13.3
Impact Factor
Theranostics 2022; 12(11):5103-5124. doi:10.7150/thno.74548 This issue Cite
Review
Silk fibroin-based biomaterials for cartilage/osteochondral repair
1. Institute of Translational Medicine, Shanghai University, Shanghai, 200444, China
2. Musculoskeletal Organoid Research Center, Shanghai University, Shanghai, 200444, China
3. School of Medicine, Shanghai University, Shanghai 200444, China
4. School of Life Sciences, Shanghai University, Shanghai 200444, China
5. Department of Orthopedics Trauma, Changhai Hospital, Second Military Medical University, Shanghai, 200433, China
6. School of Environmental and Chemical Engineering, Shanghai University, Shanghai 200444, China
*These authors contributed equally to this study.
Received 2022-4-29; Accepted 2022-6-18; Published 2022-7-4
Abstract
Osteoarthritis (OA) is a common joint disease with a high disability rate. In addition, OA not only causes great physiological and psychological harm to patients, but also puts great pressure on the social healthcare system. Pathologically, the disintegration of cartilage and the lesions of subchondral bone are related to OA. Currently, tissue engineering, which is expected to overcome the defects of existing treatment methods, had a lot of research in the field of cartilage/osteochondral repair. Silk fibroin (SF), as a natural macromolecular material with good biocompatibility, unique mechanical properties, excellent processability and degradability, holds great potential in the field of tissue engineering. Nowadays, SF had been prepared into various materials to adapt to the demands of cartilage/osteochondral repair. SF-based biomaterials can also be functionally modified to enhance repair performance further. In this review, the preparation methods, types, structures, mechanical properties, and functional modifications of SF-based biomaterials used for cartilage/osteochondral repair are summarized and discussed. We hope that this review will provide a reference for the design and development of SF-based biomaterials in cartilage/osteochondral repair field.
Keywords: Silk fibroin, Tissue engineering, Biomaterials, Cartilage repair, Osteochondral repair.
1. Introduction
Osteoarthritis (OA) is a degenerative disease, which involves the whole joint (including articular cartilage, subchondral bone, ligaments, joint capsule, synovium, and muscles around the joint) [1]. The pathogenesis of OA is complex and its prevalence is correlated with age, gender and obesity status [2, 3]. Patients suffer from severe pain and even amputation caused by OA, resulting in a sharp decline in quality of life [4]. At present, about 250 million people around the world are suffered from OA [5]. With the aging of the global population, increasing obesity, and joint injuries, the number will increase by about 50% in the next decade [6]. Statistically, OA costs about $303 billion a year in medical expenses and lost income, which is a massive burden on suffered individuals, health systems, and the wider socio-economy [7]. Typically, OA will occur when the dynamic balance between cartilage destruction and repair is broken, due to excessive mechanical load and pathological factors [8-11]. Furthermore, untimely repair of cartilage defects can lead to further deterioration of OA [12, 13]. Conversely, under osteoarthritic conditions, the rising activity of multiple proteases gives rise to augmented cartilage destruction [14]. In brief, the development of OA and cartilage destruction is a vicious cycle. Therefore, cartilage repair is the essential element in the treatment of OA [15, 16].
Currently, the treatment strategies of cartilage repair include microfracture (bone marrow stimulation), autologous chondrocyte transplantation, and allogeneic/autologous cartilage transplantation [17, 18]. Although these treatment strategies are widely used in the clinic, they still have several shortcomings. For example, microfractures often cause the formation of fibrocartilage [19]. In addition, the application of allogeneic/autologous cartilage transplantation is limited by donor shortage, poor integration, and surgical infection [20, 21]. Recently, tissue engineering has become a promising strategy for repairing damaged tissue, such as skin, heart, and bone [22-24]. In general, cartilage defects are caused by trauma, long-term wear, disease, and aging. The self-repairing ability of cartilage is limited due to the absence of blood vessels and the poor chondrocytes proliferation [25, 26]. Therefore, introducing nutrients or stem cells from adjacent tissues is an effective strategy to accelerate cartilage repair [27]. Subchondral bone is adjacent to and underneath the cartilage and is rich in vascular tissue and a variety of stem cells [28-30]. Therefore, constructing osteochondral defects (so that nutrients and a variety of stem cells in the subchondral bone can arrive at the cartilage region) to achieve integrated osteochondral repair is expected to accelerate cartilage repair [31, 32].
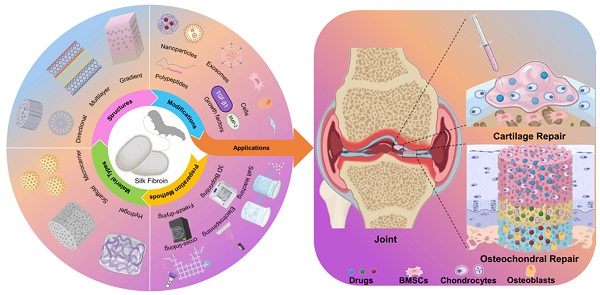
Silk fibroin (SF) is a kind of natural protein material that has been used in the clinic for decades. Compared with synthetic and natural materials, SF exhibits superior toughness, biocompatibility, biodegradability, and thermal stability. Additionally, SF can also significantly promote bone and cartilage regeneration [33, 34]. Based on these, SF-based biomaterials have been widely studied and used in the biomedical field, especially in tissue engineering [35]. Moreover, SF-based biomaterials have more incomparable advantages than other materials in the cartilage/osteochondral repair, including: (1) Excellent cytocompatibility (suitable growth environment could accelerate cell proliferation and differentiation); (2) unique mechanical property (cell adhesion and growth require adequate mechanical support); (3) non-toxic degradation products and controllable biodegradability (the space left by the degradation of biomaterials is essential for the growth of regenerative tissues); (4) desirable processability (SF-based biomaterials are satisfactory for a variety of fabricating methods and modifications) [36, 37]. Therefore, SF-based biomaterials have great research value and application prospects in the field of cartilage/osteochondral repair (Figure 1).
Preparation methods, materials types, structures, and modifications of SF-based biomaterials for cartilage/osteochondral repair. (I) Preparation methods including 3D bioprinting, electrospinning, and freeze-drying. (II) Representative types including hydrogel, scaffold, and microcarrier. (III) Directional structure, multilayer structure, and gradient structure mimic natural cartilage or subchondral bone tissue. (IV) Functional modification of SF-based biomaterials by nanoparticles, exosomes, cells, polypeptides, and growth factors. (V) The application of SF-based biomaterials for cartilage/osteochondral repair.
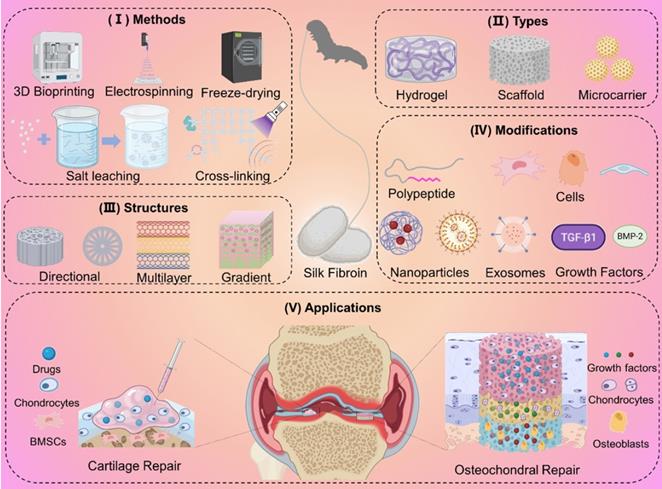
Herein, we review the research of SF-based biomaterials in cartilage/osteochondral repair in the past decade. We hope this review can provide a theoretical basis for the design and preparation of SF-based biomaterials for cartilage/osteochondral repair.
2. Silk fibroin: source, composition, structure, and features
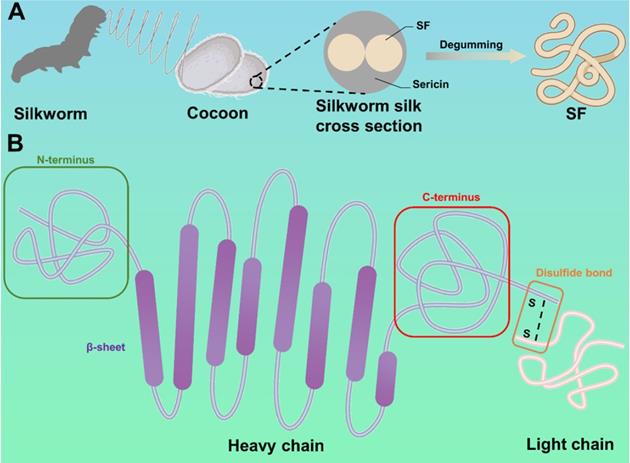
Generally, silk is secreted by various animals, such as silkworms, spiders, scorpions, mites, and bees. Compared with other silk, silkworm silk is the only silk that can be produced on a large scale and has been applied in the clinic. Hence, this review focuses on the description and discussion of silkworm silk. As shown in Figure 2A, a silkworm silk consists of two SF fibers coated with sericin. To obtain pure SF, silkworm silk needs to be degummed, rinsed, and dried [38].
SF has a complex molecular structure, which is composed of disulfide bond-linked light chain (molecular weight 26KDa) and heavy chain (molecular weight 390KDa), as shown in Figure 2B [39]. The light chain, which has excellent elasticity, is formed of disordered amino acid sequences and does not participate in the formation of crystal structure. The heavy chain consists of several repetitive sequences, including Gly-Ala-Gly-Ala-Gly-Ser sequences, Gly-Ala-Gly-Ala-Gly-Tyr sequences, and Gly-Ala-Gly-Ala-Gly-Ser-Gly-Ala-Ala-Ser sequences. The heavy chain has remarkable tensile strength due to these sequences. Therefore, attributing to the heavy and light chains, SF has better mechanical properties than other natural and synthetic materials (maximum fracture elongation, ultimate Young's modulus, and toughness are 4%, 26%, 300-700MPa, and 70-78MJ/m3, respectively) [40]. In addition, the mechanical properties of SF can be adjusted by changing the size, quantity, orientation, and arrangement of crystalline (silk Ⅱ) and amorphous structures.
The chemical composition and structure of SF endow it with excellent biocompatibility and biodegradability. The degradation products of SF (mainly amino acids and peptides) not only have no cytotoxicity, but also can provide nutrition for tissue regeneration. This makes SF have a good prospect of clinical application [40]. Actually, surgical sutures and wound dressings prepared by SF have been widely used in biomedical fields. Currently, SF is attracting more and more attention in the field of cartilage/osteochondral repair [41].
3. Articular cartilage and subchondral bone
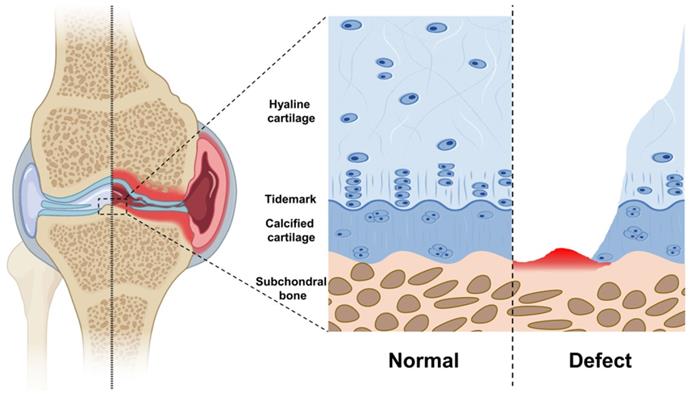
The articular cartilage is a multilayer tissue, including the hyaline cartilage layer and calcified cartilage layer. In addition, the subchondral bone is located directly below the articular cartilage (Figure 2). Hyaline cartilage, calcified cartilage, and subchondral bone form a massive complex with different components, structures, and properties [42].
Schematic illustration of the SF: (A) The source of SF. (B) The composition and structure of SF. SF consists of heavy chain and light chains connected by disulfide bonds.
Hyaline cartilage is a non-blood supply tissue composed mainly of water, type II collagen and aggregates [43]. The core protein of aggrecan has covalent binding and powerfully negatively charged glycosaminoglycan side chain. It is non-covalently linked to hyaluronic acid through connexin to form a proteoglycan polymer [44]. Highly cross-linked type II collagen fibers form an organized network that captures negatively charged proteoglycan aggregates and interacts with other collagen, small proteoglycan, and other cartilage-specific/non-specific matrix proteins [45]. Specially, chondrocytes are the only cell type in cartilage, accounting for only 1-2% of the total volume of cartilage [46]. However, chondrocytes maintain basic metabolism and proliferate slowly due to the insufficient mitotic activity of mature articular chondrocytes [47].
Calcified cartilage is a thin layer of tissue between hyaline cartilage and subchondral bone. It is produced by endochondral ossification and still exists after the growth plate is closed. Calcified cartilage is denser and more mineralized than the adjacent cartilage. Besides, there is a histologically defined tidal mark (tidemark) between calcified cartilage and articular cartilage [48]. In addition, the biological composite formed by calcified cartilage and subchondral bone is especially suitable for transmitting and distributing mechanical force under physiological load. Compared with cartilage, most of the mechanical load is borne by the composite.
Subchondral bone is located directly below the calcified cartilage with unique structural, biological, and mechanical properties [49]. It forms a plate-like structure similar to cortical bone in other skeletal parts. In comparison to cortical bone, subchondral bone has more porous and metabolic activity. In addition, the bones in the subchondral bone layer combine to form a trabecular network (also known as cancellous bone). The trabeculae in cancellous bone are oriented in different directions according to their location, and they provide a unique structural network to adapt to local mechanical effects. Moreover, the periosteum forming the edge of the joint is in direct contact with the joint capsule and synovium. Under the joint capsule and synovium, ligaments and tendons are inserted into the bone to form a unique structure at the end [14].
In brief, hyaline cartilage, calcified cartilage and subchondral bone have different characteristics. It is worth noting that the species of silkworm affects the structure of SF molecule, and then affects the biological properties of SF-based biomaterials [50]. For example, Saha et al. compared the performance of mulberry SF and non-mulberry SF in the field of bone and cartilage repair [51]. Under similar conditions, they found that mulberry SF was more osteoinductive, while non-mulberry SF was more chondroinductive. Meanwhile, Singh et al. compared cartilage repair properties of mulberry SF hydrogels and non-mulberry SF hydrogels [34]. Compared with mulberry SF hydrogels, non-mulberry SF hydrogels showed better-promoting effects on sulfated glycosaminoglycans and collagen deposition. This proved that non-mulberry SF is more suitable for cartilage repair. Additionally, the characteristics of hyaline cartilage, calcified cartilage and subchondral bone should be taken into account in the design of SF-based biomaterials for cartilage/osteochondral repair. For the cartilage layer, there is only one kind of chondrocyte with a gradient distribution. On the one hand, the SF-based biomaterial should be modified to promote the proliferation and differentiation of chondrocytes. On the other hand, the SF-based biomaterial should be endowed with a gradient structure to ensure the gradient distribution of chondrocytes (similar to primary cartilage). For the calcified cartilage layer, SF-based biomaterials should be designed as dense structure. The dense structure could prevent the migration of angiogenic cells from the subchondral bone layer to the cartilage layer and avoid cartilage layer fibrosis. For the subchondral bone layer, the biomineralization and antibacterial ability of SF-based biomaterials could be enhanced by adding metal ions [52-55]. It is worth mentioning the synergistic role of osteogenesis and angiogenesis in promoting subchondral bone repair [56]. Therefore, SF-based materials could add angiogenic factors to accelerate the repair of subchondral bone.
4. The application of SF-based biomaterials for cartilage/osteochondral repair
4.1. Preparation methods of SF-based biomaterials
4.1.1. 3D bioprinting
3D bioprinting is a controllable rapid prototyping and additive manufacturing technology [57, 58]. It allows personalized adjustment of size parameters and layer-by-layer printing of biomaterials with complex structures via computer assistance [59]. In addition, 3D printed biomaterials show great potential in meeting the mechanical, structural and biological requirements for cartilage/osteochondral repair [60-62]. Generally, printing equipment (biological printer) and ink (biological ink) are the two main components of 3D bioprinting technology.
Currently, the main types of 3D bioprinting equipment include inkjet printing, extrusion printing, and laser-assisted printing. Among them, the extrusion strategy is suitable for a wide range of materials and curing methods. Furthermore, compared with the inkjet printing method and laser-assisted method, the extrusion printing technology is able to process higher-resolution patterns and is more cost-effective [63]. Additionally, extrusion 3D printing can effectively overcome the problem that SF-based biomaterials fail to be commercialized due to the insufficiency of standardization and industrialization in the preparation process. For instance, Trucco et al. constructed and verified the analytical model of a 3D extrusion bioprinting system based on extrusion to predict the width of deposited SF filaments [64]. The model took into account the key printing process parameters (pressure and speed of biological ink) to predict the SF filament width from the various rheological properties of different hydrogels. This model helped researchers to define the most appropriate SF printing parameters to maximize the fidelity of the manufactured structure to the design parameters. In addition, in order to improve the affinity of SF-based biomaterials for chondrogenic cells, other natural macromolecular materials and stem cell-specific affinity peptides can be added when printing SF-based biomaterials. For example, Thunsiri et al. prepared bilayer bioactive biomaterial scaffolds with polylactic acid (PLA), polycaprolactone (PCL), hydroxyapatite (HA), chitosan (CS), and SF by 3D extrusion bioprinting [68]. The experimental results showed that the scaffolds had the potential to treat full-thickness articular cartilage defects. Meanwhile, Shi et al. constructed SF-gelatin-E7 (SFG-E7) scaffolds by integrating SF with gelatin in combination with bone mesenchymal stem cell (BMSC)-specific-affinity peptide using 3D bioprinting technology (Figure 4A) [65]. They found that E7 BMSCs specific affinity peptides significantly promoted the proliferation and differentiation of BMSC and the production of extracellular matrix (ECM). These works proved that extrusion 3D bioprinting had strong controllability and laid a foundation for industrialized and standardized production of SF-based biomaterials.
Schematic diagram of each layer of articular cartilage. Normal: From surface to interior, hyaline cartilage, calcified cartilage, and subchondral bone. The tidemark is between the hyaline cartilage and the calcified cartilage. Defect: Hyaline cartilage and calcified cartilage are worn or decomposed, subchondral bone hemorrhage.
According to the reports, choosing the appropriate biological ink is the key to successful biological printing [69]. Biological inks need to meet several basic standards, such as excellent bioactive, appropriate viscosity, suitable flexibility and great stability [70, 71]. Generally, modification of SF biological ink is required to strengthen the bioactivity of SF when preparing SF-based biomaterials using 3D bioprinting. For example, Li et al. fabricated SF-based biological ink with platelet-rich plasma (PRP), and used 3D bioprinting technology to prepare SF-PRP scaffolds. The addition of PRP effectively enhanced the bioactivity of SF-based biological ink and accelerated cartilage repair [72]. However, SF-based biomaterials still face the problem that the mechanical strength is inadequate to meet the needs of cartilage/osteochondral repair. In order to overcome this problem, SF biological ink can be modified with dual functions. For instance, Deng et al. designed SF-parathyroid hormone (SF-PTH) biological ink and mechanically modified SF-methacrylic anhydride (SF-MA) biological ink [73]. Then, successfully constructed the integrated gelatin methacryloyl (GM) + SF-PTH/GM + SF-MA biphasic scaffold with excellent biological activity and a mechanical gradient via 3D bioprinting technology. In addition, in vivo experiments showed that the scaffold had desirable osteochondral repair ability.
Preparation methods of SF-based biomaterials. (A) 3D bioprinting: schematic diagram of preparing SFG-E7 scaffolds by mixing SF and gelatin. Adapted with permission from [65], copyright 2017, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (B) Electrospinning: schematic diagram of preparing SP-Sr scaffolds by electrospinning PCL, SF, and strontium mixed solution. Adapted with permission from [66], copyright 2020, Elsevier Ltd. (C) Freeze-drying: fabricating 3D SF scaffolds with radially co-aligned nanofibers and interconnected macro-channels by a facilely guided ice-crystal growth and nanofiber assembly strategy. Adapted with permission from [67], copyright 2018, American Chemical Society. (D) Schematic diagram of fabricating SF-based biomaterials by salt leaching method. (E) Schematic diagram of preparing SF-based hydrogel prepared by various cross-linking methods.
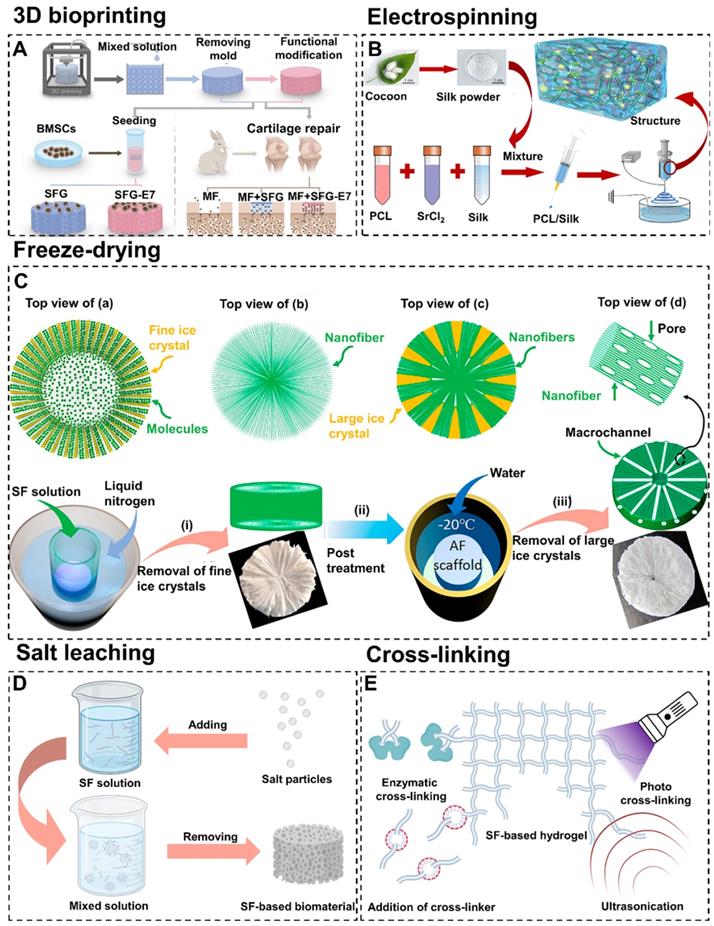
4.1.2. Electrospinning
Electrospinning is a highly controllable technology that allows fine-tuning of multiple parameters. Compared with other fiber spinning processes, electrospinning is able to produce long fibers with a smaller diameter and a higher ratio of surface area to volume. In addition, electrospun scaffolds prepared by assembling electrospun fibers have the advantages of low cost, simple process, and similar nanofibrous structure to ECM with interconnected pores compared with other tissue scaffolds [74]. Moreover, due to the highly controllable of electrostatic spinning technology, it is available to prepare SF-based scaffolds with a wide range of pore sizes [75]. For example, Huang et al. prepared SF scaffolds with gradient pore size under appropriate relative humidity by adjusting fiber diameter (concentration of electrospinning solution) and collector temperature [76]. The experimental data showed that the large pore layer scaffolds with 37.2±12.9 μm pore size significantly promoted the migration of cells into the scaffold. The medium pore layer scaffold with 11.6±1.4 μm pore size was more beneficial to cell proliferation. Additionally, the small pore layer scaffold with a micropore size of 5.9±1.4 μm had higher mechanical properties.
In recent years, electrospinning has been widely used in the field of cartilage tissue engineering [77, 78]. For instance, Li et al. constructed acellular three-dimensional meniscus scaffolds composed of PCL, SF, and strontium by wet electrospinning (Figure 4B) [66]. The functional scaffolds had been successfully used for meniscus regeneration in the rabbit meniscectomy model. This study suggested that electrospinning could mix a variety of substrates and combine the characteristics of substrates to fit the needs of cartilage tissue engineering. Furthermore, SF-based scaffolds for full-thickness repair of hyaline cartilage, calcified cartilage and subchondral bone could also be prepared by electrospinning technique. For example, Liu et al. designed and synthesized CS/SF/hydroxyapatite three-layer scaffolds via electrospinning [79]. Results confirmed that this CS/SF/hydroxyapatite three-layer scaffolds could mimic the chondral layer, calcified layer, and subchondral bone layer respectively to promote osteochondral repair. Generally, ordinary electrospinning usually produces two-dimensional films with tightly packed layers which hinder osteoid cells migration. To break through this limitation, electrospinning could be combined with other preparation methods to improve the cartilage/osteochondral repair effect of SF-based biomaterials. For instance, Chen et al. prepared three-dimensional/hyaluronic acid/SF scaffold (3DHAS) in a stepwise design by combining electrospinning, gas foaming and freeze-drying techniques [80]. They found that 3DHAS had the characteristics of low density, large pore size, high porosity, strong water absorption capacity, and good mechanical stability. Importantly, there was excellent cartilage regeneration after subcutaneous transplantation of 3DHAS. Moreover, electrospinning could maintain the elasticity of SF (which is essential for cartilage/osteochondral tissue engineering) [81]. For instance, Christakiran et al. reported a simple, scalable, and repeatable strategy to develop electrospun double-layer bioglass/SF composites for the repair of osteochondral defects [82]. The biphasic composites exhibited both an elastic region pertinent for cartilage tissue and a stiff compression resistant region simulating the bone phase.
4.1.3. Freeze-drying
Freeze-drying is a flexible and controllable technology for the preparation of biomaterials. Freeze-drying has attracted wide attention because it could control the growth of ice nuclei to adjust the pore size of biomaterials [83, 84]. This method has shown certain advantages, such as process adaptability, excellent pore-making capacity, and environmental friendliness [85].
It is reported that the pore shape and orientation of biomaterials can be controlled by adjusting the freezing direction [86, 87]. For example, Fan et al. prepared biomimetic anisotropic 3D SF scaffolds with the radial channel via directional freeze-drying technology (Figure 4C) [67]. During the preparation process, heat is transmitted from the center to all around. Therefore, the growth direction of ice crystals is from the periphery to the center. Compared with other SF scaffolds, the freeze-drying scaffolds had a higher cell capture and growth promotion ability. More importantly, the scaffolds also regulated cell migration, arrangement, elongation, and interaction. In addition, the unidirectional pore could be prepared by changing the heat transfer direction from radial direction to the single direction via freeze-drying technology. For instance, Zhang et al. fabricated biomimetic SF-based scaffolds with cartilage ECM-like configurations (horizontally aligned structure of the surface layer, vertically aligned structure of the bottom layer and random pore structure of the middle layer) by directional freezing [88]. The above two examples showed that freeze-drying technology can control the morphology of SF-based biomaterials. The radial pore structure and directional pore structure will be discussed in detail in 4.3.1. Directional structure.
Notably, the process of preparing biomaterials by freeze-drying technique causes the solvent to freeze and then sublimate, while having little effect on the solute. Therefore, freeze-drying is also convenient for SF-based biomaterials to carry drugs and growth factors [89]. For instance, Sun et al. successfully prepared oriented microtubule collagen/CS/SF-transforming growth factor-β1 (TGF-β1) scaffolds with good hydrophilicity by freeze-drying technique [90]. The scaffolds had good physical and chemical properties, mechanical properties, and biocompatibility. Furthermore, the scaffolds induced cartilage and subchondral bone regeneration by releasing TGF-β1.
4.1.4. Salt leaching
Salt leaching is widely used to prepare SF-based biomaterials due to its high effectiveness and low cost. This method induces SF aggregation by adding salt particles to the SF solution. Porous SF biomaterials can be obtained by removing the undissolved salt particles (Figure 4D) [91]. The properties of SF-based materials fabricated by salt-leaching are influenced by the size of salt particles and the concentration of SF solution [92]. In general, salt-leaching is combined with other methods to fabricate SF-based biomaterials. For example, Ribeiro et al. prepared a novel SF-based material by combining salt-leaching and freeze-drying methods [93]. This material had suitable porosity and mechanical properties for cartilage repair. In addition, Zhou et al. prepared a SF-CS material by combining salt-leaching and cross-linking methods [94]. This material could maintain the chondrocyte phenotype, attenuate the interleukin (IL)-1β-induced inflammatory response of chondrocytes in vitro, and accelerate osteochondral repair in vivo.
4.1.5. Cross-linking
As mentioned in section 2, SF contains hydrophobic residue repetitive sequences and a large number of polar groups [95]. Therefore, SF has excellent cross-linking properties. The commonly used cross-linking methods for preparing SF-based hydrogels include enzymatic cross-linking, addition of cross-linker, photo cross-linking and ultrasonication (Figure 4E). Enzymatic cross-linking is a chemical cross-linking method which induce cross-linking reaction through activating certain groups by utilizing the catalysis of enzymes. This method can maintain the mechanical properties and biocompatibility of SF due to its mild reaction condition. For example, Zhang et al. constructed a novel enzymatically cross-linked SF-laponite (LAP) nanocomposite hydrogel [96]. This hydrogel had good biocompatibility and excellent mechanical properties. Both the in vitro and in vivo experiments showed that this hydrogel could stimulate osteogenesis and chondrogenic differentiation of BMSCs, and accelerate the regeneration of osteochondral defects. Meanwhile, Li et al. fabricated a SF-tyramine-substituted gelatin (GT) hydrogel by combining enzymatic cross-linking method and 3D bioprinting [97]. This hydrogel had good biocompatibility and could be used as a cell delivery vehicle to accelerate cartilage repair. However, the high price of enzyme limits the development of enzymatic cross-linking. Cross-linkers are a series of substances that can bond multiple linear molecules into a network structure. In addition, cross-linkers are cheaper than enzymes and can enhance the mechanical properties of SF-based hydrogels by forming stable covalent bonds. For example, Wang et al. constructed a SF/diglycidyl ether (BDDE) hydrogel with high elasticity and mechanical stability by using BDDE as cross-linker [98]. The hydrogel had remarkable biocompatibility and the ability of long-term retention in the articular cavity. Photo cross-linking refers to control the cross-linking process through using ultraviolet, visible light or gamma rays by adding photo-initiators. Compared with other methods, the photo cross-linking has the fastest cross-linking rate. For instance, Piluso et al. prepared a cell-laden SF hydrogel in a few minutes by using riboflavin as photo-initiators [99]. This hydrogel showed excellent cytocompatibility with cartilage derived progenitor cells, mesenchymal stem cells, and dental pulp derived stem cells and was expected to be a promising tissue engineering scaffold material for cartilage repair. However, the residues of photo-initiators and cross-linkers may lead to the cytotoxicity of SF-based biomaterials [100]. Ultrasonication is a physical cross-linking method. This method accelerates cross-linking by increasing local temperature and shear-force, which can avoid the cytotoxicity problems caused by photo-initiators and cross-linkers. For instance, Yuan et al. prepared an ultrasonication-induced SF hydrogel [101]. This hydrogel showed satisfactory physicochemical and biomechanical properties and could promote cartilage regeneration.
4.2. Types of SF-based biomaterials
4.2.1. Hydrogels
Hydrogel is a hydrophilic three-dimensional network polymer material formed by physical or chemical cross-linking [102-104]. The physical properties of the hydrogels are similar to biological tissues, such as high water content, rubber texture, and low interfacial tension. Therefore, hydrogels have excellent biocompatibility with surrounding tissues due to their physical properties are similar to natural tissues. Additionally, hydrogels are ideal for imitating the complex and organized network of natural ECM [105, 106]. Besides, the natural EMC-like microenvironments of hydrogels are suitable for loading cells to promote cartilage/osteochondral repair [107]. For example, Cui et al. presented a rapid and cyto-compatible photo cross-linking process to produce cell-encapsulated SF hydrogels cross-linked Through the formation of di-tyrosine bonds [108]. The high density of chondrocytes in this hydrogel effectively accelerated cartilage repair.
It was reported that the mechanical properties, shape, and swelling properties of hydrogels could change with the alternation of external conditions, such as temperature, pH value, and ion concentration [109]. The environment-responsive hydrogel designed based on this characteristic can realize the intelligent release of chondrogenic and osteochondrogenic drugs [110, 111]. For example, Xu et al. prepared a series of stimulus-responsive composite hydrogels using SF and CS as raw materials and 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide/N-Hydroxysuccinimide as cross-linking agent [112]. The swelling properties of hydrogels showed stimulus-response under different pH and ion concentrations. This work indicated that hydrogels have strong plasticity.
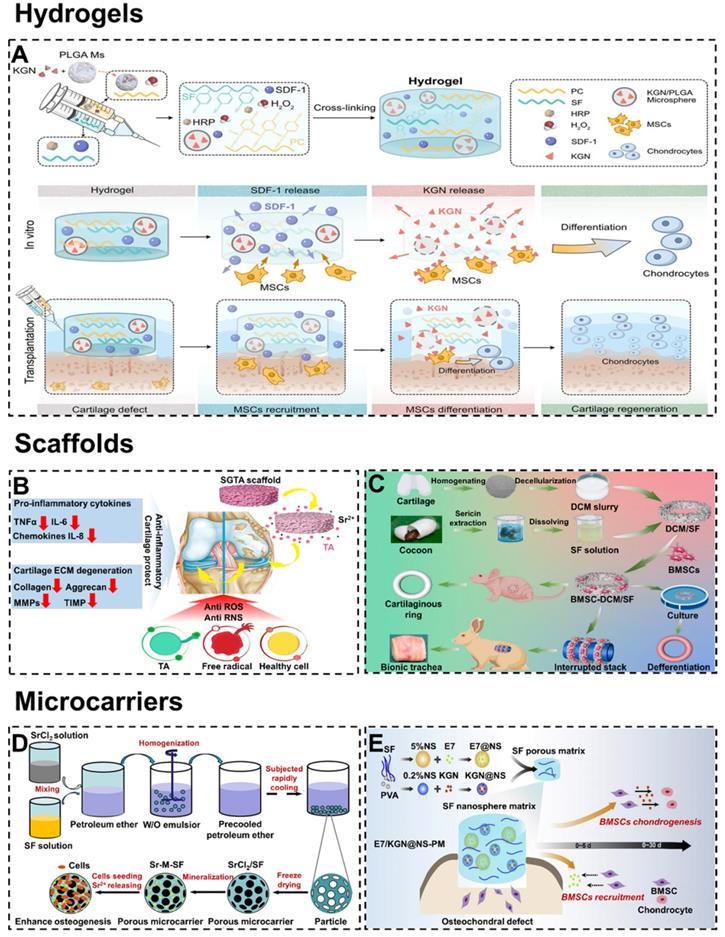
Importantly, the injectability exhibited by hydrogels is very practical in tissue engineering. This feature allows the hydrogel to fit tightly at the interface of cartilage defects to elevate the integration effect. Besides, injectable hydrogels as drug carriers adapt easily to defects of any size or shape and allow drugs to be evenly distributed [113, 114]. For example, Dong et al. designed an injectable p-hydroxybenzene propanoic acid-modified chitosan/SF hydrogel with loading kartogenin (KGN)/poly(lactic-co-glycolic acid) microsphere (Figure 5A) [115]. The hydrogel was injected in situ to recruit endogenous stem cells and induce them to differentiate into chondrocytes. In addition, after injection of the hydrogel into the cartilage defect site, there was a transition from solution to gel state. Unfortunately, the complicated procedures and toxicity of physicochemical transition methods still hinder further applications of injectable hydrogels. To overcome this problem, Yuan et al. developed an injectable SF hydrogel in a novel one-step ultrasonication cross-linking method [101]. In this way, the toxic side effects caused by the gel transformation of injectable hydrogels in cartilage/osteochondral defects could be avoided.
4.2.2. Scaffolds
Scaffolds are 3D porous matrices that promote cartilage/osteochondral repair and regeneration by providing a specific microenvironment. Ideally, scaffolds should be able to: (1) Promote transport of nutrients, and cell survival, proliferation, and differentiation; (2) provide suitable structural mechanical support; (3) degrade at a controllable rate; (4) present minimal inflammation and toxicity in vivo [120-123].
Nowadays, SF scaffolds have been widely used in cartilage/osteochondral engineering [124, 125]. Compared with hydrogels, the scaffolds have fixed shapes and higher mechanical strength. In addition, the stable surface of the scaffolds could be modified by functional coating to improve cartilage/osteochondral repair performance [126, 127]. For example, Li et al. prepared SF/graphene oxide (GO) meniscus scaffolds with tannic acid/Sr2+ functional coating (Figure 5B) [116]. According to the results of tissue staining and the Osteoarthritis Research Society International scoring system, the expression of inflammatory cytokines (such as IL-6, IL-8, and MMPs) in the rat knee joint tissue was significantly down-regulated. Meanwhile, cartilage degeneration and OA injury were also inhibited. In addition, scaffolds could be designed into different 3D shapes to fit the notch of cartilage/osteochondral. For instance, Gao et al. have successfully fabricated porous decellularized cartilaginous matrix/silk fibroin (DCM/SF) scaffolds (Figure 5C) [117]. It had been confirmed that DCM/SF scaffolds supported cartilage ring regeneration of BMSCs both in vivo and in vitro. Compared with DCM scaffolds and SF scaffolds, DCM/SF scaffolds significantly promoted the chondrogenesis of implanted BMSCs. In a rabbit model, the scaffold accomplished the restoration of the cricoid tracheal cartilage.
Scaffolds could serve as an adjunct to cell therapy to promote cell attachment, growth and differentiation. For example, Yang et al. synthesized CS/SF porous scaffolds loaded with C-type natriuretic peptide gene-modified BMSCs to accelerate the repair of articular cartilage defects [128]. Generally, it is difficult to preserve scaffold-cell composites for a long time, which limits the applications of SF-based scaffolds. To address this difficulty, ran et al. developed a SF scaffold loaded with cartilage stem/progenitor cells [129]. The SF scaffold-cell composites could be preserved in liquid nitrogen, and their activity, migration, and chondrogenic capacity were unaffected for up to 3 months.
Representative types of SF-based biomaterials. Hydrogel: (A) Injectable PC-SF hydrogels loading with SDF-1, PLGA, and KGN promote recruitment and differentiation of stem cells for cartilage regeneration. Adapted with permission from [115], copyright 2021, Springer Nature. Scaffolds: (B) Mechanism of the SF/GO scaffolds delay OA and protect cartilage. Adapted with permission from [116], copyright 2021, Elsevier Ltd. (C) Fabrication of DCM/SF scaffold for repairing cartilaginous tissue. Adapted with permission from [117], copyright 2021, Elsevier Ltd. Microcarriers: (D) Schematic diagram of synthesizing Sr-M-SF porous microcarriers for enhancing osteogenesis. Adapted with permission from [118], copyright 2021, Elsevier Ltd. (E) Schematic diagram of producing SF nanospheres microcarriers for osteochondral repair. Adapted with permission from [119], copyright 2020, Elsevier Ltd
4.2.3. Microcarriers
Microcarriers usually refer to small spherical scaffolds suitable for cell culture, growth, and transport [130, 131]. High specific surface area (promote cell growth and maintain cell differentiation phenotype) and injectability (realize tissue regeneration by directly injecting into the target site) enable microcarriers to accelerate cartilage/osteochondral repair [132, 133]. In particular, porous microcarriers could provide a protected environment for seed cells attachment, proliferation, migration, nutrient exchange, and metabolic waste excretion [134]. For example, Galuzzi et al. compared the activity of chondrocytes under the three models (pellet, alginate beads, and SF/alginate microcarriers) [135]. The experiments proved that chondrocytes in the SF/alginate microcarriers were the most active and secreted the most ECM. Meanwhile, Fang et al. prepared strontium-containing SF porous microcarriers with the dual functions of bioactive factor and cell delivery (Figure 5D) [118]. Cytobiological analysis showed that the porous microcarriers allowed attachment and proliferation of seeded cells. Besides, the released Sr2+ could stimulate the osteogenic differentiation of BMSCs.
To circumvent the problem of scarce cell sources, microcarriers could be loaded with growth factors to promote homing, adhesion, and growth of chondrogenic/osteogenesis cells [136]. For instance, He et al. synthesized the SF/CS microcarriers by loading TGF-β1 to accelerated chondrogenesis [137]. In addition, microcarriers could be loaded with active polypeptides and drugs to stimulate cartilage/osteochondral repair. For example, Zhang et al. prepared functional SF nanospheres microcarriers with peptides E7 (7-amino acid (EPLQLKM) small peptide) and KGN (Figure 5E) [119]. The microcarriers rapidly release peptides E7 in the early stage to recruit BMSCs and provide relatively slow and sustained KGN release to induce cartilage formation of BMSCs, thus promoting osteochondral regeneration.
4.3. Structures of SF-based biomaterials
At present, different kinds of SF-based biomaterials for cartilage repair have their characteristics and advantages. It is worth mentioning that the role of the internal structure of SF-based biomaterials in cartilage/osteochondral repair cannot be ignored. Furthermore, SF-based biomaterials were designed in different structures, including directional structure, layered structure, and gradient structure. For cartilage/osteochondral tissue engineering, these elaborate structures were excellent in terms of improving the repair effects.
4.3.1. Directional structure
In recent years, bioactive SF-based scaffolds with radial or axial pore structures have been developed to enhance cell migration and infiltration in vivo and in vitro, and further accelerate cartilage/osteochondral regeneration [138, 139]. For example, Yang et al. combined SF and decellularized cartilage ECM by temperature gradient-guided thermal induced phase separation to produce composite scaffolds (axial pore structures scaffolds) [140]. The results showed that the axial pore structures endowed the scaffolds with remarkable mechanical properties and were suitable for adipose stem cell attachment and proliferation. Meanwhile, Chen et al. prepared three-dimensional SF/bacterial cellulose nanoribbon (SF/BCNR) composite scaffolds with a radial layered structure by directional freezing technology (Figure 6A) [141]. The radial interconnected pores promoted the flow of nutrients and waste to maintain cells vitality and provided robust mechanical performance. Therefore, the radial pore structures SF/BCNR scaffolds had high application potential in the field of cartilage/osteochondral regeneration.
Although there have been numerous studies demonstrating that the directional structure of scaffolds could accelerate cartilage/osteochondral regeneration. However, the underlying mechanism of directional structure promoting cartilage/osteochondral repair is still unclear. At present, only comparative studies between different structures are available. For instance, Feng et al. constructed three kinds of biomimetic SF/collagen composite scaffolds with different pore structures (random pore, radial pore, and axially arranged pore), and evaluated their ability to regenerate osteochondral defects in the rabbit model (Figure 6B) [142]. The experimental data showed that the radial and axially arranged scaffolds had structural and mechanical anisotropy. Due to their high porosity, appropriate elastic modulus, good biocompatibility, and favorable three-dimensional microenvironment, these scaffolds enhanced cell activity in vitro and in vivo. Compared with random scaffolds, these properties contributed to a faster regeneration rate and better regeneration effect of osteochondral defects. Furthermore, in the comparison between radial pore structures and axial structures, the radial pore structures had a more significant effect on accelerating osteochondral repair.
4.3.2. Multilayer structure
For cartilage/osteochondral tissue engineering, the key to the preparation of suitable scaffolds lies in the design of the structure [148]. Meanwhile, scaffolds should closely simulate the physiological structure and environment of natural osteochondral tissue. In addition, on the basis of the physiological structure of natural subchondral bone and cartilage, many researchers have designed double-layer scaffolds with a cartilage layer and a subchondral bone layer to regenerate cartilage and subchondral bone at the same time [149-152]. For example, Ribeiro et al. constructed a new type of double-layer composite scaffold with multiple functional modifications for osteochondral regeneration (Figure 6C) [143]. The double-layer scaffolds included horseradish peroxidase-cross-linked SF (HRP-SF) cartilage layer, HRP-SF/ZnSr-doped β-tricalcium phosphate (β-TCP) subchondral bone-like layer (HRP-SF/dTCP layer).
Mimic Cartilage/Osteochondral structures of SF-based biomaterials. Directional structure: (A) Formation mechanism of radial lamellae and intercalation structure, and the cross-section SEM images. Adapted with permission from [141], copyright 2017, The Royal Society of Chemistry. (B) Osteochondral repair of SF scaffolds with random structure, radially aligned structure, and axially aligned structure, and the cross section and vertical section SEM images. Adapted with permission from [142], copyright 2020, The Royal Society of Chemistry. Multilayer structure: (C) Schematic illustration of preparing BdTCP scaffold and BTCP scaffold in osteochondral regeneration. Adapted with permission from [143], copyright 2019, American Chemical Society. (D) Preparation and mechanism of the integral bilayer SF scaffold combined with Sil-MA hydrogel in osteochondral repair. Adapted with permission from [144], copyright 2021, Elsevier Ltd. (E) Preparation process of integrated three-layer osteochondral scaffolds. Adapted with permission from [145], copyright 2014, American Chemical Society. Gradient structure: (F) Synthesis procession of biomimetic GSSR5 composites: biosilica particles and R5 peptide self-assembly on SF to form gradient structure. Adapted with permission from [146], copyright 2017, Elsevier Ltd. (G) Schematic illustration of preparing SF hydrogels with gradient structure and the control of cell differentiation. Adapted with permission from [147], copyright 2020, Springer Nature.
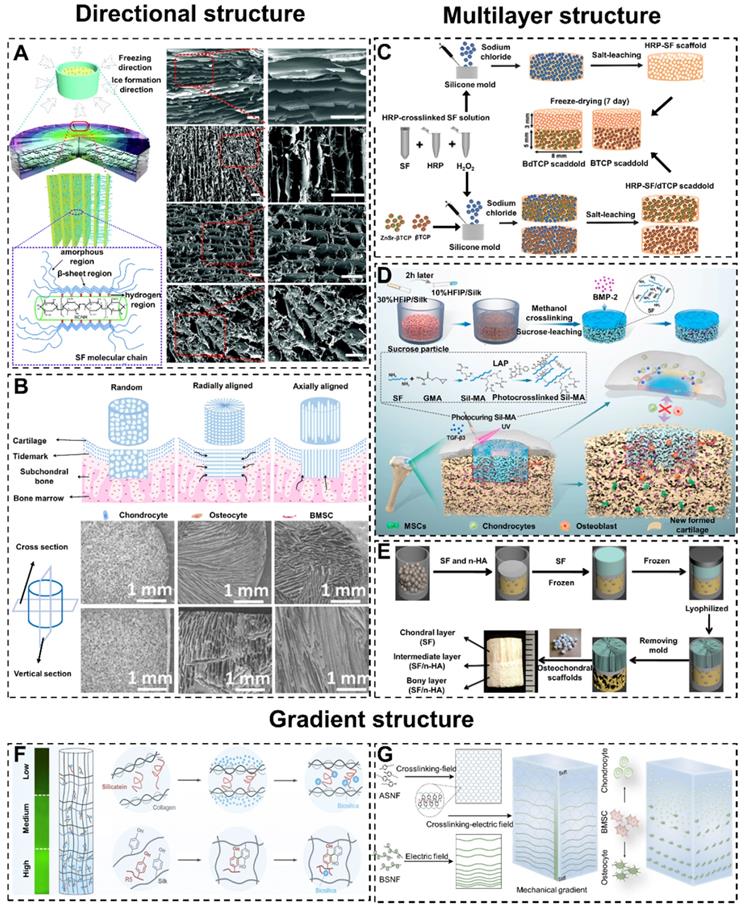
They found that different layers in the bilayer scaffolds stimulated the expression of different genes in cells to promote the repair of osteochondral. In order to overcome the poor integration of regenerated tissue and primary tissue, Wu et al. constructed a kind of double-layer SF composite scaffolds with a peripheral package (Figure 6D) [144]. The scaffold consisted of a dense and smooth biomimetic cartilage layer, a porous layer loaded with bone morphogenetic protein-2 (BMP-2) and a hydrogel coating loaded with transforming growth factor-β3 (TGF-β3). The experimental results showed that the double-layer SF scaffolds accelerated the regeneration of osteochondral, and the encapsulation hydrogel strengthened the integration of tissues. This special multi-layer design pointed out that researchers could achieve better cartilage/osteochondral repair by combining different types of SF-based biomaterials.
With the deepening of the study of double-layer scaffolds, the inherent defects in osteochondral repair become more apparent. The poor integration of the repaired cartilage layer and subchondral bone layer leads to a clear boundary between them [153, 154]. As we mentioned earlier in 3. Articular cartilage and subchondral bone, the calcified cartilage layer is an important physical barrier between cartilage and bone in different oxygen and nutrient environments. Without the calcified cartilage layer, the formation of regenerated cartilage and osteochondral tissue will be damaged [155]. In order to closely simulate the natural osteochondral structure and regeneration environment, three-layer scaffolds have become available [156, 157]. For instance, Ding et al. have successfully prepared biomimetic three-layer osteochondral scaffolds composed of SF/HA (Figure 6E) [145]. Significantly, the intermediate layer could play a role in preventing the cells within the chondral and the bony layers from mixing with each other. Meanwhile, the intermediate layer enhanced the integration of articular cartilage and subchondral bone.
4.3.3. Gradient structure
Many natural tissues, including bones, have multiple gradient structures to regulate cell behavior and guide tissue function [158-161]. Generally, if biomaterials misfit with the structure of cartilage/osteochondral, the long-term repair effect will be unsatisfactory [162, 163]. To mimic the progressive osteochondral structure, researchers could design SF-based scaffolds with gradient pore sizes [164, 165]. For example, Xiao et al. designed and prepared SF/CS/nano-hydroxyapatite (NHA) osteochondral scaffolds with gradient pore sizes [166]. The gradient pore size structure achieved better proliferation and differentiation of the stem cells to complete the repair of osteochondral defects.
The gradient modification of SF-based biomaterials could promote the gradient differentiation and distribution of cells to accelerate the repair of osteochondral [167]. For example, Guo et al. prepared gradient silicified SF R5 peptide (GSSR5) composite biomaterials (Figure 6F) [146]. The GSSR5 composite biomaterials regulated the osteogenic differentiation of hBMSCs in the bone induction environment. The material facilitated the differentiation of cells in a manner consistent with the R5 gradient distribution. This cellular differentiation and distribution consistent with native tissue facilitated osteochondral repair.
Generally, introducing gradient cues when preparing biomaterials requires special instruments and methods. Moreover, these instruments and methods are suitable for special materials only. To break through this limitation, Xu et al. prepared multifunctional building block hydrogels with tunable gradients using a simple low voltage electric field (Figure 6G) [147]. This novel approach enables this hydrogel to exhibit multiple gradients. These gradient structures drove cells to differentiate in different directions and accelerated osteochondral repair.
4.4. Physical and chemical properties of SF-based biomaterials
4.4.1. Mechanical properties of SF-based biomaterials
Articular cartilage is a special load-bearing tissue that provides friction-free movement in synovial joints such as the knee joint, hip joint, or shoulder. The elastic modulus of articular cartilage is between 1-10kPa, which requires the biomaterials to repair cartilage need to match its mechanical properties [168]. Therefore, when preparing biomaterials for osteochondral repair, researchers should regulate the mechanical properties of SF [169]. For example, Chameettachal et al. studied the effect of adding micronized CS into freeze-dried porous SF scaffolds [170]. According to the experimental data, micronized CS significantly enhanced the mechanical properties of SF scaffolds when the ratio of SF to CS microparticles was 2: 1 (the compressive strength of the non-reinforced SF scaffolds and reinforced scaffolds for SF:CS=1:2, 1:1 and 2:1 were 14.40 ± 3.2, 25.40 ± 5.4, 28.10 ± 6.3 and 30.10 ± 7.5 MPa, respectively). Meanwhile, Mirmusavi et al. added multi-walled carbon nanotubes (MWNTs) based on CS/SF scaffolds [171]. Characterization found that CS and MWNTs improved the tensile strength compared with pure SF scaffolds (the ultimate tensile strength of pure SF scaffolds, unsaturated CS/SF scaffolds, saturated CS/SF scaffolds and saturated CS-MWNTs/SF scaffolds were 28.32 ± 3.27, 39.21 ± 2.06, 41.18 ± 2.75 and 46.68 ± 2.20 MPa, respectively).
Normally, the articular cartilage is in a constant cycle of compression-rebound. In addition, during the process of cartilage/osteochondral repair, chondrocytes are more active in the dynamic environment. To prevent SF-based biomaterials premature damage after implantation in a dynamic environment of cartilage/osteochondral defect sites, SF-based biomaterials should exhibit excellent anti-fatigue ability [172]. For instance, Huang et al. modified the SF with cholesterol or β-cyclodextrin, and prepared a kind of SF hydrogel with high mechanical strength (the compressive stress of SF hydrogels and modified-SF hydrogels were 0.1 and 3.16 MPa, respectively), high toughness and remarkable fatigue resistance (the modified-SF hydrogels remained in the original state without any deformation or strength degradation after 10 loading-unloading cycles at 60% strain) [173]. Interestingly, the hydrogel could achieve self-repair after stress damage. This indicated that the self-repair hydrogels had great potential in cartilage/osteochondral repair.
4.4.2. Degradation properties of SF-based biomaterials
The degradation properties of biomaterials directly affect the speed and quality of cartilage/osteochondral repair. Specifically, in the early stage of implantation, biomaterials should be stable to provide mechanical support for cells and tissues. After that, biomaterials need to be gradually degraded to match the new tissue growth [41].
As a kind of protein material, the degradation rate of SF-based biomaterials is mainly affected by proteases. Most proteolytic enzymes tend to degrade non-crystalline SF. This suggests that SF-based biomaterials with controlled degradability can be prepared by changing the content of crystalline structures [101]. For instance, Jin et al. constructed water-stable SF films through increasing the content of crystalline structures by methanol treatment [174]. Additionally, the degradation rate of SF-based materials can also be regulated by the introduction of proteinase inhibitors or protease binding sequence SF. For example, Pritchard et al fabricated a slowly degraded SF-based biomaterial by incorporating protein inhibitors [175]. This material can realize the controlled release of drugs. On the contrary, Huang et al. prepared a novel kind of transgenic SF by introducing matrix metalloproteinase-2-sensitive sequences into SF [176]. The transgenic SF had a quicker degradation rate and was non-toxic to BMSCs.
4.5. Functional modification of SF-based biomaterials
At present, tissue engineering has been considered as the primary therapeutic strategy for osteochondral regeneration and cartilage defect repair. However, the therapeutic effect of SF-based biomaterials on cartilage/osteochondral defects is still far from the expected effect. Consequently, it is necessary to functional modification of SF to improve the repair ability of SF-based biomaterials (Table 1).
Summary of the functional modifications of SF-based biomaterials.
| Types | Modifications | Functions | Reference |
|---|---|---|---|
| Bioactive molecules | E7 | Promote recruit BMSCs | [65, 119] |
| R5 | Regulate osteogenic differentiation of hBMSCs | [146] | |
| L7 | Promote the proliferation and differentiation of SMSCs | [178] | |
| Elastin-like peptide | Support the adhesion, proliferation and differentiation of BMSCs and/or chondrocytes | [179] | |
| Apt19s | improve the cell recruitment ability of SF/HA scaffolds; accelerate the repair of osteochondral | [182] | |
| Growth factors | SDF-1 | Promote the migration and differentiation of MSCs into chondrocytes | [115] |
| SDF-1α | Enhancing cartilage forming in vitro and in vivo | [184] | |
| TGF-β1 | Accelerate chondrogenesis; Stimulate cartilage and subchondral bone regeneration | [90, 137, 184] | |
| TGF-β3 | Facilitate chondrocyte growth and regeneration and lateral integration with the adjoining cartilage sealed | [144] | |
| PRP | Promote the proliferation of chondrocytes | [72] | |
| PTH | Inhibit the hypertrophy of chondrocytes to maintain the phenotype of hyaline cartilage | [73] | |
| BMP-2 | Stimulates the osteogenic differentiation of BMSCs | [144] | |
| Cells | BMSCs | Promote bone repair | [179] |
| Chondrocytes | Promote cartilage repair | [179] | |
| C-type natriuretic peptide gene-modified BMSCs | Accelerate the repair of articular cartilage defects | [128] | |
| Others | BMP-2/ TGF-β1@CS NPs | Release BMP-2/ TGF-β1 to accelerate repair of articular cartilage | [191] |
| HA/CH-PDO NPs | Slow-release BMP-7 to promote SMSCs differentiation into chondrocytes | [192] | |
| BMSC derived exosomes | Enhance the recruitment of chondrocytes; Accelerate cartilage repair | [180] |
4.5.1. Bioactive molecules
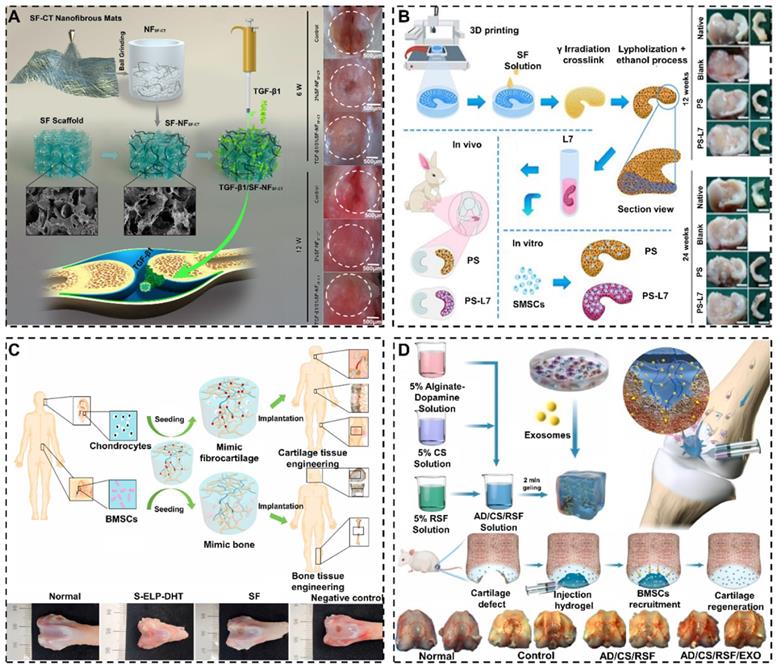
It was reported that SF-based biomaterials could enhance cartilage/osteochondral repair by loading bioactive materials [181]. For example, Wang et al. synthesized SF/HA scaffolds functionalized with non-immunogenic aptamer (Apt19s) [182]. The Apt19s significantly improved the cell recruitment ability of SF/HA scaffolds and accelerated the repair of osteochondral. In order to promote cell adhesion, Cheng et al. hybridized SF with chitin to prepare 3D composite scaffolds (Figure 7A) [177]. The hybrid treatment of SF formed a rough surface that well mimicked the ECM to facilitate cell attachment and proliferation. In addition, SF-based biomaterials are able to carry active molecules to treat cartilage/osteochondral defects. For example, Li et al. fabricated PCL/SF scaffolds loaded with synovium-derived mesenchymal stem cells-specific affinity peptide (L7) via 3D bioprinting technology (Figure 7B) [178]. L7 peptide promoted the proliferation and differentiation of endogenous synovium-derived mesenchymal stem cells (SMSCs). Meanwhile, the L7 peptide stimulated the ECM production to provide a favorable microenvironment for meniscus regeneration and cartilage protection.
4.5.2. Growth factors
Cartilage/osteochondral regeneration is regulated by a variety of growth factors. SF-based biomaterials loaded with growth factors can enhance the interaction with chondrocytes and osteoblasts. For example, Wu et al. fabricated biodegradable SF-gelatin porous scaffolds loading with ginsenoside Rb1 and TGF-β1 [183]. The results showed that the scaffolds loaded with Rb1 and TGF-β1 could create a good microenvironment for cartilage regeneration by promoting cartilage formation and inhibiting inflammation in vivo. Meanwhile, Chen et al. prepared SF-porous gelatin scaffolds loading with SDF-1α and TGF-β1 [184]. They found the scaffolds could accelerate cartilage repair by sustained release of SDF-1α and TGF-β1.
SF-based biomaterials enhance cartilage/osteochondral repair effect by functional modifications: (A) Schematic illustration of SF-NFSF-CT scaffolds improve cell attachment and proliferation by hybrid treatment of SF. Adapted with permission from [177], copyright 2021, Elsevier Ltd. (B) Schematic illustration of PCL/SF scaffolds strengthen meniscus regeneration and cartilage protection by compositing with L7- peptide. Adapted with permission from [178], copyright 2020, Ivyspring International Publisher. (C) Schematic illustration of SF composite scaffolds intensifies bone repair and cartilage repair by seeding chondrocytes and BMSCs. Adapted with permission from [179], copyright 2020, Elsevier Ltd. (D) Schematic illustration of SF hydrogels enhanced cartilage repair by carrying exosomes. Adapted with permission from [180], copyright 2021, Elsevier Ltd.
4.5.3. Cells
SF-based biomaterials also could carry different cells to achieve the repair of different tissues. For example, Chen et al. designed a new type of elastin-like peptide modified SF composite scaffolds through simple and green dehydration treatment (Figure 7C) [179]. They found that the SF composite scaffolds enhanced the adhesion, proliferation, and differentiation of BMSCs or chondrocytes in vitro. Additionally, the SF composite scaffolds containing BMSCs or chondrocytes could promote bone repair and cartilage repair, respectively.
4.5.4. Others
In addition to bioactive molecules, growth factors and cells, loading exosomes can enhance the interaction between SF-based biomaterials and cells [185-188]. For instance, Zhang et al. prepared a composite SF hydrogel loaded with BMSC derived exosomes (Figure 7D) [180]. It was experimentally demonstrated that exosomes enhanced the recruitment of chondrocytes and accelerated cartilage repair. Furthermore, it is feasible to improve the performance of SF-based biomaterials for cartilage/osteochondral repair by loading a variety of nanoparticles (NPs) [189, 190]. For instance, Li et al. prepared SF hydrogels containing BMP-2/TGF-β1@CS NPs [191]. The experimental results showed hydrogels released BMP-2/TGF-β1 from CS NPs to accelerate articular cartilage repair. Meanwhile, Min et al. contrasted alginate-poloxamer/SF dual network hydrogels loading with hyaluronic acid/chitosan-poly(dioxanone) complex nanoparticles (HA/CH-PDO NPs) [192]. They found that HA/CH-PDO NPs could slow-release bone morphogenic protein-7(BMP-7) to promote SMSCs differentiation into chondrocytes.
5. Conclusion and outlook
Cartilage and osteochondral repair have always been a clinical challenge. In recent years, tissue engineering techniques have shown certain advantages in cartilage/osteochondral repair. As a natural macromolecular material with unique mechanical properties, superior processability and excellent biocompatibility, SF is regarded as an excellent tissue engineering material. This paper reviews the research on SF-based biomaterials for cartilage/osteochondral repair. From these studies, it is concluded that SF-based biomaterials have promising applications in cartilage/osteochondral repair. For example, due to the amorphous feature of SF-based hydrogels, they could closely fit irregular cartilage/osteochondral defects [115]. In addition, the controllable degradation of SF-based hydrogels enables them to adapt to different pathological conditions and provides the basis for personalized treatment. Besides, SF-based scaffolds possess more robust mechanical properties compared to other biomaterial scaffolds, which could support cell adhesion and growth better [116]. In addition, the cartilage/osteochondral repair ability of SF-based biomaterials can also be improved by loading polypeptides, cells, exosomes, nanoparticles and growth factors. These functional modifications combined with bioactive substances enhance the ability of SF-based biomaterials to recruit endogenous stem cells or chondrogenesis/osteogenesis. The functional modification of SF-based biomaterials also includes the strengthening of SF itself. For example, the tyrosine-specific diazonium coupling chemistry is used to design the surface chemistry and hydrophilicity of SF for promoting the adhesion, proliferation and differentiation of BMSCs on SF-based biomaterials [193]. Genetic modification of silkworms by means of gene editing can also achieve the purpose of functional modifying SF. For example, insert RGD gene sequence into mulberry silkworm SF gene to produce mulberry silkworm SF containing RGD sequence [194].
Currently, the clinical application researches (data from www.ClinicTrials.gov) demonstrate that the clinical applications of SF-based biomaterials are relatively mature in surgical sutures and wound dressings. In addition, SF-based scaffolds applied to breast reconstruction have shown remarkable improvements. It is worth noting that clinical studies on the use of SF-based scaffolds for meniscal cartilage repair have also been conducted in recent years (Table 2). Although SF-based biomaterials have promising applications, they still face several challenges in sericulture [195]. On the one hand, the production standardization of cocoon (SF source) is insufficient due to the lack of international testing standards. On the other hand, the quality of silkworm cocoons is affected by several production factors, including the silkworm species, breeding method, temperature, humidity, light and hygiene conditions of the breeding environment, transportation and storage methods. Additionally, the inadequate mechanization of the sericulture industry is caused by the complex breeding conditions of silkworms and the seasonal supply of mulberry leaves. Meanwhile, the low degree of mechanization requires a lot of manual operation and leads to the high price of SF. Besides, in the aspects of SF-based biomaterials preparation, there are several factors that inhibit their clinical transformation and commercialization for cartilage/osteochondral repair. (1) The preparation process of SF-based biomaterials is tedious. The LiBr dissolution system is the most commonly used system for dissolving SF. However, the dissolution of SF by this system is time-consuming (SF is slow to dissolve and requires further dialysis, ultrasound and centrifugation after dissolution), and the obtained SF solution is insufficiently concentrated. (2) The forms and preparation methods of completed SF-based biomaterials are undiversified. (3) Most SF-based biomaterials are non-intelligent and difficult to re-intervene after implantation. (4) The entire field of cartilage/osteochondral repair is at a bottleneck stage, and it is difficult to make significant breakthroughs by only aiming to construct new SF-based biomaterials. Fortunately, some scholars have explored and discussed these limiting factors, and made certain improvements. For example, Wang et al. used CaCl2/formic acid solution (weight ratio of 1:20) instead of LiBr solution to dissolve SF [196]. By this dissolution system, the stable and homogeneous SF solution could be obtained in 5 min. This work significantly optimized the preparation process of SF-based biomaterials. To promote the diversification of SF-based biomaterials, it is necessary to combine the advantages of various preparation methods. For instance, Chen et al. combined the advantages of electrostatic spinning, freeze-drying, and gas foaming to prepare SF-based scaffolds with excellent performance [80]. Meanwhile, combining the advantages of various types of SF-based biomaterials is equally important to promote diversification. For example, Wu et al. have combined SF-based scaffolds and SF-based hydrogels to construct new SF-based composite biomaterials that integrate the advantages of scaffolds and hydrogels [144]. In addition, designing SF-based biomaterials with magnetic field response is an effective way to advance the intelligence process. For instance, Wang et al. prepared a thermosensitive and pH-sensitive SF-based biomaterial that would morphological transform in alternating magnetic fields [197]. Notably, Chen et al. discussed the bottleneck of bone tissue repair and the construction of bone organoids [198]. Similarly, SF-based biomaterials may construct the cartilage/osteochondral-like tissues in vitro, which could be transplanted to achieve cartilage/osteochondral repair.
Clinical application research of SF-based biomaterial.
| Title | Year | Tpyes | Applications | ClinicalTrials.gov Identifier |
|---|---|---|---|---|
| Porous Tissue Regenerative Silk Scaffold for Human Meniscal Cartilage Repair (REKREATE) | 2021 | Scaffold | Meniscal cartilage repair | NCT02732873 |
| Initial Safety Evaluation of FibroFix™ Meniscus | 2017 | Scaffold | Meniscal cartilage repair | NCT02205645 |
| SeriACL™ Device Trial for Anterior Cruciate Ligament (ACL) Reconstruction | 2008 | Scaffold | Anterior cruciate ligament repair | NCT00490594 |
| SERI® Surgical Scaffold Postmarket Study of Soft Tissue Support in Ventral Hernia Repair | 2016 | Scaffold | Soft tissue support and repair | NCT01981044 |
| SERI® Surgical Scaffold Postmarket Study of Soft Tissue Support and Repair in Breast Reconstruction | 2016 | Scaffold | Soft tissue support and repair | NCT01914653 |
| Circumferential Periareolar Mastopexy Using SERI Surgical Scaffold | 2016 | Scaffold | Soft tissue support and repair | NCT02293798 |
| Clinical and Economic Outcomes With the Use of SERI® Surgical Scaffold in Direct-to-implant Breast Reconstruction | 2014 | Scaffold | Soft tissue support and repair | NCT02033590 |
| Seri Surgical Scaffold Support of the Lower Pole of the Breast (SeriSupport) | 2016 | Scaffold | Soft tissue support and repair | NCT02016612 |
| The SeriScaffold® Use in Reconstruction Post Market Study for Tissue Support and Repair in Breast Reconstruction Surgery in Europe | 2015 | Scaffold | Soft tissue support and repair | NCT01389232 |
| SERI® Surgical Scaffold for Soft Tissue Support in Revision Augmentation Surgery | 2015 | Scaffold | Soft tissue support and repair | NCT02030938 |
| Evaluation of HQ® Matrix Soft Tissue Mesh for the Treatment of Inguinal Hernia | 2016 | Scaffold | Treatment of inguinal hernia | NCT02487628 |
| AUTOLOGOUS FIBRIN GLUE VERSES 4-0 SILK SUTURES IN PERIODONTAL FLAP CLOSURE | 2020 | Suture | Wound closure | NCT03792113 |
| Coated VICRYL* Plus Suture Compared to Chinese Silk in Scheduled Breast Cancer Surgery | 2009 | Suture | Wound closure | NCT00768222 |
| Cyanoacrylate Tissue Adhesives Versus Silk Suture at the Palatal Donor Site of Sub Epithelial Connective Tissue Graft | 2021 | Suture | Wound closure | NCT04780360 |
| The Comparison of Microbial Adherence to Various Sutures in Patients Undergoing Oral Surgery | 2017 | Suture | Wound closure | NCT02653924 |
| Suture Materials: an Evaluation | 2016 | Suture | Wound closure | NCT03410433 |
| Clinical Evaluation of Sutures in Periodontal Surgery | 2012 | Suture | Wound closure | NCT02013661 |
| Performance of Safety of SILKAM® Suture Material in Oral Surgery (SILKOS) | 2022 | Suture | Mucosal closure | NCT05296902 |
| Efficacy and Safety of Silk Fibroin With Bioactive Coating Layer Dressing | 2015 | Wound dressing | Wound healing | NCT02091076 |
| Evaluation of HQ® Matrix Medical Wound Dressing for Healing of Donor Site Wounds | 2014 | wound dressing | Wound healing | NCT01993030 |
| Manufacturing, Characterization and Evaluation of the Effect of Silk Fibroin Membranes, Loaded or Not With Neurotensins on Open Wounds in the Palate | 2022 | Film | Wound healing | NCT05191082 |
In conclusion, SF-based biomaterials have been widely studied in the field of cartilage/osteochondral repair. In the future, Researchers should develop special mechanical equipment suitable for silkworm rearing and promote mechanization to reduce the production cost of SF-based biomaterials. Meanwhile, it is also necessary to improve the preparation process of SF-based biomaterials. With the assistance of a variety of preparation methods and functional modifications, SF-based biomaterials should be more diversified and intelligent. It is important that SF-based biomaterials transform from 3D biomaterials to 4D biomaterials in combination with the fourth dimension "time". With the progress of cartilage/osteochondral repair process, 4D SF-based biomaterials can be adaptively adjusted to ensure the rapid repair of cartilage/osteochondral. We believe that SF-based biomaterials will be promising for clinical applications in cartilage/osteochondral repair.
Abbreviations
OA: osteoarthritis; SF: silk fibroin; PLA: polylactic acid; PCL: polycaprolactone; HA: hydroxyapatite; CS: chitosan; SFG-E7: silk fibroin-gelatin-E7; BMSC: bone mesenchymal stem cell; ECM: extracellular matrix; PRP: platelet-rich plasma; PTH: parathyroid hormone; MA: methacrylic anhydride; GM: gelatin methacryloyl; 3DHAS: three-dimensional/hyaluronic acid/SF scaffold; TGF-β1: transforming growth factor-β1; KGN: kartogenin; GO: graphene oxide; DCM: decellularized cartilaginous matrix; BCNR: bacterial cellulose nanoribbon; HRP-SF: horseradish peroxidase-cross-linked SF; β-TCP: β-tricalcium; BMP-2: bone morphogenetic protein-2; TGF-β3: transforming growth factor-β3; NHA: nano-hydroxyapatite; GSSR5: gradient silicified silk fibroin R5 peptide; MWNTs: multi-walled carbon nanotubes; Apt19s: non-immunogenic aptamer; NPs: nanoparticles; L7: synovium-derived mesenchymal stem cells-specific affinity peptide; HA/CH-PDO NPs: hyaluronic acid/chitosan-poly(dioxanone) complex nanoparticles; SMSCs: synovium-derived mesenchymal stem cells; LAP: laponite; BDDE: diglycidyl ether.
Acknowledgements
The authors acknowledge the financial support from National Key R&D Program of China (2018YFC2001500); National Natural Science Foundation of China (82172098, 32101084).
Author Contributions
Ziyang Zhou: Resources, Visualization, Writing-original draft. Jin Cui: Resources, Visualization, Writing-original draft. Shunli Wu: Resources, Visualization, Writing-original draft. Zhen Geng: Writing-review & editing, Supervision, Funding acquisition. Jiacan Su: Writing- review & editing, Supervision, Funding acquisition.
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Mandl LA. Osteoarthritis year in review 2018: clinical. Osteoarthritis Cartilage. 2019;27:359-64
2. Xue X, Liu H, Wang S, Hu Y, Huang B, Li M. et al. Neutrophil-erythrocyte hybrid membrane-coated hollow copper sulfide nanoparticles for targeted and photothermal/ anti-inflammatory therapy of osteoarthritis. Compos B Eng. 2022;237:109855
3. Tan J, Wang C, Wang D, Jiang H, Qiao Y, Zhang D. et al. Tailoring time-varying alkaline microenvironment on titanium for sequential anti-infection and osseointegration. Chem Eng J. 2022;431:133940
4. Abramoff B, Caldera FE. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med Clin North Am. 2020;104:293-311
5. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745-59
6. Hunter DJ. Lower extremity osteoarthritis management needs a paradigm shift. Br J Sports Med. 2011;45:283-8
7. Murphy LB, Cisternas MG, Pasta DJ, Helmick CG, Yelin EH. Medical Expenditures and Earnings Losses Among US Adults With Arthritis in 2013. Arthritis Care Res (Hoboken). 2018;70:869-76
8. Hu Y, Chen X, Wang S, Jing Y, Su J. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res. 2021;9:20
9. Makris EA, Gomoll AH, Malizos KN, Hu JC, Athanasiou KA. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11:21-34
10. Li J, Yin Z, Huang B, Xu K, Su J. Stat3 Signaling Pathway: A Future Therapeutic Target for Bone-Related Diseases. Front Pharmacol. 2022;13:897539
11. Chen H, Fang C, Zhi X, Song S, Gu Y, Chen X. et al. Neobavaisoflavone inhibits osteoclastogenesis through blocking RANKL signalling-mediated TRAF6 and c-Src recruitment and NF-kappaB, MAPK and Akt pathways. J Cell Mol Med. 2020;24:9067-84
12. Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6:625-35
13. Fang C, Guo JW, Wang YJ, Li XQ, Zhang H, Cui J. et al. Diterbutyl phthalate attenuates osteoarthritis in ACLT mice via suppressing ERK/c-fos/NFATc1 pathway, and subsequently inhibiting subchondral osteoclast fusion. Acta Pharmacol Sin. 2021;43:1299-1310
14. Goldring SR, Goldring MB. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. Nat Rev Rheumatol. 2016;12:632-44
15. Scanzello CR. Chemokines and inflammation in osteoarthritis: Insights from patients and animal models. J Orthop Res. 2017;35:735-9
16. Geng Z, Sang S, Wang S, Meng F, Li Z, Zhu S. et al. Optimizing the strontium content to achieve an ideal osseointegration through balancing apatite-forming ability and osteogenic activity. Biomater Adv. 2022;133:112647
17. Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37:2053-63
18. Haene R, Qamirani E, Story RA, Pinsker E, Daniels TR. Intermediate outcomes of fresh talar osteochondral allografts for treatment of large osteochondral lesions of the talus. J Bone Joint Surg Am. 2012;94:1105-10
19. Galperin A, Oldinski RA, Florczyk SJ, Bryers JD, Zhang M, Ratner BD. Integrated bi-layered scaffold for osteochondral tissue engineering. Adv Healthc Mater. 2013;2:872-83
20. Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338:917-21
21. Bal BS, Rahaman MN, Jayabalan P, Kuroki K, Cockrell MK, Yao JQ. et al. In vivo outcomes of tissue-engineered osteochondral grafts. J Biomed Mater Res B Appl Biomater. 2010;93:164-74
22. Su T, Zhang M, Zeng Q, Pan W, Huang Y, Qian Y. et al. Mussel-inspired agarose hydrogel scaffolds for skin tissue engineering. Bioact Mater. 2021;6:579-88
23. Huang B, Tan L, Liu X, Li J, Wu S. A facile fabrication of novel stuff with antibacterial property and osteogenic promotion utilizing red phosphorus and near-infrared light. Bioact Mater. 2019;4:17-21
24. Chen X, Zhang Z, Hu Y, Cui J, Zhi X, Li X. et al. Lactulose Suppresses Osteoclastogenesis and Ameliorates Estrogen Deficiency-Induced Bone Loss in Mice. Aging Dis. 2020;11:629-41
25. Vilela CA, Correia C, Oliveira JM, Sousa RA, Espregueira-Mendes J, Reis RL. Cartilage Repair Using Hydrogels: A Critical Review of in vivo Experimental Designs. ACS Biomater Sci Eng. 2015;1:726-39
26. Zhang W, Ouyang H, Dass CR, Xu J. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res. 2016;4:15040
27. Gu X, Zha Y, Li Y, Chen J, Liu S, Du Y. et al. Integrated polycaprolactone microsphere-based scaffolds with biomimetic hierarchy and tunable vascularization for osteochondral repair. Acta Biomater. 2022;141:190-7
28. Chen X, Hu Y, Geng Z, Su J. The “Three in One” Bone Repair Strategy for Osteoporotic Fractures. Front Endocrinol (Lausanne). 2022;13:910602
29. Chen X, Zhi X, Wang J, Su J. RANKL signaling in bone marrow mesenchymal stem cells negatively regulates osteoblastic bone formation. Bone Res. 2018;6:34
30. Hu Y, Li X, Zhi X, Cong W, Huang B, Chen H. et al. RANKL from bone marrow adipose lineage cells promotes osteoclast formation and bone loss. EMBO Rep. 2021;22:e52481
31. Luo Y, Cao X, Chen J, Gu J, Yu H, Sun J. et al. Platelet-Derived Growth Factor-Functionalized Scaffolds for the Recruitment of Synovial Mesenchymal Stem Cells for Osteochondral Repair. Stem Cells Int. 2022;2022:2190447
32. Qin C, Ma J, Chen L, Ma H, Zhuang H, Zhang M. et al. 3D bioprinting of multicellular scaffolds for osteochondral regeneration. Mater Today (Kidlington). 2021;49:68-84
33. Patil S, Dhyani V, Kaur T, Singh N. Spatiotemporal Control over Cell Proliferation and Differentiation for Tissue Engineering and Regenerative Medicine Applications Using Silk Fibroin Scaffolds. ACS Appl Bio Mater. 2020;3:3476-93
34. Singh YP, Bhardwaj N, Mandal BB. Potential of Agarose/Silk Fibroin Blended Hydrogel for in vitro Cartilage Tissue Engineering. ACS Appl Mater Interfaces. 2016;8:21236-49
35. Kundu SC, Kundu B, Talukdar S, Bano S, Nayak S, Kundu J. et al. Invited review nonmulberry silk biopolymers. Biopolymers. 2012;97:455-67
36. Ni T, Liu M, Zhang Y, Cao Y, Pei R. 3D Bioprinting of Bone Marrow Mesenchymal Stem Cell-Laden Silk Fibroin Double Network Scaffolds for Cartilage Tissue Repair. Bioconjug Chem. 2020;31:1938-47
37. Kim SH, Seo YB, Yeon YK, Lee YJ, Park HS, Sultan MT. et al. 4D-bioprinted silk hydrogels for tissue engineering. Biomaterials. 2020;260:120281
38. Gholipourmalekabadi M, Sapru S, Samadikuchaksaraei A, Reis RL, Kaplan DL, Kundu SC. Silk fibroin for skin injury repair: Where do things stand? Adv Drug Deliv Rev. 2020;153:28-53
39. Holland C, Numata K, Rnjak-Kovacina J, Seib FP. The Biomedical Use of Silk: Past, Present, Future. Adv Healthc Mater. 2019;8:e1800465
40. Sun W, Gregory DA, Tomeh MA, Zhao X. Silk Fibroin as a Functional Biomaterial for Tissue Engineering. Int J Mol Sci. 2021;22:1499
41. Cao Y, Wang BC. Biodegradation of Silk Biomaterials. Int J Mol Sci. 2009;10:1514-24
42. Messaoudi O, Henrionnet C, Bourge K, Loeuille D, Gillet P, Pinzano A. Stem Cells and Extrusion 3D Printing for Hyaline Cartilage Engineering. Cells. 2020;10:2
43. Onnerfjord P, Khabut A, Reinholt FP, Svensson O, Heinegard D. Quantitative proteomic analysis of eight cartilaginous tissues reveals characteristic differences as well as similarities between subgroups. J Biol Chem. 2012;287:18913-24
44. Heinegard D, Saxne T. The role of the cartilage matrix in osteoarthritis. Nat Rev Rheumatol. 2011;7:50-6
45. Quinn TM, Häuselmann HJ, Shintani N, Hunziker EB. Cell and matrix morphology in articular cartilage from adult human knee and ankle joints suggests depth-associated adaptations to biomechanical and anatomical roles. Osteoarthritis Cartilage. 2013;21:1904-12
46. Hunziker EB, Lippuner K, Shintani N. How best to preserve and reveal the structural intricacies of cartilaginous tissue. Matrix Biol. 2014;39:33-43
47. Hunziker EB, Kapfinger E, Geiss J. The structural architecture of adult mammalian articular cartilage evolves by a synchronized process of tissue resorption and neoformation during postnatal development. Osteoarthritis Cartilage. 2007;15:403-13
48. Simkin PA. Consider the tidemark. J Rheumatol. 2012;39:890-2
49. Goldring MB, Goldring SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010;1192:230-7
50. Ribeiro VP, Pina S, Oliveira JM, Reis RL. Silk Fibroin-Based Hydrogels and Scaffolds for Osteochondral Repair and Regeneration. Adv Exp Med Biol. 2018;1058:305-25
51. Saha S, Kundu B, Kirkham J, Wood D, Kundu SC, Yang XB. Osteochondral tissue engineering in vivo: a comparative study using layered silk fibroin scaffolds from mulberry and nonmulberry silkworms. PLoS One. 2013;8:e80004
52. Mao C, Xiang Y, Liu X, Cui Z, Yang X, Yeung KWK. et al. Photo-Inspired Antibacterial Activity and Wound Healing Acceleration by Hydrogel Embedded with Ag/Ag@AgCl/ZnO Nanostructures. ACS Nano. 2017;11:9010-21
53. Xiang Y, Zhou Q, Li Z, Cui Z, Liu X, Liang Y. et al. A Z-scheme heterojunction of ZnO/CDots/C3N4 for strengthened photoresponsive bacteria-killing and acceleration of wound healing. J Mater Sci Technol. 2020;57:1-11
54. Wan L, Song H, Chen X, Zhang Y, Yue Q, Pan P. et al. A Magnetic-Field Guided Interface Coassembly Approach to Magnetic Mesoporous Silica Nanochains for Osteoclast-Targeted Inhibition and Heterogeneous Nanocatalysis. Adv Mater. 2018;30:e1707515
55. Yue Q, Li J, Zhang Y, Cheng X, Chen X, Pan P. et al. Plasmolysis-Inspired Nanoengineering of Functional Yolk-Shell Microspheres with Magnetic Core and Mesoporous Silica Shell. J Am Chem Soc. 2017;139:15486-93
56. Li XQ, Wang LP, Huang BT, Gu YQ, Luo Y, Zhi X. et al. Targeting actin-bundling protein L-plastin as an anabolic therapy for bone loss. Sci Adv. 2020;6:eabb7135
57. Yuan Z, Long T, Zhang J, Lyu Z, Zhang W, Meng X. et al. 3D printed porous sulfonated polyetheretherketone scaffold for cartilage repair: Potential and limitation. J Orthop Translat. 2022;33:90-106
58. Xu Y, Peng J, Richards G, Lu S, Eglin D. Optimization of electrospray fabrication of stem cell-embedded alginate-gelatin microspheres and their assembly in 3D-printed poly(epsilon-caprolactone) scaffold for cartilage tissue engineering. J Orthop Translat. 2019;18:128-41
59. Bilgen B, Jayasuriya CT, Owens BD. Current Concepts in Meniscus Tissue Engineering and Repair. Adv Healthc Mater. 2018;7:e1701407
60. Rathan S, Dejob L, Schipani R, Haffner B, Mobius ME, Kelly DJ. Fiber Reinforced Cartilage ECM Functionalized Bioinks for Functional Cartilage Tissue Engineering. Adv Healthc Mater. 2019;8:e1801501
61. Xia H, Zhao D, Zhu H, Hua Y, Xiao K, Xu Y. et al. Lyophilized Scaffolds Fabricated from 3D-Printed Photocurable Natural Hydrogel for Cartilage Regeneration. ACS Appl Mater Interfaces. 2018;10:31704-15
62. Yang X, Lu Z, Wu H, Li W, Zheng L, Zhao J. Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater Sci Eng C Mater Biol Appl. 2018;83:195-201
63. Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32:773-85
64. Trucco D, Sharma A, Manferdini C, Gabusi E, Petretta M, Desando G. et al. Modeling and Fabrication of Silk Fibroin-Gelatin-Based Constructs Using Extrusion-Based Three-Dimensional Bioprinting. ACS Biomater Sci Eng. 2021;7:3306-20
65. Shi W, Sun M, Hu X, Ren B, Cheng J, Li C. et al. Structurally and Functionally Optimized Silk-Fibroin-Gelatin Scaffold Using 3D Printing to Repair Cartilage Injury In vitro and In vivo. Adv Mater. 2017;29:1701089
66. Li Y, Chen M, Zhou W, Gao S, Luo X, Peng L. et al. Cell-free 3D wet-electrospun PCL/silk fibroin/Sr(2+) scaffold promotes successful total meniscus regeneration in a rabbit model. Acta Biomater. 2020;113:196-209
67. Fan L, Li JL, Cai Z, Wang X. Creating Biomimetic Anisotropic Architectures with Co-Aligned Nanofibers and Macrochannels by Manipulating Ice Crystallization. ACS Nano. 2018;12:5780-90
68. Thunsiri K, Pitjamit S, Pothacharoen P, Pruksakorn D, Nakkiew W, Wattanutchariya W. The 3D-Printed Bilayer's Bioactive-Biomaterials Scaffold for Full-Thickness Articular Cartilage Defects Treatment. Materials (Basel). 2020;13:3417
69. Jia L, Hua Y, Zeng J, Liu W, Wang D, Zhou G. et al. Bioprinting and regeneration of auricular cartilage using a bioactive bioink based on microporous photocrosslinkable acellular cartilage matrix. Bioact Mater. 2022;16:66-81
70. Nguyen D, Hagg DA, Forsman A, Ekholm J, Nimkingratana P, Brantsing C. et al. Cartilage Tissue Engineering by the 3D Bioprinting of iPS Cells in a Nanocellulose/Alginate Bioink. Sci Rep. 2017;7:658
71. Singh YP, Bandyopadhyay A, Mandal BB. 3D Bioprinting Using Cross-Linker-Free Silk-Gelatin Bioink for Cartilage Tissue Engineering. ACS Appl Mater Interfaces. 2019;11:33684-96
72. Li Z, Zhang X, Yuan T, Zhang Y, Luo C, Zhang J. et al. Addition of Platelet-Rich Plasma to Silk Fibroin Hydrogel Bioprinting for Cartilage Regeneration. Tissue Eng Part A. 2020;26:886-95
73. Deng C, Yang J, He H, Ma Z, Wang W, Zhang Y. et al. 3D bio-printed biphasic scaffolds with dual modification of silk fibroin for the integrated repair of osteochondral defects. Biomater Sci. 2021;9:4891-903
74. Jeong SI, Burns NA, Bonino CA, Kwon IK, Khan SA, Alsberg E. Improved cell infiltration of highly porous nanofibrous scaffolds formed by combined fiber-fiber charge repulsions and ultra-sonication. J Mater Chem B. 2014;2:8116-22
75. Chen Y, Shafiq M, Liu M, Morsi Y, Mo X. Advanced fabrication for electrospun three-dimensional nanofiber aerogels and scaffolds. Bioact Mater. 2020;5:963-79
76. Huang L, Huang J, Shao H, Hu X, Cao C, Fan S. et al. Silk scaffolds with gradient pore structure and improved cell infiltration performance. Mater Sci Eng C Mater Biol Appl. 2019;94:179-89
77. Garrigues NW, Little D, Sanchez-Adams J, Ruch DS, Guilak F. Electrospun cartilage-derived matrix scaffolds for cartilage tissue engineering. J Biomed Mater Res A. 2014;102:3998-4008
78. Kuo YC, Hung SC, Hsu SH. The effect of elastic biodegradable polyurethane electrospun nanofibers on the differentiation of mesenchymal stem cells. Colloids Surf B Biointerfaces. 2014;122:414-22
79. Liu J, Fang Q, Yu X, Wan Y, Xiao B. Chitosan-Based Nanofibrous Membrane Unit with Gradient Compositional and Structural Features for Mimicking Calcified Layer in Osteochondral Matrix. Int J Mol Sci. 2018 19
80. Chen Y, Xu W, Shafiq M, Tang J, Hao J, Xie X. et al. Three-dimensional porous gas-foamed electrospun nanofiber scaffold for cartilage regeneration. J Colloid Interface Sci. 2021;603:94-109
81. Zhang F, Zuo B, Fan Z, Xie Z, Lu Q, Zhang X. et al. Mechanisms and control of silk-based electrospinning. Biomacromolecules. 2012;13:798-804
82. M JC, Reardon PJ, Konwarh R, Knowles JC, Mandal BB. Mimicking Hierarchical Complexity of the Osteochondral Interface Using Electrospun Silk-Bioactive Glass Composites. ACS Appl Mater Interfaces. 2017;9:8000-13
83. Pot MW, Faraj KA, Adawy A, van Enckevort WJ, van Moerkerk HT, Vlieg E. et al. Versatile wedge-based system for the construction of unidirectional collagen scaffolds by directional freezing: practical and theoretical considerations. ACS Appl Mater Interfaces. 2015;7:8495-505
84. Arora A, Kothari A, Katti DS. Pore orientation mediated control of mechanical behavior of scaffolds and its application in cartilage-mimetic scaffold design. J Mech Behav Biomed Mater. 2015;51:169-83
85. Ren L, Zeng Y-P, Jiang D. Preparation of porous TiO2 by a novel freeze casting. Ceram Int. 2009;35:1267-70
86. Farhangdoust S, Zamanian A, Yasaei M, Khorami M. The effect of processing parameters and solid concentration on the mechanical and microstructural properties of freeze-casted macroporous hydroxyapatite scaffolds. Mater Sci Eng C Mater Biol Appl. 2013;33:453-60
87. Zhang Y, Hu L, Han J, Jiang Z. Freeze casting of aqueous alumina slurries with glycerol for porous ceramics. Ceram Int. 2010;36:617-21
88. Zhang W, Ling C, Li X, Sheng R, Liu H, Zhang A. et al. Cell-Free Biomimetic Scaffold with Cartilage Extracellular Matrix-Like Architectures for In situ Inductive Regeneration of Osteochondral Defects. ACS Biomater Sci Eng. 2020;6:6917-25
89. Kankala RK, Zhu K, Li J, Wang CS, Wang SB, Chen AZ. Fabrication of arbitrary 3D components in cardiac surgery: from macro-, micro- to nanoscale. Biofabrication. 2017;9:032002
90. Sun X, Wang J, Wang Y, Huang C, Yang C, Chen M. et al. Scaffold with Orientated Microtubule Structure Containing Polylysine-Heparin Sodium Nanoparticles for the Controlled Release of TGF-β1 in Cartilage Tissue Engineering. ACS Appl Bio Mater. 2018;1:2030-40
91. Lim J, You M, Li J, Li Z. Emerging bone tissue engineering via Polyhydroxyalkanoate (PHA)-based scaffolds. Mater Sci Eng C Mater Biol Appl. 2017;79:917-29
92. Kim UJ, Park J, Kim HJ, Wada M, Kaplan DL. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials. 2005;26:2775-85
93. Ribeiro VP, da Silva Morais A, Maia FR, Canadas RF, Costa JB, Oliveira AL. et al. Combinatory approach for developing silk fibroin scaffolds for cartilage regeneration. Acta Biomater. 2018;72:167-81
94. Zhou F, Zhang X, Cai D, Li J, Mu Q, Zhang W. et al. Silk fibroin-chondroitin sulfate scaffold with immuno-inhibition property for articular cartilage repair. Acta Biomater. 2017;63:64-75
95. Li GY, Zhou P, Shao ZZ, Xie X, Chen X, Wang HH. et al. The natural silk spinning process - A nucleation-dependent aggregation mechanism? Eur J Biochem. 2001;268:6600-6
96. Zhang W, Zhang Y, Zhang A, Ling C, Sheng R, Li X. et al. Enzymatically crosslinked silk-nanosilicate reinforced hydrogel with dual-lineage bioactivity for osteochondral tissue engineering. Mater Sci Eng C Mater Biol Appl. 2021;127:112215
97. Li Q, Xu S, Feng Q, Dai Q, Yao L, Zhang Y. et al. 3D printed silk-gelatin hydrogel scaffold with different porous structure and cell seeding strategy for cartilage regeneration. Bioact Mater. 2021;6:3396-410
98. Wang T, Li Y, Liu J, Fang Y, Guo W, Liu Y. et al. Intraarticularly injectable silk hydrogel microspheres with enhanced mechanical and structural stability to attenuate osteoarthritis. Biomaterials. 2022;286:121611
99. Piluso S, Flores Gomez D, Dokter I, Moreira Texeira L, Li Y, Leijten J. et al. Rapid and cytocompatible cell-laden silk hydrogel formation via riboflavin-mediated crosslinking. J Mater Chem B. 2020;8:9566-75
100. Farokhi M, Aleemardani M, Solouk A, Mirzadeh H, Teuschl AH, Redl H. Crosslinking strategies for silk fibroin hydrogels: promising biomedical materials. Biomed Mater. 2021;16:022004
101. Yuan T, Li Z, Zhang Y, Shen K, Zhang X, Xie R. et al. Injectable Ultrasonication-Induced Silk Fibroin Hydrogel for Cartilage Repair and Regeneration. Tissue Eng Part A. 2021;27:1213-24
102. Xue X, Hu Y, Wang S, Chen X, Jiang Y, Su J. Fabrication of physical and chemical crosslinked hydrogels for bone tissue engineering. Bioact Mater. 2022;12:327-39
103. Varaprasad K, Raghavendra GM, Jayaramudu T, Yallapu MM, Sadiku R. A mini review on hydrogels classification and recent developments in miscellaneous applications. Mater Sci Eng C Mater Biol Appl. 2017;79:958-71
104. Chen W, Zhou Z, Chen D, Li Y, Zhang Q, Su J. Bone Regeneration Using MMP-Cleavable Peptides-Based Hydrogels. Gels. 2021;7:199
105. Pan P, Chen X, Xing H, Deng Y, Chen J, Alharthi FA. et al. A fast on-demand preparation of injectable self-healing nanocomposite hydrogels for efficient osteoinduction. Chin Chem Lett. 2021;32:2159-63
106. Xue X, Hu Y, Deng Y, Su J. Recent Advances in Design of Functional Biocompatible Hydrogels for Bone Tissue Engineering. Adv Funct Mater. 2021;31:2009432
107. Guvendiren M, Burdick JA. Engineering synthetic hydrogel microenvironments to instruct stem cells. Curr Opin Biotechnol. 2013;24:841-6
108. Cui X, Soliman BG, Alcala-Orozco CR, Li J, Vis MAM, Santos M. et al. Rapid Photocrosslinking of Silk Hydrogels with High Cell Density and Enhanced Shape Fidelity. Adv Healthc Mater. 2020;9:e1901667
109. Koetting MC, Peters JT, Steichen SD, Peppas NA. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater Sci Eng R Rep. 2015;93:1-49
110. Li J, Mooney DJ. Designing hydrogels for controlled drug delivery. Nat Rev Mater. 2016;1:16071
111. Cui J, Li X, Wang S, Su Y, Chen X, Cao L. et al. Triptolide prevents bone loss via suppressing osteoclastogenesis through inhibiting PI3K-AKT-NFATc1 pathway. J Cell Mol Med. 2020;24:6149-61
112. Xu Z, Tang E, Zhao H. An Environmentally Sensitive Silk Fibroin/Chitosan Hydrogel and Its Drug Release Behaviors. Polymers (Basel). 2019;11:1980
113. Baghaei B, Jafari SH, Khonakdar HA, Wagenknecht U, Heinrich G. Novel thermosensitive hydrogel composites based on poly(d,l-lactide-co-glycolide) nanoparticles embedded in poly(n-isopropyl acrylamide) with sustained drug-release behavior. J Appl Polym Sci. 2014;131:40625
114. Jeong B, Kim SW, Bae YH. Thermosensitive sol-gel reversible hydrogels. Adv Drug Deliv Rev. 2012;64:154-62
115. Dong Y, Liu Y, Chen Y, Sun X, Zhang L, Zhang Z. et al. Spatiotemporal regulation of endogenous MSCs using a functional injectable hydrogel system for cartilage regeneration. NPG Asia Mater. 2021;13:71
116. Li Y, Chen M, Yan J, Zhou W, Gao S, Liu S. et al. Tannic acid/Sr(2+)-coated silk/graphene oxide-based meniscus scaffold with anti-inflammatory and anti-ROS functions for cartilage protection and delaying osteoarthritis. Acta Biomater. 2021;126:119-31
117. Gao E, Li G, Cao R, Xia H, Xu Y, Jiang G. et al. Bionic tracheal tissue regeneration using a ring-shaped scaffold comprised of decellularized cartilaginous matrix and silk fibroin. Compos B Eng. 2022;229:109470
118. Fang J, Wang D, Hu F, Li X, Zou X, Xie J. et al. Strontium mineralized silk fibroin porous microcarriers with enhanced osteogenesis as injectable bone tissue engineering vehicles. Mater Sci Eng C Mater Biol Appl. 2021;128:112354
119. Zhang W, Ling C, Zhang A, Liu H, Jiang Y, Li X. et al. An all-silk-derived functional nanosphere matrix for sequential biomolecule delivery and in situ osteochondral regeneration. Bioact Mater. 2020;5:832-43
120. Thorrez L, Shansky J, Wang L, Fast L, VandenDriessche T, Chuah M. et al. Growth, differentiation, transplantation and survival of human skeletal myofibers on biodegradable scaffolds. Biomaterials. 2008;29:75-84
121. Zou Y, Huang B, Cao L, Deng Y, Su J. Tailored Mesoporous Inorganic Biomaterials: Assembly, Functionalization, and Drug Delivery Engineering. Adv Mater. 2021;33:e2005215
122. Weng W, He S, Song H, Li X, Cao L, Hu Y. et al. Aligned Carbon Nanotubes Reduce Hypertrophic Scar via Regulating Cell Behavior. ACS Nano. 2018;12:7601-12
123. Metavarayuth K, Maturavongsadit P, Chen X, Sitasuwan P, Lu L, Su J. et al. Nanotopographical Cues Mediate Osteogenesis of Stem Cells on Virus Substrates through BMP-2 Intermediate. Nano Lett. 2019;19:8372-80
124. Zhou L, Gjvm VO, Malda J, Stoddart MJ, Lai Y, Richards RG. et al. Innovative Tissue-Engineered Strategies for Osteochondral Defect Repair and Regeneration: Current Progress and Challenges. Adv Healthc Mater. 2020: e2001008.
125. Nie W, Peng C, Zhou X, Chen L, Wang W, Zhang Y. et al. Three-dimensional porous scaffold by self-assembly of reduced graphene oxide and nano-hydroxyapatite composites for bone tissue engineering. Carbon. 2017;116:325-37
126. Li B, Huang R, Ye J, Liu L, Qin L, Zhou J. et al. A self-healing coating containing curcumin for osteoimmunomodulation to ameliorate osseointegration. Chem Eng J. 2021;403:126323
127. Wenhao Z, Zhang T, Yan J, Li Q, Xiong P, Li Y. et al. In vitro and in vivo evaluation of structurally-controlled silk fibroin coatings for orthopedic infection and in-situ osteogenesis. Acta Biomater. 2020;116:223-45
128. Yang S, Qian Z, Liu D, Wen N, Xu J, Guo X. Integration of C-type natriuretic peptide gene-modified bone marrow mesenchymal stem cells with chitosan/silk fibroin scaffolds as a promising strategy for articular cartilage regeneration. Cell Tissue Bank. 2019;20:209-20
129. Ran J, Fei Y, Wang C, Ruan D, Hu Y, Zheng Z. et al. An Off-the-Shelf Tissue Engineered Cartilage Composed of Optimally Sized Pellets of Cartilage Progenitor/Stem Cells. ACS Biomater Sci Eng. 2021;7:881-92
130. Zhou Z, Wu W, Fang J, Yin J. Polymer-based porous microcarriers as cell delivery systems for applications in bone and cartilage tissue engineering. Int Mater Rev. 2020;66:77-113
131. Pan P, Yue Q, Yang X, Ren Y, Alharthi FA, Alghamdi A. et al. Structure Engineering of Yolk-Shell Magnetic Mesoporous Silica Microspheres with Broccoli-Like Morphology for Efficient Catalysis and Enhanced Cellular Uptake. Small. 2021;17:e2006925
132. Kankala RK, Zhao J, Liu CG, Song XJ, Yang DY, Zhu K. et al. Highly Porous Microcarriers for Minimally Invasive In situ Skeletal Muscle Cell Delivery. Small. 2019;15:e1901397
133. Liao S, Meng H, Li J, Zhao J, Xu Y, Wang A. et al. Potential and recent advances of microcarriers in repairing cartilage defects. J Orthop Translat. 2021;27:101-9
134. Fang J, Zhang Y, Yan S, Liu Z, He S, Cui L. et al. Poly(L-glutamic acid)/chitosan polyelectrolyte complex porous microspheres as cell microcarriers for cartilage regeneration. Acta Biomater. 2014;10:276-88
135. Galuzzi M, Perteghella S, Antonioli B, Tosca MC, Bari E, Tripodo G. et al. Human Engineered Cartilage and Decellularized Matrix as an Alternative to Animal Osteoarthritis Model. Polymers (Basel). 2018;10:738
136. Wang X, Yucel T, Lu Q, Hu X, Kaplan DL. Silk nanospheres and microspheres from silk/pva blend films for drug delivery. Biomaterials. 2010;31:1025-35
137. He JM, Zhu PF, Li LH, Wang Z, Li XL, Wang S. et al. Silk fibroin/chitosan/TGF-beta1-loaded microsphere scaffolds for cartilage reparation. Biomed Mater Eng. 2021;32:347-58
138. Jia S, Liu L, Pan W, Meng G, Duan C, Zhang L. et al. Oriented cartilage extracellular matrix-derived scaffold for cartilage tissue engineering. J Biosci Bioeng. 2012;113:647-53
139. Shen H, Niu Y, Hu X, Yang F, Wang S, Wu D. A biomimetic 3D microtubule-orientated poly(lactide-co-glycolide) scaffold with interconnected pores for tissue engineering. J Mater Chem B. 2015;3:4417-25
140. Yang Q, Teng BH, Wang LN, Li K, Xu C, Ma XL. et al. Silk fibroin/cartilage extracellular matrix scaffolds with sequential delivery of TGF-beta3 for chondrogenic differentiation of adipose-derived stem cells. Int J Nanomedicine. 2017;12:6721-33
141. Chen J, Zhuang A, Shao H, Hu X, Zhang Y. Robust silk fibroin/bacterial cellulose nanoribbon composite scaffolds with radial lamellae and intercalation structure for bone regeneration. J Mater Chem B. 2017;5:3640-50
142. Feng X, Xu P, Shen T, Zhang Y, Ye J, Gao C. Influence of pore architectures of silk fibroin/collagen composite scaffolds on the regeneration of osteochondral defects in vivo. J Mater Chem B. 2020;8:391-405
143. Ribeiro VP, Pina S, Costa JB, Cengiz IF, Garcia-Fernandez L, Fernandez-Gutierrez MDM. et al. Enzymatically Cross-Linked Silk Fibroin-Based Hierarchical Scaffolds for Osteochondral Regeneration. ACS Appl Mater Interfaces. 2019;11:3781-99
144. Wu X, Zhou M, Jiang F, Yin S, Lin S, Yang G. et al. Marginal sealing around integral bilayer scaffolds for repairing osteochondral defects based on photocurable silk hydrogels. Bioact Mater. 2021;6:3976-86
145. Ding X, Zhu M, Xu B, Zhang J, Zhao Y, Ji S. et al. Integrated trilayered silk fibroin scaffold for osteochondral differentiation of adipose-derived stem cells. ACS Appl Mater Interfaces. 2014;6:16696-705
146. Guo J, Li C, Ling S, Huang W, Chen Y, Kaplan DL. Multiscale design and synthesis of biomimetic gradient protein/biosilica composites for interfacial tissue engineering. Biomaterials. 2017;145:44-55
147. Xu G, Ding Z, Lu Q, Zhang X, Zhou X, Xiao L. et al. Electric field-driven building blocks for introducing multiple gradients to hydrogels. Protein Cell. 2020;11:267-85
148. Yang T, Tamaddon M, Jiang L, Wang J, Liu Z, Liu Z. et al. Bilayered scaffold with 3D printed stiff subchondral bony compartment to provide constant mechanical support for long-term cartilage regeneration. J Orthop Translat. 2021;30:112-21
149. Cengiz IF, Oliveira JM, Reis RL. Tissue Engineering and Regenerative Medicine Strategies for the Treatment of Osteochondral Lesions. 3D Multiscale Physiological Human. 2014 p. 25-47
150. Nooeaid P, Salih V, Beier JP, Boccaccini AR. Osteochondral tissue engineering: scaffolds, stem cells and applications. J Cell Mol Med. 2012;16:2247-70
151. Shimomura K, Moriguchi Y, Murawski CD, Yoshikawa H, Nakamura N. Osteochondral tissue engineering with biphasic scaffold: current strategies and techniques. Tissue Eng Part B Rev. 2014;20:468-76
152. Nukavarapu SP, Dorcemus DL. Osteochondral tissue engineering: current strategies and challenges. Biotechnol Adv. 2013;31:706-21
153. Kon E, Delcogliano M, Filardo G, Busacca M, Di Martino A, Marcacci M. Novel nano-composite multilayered biomaterial for osteochondral regeneration: a pilot clinical trial. Am J Sports Med. 2011;39:1180-90
154. Dormer NH, Berkland CJ, Detamore MS. Emerging techniques in stratified designs and continuous gradients for tissue engineering of interfaces. Ann Biomed Eng. 2010;38:2121-41
155. Hoemann CD, Lafantaisie-Favreau CH, Lascau-Coman V, Chen G, Guzman-Morales J. The cartilage-bone interface. J Knee Surg. 2012;25:85-97
156. Jeon JE, Vaquette C, Klein TJ, Hutmacher DW. Perspectives in multiphasic osteochondral tissue engineering. Anat Rec (Hoboken). 2014;297:26-35
157. Nooeaid P, Roether JA, Weber E, Schubert DW, Boccaccini AR. Technologies for Multilayered Scaffolds Suitable for Interface Tissue Engineering. Adv Eng Mater. 2014;16:319-27
158. Di Donato V, De Santis F, Albadri S, Auer TO, Duroure K, Charpentier M. et al. An Attractive Reelin Gradient Establishes Synaptic Lamination in the Vertebrate Visual System. Neuron. 2018;97:1049-62 e6
159. Li C, Armstrong JP, Pence IJ, Kit-Anan W, Puetzer JL, Correia Carreira S. et al. Glycosylated superparamagnetic nanoparticle gradients for osteochondral tissue engineering. Biomaterials. 2018;176:24-33
160. Radhakrishnan J, Manigandan A, Chinnaswamy P, Subramanian A, Sethuraman S. Gradient nano-engineered in situ forming composite hydrogel for osteochondral regeneration. Biomaterials. 2018;162:82-98
161. Wu T, Xue J, Li H, Zhu C, Mo X, Xia Y. General Method for Generating Circular Gradients of Active Proteins on Nanofiber Scaffolds Sought for Wound Closure and Related Applications. ACS Appl Mater Interfaces. 2018;10:8536-45
162. Guo X, Wang C, Duan C, Descamps M, Zhao Q, Dong L. et al. Repair of osteochondral defects with autologous chondrocytes seeded onto bioceramic scaffold in sheep. Tissue Eng. 2004;10:1830-40
163. Wang X, Grogan SP, Rieser F, Winkelmann V, Maquet V, Berge ML. et al. Tissue engineering of biphasic cartilage constructs using various biodegradable scaffolds: an in vitro study. Biomaterials. 2004;25:3681-8
164. Naskar D, Ghosh AK, Mandal M, Das P, Nandi SK, Kundu SC. Dual growth factor loaded nonmulberry silk fibroin/carbon nanofiber composite 3D scaffolds for in vitro and in vivo bone regeneration. Biomaterials. 2017;136:67-85
165. Hubka KM, Carson DD, Harrington DA, Farach-Carson MC. Perlecan domain I gradients establish stable biomimetic heparin binding growth factor gradients for cell migration in hydrogels. Acta Biomater. 2019;97:385-98
166. Xiao H, Huang W, Xiong K, Ruan S, Yuan C, Mo G. et al. Osteochondral repair using scaffolds with gradient pore sizes constructed with silk fibroin, chitosan, and nano-hydroxyapatite. Int J Nanomedicine. 2019;14:2011-27
167. Oh SH, An DB, Kim TH, Lee JH. Wide-range stiffness gradient PVA/HA hydrogel to investigate stem cell differentiation behavior. Acta Biomater. 2016;35:23-31
168. Ribeiro VP, Pina S, Gheduzzi S, Araújo AC, Reis RL, Oliveira JM. Hierarchical HRP-Crosslinked Silk Fibroin/ZnSr-TCP Scaffolds for Osteochondral Tissue Regeneration: Assessment of the Mechanical and Antibacterial Properties. Front Mater. 2020;7:49
169. Bachmann B, Spitz S, Schadl B, Teuschl AH, Redl H, Nurnberger S. et al. Stiffness Matters: Fine-Tuned Hydrogel Elasticity Alters Chondrogenic Redifferentiation. Front Bioeng Biotechnol. 2020;8:373
170. Chameettachal S, Murab S, Vaid R, Midha S, Ghosh S. Effect of visco-elastic silk-chitosan microcomposite scaffolds on matrix deposition and biomechanical functionality for cartilage tissue engineering. J Tissue Eng Regen Med. 2017;11:1212-29
171. Mirmusavi MH, Zadehnajar P, Semnani D, Karbasi S, Fekrat F, Heidari F. Evaluation of physical, mechanical and biological properties of poly 3-hydroxybutyrate-chitosan-multiwalled carbon nanotube/silk nano-micro composite scaffold for cartilage tissue engineering applications. Int J Biol Macromol. 2019;132:822-35
172. Sawatjui N, Limpaiboon T, Schrobback K, Klein T. Biomimetic scaffolds and dynamic compression enhance the properties of chondrocyte- and MSC-based tissue-engineered cartilage. J Tissue Eng Regen Med. 2018;12:1220-9
173. Huang X, Zhang M, Ming J, Ning X, Bai S. High-Strength and High-Toughness Silk Fibroin Hydrogels: A Strategy Using Dynamic Host-Guest Interactions. ACS Appl Bio Mater. 2020;3:7103-12
174. Jin HJ, Park J, Karageorgiou V, Kim UJ, Valluzzi R, Kaplan DL. Water-stable silk films with reduced beta-sheet content. Adv Funct Mater. 2005;15:1241-7
175. Pritchard EM, Valentin T, Boison D, Kaplan DL. Incorporation of proteinase inhibitors into silk-based delivery devices for enhanced control of degradation and drug release. Biomaterials. 2011;32:909-18
176. Huang GP, Yang DF, Sun CF, Huang JP, Chen KP, Zhang CX. et al. A quicker degradation rate is yielded by a novel kind of transgenic silk fibroin consisting of shortened silk fibroin heavy chains fused with matrix metalloproteinase cleavage sites. J Mater Sci-Mater M. 2014;25:1833-42
177. Cheng G, Dai J, Dai J, Wang H, Chen S, liu Y. et al. Extracellular matrix imitation utilizing nanofibers-embedded biomimetic scaffolds for facilitating cartilage regeneration. Chem Eng J. 2021;410:128379
178. Li Z, Wu N, Cheng J, Sun M, Yang P, Zhao F. et al. Biomechanically, structurally and functionally meticulously tailored polycaprolactone/silk fibroin scaffold for meniscus regeneration. Theranostics. 2020;10:5090-106
179. Chen Z, Zhang Q, Li H, Wei Q, Zhao X, Chen F. Elastin-like polypeptide modified silk fibroin porous scaffold promotes osteochondral repair. Bioact Mater. 2021;6:589-601
180. Zhang FX, Liu P, Ding W, Meng QB, Su DH, Zhang QC. et al. Injectable Mussel-Inspired highly adhesive hydrogel with exosomes for endogenous cell recruitment and cartilage defect regeneration. Biomaterials. 2021;278:121169
181. Hu Y, Wu B, Xiong Y, Tao R, Panayi AC, Chen L. et al. Cryogenic 3D printed hydrogel scaffolds loading exosomes accelerate diabetic wound healing. Chem Eng J. 2021;426:130634
182. Wang X, Song X, Li T, Chen J, Cheng G, Yang L. et al. Aptamer-Functionalized Bioscaffold Enhances Cartilage Repair by Improving Stem Cell Recruitment in Osteochondral Defects of Rabbit Knees. Am J Sports Med. 2019;47:2316-26
183. Wu T, Chen Y, Liu W, Tong KL, Suen CW, Huang S. et al. Ginsenoside Rb1/TGF-beta1 loaded biodegradable silk fibroin-gelatin porous scaffolds for inflammation inhibition and cartilage regeneration. Mater Sci Eng C Mater Biol Appl. 2020;111:110757
184. Chen Y, Wu T, Huang S, Suen CW, Cheng X, Li J. et al. Sustained Release SDF-1alpha/TGF-beta1-Loaded Silk Fibroin-Porous Gelatin Scaffold Promotes Cartilage Repair. ACS Appl Mater Interfaces. 2019;11:14608-18
185. Hu Y, Li X, Zhang Q, Gu Z, Luo Y, Guo J. et al. Exosome-guided bone targeted delivery of Antagomir-188 as an anabolic therapy for bone loss. Bioact Mater. 2021;6:2905-13
186. Song H, Li X, Zhao Z, Qian J, Wang Y, Cui J. et al. Reversal of Osteoporotic Activity by Endothelial Cell-Secreted Bone Targeting and Biocompatible Exosomes. Nano Lett. 2019;19:3040-8
187. Liu H, Zhang Q, Wang S, Weng W, Jing Y, Su J. Bacterial extracellular vesicles as bioactive nanocarriers for drug delivery: Advances and perspectives. Bioact Mater. 2021;14:169-181
188. Jiang C, Fu Y, Liu G, Shu B, Davis J, Tofaris GK. Multiplexed Profiling of Extracellular Vesicles for Biomarker Development. Nanomicro Lett. 2021;14:3
189. Xu Z, Shi L, Yang M, Zhu L. Preparation and biomedical applications of silk fibroin-nanoparticles composites with enhanced properties - A review. Mater Sci Eng C Mater Biol Appl. 2019;95:302-11
190. Xue X, Zhang H, Liu H, Wang S, Li J, Zhou Q. et al. Rational Design of Multifunctional CuS Nanoparticle-PEG Composite Soft Hydrogel-Coated 3D Hard Polycaprolactone Scaffolds for Efficient Bone Regeneration. Adv Funct Mater. 2022 2202470
191. Li Y, Liu Y, Guo Q. Silk fibroin hydrogel scaffolds incorporated with chitosan nanoparticles repair articular cartilage defects by regulating TGF-beta1 and BMP-2. Arthritis Res Ther. 2021;23:50
192. Min Q, Liu J, Zhang Y, Yang B, Wan Y, Wu J. Dual Network Hydrogels Incorporated with Bone Morphogenic Protein-7-Loaded Hyaluronic Acid Complex Nanoparticles for Inducing Chondrogenic Differentiation of Synovium-Derived Mesenchymal Stem Cells. Pharmaceutics. 2020;12:613
193. Kundu B, Kurland NE, Bano S, Patra C, Engel FB, Yadavalli VK. et al. Silk proteins for biomedical applications: Bioengineering perspectives. Prog Polym Sci. 2014;39:251-67
194. Kambe Y, Yamamoto K, Kojima K, Tamada Y, Tomita N. Effects of RGDS sequence genetically interfused in the silk fibroin light chain protein on chondrocyte adhesion and cartilage synthesis. Biomaterials. 2010;31:7503-11
195. Raj ANJ, Sundaram R, Mahesh VGV, Zhuang ZM, Simeone A. A Multi-Sensor System for Silkworm Cocoon Gender Classification via Image Processing and Support Vector Machine. Sensors (Basel). 2019;19:2656
196. Wang Q, Ling S, Liang X, Wang H, Lu H, Zhang Y. Self-Healable Multifunctional Electronic Tattoos Based on Silk and Graphene. Adv Funct Mater. 2019;29:1808695
197. Wang Y, Boero G, Zhang X, Brugger J. Thermal and pH Sensitive Composite Membrane for On-Demand Drug Delivery by Applying an Alternating Magnetic Field. Adv Mater Interfaces. 2020;7:2000733
198. Chen S, Chen X, Geng Z, Su J. The horizon of bone organoid: A perspective on construction and application. Bioact Mater. 2022;18:15-25
Author contact
![]() Corresponding authors: Zhen Geng, nanboshan1987com; Jiacan Su, drsujiacancom.
Corresponding authors: Zhen Geng, nanboshan1987com; Jiacan Su, drsujiacancom.
 Global reach, higher impact
Global reach, higher impact