13.3
Impact Factor
Theranostics 2022; 12(10):4791-4801. doi:10.7150/thno.69168 This issue Cite
Research Paper
Ultrasound-directed enzyme-prodrug therapy (UDEPT) using self-immolative doxorubicin derivatives
1. Department of Nanomedicine and Theranostics, Institute for Experimental Molecular Imaging, Uniklinik RWTH Aachen and Helmholtz Institute for Biomedical Engineering, Faculty of Medicine, RWTH Aachen University, 52074 Aachen, Germany.
2. Institute of Pathology, Uniklinik RWTH Aachen, Faculty of Medicine, RWTH Aachen University, 52074 Aachen, Germany.
3. Division of Imaging and Oncology, University Medical Center Utrecht, 3584 CX Utrecht, the Netherlands.
4. Department of Pharmaceutics, Utrecht University, Utrecht, 3584 CG, The Netherlands.
5. Laboratory of Drug and Gene Delivery Research, Faculty of Pharma-Science, Teikyo University, Tokyo, Japan.
6. Department of Targeted Therapeutics, University of Twente, Enschede, The Netherlands.
7. Department of Surgery, Yong Loo Lin School of Medicine, National University of Singapore, 119074 Singapore, Singapore.
8. State Key Laboratory of Drug Research & Center of Pharmaceutics, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, 201203, China.
*These authors contributed equally to this work.
Abstract
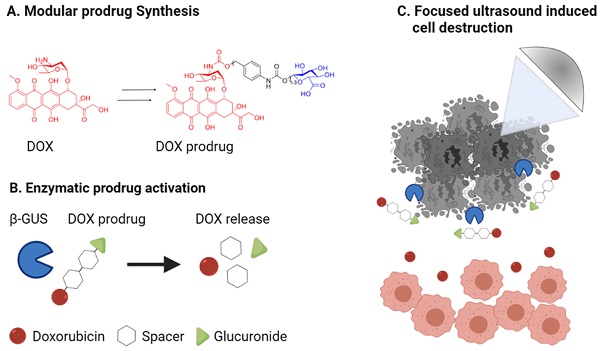
Background: Enzyme-activatable prodrugs are extensively employed in oncology and beyond. Because enzyme concentrations and their (sub)cellular compartmentalization are highly heterogeneous in different tumor types and patients, we propose ultrasound-directed enzyme-prodrug therapy (UDEPT) as a means to increase enzyme access and availability for prodrug activation locally.
Methods: We synthesized β-glucuronidase-sensitive self-immolative doxorubicin prodrugs with different spacer lengths between the active drug moiety and the capping group. We evaluated drug conversion, uptake and cytotoxicity in the presence and absence of the activating enzyme β-glucuronidase. To trigger the cell release of β-glucuronidase, we used high-intensity focused ultrasound to aid in the conversion of the prodrugs into their active counterparts.
Results: More efficient enzymatic activation was observed for self-immolative prodrugs with more than one aromatic unit in the spacer. In the absence of β-glucuronidase, the prodrugs showed significantly reduced cellular uptake and cytotoxicity compared to the parent drug. High-intensity focused ultrasound-induced mechanical destruction of cancer cells resulted in release of intact β-glucuronidase, which activated the prodrugs, restored their cytotoxicity and induced immunogenic cell death.
Conclusion: These findings shed new light on prodrug design and activation, and they contribute to novel UDEPT-based mechanochemical combination therapies for the treatment of cancer.
Keywords: Cancer, Prodrugs, Focused ultrasound, HIFU, β-glucuronidase
 Global reach, higher impact
Global reach, higher impact