13.3
Impact Factor
Theranostics 2022; 12(11):4834-4850. doi:10.7150/thno.68966 This issue Cite
Research Paper
A multimodal imaging workflow for monitoring CAR T cell therapy against solid tumor from whole-body to single-cell level
1. Miltenyi Biotec B.V. & Co. KG, R&D Reagents, Bergisch Gladbach, North Rhine-Westphalia, Germany.
2. University Medical Center Göttingen, Translational Molecular Imaging, Institute for Diagnostic and Interventional Radiology & Clinic for Haematology and Medical Oncology, Göttingen, Lower Saxony, Germany.
3. Institute of Medical Statistics and Computational Biology, University of Cologne, Cologne, North Rhine-Westphalia, Germany.
4. Gremse-IT GmbH, Aachen, North Rhine-Westphalia, Germany.
5. Max-Planck-Institute for Multidisciplinary Science, Translational Molecular Imaging, Göttingen, Lower Saxony, Germany.
6. Ossium Health Inc, Indianapolis, Indiana, United States of America.
*Shared first authorship.
Abstract
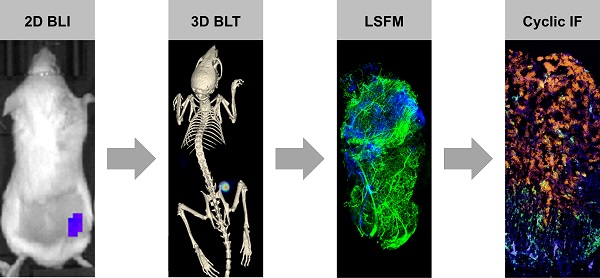
CAR T cell research in solid tumors often lacks spatiotemporal information and therefore, there is a need for a molecular tomography to facilitate high-throughput preclinical monitoring of CAR T cells. Furthermore, a gap exists between macro- and microlevel imaging data to better assess intratumor infiltration of therapeutic cells. We addressed this challenge by combining 3D µComputer tomography bioluminescence tomography (µCT/BLT), light-sheet fluorescence microscopy (LSFM) and cyclic immunofluorescence (IF) staining.
Methods: NSG mice with subcutaneous AsPC1 xenograft tumors were treated with EGFR CAR T cell (± IL-2) or control BDCA-2 CAR T cell (± IL-2) (n = 7 each). Therapeutic T cells were genetically modified to co-express the CAR of interest and the luciferase CBR2opt. IL-2 was administered s.c. under the xenograft tumor on days 1, 3, 5 and 7 post-therapy-initiation at a dose of 25,000 IU/mouse. CAR T cell distribution was measured in 2D BLI and 3D µCT/BLT every 3-4 days. On day 6, 4 tumors were excised for cyclic IF where tumor sections were stained with a panel of 25 antibodies. On day 6 and 13, 8 tumors were excised from rhodamine lectin-preinjected mice, permeabilized, stained for CD3 and imaged by LSFM.
Results: 3D µCT/BLT revealed that CAR T cells pharmacokinetics is affected by antigen recognition, where CAR T cell tumor accumulation based on target-dependent infiltration was significantly increased in comparison to target-independent infiltration, and spleen accumulation was delayed. LSFM supported these findings and revealed higher T cell accumulation in target-positive groups at day 6, which also infiltrated the tumor deeper. Interestingly, LSFM showed that most CAR T cells accumulate at the tumor periphery and around vessels. Surprisingly, LSFM and cyclic IF revealed that local IL-2 application resulted in early-phase increased proliferation, but long-term overstimulation of CAR T cells, which halted the early added therapeutic effect.
Conclusion: Overall, we demonstrated that 3D µCT/BLT is a valuable non-isotope-based technology for whole-body cell therapy monitoring and investigating CAR T cell pharmacokinetics. We also presented combining LSFM and MICS for ex vivo 3D- and 2D-microscopy tissue analysis to assess intratumoral therapeutic cell distribution and status.
Keywords: CAR T Cells, Cell Tracking, Optical tomography, 3D µCT/BLT, Light-Sheet Fluorescence microscopy
 Global reach, higher impact
Global reach, higher impact