove the ICB potency by PD-L1 KD, Wang et al. prepared the POP micelles loaded with siRNA/PD-L1 (POP/PD-L1) and POP/NC for synergistic PDIT. Indeed, it was found that POP/PD-L1 could present stronger capacity of PD-L1 KD with increasing concentration of siRNA, thus dramatically inhibiting PD-L1 expression. Particularly, while the concentration of siRNA in the POP micelles remained 160 nM, more than 50% of PD-L1 expression presented downregulated (Figure 7C). During tumor PDIT, siRNA-caused PD-L1 downregulation resulted in the inability of the tumor cell membrane surface to produce PD-L1 ligands, thus indirectly blocking PD-L1 followed by an increase in activated T cell (Figure 7D). In in vivo assessment of antitumor potency, compared to other groups, POP/PD-L1 with laser irradiation group completely eliminated the B16-F10 tumors without body weight loss of mice (Figure 7E). Moreover, TUNEL and H&E staining of the tumor sections showed that PDT combined with PD-L1 KD significantly induced apoptosis of the tumor cells (Figure 7F), which reflected the stronger antitumor effect generated by the cooperation with ICB-based immunotherapy.
CTLA4 blockade
During the immune response of PDIT, in addition to the engagement of antigen-major histocompatibility complex (MHC) complexes with T cell receptors, extra costimulatory signals are also necessary for T cell activation [163, 164]. Among them, B7 molecules expressed on APCs (e.g., DCs, B cells) and CD28 on T cells are usually identified as two important costimulatory molecules, which can generate costimulatory signals by mutual combination [165]. Notably, as a second counter-receptor for the B7 family of costimulatory molecules, CTLA4 can negatively regulate the activation of T cells by displacing CD28 costimulation [166], thus suppressing antitumor immunity. Accordingly, the anti-CTLA4 antibody can be usually considered as a common ICB agent for improved PDIT, which can inhibit tumor immune escape by effectively blocking CTLA4 on the surface of T cells, further activating a large number of CD8+ T cells to eliminate tumors. For example, Xu et al. designed an UCNP that co-loaded the Ce6 and the adjuvant R837 for combined PDIT (Figure 8A) [9]. While injected into mice and given light, the NPs triggered PDT for destroying tumor cells. Subsequently, after injection of anti-CTLA4 antibodies into mice, CTLA4 molecules on the surface of T cells could effectively bound to anti-CTLA4 antibodies, which prevented CTLA4 from competitively binding to mature DCs, thereby enabling the increased levels of CD8+ T cells and decreased levels of Tregs for boosting antitumor effect (Figure 8B-D). Additionally, Chen et al. constructed a pH-responsive dextran NP co-loaded with the PS zinc phthalocyanine and anti-CTLA4 antibody to apply in 4T1 tumor-bearing mouse models, which could induce an abundant activation of T cells through blocking CTLA4, effectively eliminating breast tumor cells during PDIT (Figure 8E) [143].
IDO blockade
Unlike other immune checkpoints, IDO belongs to a special class of small molecule inhibitors [167], such as NLG919. Endogenous IDO is often defined as an immune-mediated enzyme that can catalyze the oxidative metabolism of tryptophan (Trp), thus accelerating the degradation of Trp into kynurenine (Kyn). Actually, the lack of Trp can usually impair the activity of CTLs, and conversely, the accumulated Kyn can heighten the activity of Tregs [168-170], thus causing a certain inhibitory effect on cancer immunotherapy. From this principle, IDO blockade becomes a feasible strategy for improved antitumor immunity in PDIT. Huang et al. constructed a liposome co-loaded with PpIX and NLG919, which could achieve the synergistic effect of PDT and IDO-based ICB, and thus apparently restraining tumor growth (Figure 9A) [47]. In this research, PpIX released from liposomes was able to destroy tumor cells by the production of ROS under light, and meanwhile, immune checkpoint IDO could be successfully blocked by small molecular NLG919. Compared with the saline and PpIX@Lipo groups, the Kyn/Trp ratios were dramatically reduced and more CD8+ T cells were generated following the treatments of NLG@Lipo and PpIX-NLG@Lipo groups (Figure 9B, C), implying that IDO was significantly inhibited by NLG919 and thus effectively stimulating T cell immunity. Notably, due to PDIT combined with IDO-blockade, it was discovered that the PpIX-NLG@Lipo with light irradiation group revealed the most CD8+ T cells and obvious inhibitory effects on both primary and distant tumors, eventually resulting in a prominent reduction in tumor volume (Figure 9D, E). Likewise, Hu et al. also designed a GSH-responsive HA NP combined with Ce6 and NLG919 via host-guest interaction for enhanced PDIT, which expectably brought about a superior antitumor immune response by the combination of Ce6-based PDT and NLG919-induced IDO blockade (Figure 9F) [144].
Conclusion and outlook
In recent years, the combination strategy of PDIT has attracted more and more attention for its promising application in the treatment of tumor recurrence and metastasis. With the rapid development of nanomedicine, a variety of multifunctional nanocarriers have been developed for antitumor PDIT. It should be noted that different types of nanocarriers have diverse structural characteristics and functions. In general, therapeutic payloads are loaded into different delivery nanoplatforms through physical hydrophilicity, hydrophobic interaction or electrostatic adsorption. During drug delivery, functional nanocarriers can be utilized to precisely target the tumor cells and responsively release the therapeutic cargoes to target site upon exposure to various physical or chemical stimuli, thus exerting the corresponding antitumor effect. Whereas, as a result of severe hypoxia, poor immunogenicity and immune escape in TME, the tumor-suppression effect of PDIT is extremely limited. Therefore, it is necessary to consider combining different treatment strategies, including increasing O2 concentration, boosting ICD effect, enhancing tumor immunogenicity, to activate mature DCs and inhibit the immune escape of tumor cells, thus strengthening the anti-tumor immune responses of PDIT.
(A) Schematic illustration showing the fabrication procedure of UCNP-Ce6-R837. (B) Mechanism of enhanced cancer PDIT by combining NIR-mediated PDT with CTLA4 checkpoint blockade therapy. (C&D) Frequency of tumor-infiltrating CD8+ CTLs (C) and Tregs (D) in the distant tumor. Adapted with permission from [9], copyright 2017 American Chemical Society. (E) Scheme of the co-delivery system loaded with CTLA4 antibodies and mechanism of antitumor immune responses induced by PDT in combination with CTLA4 blockade. Adapted with permission from [143], copyright 2020 Elsevier B.V. Abbreviations: UCNP: upconversion nanoparticle; TCR: T cell receptor; mDC: mature dendritic cell; ZnPc: zinc phthalocyanine.
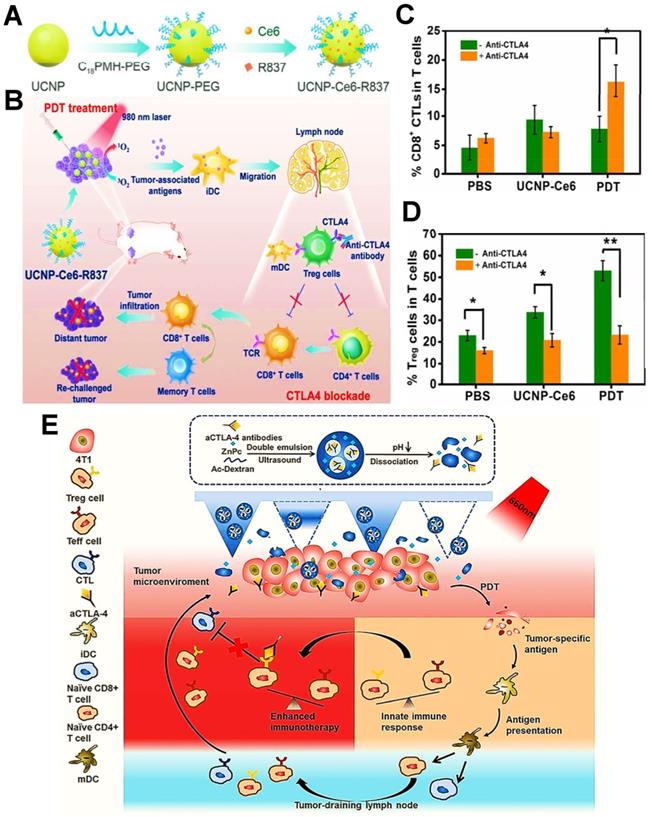
However, there are still many challenges in anti-tumor PDIT. First, some certain nanomaterials own inherent toxicity, which may produce obvious inhibitory effects on other normal tissue cells in the body. Hence, we need to design more hypotoxic nanomaterials such as liposomes to ensure systematic safety during tumor PDIT. Second, due to the insufficient light penetration, the PSs cannot be effectively stimulated for more production of ROS, thus leading to a great impact on the efficacy of PDIT. At present, some high penetration light sources like X-rays have been utilized to enhance PDIT by augmenting photodynamic conversion efficiency [171]. Even so, there is a still concern here that high penetration light-based PDIT should ensure the less damage to normal tissues. Furthermore, it may become a feasible strategy that PSs with high photoconversion capacity, such as IR780 or RB, can also be abundantly developed for improved tumor PDIT [172, 173]. Third, to a certain extent, tumor hypoxia can also limit the efficacy of oxygen-driven PDT. Usually, we can solve this problem by nanoformulations loading oxygen catalysts (e.g., CAT and MnO2) or oxygen carriers (e.g., PFCs and Hb). The underlying mechanisms of these catalysts and oxygen carriers were concretely discussed in Section “Oxygen-increasing PDIT”. Fourth, immune escape of tumor cells can directly contribute to a poor antitumor immune response, which also become a tough challenge in tumor PDIT. To address it, diverse strategies, especially ICB-combined therapy, have been extensively developed for preventing checkpoints-based immunosuppression of tumor. In addition, the development of new-type immune checkpoints and relevant inhibitors may bring about a more powerful anti-tumor immune response, which also requires extensive laboratory exploration and research. Fifth, in basic or clinical studies, ICB-based antibodies such as anti-CTLA4 and anti-PD-1/PD-L1 are usually administered individually instead of the development of nano-preparation, one possible reason attributed to a certain decrease in antibody activity during NP delivery. Thus, it is urgent to develop different functional some small molecule reagents such as siRNAs or compounds to replace macromolecule ICB antibodies, which may hold great potential in nanotherapeutics-based PDIT for achieving the effective delivery of ICIs.
(A) Schematic illustration of preparation of PpIX-NLG@Lipo and mechanism of enhanced PDIT. (B) Kyn/Trp ratios in plasma of 4T1 tumor-bearing mice by different formulations. (C) Frequency of CD8+ T cells infiltration in the distant tumors detected by flow cytometric analysis. (D) Primary and (E) distant tumor growth curves of mice after receiving different treatments during PDIT. Adapted with permission from [47], copyright 2019 Ivyspring International Publisher. (F) Schematic illustration of fabrication of HCNSP nanocarrier via host-guest interaction and working principle of combination antitumor immunotherapy by simultaneous ICD induction and IDO-1 inhibition. Adapted with permission from [144], copyright 2020 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim. Abbreviations: PpIX: protoporphyrin IX; Kyn: kynurenine; Trp: tryptophan; HA: hyaluronic acid; CD: cyclodextrin; CRT: calreticulin.
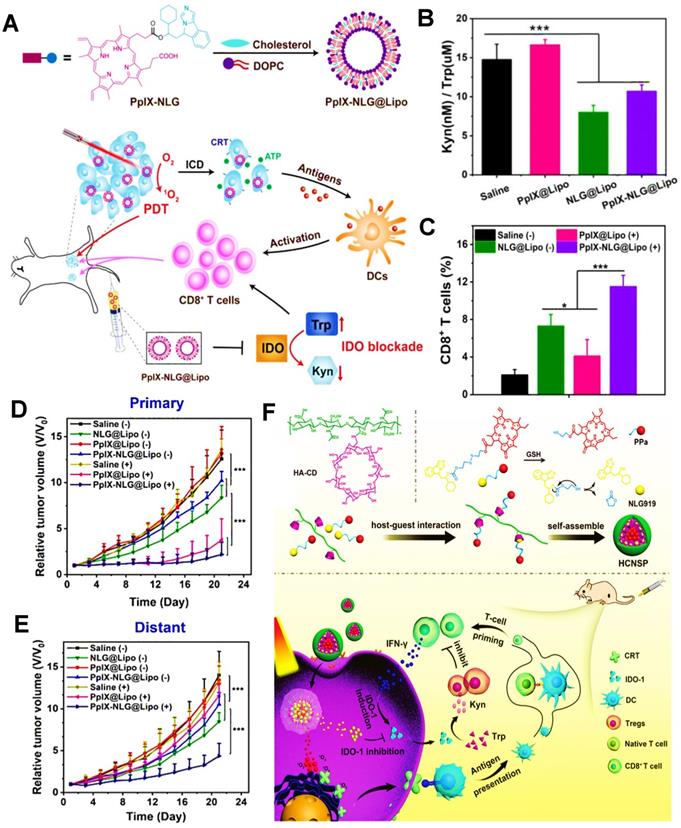
Although some advances have been achieved in basic research on tumor PDIT, it still faces some difficulties in its clinical transformation. First of all, the antitumor effect of PDIT exhibits as poorly as that of clinical traditional treatments. Additionally, it is difficult to accurately control the light conditions that trigger the photodynamic conversion of PSs in clinical practice. What's more, the anti-tumor effect of PDIT is mostly evaluated in ordinary mice, which makes us doubt whether PDIT can also have a strong therapeutic effect on clinical patients. So more researches should be assessed in humanized mice, non-human primates, pigs and so on. Altogether there are many limitations in preclinical studies of PDIT, one thing for sure is that PDIT can be considered as an effective and minimally invasive strategy for tumor therapy, which exhibits good clinical development prospects in colorectal cancer, breast cancer, lung cancer, skin cancer, etc. [174-177]. Therefore, there is an urgent need for improvement and extensive studies in light conditions, animal models, material design, and drug combinations. It is deeply believed that PDIT can play a central role in the clinical cancer therapy in the future.
Abbreviations
PDT: photodynamic therapy; ROS: reactive oxygen species; ICD: immunogenic cell death; PDIT: photodynamic immunotherapy; TME: tumor microenvironment; ICIs: immune checkpoint inhibitors; O2: oxygen; PSs: photosensitizers; DAMPs: damage- associated molecular patterns; CRT: calreticulin; HMGB1: high mobility group box 1; ATP: adenosine triphosphate; DOX: doxorubicin; Ce6: chlorin e6; PpIX: protoporphyrin IX; R837: imiquimod; EPR: enhanced permeability and retention; APCs: antigen-presenting cells; DCs: dendritic cells; CAT: catalase; MnO2: manganese dioxide; H2O2: hydrogen peroxide; PFCs: perfluorocarbons; Hb: hemoglobin; PTT: photothermal therapy; CpG: cytosine-phosphate-guanine; TLRs: toll-like receptors; PD-1: programmed cell death protein 1; PD-L1: programmed death-ligand 1; CTLA4: cytotoxic T-lymphocyte-associated protein 4; IDO: indoleamine 2,3-dioxygenase; ICB: immune checkpoint blockade; MOFs: metal-organic frameworks; UCNPs: upconversion nanoparticles; NCPs: nanoscale coordination polymers; siRNA: small interfering RNA; PI3Kγ: phosphoinositide 3-kinase gamma; MDSCs: myeloid- derived suppressive cells; TIME: tumor immune microenvironment; GEM: gemcitabine; KD: knockdown; TPP: triphenylphosphonium; PLGA: poly(lactic-co-glycolic acid); NPs: nanoparticles; FRT: ferritin; ZnF16Pc: zinc hexadecafuoro- phthalocyanine; TNF: tumor necrosis factor; IMT: imatinib; HA: hyaluronic acid; CD: cyclodextrin; AD: adamantine; BRD4: bromodomain and extraterminal protein 4; PPa: pyropheophorbidea; MMP-2: matrix metalloproteinase-2; MET: metformin; PDA: polydopamine; BP: black phosphorus; BPNV: BPQDs nanovesicle; 2D: two- dimensional; SiO2: silica; bMSN: biodegradable mesoporous SiO2 NP; Fe3O4: ferriferrous oxide; M-MONs: magnetic mesoporous organosilicon NPs; TBP: 5,10,15,20-tetra(p-benzoato)porphyrin; EpCAM: epithelial cell adhesion molecule; MX: mitoxantrone; CAFs: cancer-associated fibroblasts; TAMs: tumor-associated macrophages; FAP: fibroblast activation protein; NRP-1: neuropilin-1; LINC: light-inducible nanocargo; CTLs: cytotoxic T lymphocytes; iPSs: induced pluripotent stem cells; CeO2: cerium oxide; ICG: indocyanine green; TiO2: titania; PEGDA: PEG double acrylate; OXA: oxaliplatin; Tregs: regulatory T cells; RB: rose bengal; MLT: melittin; ER: endoplasmic reticulum; ACQ: aggregation-caused-quenching; TAAs: tumor associated antigens; TABNs: thiol-activated bovine serum albumin NPs; HSP70: heat shock protein 70; MHC: major histocompatibility complex; Trp: tryptophan; Kyn: kynurenine.
Acknowledgements
This study was partially supported by the Natural Science Foundation of Sichuan Province (NO. 2022NSFSC1925), the Introduction of Talents Research Project of Chengdu University (NO. 2081921049), and the Health and Family Planning Commission of Chengdu-Key disciplines of clinical pharmacy.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Ng CW, Li J, Pu K. Recent progresses in phototherapy-synergized cancer immunotherapy. Adv Funct Mater. 2018;28:1804688
2. Kim J, Cho HR, Jeon H, Kim D, Song C, Lee N. et al. Continuous O2-evolving MnFe2O4 nanoparticle-anchored mesoporous silica nanoparticles for efficient photodynamic therapy in hypoxic cancer. J Am Chem Soc. 2017;139:10992-10995
3. Yang H, Liu R, Xu Y, Qian L, Dai Z. Photosensitizer nanoparticles boost photodynamic therapy for pancreatic cancer treatment. Nanomicro Lett. 2021;13:35
4. Wan Y, Fu LH, Li C, Lin J, Huang P. Conquering the hypoxia limitation for photodynamic therapy. Adv Mater. 2021;33:e2103978
5. Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860-875
6. Zhao X, Yang K, Zhao R, Ji T, Wang X, Yang X. et al. Inducing enhanced immunogenic cell death with nanocarrier-based drug delivery systems for pancreatic cancer therapy. Biomaterials. 2016;102:187-197
7. Mai X, Zhang Y, Fan H, Song W, Chang Y, Chen B. et al. Integration of immunogenic activation and immunosuppressive reversion using mitochondrial-respiration-inhibited platelet-mimicking nanoparticles. Biomaterials. 2020;232:119699
8. Chen H, Wu F, Xie X, Wang W, Li Q, Tu L. et al. Hybrid nanoplatform: enabling a precise antitumor strategy via dual-modal imaging-guided photodynamic/chemo-/immunosynergistic therapy. ACS Nano. 2021;15:20643-20655
9. Xu J, Xu L, Wang C, Yang R, Zhuang Q, Han X. et al. Near-infrared-triggered photodynamic therapy with multitasking upconversion nanoparticles in combination with checkpoint blockade for immunotherapy of colorectal cancer. ACS Nano. 2017;11:4463-4474
10. Yang C, Fu Y, Huang C, Hu D, Zhou K, Hao Y. et al. Chlorin e6 and CRISPR-Cas9 dual-loading system with deep penetration for a synergistic tumoral photodynamic-immunotherapy. Biomaterials. 2020;255:120194
11. Xi D, Xu N, Xia X, Shi C, Li X, Wang D. et al. Strong π-π stacking stabilized nanophotosensitizers: improving tumor retention for enhanced therapy for large tumors in mice. Adv Mater. 2022;34:e2106797
12. Srivatsan A, Missert JR, Upadhyay SK, Pandey RK. Porphyrin-based photosensitizers and the corresponding multifunctional nanoplatforms for cancer-imaging and phototherapy. J Porphyrins Phthalocyanines. 2015;19:109-134
13. Maeda H, Nakamura H, Fang J. The EPR effect for macromolecular drug delivery to solid tumors: improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv Drug Delivery Rev. 2013;65:71-79
14. Zhang X, Tang J, Li C, Lu Y, Cheng L, Liu J. A targeting black phosphorus nanoparticle based immune cells nano-regulator for photodynamic/photothermal and photo-immunotherapy. Bioact Mater. 2021;6:472-489
15. Liu Z, Xie Z, Li W, Wu X, Jiang X, Li G. et al. Photodynamic immunotherapy of cancers based on nanotechnology: recent advances and future challenges. J Nanobiotechnol. 2021;19:160
16. Cheng X, Gao J, Ding Y, Lu Y, Wei Q, Cui D. et al. Multi-functional liposome: a powerful theranostic nano-platform enhancing photodynamic therapy. Adv Sci. 2021;8:e2100876
17. Li X, Kwon N, Guo T, Liu Z, Yoon J. Innovative strategies for hypoxic-tumor photodynamic therapy. Angew Chem Int Ed. 2018;57:11522-11531
18. Li Q, Ren J, Chen Q, Liu W, Xu Z, Cao Y. et al. A HMCuS@MnO2 nanocomplex responsive to multiple tumor environmental clues for photoacoustic/fluorescence/magnetic resonance trimodal imaging-guided and enhanced photothermal/photodynamic therapy. Nanoscale. 2020;12:12508-12521
19. Cheng H, Zhu J-Y, Li S-Y, Zeng J-Y, Lei Q, Chen K-W. et al. An O2 self-sufficient biomimetic nanoplatform for highly specific and efficient photodynamic therapy. Adv Funct Mater. 2016;26:7847-7860
20. Zhang G, Wang N, Sun H, Fu X, Zhai S, Cui J. Self-adjuvanting photosensitizer nanoparticles for combination photodynamic immunotherapy. Biomater Sci. 2021;9:6940-6949
21. Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL. et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54-61
22. Prendergast GC. Immune escape as a fundamental trait of cancer: focus on IDO. Oncogene. 2008;27:3889-3900
23. Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20:651-668
24. Xia A, Zhang Y, Xu J, Yin T, Lu XJ. T cell dysfunction in cancer immunity and immunotherapy. Front Immunol. 2019;10:1719
25. Xu J, Yu S, Wang X, Qian Y, Wu W, Zhang S. et al. High affinity of chlorin e6 to immunoglobulin G for intraoperative fluorescence image-guided cancer photodynamic and checkpoint blockade therapy. ACS Nano. 2019;13:10242-10260
26. Guo Y, Liu Y, Wu W, Ling D, Zhang Q, Zhao P. et al. Indoleamine 2,3-dioxygenase (IDO) inhibitors and their nanomedicines for cancer immunotherapy. Biomaterials. 2021;276:121018
27. Wu M, Huang Q, Xie Y, Wu X, Ma H, Zhang Y. et al. Improvement of the anticancer efficacy of PD-1/PD-L1 blockade via combination therapy and PD-L1 regulation. J Hematol Oncol. 2022;15:24
28. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252-264
29. Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S. et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378:1789-1801
30. Wang D, Lin J, Yang X, Long J, Bai Y, Yang X. et al. Combination regimens with PD-1/PD-L1 immune checkpoint inhibitors for gastrointestinal malignancies. J Hematol Oncol. 2019;12:42
31. Lommatzsch M, Bratke K, Stoll P. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;379:e14
32. Meng Z, Zhou X, Xu J, Han X, Dong Z, Wang H. et al. Light-triggered in situ gelation to enable robust photodynamic-immunotherapy by repeated stimulations. Adv Mater. 2019;31:e1900927
33. Cai Z, Xin F, Wei Z, Wu M, Lin X, Du X. et al. Photodynamic therapy combined with antihypoxic signaling and CpG adjuvant as an in situ tumor vaccine based on metal-organic framework nanoparticles to boost cancer immunotherapy. Adv Healthc Mater. 2019;9:e1900996
34. Fan W, Bu W, Shi J. On the latest three-stage development of nanomedicines based on upconversion nanoparticles. Adv Mater. 2016;28:3987-4011
35. Li Z, Hu Y, Fu Q, Liu Y, Wang J, Song J. et al. NIR/ROS-responsive black phosphorus QD vesicles as immunoadjuvant carrier for specific cancer photodynamic immunotherapy. Adv Funct Mater. 2019;30:1905758
36. Chen Z, Liu L, Liang R, Luo Z, He H, Wu Z. et al. Bioinspired hybrid protein oxygen nanocarrier amplified photodynamic therapy for eliciting anti-tumor immunity and abscopal effect. ACS Nano. 2018;12:8633-8645
37. Yang W, Zhu G, Wang S, Yu G, Yang Z, Lin L. et al. In situ dendritic cell vaccine for effective cancer immunotherapy. ACS Nano. 2019;13:3083-3094
38. Liu D, Chen B, Mo Y, Wang Z, Qi T, Zhang Q. et al. Redox-activated porphyrin-based liposome remote-loaded with indoleamine 2,3-dioxygenase (IDO) inhibitor for synergistic photoimmunotherapy through induction of immunogenic cell death and blockage of IDO pathway. Nano Lett. 2019;19:6964-6976
39. Wang D, Wang T, Liu J, Yu H, Jiao S, Feng B. et al. Acid-activatable versatile micelleplexes for PD-L1 blockade-enhanced cancer photodynamic immunotherapy. Nano Lett. 2016;16:5503-5513
40. He C, Liu D, Lin W. Self-assembled core-shell nanoparticles for combined chemotherapy and photodynamic therapy of resistant head and neck cancers. ACS Nano. 2015;9:991-1003
41. Shao Y, Liu B, Di Z, Zhang G, Sun LD, Li L. et al. Engineering of upconverted metal-organic frameworks for near-infrared light-triggered combinational photodynamic/chemo-/immunotherapy against hypoxic tumors. J Am Chem Soc. 2020;142:3939-3946
42. Xu C, Nam J, Hong H, Xu Y, Moon JJ. Positron emission tomography-guided photodynamic therapy with biodegradable mesoporous silica nanoparticles for personalized cancer immunotherapy. ACS Nano. 2019;13:12148-12161
43. Li W, Yang J, Luo L, Jiang M, Qin B, Yin H. et al. Targeting photodynamic and photothermal therapy to the endoplasmic reticulum enhances immunogenic cancer cell death. Nat Commun. 2019;10:3349
44. He C, Duan X, Guo N, Chan C, Poon C, Weichselbaum RR. et al. Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nat Commun. 2016;7:12499
45. Shu G, Zhu W, Jiang Y, Li X, Pan J, Zhang X. et al. Persistent luminescence immune hydrogel for photodynamic-immunotherapy of tumors in vivo. Adv Funct Mater. 2021;31:2104472
46. Sun F, Zhu Q, Li T, Saeed M, Xu Z, Zhong F. et al. Regulating glucose metabolism with prodrug nanoparticles for promoting photoimmunotherapy of pancreatic cancer. Adv Sci. 2021;8:2002746
47. Huang Z, Wei G, Zeng Z, Huang Y, Huang L, Shen Y. et al. Enhanced cancer therapy through synergetic photodynamic/immune checkpoint blockade mediated by a liposomal conjugate comprised of porphyrin and IDO inhibitor. Theranostics. 2019;9:5542-5557
48. Ou W, Jiang L, Thapa RK, Soe ZC, Poudel K, Chang JH. et al. Combination of NIR therapy and regulatory T cell modulation using layer-by-layer hybrid nanoparticles for effective cancer photoimmunotherapy. Theranostics. 2018;8:4574-4590
49. Liu Y, Yang J, Liu B, Cao W, Zhang J, Yang Y. et al. Human iPS cells loaded with MnO2-based nanoprobes for photodynamic and simultaneous enhanced immunotherapy against cancer. Nanomicro Lett. 2020;12:127
50. Liang R, Liu L, He H, Chen Z, Han Z, Luo Z. et al. Oxygen-boosted immunogenic photodynamic therapy with gold nanocages@manganese dioxide to inhibit tumor growth and metastases. Biomaterials. 2018;177:149-160
51. Desai MP, Labhasetwar V, Amidon GL, Levy RJ. Gastrointestinal uptake of biodegradable microparticles: effect of particle size. Pharm Res. 1996;13:1838-1845
52. Raj S, Khurana S, Choudhari R, Kesari KK, Kamal MA, Garg N. et al. Specific targeting cancer cells with nanoparticles and drug delivery in cancer therapy. Semin Cancer Biol. 2021;69:166-177
53. Aslan B, Ozpolat B, Sood AK, Lopez-Berestein G. Nanotechnology in cancer therapy. J Drug Targeting. 2013;21:904-913
54. Talelli M, Oliveira S, Rijcken CJ, Pieters EH, Etrych T, Ulbrich K. et al. Intrinsically active nanobody-modified polymeric micelles for tumor-targeted combination therapy. Biomaterials. 2013;34:1255-1260
55. Ding D, Zhong H, Liang R, Lan T, Zhu X, Huang S. et al. Multifunctional nanodrug mediates synergistic photodynamic therapy and MDSCs-targeting immunotherapy of colon cancer. Adv Sci. 2021;8:e2100712
56. Kim DH, Im BN, Hwang HS, Na K. Gemcitabine-loaded DSPE-PEG-PheoA liposome as a photomediated immune modulator for cholangiocarcinoma treatment. Biomaterials. 2018;183:139-150
57. Han Y, An Y, Jia G, Wang X, He C, Ding Y. et al. Theranostic micelles based on upconversion nanoparticles for dual-modality imaging and photodynamic therapy in hepatocellular carcinoma. Nanoscale. 2018;10:6511-6523
58. Hu C, He X, Chen Y, Yang X, Qin L, Lei T. et al. Metformin mediated PD-L1 downregulation in combination with photodynamic-immunotherapy for treatment of breast cancer. Adv Funct Mater. 2021;31:2007149
59. Zhang C, Gao F, Wu W, Qiu WX, Zhang L, Li R. et al. Enzyme-driven membrane-targeted chimeric peptide for enhanced tumor photodynamic immunotherapy. ACS Nano. 2019;13:11249-11262
60. Nafiujjaman M, Revuri V, Park H-K, Kwon IK, Cho K-J, Lee Y-k. Enhanced photodynamic properties of graphene quantum dot conjugated Ce6 nanoparticles for targeted cancer therapy and imaging. Chem Lett. 2016;45:997-999
61. Wu C, Wang L, Tian Y, Guan X, Liu Q, Li S. et al. "Triple-punch" anticancer strategy mediated by near-infrared photosensitizer/CpG oligonucleotides dual-dressed and mitochondria-targeted nanographene. ACS Appl Mater Interfaces. 2018;10:6942-6955
62. Wang Z, Zhang F, Shao D, Chang Z, Wang L, Hu H. et al. Janus nanobullets combine photodynamic therapy and magnetic hyperthermia to potentiate synergetic anti-metastatic immunotherapy. Adv Sci. 2019;6:1901690
63. Lukowski JK, Weaver EM, Hummon AB. Analyzing liposomal drug delivery systems in three-dimensional cell culture models using MALDI imaging mass spectrometry. Anal Chem. 2017;89:8453-8458
64. Lombardo D, Kiselev MA. Methods of liposomes preparation: formation and control factors of versatile nanocarriers for biomedical and nanomedicine application. Pharmaceutics. 2022;14:543
65. Tan C, Wang J, Sun B. Biopolymer-liposome hybrid systems for controlled delivery of bioactive compounds: recent advances. Biotechnol Adv. 2021;48:107727
66. Demetzos C, Pippa N. Advanced drug delivery nanosystems (aDDnSs): a mini-review. Drug Delivery. 2014;21:250-257
67. Yang T, Li B, Qi S, Liu Y, Gai Y, Ye P. et al. Co-delivery of doxorubicin and Bmi1 siRNA by folate receptor targeted liposomes exhibits enhanced anti-tumor effects in vitro and in vivo. Theranostics. 2014;4:1096-1111
68. Lang J, Zhao X, Wang X, Zhao Y, Li Y, Zhao R. et al. Targeted co-delivery of the iron chelator deferoxamine and a HIF1α inhibitor impairs pancreatic tumor growth. ACS Nano. 2019;13:2176-2189
69. Gu L, Faig A, Abdelhamid D, Uhrich K. Sugar-based amphiphilic polymers for biomedical applications: from nanocarriers to therapeutics. Acc Chem Res. 2014;47:2867-2877
70. Ghezzi M, Pescina S, Padula C, Santi P, Del Favero E, Cantu L. et al. Polymeric micelles in drug delivery: an insight of the techniques for their characterization and assessment in biorelevant conditions. J Controlled Release. 2021;332:312-336
71. Zhang Y, Ren T, Gou J, Zhang L, Tao X, Tian B. et al. Strategies for improving the payload of small molecular drugs in polymeric micelles. J Controlled Release. 2017;261:352-366
72. Deng C, Jiang Y, Cheng R, Meng F, Zhong Z. Biodegradable polymeric micelles for targeted and controlled anticancer drug delivery: Promises, progress and prospects. Nano Today. 2012;7:467-480
73. Dai L, Li K, Li M, Zhao X, Luo Z, Lu L. et al. Size/charge changeable acidity-responsive micelleplex for photodynamic-improved PD-L1 immunotherapy with enhanced tumor penetration. Adv Funct Mater. 2018 28
74. Peng N, Yu H, Yu W, Yang M, Chen H, Zou T. et al. Sequential-targeting nanocarriers with pH-controlled charge reversal for enhanced mitochondria-located photodynamic-immunotherapy of cancer. Acta Biomater. 2020;105:223-238
75. Guo S, Huang L. Nanoparticles containing insoluble drug for cancer therapy. Biotechnol Adv. 2014;32:778-788
76. Patil Y, Panyam J. Polymeric nanoparticles for siRNA delivery and gene silencing. Int J Pharmacol. 2009;367:195-203
77. Vrignaud S, Benoit JP, Saulnier P. Strategies for the nanoencapsulation of hydrophilic molecules in polymer-based nanoparticles. Biomaterials. 2011;32:8593-8604
78. Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf, B. 2010;75:1-18
79. Yang X, Zhang W, Jiang W, Kumar A, Zhou S, Cao Z. et al. Nanoconjugates to enhance PDT-mediated cancer immunotherapy by targeting the indoleamine-2,3-dioxygenase pathway. J Nanobiotechnol. 2021;19:182
80. Yan S, Zeng X, Tang Y, Liu BF, Wang Y, Liu X. Activating antitumor immunity and antimetastatic effect through polydopamine-encapsulated core-shell upconversion nanoparticles. Adv Mater. 2019;31:e1905825
81. Qin L, Jiang S, He H, Ling G, Zhang P. Functional black phosphorus nanosheets for cancer therapy. J Controlled Release. 2020;318:50-66
82. Gazzi A, Fusco L, Khan A, Bedognetti D, Zavan B, Vitale F. et al. Photodynamic therapy based on graphene and MXene in cancer theranostics. Front Bioeng Biotechnol. 2019;7:295
83. Youssef Z, Vanderesse R, Colombeau L, Baros F, Roques-Carmes T, Frochot C. et al. The application of titanium dioxide, zinc oxide, fullerene, and graphene nanoparticles in photodynamic therapy. Cancer Nanotechnol. 2017;8:6
84. Yang B, Chen Y, Shi J. Exogenous/endogenous-triggered mesoporous silica cancer nanomedicine. Adv Healthc Mater. 2018;7:e1800268
85. Wen J, Yang K, Sun S. MnO2-based nanosystems for cancer therapy. Chem Commun. 2020;56:7065-7079
86. Zhou L, Chen L, Hu X, Lu Y, Liu W, Sun Y. et al. A Cu9S5 nanoparticle-based CpG delivery system for synergistic photothermal-, photodynamic- and immunotherapy. Commun Biol. 2020;3:343
87. Qin X, Zhang H, Wang Z, Jin Y. Fe3O4@SiO2 mesoporous spheres as Fe(ii) donors loaded with artemisinin and a photosensitizer to alleviate tumor hypoxia in PDT for enhanced anticancer therapy. New J Chem. 2019;43:8761-8773
88. Ibrahim M, Sabouni R, Husseini GA. Anti-cancer drug delivery using metal organic frameworks (MOFs). Curr Med Chem. 2017;24:193-214
89. Lan G, Ni K, Xu Z, Veroneau SS, Song Y, Lin W. Nanoscale metal-organic framework overcomes hypoxia for photodynamic therapy primed cancer immunotherapy. J Am Chem Soc. 2018;140:5670-5673
90. Xu J, Lv R, Du S, Gai S, He F, Yang D. et al. UCNPs@gelatin-ZnPc nanocomposite: synthesis, imaging and anticancer properties. J Mater Chem B. 2016;4:4138-4146
91. Wang C, Cheng L, Liu Z. Upconversion nanoparticles for photodynamic therapy and other cancer therapeutics. Theranostics. 2013;3:317-330
92. Yang Y, Zhu W, Feng L, Chao Y, Yi X, Dong Z. et al. G-quadruplex-based nanoscale coordination polymers to modulate tumor hypoxia and achieve nuclear-targeted drug delivery for enhanced photodynamic therapy. Nano Lett. 2018;18:6867-6875
93. Wang J-L, Wang X-Y, Wang Y-H, Hu X-Y, Lian J-R, Guan Y-L. et al. Room-temperature preparation of coordination polymers for biomedicine. Coord Chem Rev. 2020;411:213256
94. Went P, Vasei M, Bubendorf L, Terracciano L, Tornillo L, Riede U. et al. Frequent high-level expression of the immunotherapeutic target Ep-CAM in colon, stomach, prostate and lung cancers. Br J Cancer. 2006;94:128-135
95. Schnell U, Cirulli V, Giepmans BN. EpCAM: structure and function in health and disease. Biochim Biophys Acta. 2013;1828:1989-2001
96. Li Y, Guo G, Song J, Cai Z, Yang J, Chen Z. et al. B7-H3 promotes the migration and invasion of human bladder cancer cells via the PI3K/Akt/STAT3 signaling pathway. J Cancer. 2017;8:816-824
97. Castellanos JR, Purvis IJ, Labak CM, Guda MR, Tsung AJ, Velpula KK. et al. B7-H3 role in the immune landscape of cancer. Am J Clin Exp Immunol. 2017;6:66-75
98. Berghoff AS, Kovanda AK, Melchardt T, Bartsch R, Hainfellner JA, Sipos B. et al. αvβ3, αvβ5 and αvβ6 integrins in brain metastases of lung cancer. Clin Exp Metastasis. 2014;31:841-851
99. Chen WH, Luo GF, Zhang XZ. Recent advances in subcellular targeted cancer therapy based on functional materials. Adv Mater. 2019;31:e1802725
100. Dutta D, Calvani R, Bernabei R, Leeuwenburgh C, Marzetti E. Contribution of impaired mitochondrial autophagy to cardiac aging: mechanisms and therapeutic opportunities. Circ Res. 2012;110:1125-1138
101. Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483-495
102. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423-1437
103. Fiori ME, Di Franco S, Villanova L, Bianca P, Stassi G, De Maria R. Cancer-associated fibroblasts as abettors of tumor progression at the crossroads of EMT and therapy resistance. Mol Cancer. 2019;18:70
104. Zhen Z, Tang W, Wang M, Zhou S, Wang H, Wu Z. et al. Protein nanocage mediated FAP-targeted photo-immunotherapy to enhance cytotoxic T cell infiltration and tumor control. Nano Lett. 2017;17:862-869
105. Petty AJ, Owen DH, Yang Y, Huang X. Targeting tumor-associated macrophages in cancer immunotherapy. Cancers. 2021;13:5318
106. Ovais M, Guo M, Chen C. Tailoring nanomaterials for targeting tumor-associated macrophages. Adv Mater. 2019;31:e1808303
107. Gao S, Liu Y, Liu M, Yang D, Zhang M, Shi K. Biodegradable mesoporous nanocomposites with dual-targeting function for enhanced anti-tumor therapy. J Controlled Release. 2022;341:383-398
108. Gries M, Thomas N, Daouk J, Rocchi P, Choulier L, Jubréaux J. et al. Multiscale selectivity and in vivo biodistribution of NRP-1-targeted theranostic AGuIX nanoparticles for PDT of glioblastoma. Int J Nanomed. 2020;15:8739-8758
109. Youssef Z, Yesmurzayeva N, Larue L, Jouan-Hureaux V, Colombeau L, Arnoux P. et al. New targeted gold nanorods for the treatment of glioblastoma by photodynamic therapy. J Clin Med. 2019;8:2205
110. Hatakeyama H. Recent advances in endogenous and exogenous stimuli-responsive nanocarriers for drug delivery and therapeutics. Chem Pharm Bull. 2017;65:612-617
111. Du JZ, Sun TM, Song WJ, Wu J, Wang J. A tumor-acidity-activated charge-conversional nanogel as an intelligent vehicle for promoted tumoral-cell uptake and drug delivery. Angew Chem Int Ed. 2010;49:3621-3626
112. Pecot CV, Calin GA, Coleman RL, Lopez-Berestein G, Sood AK. RNA interference in the clinic: challenges and future directions. Nat Rev Cancer. 2011;11:59-67
113. Wang X, Chen C, Huo D, Qian H, Ding Y, Hu Y. et al. Synthesis of β-cyclodextrin modified chitosan-poly(acrylic acid) nanoparticles and use as drug carriers. Carbohydr Polym. 2012;90:361-369
114. Rayamajhi S, Marchitto J, Nguyen TDT, Marasini R, Celia C, Aryal S. pH-responsive cationic liposome for endosomal escape mediated drug delivery. Colloids Surf, B. 2020;188:110804
115. Guo F, Wang J, Ma M, Tan F, Li N. Skin targeted lipid vesicles as novel nano-carrier of ketoconazole: characterization, in vitro and in vivo evaluation. J Mater Sci Mater Med. 2015;26:175
116. Yang W, Zhang F, Deng H, Lin L, Wang S, Kang F. et al. Smart nanovesicle-mediated immunogenic cell death through tumor microenvironment modulation for effective photodynamic immunotherapy. ACS Nano. 2020;14:620-631
117. Maher P. The effects of stress and aging on glutathione metabolism. Ageing Res Rev. 2005;4:288-314
118. Feng B, Hou B, Xu Z, Saeed M, Yu H, Li Y. Self-amplified drug delivery with light-inducible nanocargoes to enhance cancer immunotherapy. Adv Mater. 2019;31:e1902960
119. Aili D, Mager M, Roche D, Stevens MM. Hybrid nanoparticle-liposome detection of phospholipase activity. Nano Lett. 2011;11:1401-1405
120. Yu F, Shang X, Zhu Y, Lou H, Liu Y, Meng T. et al. Self-preparation system using glucose oxidase-inspired nitroreductase amplification for cascade-responsive drug release and multidrug resistance reversion. Biomaterials. 2021;275:120927
121. Zhu L, Kate P, Torchilin VP. Matrix metalloprotease 2-responsive multifunctional liposomal nanocarrier for enhanced tumor targeting. ACS Nano. 2012;6:3491-3498
122. Song W, Kuang J, Li CX, Zhang M, Zheng D, Zeng X. et al. Enhanced immunotherapy based on photodynamic therapy for both primary and lung metastasis tumor eradication. ACS Nano. 2018;12:1978-1989
123. Sun X, Cao Z, Mao K, Wu C, Chen H, Wang J. et al. Photodynamic therapy produces enhanced efficacy of antitumor immunotherapy by simultaneously inducing intratumoral release of sorafenib. Biomaterials. 2020;240:119845
124. Hu L, Cao Z, Ma L, Liu Z, Liao G, Wang J. et al. The potentiated checkpoint blockade immunotherapy by ROS-responsive nanocarrier-mediated cascade chemo-photodynamic therapy. Biomaterials. 2019;223:119469
125. Fan YT, Zhou TJ, Cui PF, He YJ, Chang X, Xing L. et al. Modulation of intracellular oxygen pressure by dual-drug nanoparticles to enhance photodynamic therapy. Adv Funct Mater. 2019;29:1806708
126. Shen Z, Ma Q, Zhou X, Zhang G, Hao G, Sun Y. et al. Strategies to improve photodynamic therapy efficacy by relieving the tumor hypoxia environment. NPG Asia Mater. 2021;13:39
127. Chaput N, De Botton S, Obeid M, Apetoh L, Ghiringhelli F, Panaretakis T. et al. Molecular determinants of immunogenic cell death: surface exposure of calreticulin makes the difference. J Mol Med. 2007;85:1069-1076
128. Liu Y, Pan Y, Cao W, Xia F, Liu B, Niu J. et al. A tumor microenvironment responsive biodegradable CaCO3/MnO2-based nanoplatform for the enhanced photodynamic therapy and improved PD-L1 immunotherapy. Theranostics. 2019;9:6867-6884
129. Zuo H, Hou Y, Yu Y, Li Z, Liu H, Liu C. et al. Circumventing myeloid-derived suppressor cell-mediated immunosuppression using an oxygen-generated and -economized nanoplatform. ACS Appl Mater Interfaces. 2020;12:55723-55736
130. Zheng T, Wang W, Wu F, Zhang M, Shen J, Sun Y. Zwitterionic polymer-gated Au@TiO2 core-shell nanoparticles for imaging-guided combined cancer therapy. Theranostics. 2019;9:5035-5048
131. Yang G, Xu L, Xu J, Zhang R, Song G, Chao Y. et al. Smart nanoreactors for pH-responsive tumor homing, mitochondria-targeting, and enhanced photodynamic-immunotherapy of cancer. Nano Lett. 2018;18:2475-2484
132. Xing L, Gong JH, Wang Y, Zhu Y, Huang ZJ, Zhao J. et al. Hypoxia alleviation-triggered enhanced photodynamic therapy in combination with IDO inhibitor for preferable cancer therapy. Biomaterials. 2019;206:170-182
133. Jin F, Qi J, Zhu M, Liu D, You Y, Shu G. et al. NIR-triggered sequentially responsive nanocarriers amplified cascade synergistic effect of chemo-photodynamic therapy with inspired antitumor immunity. ACS Appl Mater Interfaces. 2020;12:32372-32387
134. Liu H, Hu Y, Sun Y, Wan C, Zhang Z, Dai X. et al. Co-delivery of bee venom melittin and a photosensitizer with an organic-inorganic hybrid nanocarrier for photodynamic therapy and immunotherapy. ACS Nano. 2019;13:12638-12652
135. Sun Y, Zhang Y, Gao Y, Wang P, He G, Blum NT. et al. Six birds with one stone: versatile nanoporphyrin for single-laser-triggered synergistic phototheranostics and robust immune activation. Adv Mater. 2020;32:e2004481
136. Liu X, Su H, Shi W, Liu Y, Sun Y, Ge D. Functionalized poly(pyrrole-3-carboxylic acid) nanoneedles for dual-imaging guided PDT/PTT combination therapy. Biomaterials. 2018;167:177-190
137. Deng H, Zhou Z, Yang W, Lin LS, Wang S, Niu G. et al. Endoplasmic reticulum targeting to amplify immunogenic cell death for cancer immunotherapy. Nano Lett. 2020;20:1928-1933
138. Zhao H, Xu J, Wang Y, Sun C, Bao L, Zhao Y. et al. A photosensitizer discretely loaded nanoaggregate with robust photodynamic effect for local treatment triggers systemic antitumor responses. ACS Nano. 2022;16:3070-3080
139. Zhao H, Xu J, Feng C, Ren J, Bao L, Zhao Y. et al. Tailoring aggregation extent of photosensitizers to boost phototherapy potency for eliciting systemic antitumor immunity. Adv Mater. 2022;34:e2106390
140. Wen Y, Liu Y, Guo F, Han Y, Qiu Q, Li Y. et al. A vaccine for photodynamic immunogenic cell death: tumor cell caged by cellular disulfide-thiol exchange for immunotherapy. Biomater Sci. 2021;9:973-984
141. Gao L, Zhang C, Gao D, Liu H, Yu X, Lai J. et al. Enhanced anti-tumor efficacy through a combination of integrin αvβ6-targeted photodynamic therapy and immune checkpoint inhibition. Theranostics. 2016;6:627-637
142. Wang N, Zhou Y, Xu Y, Ren X, Zhou S, Shang Q. et al. Molecular engineering of anti-PD-L1 peptide and photosensitizer for immune checkpoint blockade photodynamic-immunotherapy. Chem Eng J. 2020;400:125995
143. Chen SX, Ma M, Xue F, Shen S, Chen Q, Kuang Y. et al. Construction of microneedle-assisted co-delivery platform and its combining photodynamic/immunotherapy. J Controlled Release. 2020;324:218-227
144. Hu X, Hou B, Xu Z, Saeed M, Sun F, Gao Z. et al. Supramolecular prodrug nanovectors for active tumor targeting and combination immunotherapy of colorectal cancer. Adv Sci. 2020;7:1903332
145. Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO. et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61:250-281
146. Ruan DY, Li T, Wang YN, Meng Q, Li Y, Yu K. et al. FTO downregulation mediated by hypoxia facilitates colorectal cancer metastasis. Oncogene. 2021;40:5168-5181
147. Gao Z, Li Y, Zhang Y, Cheng K, An P, Chen F. et al. Biomimetic platinum nanozyme immobilized on 2D metal-organic frameworks for mitochondrion-targeting and oxygen self-supply photodynamic therapy. ACS Appl Mater Interfaces. 2020;12:1963-1972
148. Sun X, Ni N, Ma Y, Wang Y, Leong DT. Retooling cancer nanotherapeutics' entry into tumors to alleviate tumoral hypoxia. Small. 2020;16:e2003000
149. Wu J, Niu S, Bremner DH, Nie W, Fu Z, Li D. et al. A tumor microenvironment-responsive biodegradable mesoporous nanosystem for anti-inflammation and cancer theranostics. Adv Healthc Mater. 2020;9:e1901307
150. Yang N, Xiao W, Song X, Wang W, Dong X. Recent advances in tumor microenvironment hydrogen peroxide-responsive materials for cancer photodynamic therapy. Nanomicro Lett. 2020;12:15
151. Wang J, Zhu W, Niu G, Jiang G, Chen Q, Gao P. et al. Selectively light-up hydrogen peroxide in hypoxic cancer cells with a novel fluorescent probe. Chem Commun. 2018;54:13957-13960
152. Yang G, Xu L, Chao Y, Xu J, Sun X, Wu Y. et al. Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat Commun. 2017;8:902
153. Shi C, Li M, Zhang Z, Yao Q, Shao K, Xu F. et al. Catalase-based liposomal for reversing immunosuppressive tumor microenvironment and enhanced cancer chemo-photodynamic therapy. Biomaterials. 2020;233:119755
154. Castro CI, Briceno JC. Perfluorocarbon-based oxygen carriers: review of products and trials. Artif Organs. 2010;34:622-634
155. Luo Z, Tian H, Liu L, Chen Z, Liang R, Chen Z. et al. Tumor-targeted hybrid protein oxygen carrier to simultaneously enhance hypoxia-dampened chemotherapy and photodynamic therapy at a single dose. Theranostics. 2018;8:3584-3596
156. Huang Z, Chen Y, Zhang J, Li W, Shi M, Qiao M. et al. Laser/GSH-activatable oxaliplatin/phthalocyanine-based coordination polymer nanoparticles combining chemophotodynamic therapy to improve cancer immunotherapy. ACS Appl Mater Interfaces. 2021;13:39934-39948
157. Yang Y, Chen F, Xu N, Yao Q, Wang R, Xie X. et al. Red-light-triggered self-destructive mesoporous silica nanoparticles for cascade-amplifying chemo-photodynamic therapy favoring antitumor immune responses. Biomaterials. 2022;281:121368
158. Shah RR, Hassett KJ, Brito LA. Overview of vaccine adjuvants: introduction, history, and current status. Methods Mol Biol. 2017;1494:1-13
159. Yu S, Wang C, Yu J, Wang J, Lu Y, Zhang Y. et al. Injectable bioresponsive gel depot for enhanced immune checkpoint blockade. Adv Mater. 2018;30:e1801527
160. Fan Q, Chen Z, Wang C, Liu Z. Toward biomaterials for enhancing immune checkpoint blockade therapy. Adv Funct Mater. 2018;28:1802540
161. Gao A, Chen B, Gao J, Zhou F, Saeed M, Hou B. et al. Sheddable prodrug vesicles combating adaptive immune resistance for improved photodynamic immunotherapy of cancer. Nano Lett. 2020;20:353-362
162. Kyi C, Postow MA. Checkpoint blocking antibodies in cancer immunotherapy. FEBS Lett. 2014;588:368-376
163. Fan Y, Zhang C, Jin S, Gao Z, Cao J, Wang A. et al. Progress of immune checkpoint therapy in the clinic (Review). Oncol Rep. 2019;41:3-14
164. Church SE, Galon J. Tumor microenvironment and immunotherapy: the whole picture is better than a glimpse. Immunity. 2015;43:631-633
165. Porciello N, Tuosto L. CD28 costimulatory signals in T lymphocyte activation: emerging functions beyond a qualitative and quantitative support to TCR signalling. Cytokine Growth Factor Rev. 2016;28:11-19
166. Hosseini A, Gharibi T, Marofi F, Babaloo Z, Baradaran B. CTLA-4: from mechanism to autoimmune therapy. Int Immunopharmacol. 2020;80:106221
167. Liu M, Wang X, Wang L, Ma X, Gong Z, Zhang S. et al. Targeting the IDO1 pathway in cancer: from bench to bedside. J Hematol Oncol. 2018;11:100
168. Bartok O, Pataskar A, Nagel R, Laos M, Goldfarb E, Hayoun D. et al. Anti-tumour immunity induces aberrant peptide presentation in melanoma. Nature. 2021;590:332-337
169. Munn DH, Mellor AL. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol. 2016;37:193-207
170. Han X, Cheng K, Xu Y, Wang Y, Min H, Zhang Y. et al. Modularly designed peptide nanoprodrug augments antitumor immunity of PD-L1 checkpoint blockade by targeting indoleamine 2,3-dioxygenase. J Am Chem Soc. 2020;142:2490-2496
171. Sun W, Shi T, Luo L, Chen X, Lv P, Lv Y. et al. Monodisperse and uniform mesoporous silicate nanosensitizers achieve low-dose X-ray-induced deep-penetrating photodynamic therapy. Adv Mater. 2019;31:e1808024
172. Alves CG, Lima-Sousa R, de Melo-Diogo D, Louro RO, Correia IJ. IR780 based nanomaterials for cancer imaging and photothermal, photodynamic and combinatorial therapies. Int J Pharmacol. 2018;542:164-175
173. Jain A, Koyani R, Munoz C, Sengar P, Contreras OE, Juarez P. et al. Magnetic-luminescent cerium-doped gadolinium aluminum garnet nanoparticles for simultaneous imaging and photodynamic therapy of cancer cells. J Colloid Interface Sci. 2018;526:220-229
174. Moghissi K, Dixon K. Is bronchoscopic photodynamic therapy a therapeutic option in lung cancer? Eur Respir J. 2003;22:535-541
175. Dos Santos AF, Terra LF, Wailemann RA, Oliveira TC, Gomes VM, Mineiro MF. et al. Methylene blue photodynamic therapy induces selective and massive cell death in human breast cancer cells. BMC Cancer. 2017;17:194
176. Lopez RF, Lange N, Guy R, Bentley MV. Photodynamic therapy of skin cancer: controlled drug delivery of 5-ALA and its esters. Adv Drug Delivery Rev. 2004;56:77-94
177. Tsai WH, Yu KH, Huang YC, Lee CI. EGFR-targeted photodynamic therapy by curcumin-encapsulated chitosan/TPP nanoparticles. Int J Nanomed. 2018;13:903-916
Author contact
![]() Corresponding authors: School of Preclinical Medicine, Chengdu University, Chengdu 610106, P. R. China (X. Wei); Key Disciplines of Clinical Pharmacy, Affiliated Hospital and Clinical Medical College of Chengdu University, Chengdu 610081, P. R. China (Z. Yang); School of Food and Bioengineering, Chengdu University, Chengdu 610106, P. R. China (L. Zou). E-mail addresses: weixiaoedu.cn (Xiao Wei); 109872931com (Zhiyong Yang); zouliangcducom (Liang Zou).
Corresponding authors: School of Preclinical Medicine, Chengdu University, Chengdu 610106, P. R. China (X. Wei); Key Disciplines of Clinical Pharmacy, Affiliated Hospital and Clinical Medical College of Chengdu University, Chengdu 610081, P. R. China (Z. Yang); School of Food and Bioengineering, Chengdu University, Chengdu 610106, P. R. China (L. Zou). E-mail addresses: weixiaoedu.cn (Xiao Wei); 109872931com (Zhiyong Yang); zouliangcducom (Liang Zou).
 Global reach, higher impact
Global reach, higher impact