13.3
Impact Factor
Theranostics 2022; 12(10):4818-4833. doi:10.7150/thno.73181 This issue Cite
Research Paper
Dual-sensitive antibacterial peptide nanoparticles prevent dental caries
1. The National and Local Joint Engineering Laboratory of Animal Peptide Drug Development, College of Life Sciences, Hunan Normal University, Changsha, Hunan, China.
2. Key Laboratory of Functional Polymer Materials, Ministry of Education, College of Chemistry, Nankai University, Tianjin, Tianjin, China.
3. Department of Periodontology and Pediatric Dentistry, Changsha Stomatological Hospital, School of Dental Medicine, Hunan University of Chinese Medicine Changsha, Hunan, China.
4. The National and Local Joint Engineering Laboratory for New Petrochemical Materials and Fine Utilization of Resources, College of Chemistry and Chemical Engineering, Hunan Normal University, Changsha, Hunan, China.
#These authors contributed equally to this paper.
Abstract
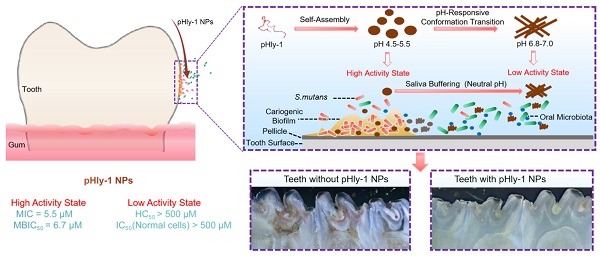
Background: Dental caries is the most prevalent bacterial biofilm-induced disease. Current clinical prevention and treatment agents often suffer from adverse effects on oral microbiota diversity and normal tissues, predominately arising from the poor biofilm-targeting property of the agents.
Methods: To address this concern, we herein report dual-sensitive antibacterial peptide nanoparticles pHly-1 NPs upon acid and lipid-binding for treatment of dental caries. Amino acid substitutions were performed to design the peptide pHly-1. The potential, morphology and secondary structure of pHly-1 were characterized to elucidate the mechanisms of its pH and lipid sensitivity. Bacterial membrane integrity assay and RNA-seq were applied to uncover the antimicrobial mechanism of peptides under acidic condition. The in vitro and ex vivo antibiofilm assays were used to determine the antibiofilm performance of pHly-1 NPs. We also carried out the in vivo anti-caries treatment by pHly-1 NPs on dental caries animal model. Oral microbiome and histopathological analyses were performed to assess the in vivo safety of pHly-1 NPs.
Results: The pHly-1 peptide underwent the coil-helix conformational transition upon binding to bacterial membranes in the acidic cariogenic biofilm microenvironment, thereby killing cariogenic bacteria. Under normal physiological conditions, pHly-1 adopted a β-sheet conformation and formed nanofibers, resulting in negligible cytotoxicity towards oral microbes. However, in acidic solution, pHly-1 NPs displayed reliable antibacterial activity against Streptococcus mutans, including standard and clinically isolated strains, mainly via cell membrane disruption, and also suppressed in vitro and human-derived ex vivo biofilm development. Compared to the clinical agent chlorhexidine, in vivo topical treatment with pHly-1 NPs showed an advanced effect on inhibiting rat dental caries development without adverse effects on oral microbiota diversity and normal oral or gastric tissues.
Conclusion: Our results demonstrated the high efficacy of dual-sensitive antimicrobial peptides for the selective damage of bacterial biofilms, providing an efficient strategy for preventing and treating dental caries.
Keywords: antimicrobial peptides, stimuli-responsive, conformational transition, nanoparticles, biofilms, dental caries
 Global reach, higher impact
Global reach, higher impact