13.3
Impact Factor
Theranostics 2022; 12(10):4734-4752. doi:10.7150/thno.69682 This issue Cite
Review
Challenges and opportunities to penetrate the blood-brain barrier for brain cancer therapy
1. Children's Cancer Institute, Lowy Cancer Research Centre, UNSW Sydney, Sydney, NSW, 2031, Australia.
2. School of Women's and Children's Health, Faculty of Medicine and Health, UNSW Sydney, Sydney, NSW, 2052, Australia.
3. Australian Centre for Nanomedicine, UNSW Sydney, Sydney, NSW 2052, Australia.
4. Kids Cancer Centre, Sydney Children's Hospital, Randwick, NSW 2031, Australia.
*Equal authorship.
Received 2021-12-2; Accepted 2022-5-10; Published 2022-6-6
Abstract
Despite significant advances in research, the prognosis for both primary and secondary brain cancers remains poor. The blood-brain barrier (BBB) is a complex and unique semi-permeable membrane that serves as a protective structure to maintain homeostasis within the brain. However, it presents a significant challenge for the delivery of therapeutics into the brain and tumor. Some brain tumors are known to compromise BBB integrity, producing a highly heterogeneous vasculature known as the blood-tumor-barrier (BTB). Identifying strategies to bypass these obstacles to improve the penetrability of anticancer therapeutics has been the focus of research in this area. In this review, we discuss the strategies that have been investigated to evade or alter the cellular and molecular barriers of both the BBB and the BTB and detail the methods currently under preclinical or clinical investigation, including molecular, biological, and physical processes to overcome the BBB or BTB. Increased understanding of the BBB and BTB and the current methods of overcoming these barriers will enable the development of new and more effective treatment strategies for brain tumors.
Keywords: brain tumors, CNS malignancies, blood brain barrier, drug delivery systems, BBB disruption
Introduction
The Blood-Brain barrier
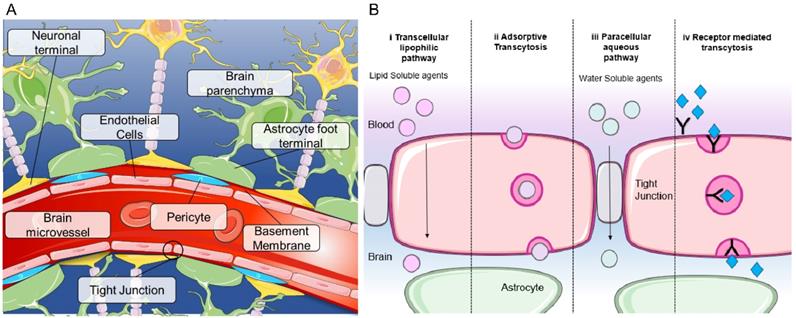
The blood-brain barrier (BBB) is a complex and unique semi-permeable membrane that serves as a protective structure to maintain homeostasis within the brain [1]. Composed of around a hundred billion capillaries, the BBB spans 20 m2 in the human brain and is approximately 600 kilometers long. Each capillary is approximately 7.5 μm in diameter, allowing for blood supply within 10μm of each brain cell [2]. This selective physical barrier prevents the passage of molecules greater than 400 Daltons from the bloodstream into the brain, thereby protecting the microenvironment of the brain from potentially harmful exogenous substances [1]. The delivery of oxygen into the brain and removal of carbon dioxide and metabolites is regulated by the BBB. The BBB cerebrovasculature is composed of a specialized endothelial cell monolayer supported by pericytes and astrocytes creating a tight interconnectivity and a unique barrier not replicated in any other organ (Figure 1A). Vascular pericytes surround 30% of the endothelial layer, controlling the vessels' diameter and regulating endothelial cell BBB-specific gene expression [2, 3]. Perivascular astrocytic end-feet attached to the external wall of the endothelium upregulate BBB properties and strengthen tight junctions [4]. Tight junctions between adjacent vascular endothelial cells function to restrict paracellular movement and favor transcellular movement [1] (Figure 1B). Small, lipophilic molecules diffuse passively into the brain, whereas larger hydrophilic molecules such as peptides or proteins require transport mechanisms (Figure 1B) [5]. The continuity of the tight junctions, coupled with lack of fenestrae and efflux transporters, results in the BBB with distinct luminal and abluminal compartments for strict regulation and control between the blood and the brain [5].
The expression of transport proteins and efflux transporters in the BBB blocks the efficacy of therapies used to treat brain tumors. Molecular size and lipophilicity determine each therapeutic's ability to enter the brain. Moreover, once in the brain, the drug must remain active and at pharmacologically therapeutic concentrations. It is known that 100% of large molecule therapeutics and 98% of all small molecule therapeutics are excluded from the brain by the BBB [6]. Hence bypassing the BBB to improve drug penetration and delivery is important for treating brain malignancies and metastatic extracranial tumors.
The Blood-tumor barrier
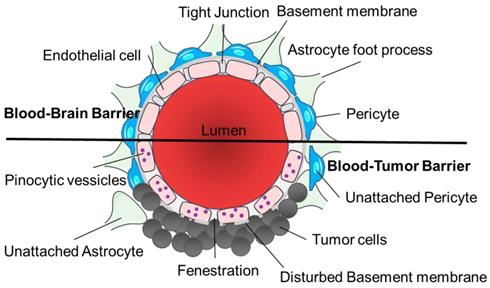
Tumors are known to compromise BBB integrity, producing a highly heterogeneous vasculature known as the blood-tumor barrier (BTB). The BTB is formed by brain tumor capillaries and is distinct from the BBB, characterized by non-uniform permeability and active efflux of molecules. The BTB encompasses both existing and newly formed blood vessels that deliver nutrients and oxygen to the tumor and facilitate cell migration to other parts of the brain and is considered in many cases to be 'leakier' than the BBB [7]. The BBB/BTB structural integrity is heterogenous between metastatic lesions and different primary tumor types. This leads to heterogeneous permeability, and due to these “leakier” and semi-dysfunctional vessels, water and metabolic waste are retained in the neuro-parenchymal space, increasing the interstitial and intracranial fluid pressure.
The BTB has three distinct microvessel populations; continuous capillaries, fenestrated capillaries, and capillaries containing inter-endothelial gaps (Figure 2). Abnormal pericyte distribution, loss of astrocytic end-feet and neuronal connections are characteristic of the BTB (Figure 2) [7]. Tumor cells can dislodge astrocytic end-feet, thereby disrupting BBB integrity [7]. Circulating immune cells such as T-cell subpopulations and peripheral monocytes are detected in brain tumors, demonstrating the permeability of the neurovascular unit to these cell types. BTB endothelial cells have reduced junctional proteins and the intra-tumoral vasculature never fully re-establishes a normal BBB in brain metastases [7]. Both the BBB and BTB have drug efflux transporters expressed in endothelial cells in common and ATP-binding cassette (ABC) transporters can be expressed in some tumor cells [8]. Brain tumor capillaries can also overexpress receptors that mediate ligand-dependent drug delivery, which has previously been exploited to enhance drug delivery directly to tumor tissues [9].
Drug delivery across the blood-brain barrier and blood-tumor barrier
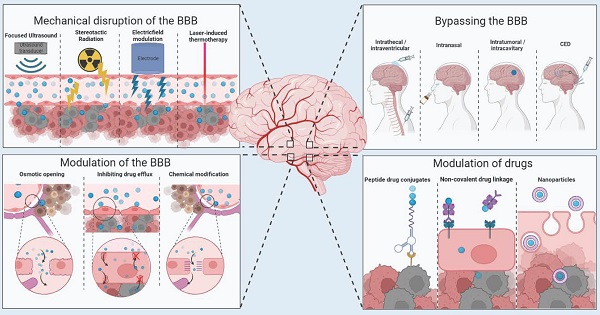
Numerous strategies have now been investigated to either evade or alter the cellular and molecular barriers of both the BBB and BTB. These methods can be sub-categorized into methods of drug modulation, methods of BBB/BTB modulation, mechanical disruption of the BBB/BTB and complete BBB/BTB bypass methods. Strategies to bypass or hijack the BBB are currently being established or optimized. In this review, we discuss all methods currently used in the clinic or under preclinical or clinical investigation, including molecular, biological, and physical methods to overcome the BBB.
Blood brain barrier structure and mechanisms of transport (A) Structure of the blood brain barrier depicting brain microvessels composed of: pericytes, endothelial cells, astrocytes and neurons. (B) Mechanisms of transport across the BBB including (i) transcellular lipophilic pathway, (ii) adsorptive transcytosis, (iii) paracellular aqueous pathway and (iv) receptor mediated transcytosis. Image was created with BioRender.
Structural differences between the blood-brain barrier and blood-tumor barrier. The architecture of the blood-brain barrier includes a non-fenestrated endothelial cell monolayer with tight junctions, contact with astrocyte foot processes and pericytes and a functional basement membrane. Tumor cells disrupt the normal vasculature to form the blood-tumor barrier causing fenestrations in the endothelial cells, disturbed basement membrane and unattached astrocytes and pericytes, with increased pinocytic vesicles. Image was created with BioRender.
Modulation of drugs to cross the BBB
Peptide-drug conjugates and modulation of transcytosis
Peptide-drug conjugates are considered as prodrugs due to the covalent coupling of a peptide to a drug via specific linkers. They typically include a cytotoxic agent, a tumor-homing peptide, and a linker between them. Peptide-drug conjugates have been produced by linking drugs with BBB permeable peptides. In recent years peptide-drug conjugates have evolved, with the large-scale production and purification of peptides being simplified while an array of different tumor-targeting peptides have been discovered for different cancer types. This allows for a somewhat personalized therapy to be designed by selecting a tumor-homing peptide and desired physiochemical properties, such as solubility and stability, necessary for conjugation with the therapeutic load.
For a peptide-drug conjugate to be effective, it requires specific features, including a receptor-specific peptide and a stable peptide-drug conjugate with a strong binding affinity for the receptor. The peptide must bind selectively to a specific receptor on the cell surface of the target tissue that is unique or overexpressed in the cancer cells and at sufficient levels to transport the drug into the tumor. The peptide-drug conjugate site and linker must not modify the binding affinity to the target receptor or the stability to ensure that it will reach the tumor site to release the drug, thus limiting off-target toxicity. Some of the most used linear and cyclic peptides are arginine-glycine-aspartic acid, gonadotropin-releasing hormone, somatostatin, epidermal growth factor and Angiopep-2. All these peptides are delivered to cells using endocytosis/adsorptive-mediated transcytosis except for Angiopep-2, which enters the cells via transcytosis via the Low-density lipoprotein receptor-related protein 10 (LRP-1) transporter. These peptides have commonly been paired with cytotoxic agents such as gemcitabine, doxorubicin (Dox), daunorubicin, paclitaxel and camptothecin.
Arginine-glycine-aspartic acid (RGD)
The tripeptide arginine-glycine-aspartic acid (RGD) motif is a widely applied peptide carrier and mediates cell attachment by targeting integrins. The RGD motif is contained in various proteins like fibrinogen, fibronectin, prothrombin, tenascin, and other glycoproteins. RGD is recognized by integrins, which play an essential role in cell proliferation, invasion and angiogenesis, and are overexpressed in brain endothelial cells, newly formed vessels and tumor cells [10].
RGD peptide drug conjugates have been used for targeted drug delivery in multiple high grade glioma models [11-16]. RGD peptides have been tested in clinical trials (Phase I/II and Phase III) in newly diagnosed, progressive, or recurrent glioma with modest anti-tumor effects observed [17-21].
Somatostatin (SST)
Somatostatin (SST) is a neuropeptide produced by neuroendocrine, inflammatory, and immune cells. SST's various physiological functions include working as an endocrine hormone, a paracrine regulator or a neurotransmitter [22]. SST is ubiquitously expressed in humans, with high concentrations in the brain, liver, lungs, pancreas, thyroid, gastrointestinal tract, and adrenal gland [22]. SST has two active forms: SST-14 and SST-28, are mediated through five distinct GPCR subtypes (somatostatin receptor 1-5; SSTR1-5). SST is thought to be transported across the BBB by both the ATP-powered efflux pump, P-glycoprotein (Pgp), on the plasmatic membranes of the endothelial cells of the BBB and the unidirectional efflux transporter, Multidrug resistance protein 2 (Mrp2), on the apical membrane of brain capillary endothelial cells of the BBB [23].
GBMs express multiple SST receptors, with SSTR1 and SSTR2 being the most frequently expressed in GBM [24]. SST analogues linked with Dox (or Dox derivatives) or novel peptides have been successfully used for drug targeting in GBM models [25, 26]. These studies demonstrate the efficacy of modifying drugs to target the SSTRs in gliomas for efficient and specific drug targeting of therapeutics however, none of these have progressed to clinical trial.
Angiopep-2
Angiopep-2 was derived from low-density lipoprotein receptor-related protein 1 (LRP1) receptor involved in the uptake and processing of amyloid precursor protein in the intracellular compartment inside endosomal vesicles [27]. Angiopep-2 has recently attracted attention due to its ability to cross the BBB via receptor-mediated transcytosis after binding to LRP1, making it an appealing drug carrier for brain malignancies. Angiopep-2 has been exploited for transporting cancer therapeutics such as Dox, paclitaxel, camptothecin and etoposide as well as proteins and genetic materials [27-31]. Additionally, nanoparticles formulated by Guo and colleagues, statin-loaded Angiopep-2-anchored nanoparticles (S@A-NPs), has been shown to increase LRP1 expression in brain endothelial cells and brain metastatic tumor cells in vitro, leading to improved transcytosis. The nanoparticles were also seen to improve median survival in a brain metastatic mouse model when loaded with Dox [32]. Other nanoparticle formulations, such as that by Khan and colleagues, are developed to evade clearance by LRP1. They reported that nanoparticles attached to an MMP1-sensitive fusion peptide containing HER2-targeting K and LRP1-targeting angiopep-2 (A), or NPs-K-s-A, showed an increased brain accumulation compared to angiopep-2-decorated NPs in breast cancer brain metastases mouse models [33]. ANG1005, consisting of paclitaxel conjugated to Angiopep-2 has been examined in clinical trials to treat brain metastases and high-grade gliomas with early signs of clinical activity [34, 35].
Mfsd2a inhibition
Other strategies to modulate transcytosis for improved penetration of the BBB have been studied. Mfsd2a is an essential fatty acid transporter and is a regulator of transcytosis, limiting BBB penetration. Inhibition of Mfsd2a would therefore allow greater permeability. Ju, et al. designed tunicamycin-loaded transcytosis-targeting-peptide-decorated-nanoparticles (TM@TTP) loaded with Dox to reversibly inhibit Mfsda2 and enhance transcytosis, showing efficacy in a breast cancer brain metastases mouse model [36]. Other nanoparticles developed to improve transcytosis and downregulate tight junction proteins have been investigated, such as the minoxidil-loaded hyaluronic acid-tethered nanoparticles (M@H-NPs), shown to improve delivery of Dox and thereby significantly improving median survival of breast cancer brain metastasis bearing mice [37]. A recent, more detailed review on modulation of efflux, modulation of transcytosis and interference of tight junctions has been published by Han in 2021 [38].
Non-covalent avidin-biotin linkage of drugs
The non-covalent interaction of avidin-biotin has been utilized as a nanoscale drug delivery system for pharmaceuticals, including small molecules, proteins, vaccines, monoclonal antibodies, and nucleic acids. Avidin is composed of four identical glycoprotein subunits that bind biotin with high affinity and specificity. The avidin-biotin non-covalent interaction is highly specific and stable against manipulation, proteolytic enzymes, temperature, pH, harsh organic reagents, and denaturing reagents [39]. Avidin-biotin technologies have been recently exploited to deliver biologics and chemotherapeutic agents across the BBB via transferrin receptors (TfR).
Avidin-biotin technology is an efficient way to deliver chemotherapeutic drugs to the site of action via monoclonal antibody carriers. TfR monoclonal antibodies function to transport the bound drug into the brain through binding to the BBB TfR. Avidin-biotin technologies have shown promise due to their ability to specifically target and accumulate in tumors, permitting non-BBB permeable drugs to efficiently reach the tumor site, minimizing off-target effects and adverse side-effects. The avidin-biotin delivery system has been used preclinically with paclitaxel (PTX) and with siRNA and clinically in GBM [40-42]. Treatment with biotinylated anti-tenascin monoclonal antibody treatment followed by avidin and 90Y-biotin showed promising survival rates in a cohort of GBM patients. Survival was 98.4% at 6 months, 79.2% at 12 months, 51.7% at 18 months, and 30.7% at 24 months after treatment [42].
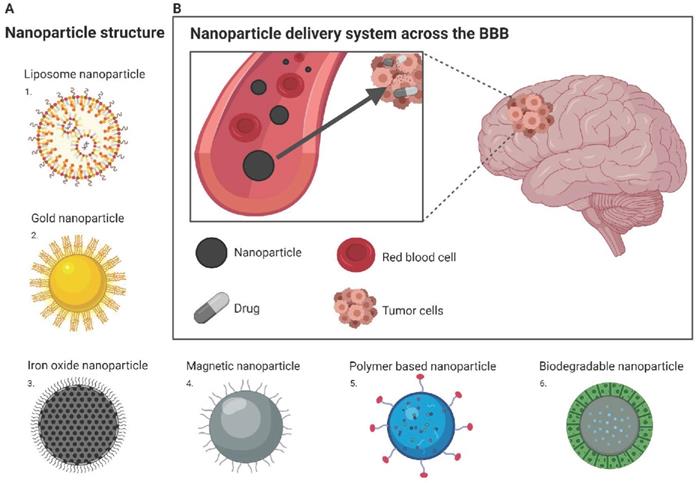
Nanoparticle drug delivery to the brain (A) Structure of different nanoparticles: 1. Liposome nanoparticle; 2. Gold nanoparticle; 3. Iron oxide nanoparticle; 4. Magnetic nanoparticle; 5. Polymer based nanoparticle; 6. Biodegradable nanoparticle (B) Nanoparticle delivery system across the BBB: Drug is encapsulated within nanoparticle and can freely travel from the blood stream to the tumor site located within the brain. Image was created with BioRender.
Nanoparticle drug carriers to cross the BBB
Recent breakthroughs and the advancement of nanotechnology offers the potential for patients suffering from CNS diseases a promising route for drug delivery. Nanotechnology is an engineering approach that can be used for medical applications including the development of nanoparticles as drug carriers to enhance delivery through the BBB (Figure 3B). The benefits of delivering nanoparticles include the ability to cross the BBB, increased permeability and retention in cancer cells, and being non-invasive for patients with limited effects on surrounding healthy tissues [43]. Nanoparticles used as drug carriers are based on stable elements (inorganic), including gold and iron, or organic nanoparticles, such as liposomes, micelles, and polymer nanoparticles.
Lipid-based nanoparticles
Lipid-based nanoparticles have either bilayer or multilayers and are capable of transporting both hydrophilic and hydrophobic drugs. Their characteristics increase the stability and solubility of the drugs being delivered across the BBB. In addition, being coated in micelles or polyethylene glycol allows efficient targeting and transportation of the nanoparticles [44, 45]. These nanoparticles are stable, an advantage that allows for detailed control of the carrier properties such as size and charge [46]. Preclinical studies have investigated lipid-based nanoparticles in combination with various agents, but with limited efficacy in brain cancer.
Liposomes are nanosized, spherical vesicles that are made up of one or more phospholipid bilayers where the polar groups of phospholipids are orientated inside, and there is an outer aqueous phase. They can also contain cholesterol can reduce the permeability of the lipid bilayer and increase liposome stability. Liposomes can be readily encapsulated with a range of drugs within the inside aqueous compartment, the lipid bilayers, and at their interfaces (Figure 3A). Due to the physical characteristics of liposomes, they can incorporate therapeutics that are hydrophilic, lipophilic, or hydrophobic. Hydrophilic agents are encapsulated into the aqueous core or are located between the lipid bilayer interface and the outer water phase of the liposomes. Lipophilic or hydrophobic agents are encapsulated in the hydrophobic core of the lipid bilayers of the liposomes. Liposomes have excellent biocompatibility and biodegradability, drug-targeted delivery, controlled drug release, and low toxicity [47]. They can also be modified to improve their blood circulation and BBB permeability by surface functionalization with macromolecules, such as polymers, polysaccharides, peptides, antibodies, or aptamers, on the liposome surface. Further to this, additional properties can also be added to improve delivery according to fluctuations in the target site microenvironment, such as temperature, pH, or externally applied stimuli, such as ultrasound intensity or magnetic fields [48]. Specific BBB targeting can occur through harnessing the low-density lipoprotein receptor expressed on brain capillary endothelial cells. Additionally, receptor-mediated endocytosis through conjugating ligands, such as peptides or antibodies (as described earlier), to the liposomes can be employed to interact with specific receptors expressed on endothelial cells. Other approaches include using electrostatic interactions between cationic charged compounds with negatively charged plasma membrane of endothelial cells, allowing for adsorption-mediated transcytosis or endocytosis into the BBB (comprehensively reviewed by Vieira & Gamarra) [48]. Currently, liposomal drug delivery is not a readily used technique in a clinical setting for brain tumors.
Clinical results of lipid-based nanoparticles applications used to improve BBB penetration in brain tumor treatment are summarized in Table 1.
Metal-based nanoparticles
Nanoparticles synthesized with metals such as gold, iron, silver, copper, and others have been studied and are of interest in the use of diagnostic probes in brain tumors. Coatings and functional groups on nanoparticles play a role in improving penetrability while limiting toxicity to surrounding tissues. Variation of the physiochemical properties of nanoparticles, including size, surface coating and charge, shape and chemical composition can significantly alter biocompatibility, distribution, and function, thus making them a potential nano-carrier in neuro-oncology. A study by Guo and colleagues tested varied sizes of different metal-based nanoparticles including cerumin oxide, iron oxide and zinc oxide, along with four different shapes of silver (spherical, disc-like, rod-shaped and nanowires). Results showed that zinc oxide was able to readily cross the in vitro BBB model and that spherical and disc-like silver nanoparticles created an easier entry pathway [70].
The use of specific coatings, such as starch, PEG, chitosan, dextran or polysorbate, can enhance penetration, bio compatibility, and systemic half-life by evading the clearance by the reticulo-endothelial system [71].
Gold nanoparticles are a promising agent for brain tumors and have gathered momentum in the advancement of modern nanotechnology (Figure 3A). They can be advantageous in that they can be engineered to target specific tumor markers, encapsulate insoluble drugs, modulate drug release, and improve bioavailability. They were initially designed to carry antineoplastics but are now considered suitable to also carry nucleic acids for gene therapy, inflammatory cytokines, flavonoids, and inhibitors. They are also used as imaging agents, radiosensitizers by inducing BBB disruption and theranostic agents.
Liposomal drug delivery in brain tumors
| Liposomal Drug Delivery | Nanoparticle Size | Functionalization | Drug | Nanoparticle Status | In vivo results | Clinical Use | Clinical Trial | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Lipid-based nanoparticles | 1-500nm | LBNP conjugate | Epirubicin [49, 50], Cisplatin [51-54], Bleomycin [55, 56], CPT-11 [57], edelfosine [58], TMZ [59, 60], vincristine [59, 61] | LBNP CPT-11 (Phase I) | Accumulation of edelfosine in xenograft glioma mouse model [58], TMZ increased cytotoxic effect and higher antitumor efficacy [60] | GBM, Gliosarcoma, Anaplastic astrocytoma, Anaplastic oligodendroglioma, HGG | NCT00734682 (completed), NCT02022644 (active, not recruiting) | No unexpected toxicities when given LBNP CPT-11 via IV |
| wheat germ agglutinin [62] | Daunorubicin [63-66] | Investigational compound in pre-clinical studies | Wheat germ agglutinin daunorubicin demonstrated strong capabilities in crossing the BBB in glioma bearing mice [62] | Not yet reported | ||||
| non-PEGylated LBNPs (Myocet®) [67] | DOX [67, 68] | Myocet® (Phase I) | Treatment of Myocet® showed higher concentration of DOX in brain and spleen, lower concentration of DOX in heart compared to DOX alone [68] | Recurrent/refractory HGG, malignant glioma | NCT02861222 (completed) | Myocet® demonstrated acceptable safety | ||
| positive-charged LBNPs [69] | PTX [69] | Investigational compound in pre-clinical studies | Not yet reported |
Abbreviations: LBNPs: lipid-based nanoparticles; PEGylation: polyethylene glycol; PTX: paclitaxel; DOX: doxorubicin; TMZ: temozolomide; CPT-11: irinotecan; GBM: glioblastoma; HGG: high-grade glioma; IV: intravenous; Myocet®: non-polyethylene glycol liposomal doxorubicin encapsulation.
Metal-based nanoparticle drug delivery in brain tumors
| Metal Drug Delivery | Nanoparticle Size | Functionalization | Drug | Nanoparticle Status | In vivo results | Clinical Use | Clinical Trial | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Gold nanoparticles | 5-400 nm | AuNP conjugate | Cisplatin [74], DOX [75, 76], L-aspartate TMZ [77] | Investigational compound in pre-clinical studies | Efficient BBB permeability of AuNP DOX and PEG TAT Gd(3+) [75] | Not yet reported | ||
| PEG TAT [75] | DOX [75], Gd(3+) [75] | |||||||
| gellan gum [78] | anthracycline ring antibiotic DOX hydrochloride [78] | Investigational compound in pre-clinical studies | Further investigation needed for in vivo experiments [78] | Not yet reported | ||||
| sperhical nucleic acid (SNA) [79] | cytotoxic agents | SNA NU-0129 (Early Phase I) | SNA penetrated BBB and BTB in GBM mouse model [79] | Recurrent GBM, Gliosarcoma | NCT03020017 (completed) | NU-0129 well tolerated in GBM, patients had no unexpected adverse effects, showed initial evidence of crossing the BBB | ||
| Iron oxide nanoparticles | 1-100 nm | DOX-EDT-IONPs [80] | DOX [80] | Investigational compound in pre-clinical studies | DOX-EDT-IONPs increased blood penetration of these nanoparticles [80] | Not yet reported | ||
| IONP conjugate | Chlorotoxin [81, 82], gemcitabine [82], PTX [83], 5-fluorouracil [84] | Investigational compound in pre-clinical studies | Efficient chlorotoxin and gemcitabine release activated by high-frequency magnetic field in GBM murine model, successful cellular transport [85] | Not yet reported | ||||
| Magnetic nanoparticles | 10-100 nm | magnetic nanoparticle conjugate | magnetic PTX [86] | Investigational compound in pre-clinical studies | Efficient BBB permeability of magnetic PTX following IV injection, survival prolonged using magnetic PTX in glioma-bearing rats [86] | Not yet reported | ||
| PEG, chitosan, dextran, polysorbate [71, 87], collagen, glycine, glutamine acid [88] | cytotoxic agents | Investigational compound in pre-clinical studies | Successful cellular transport, pronounced cytotoxic action of magnetic nanoparticle therapeutics in brain astrocytoma-bearing mice [89] | Not yet reported |
Abbreviations: AuNPs: gold nanoparticles; TAT: transactivator of transcription; SNA: spherical nucleic acid; DOX: doxorubicin; TMZ: temozolomide; GBM: glioblastoma; PTX: paclitaxel; PEG: polyethylene glycol; DOX-EDT-IONPs: DOX-chlorotoxin stabilized with trimethyoxysiylpropyl-ethylenediamine triacetic acid; Gd(3+): gadolinium; IV: intravenous; BBB: blood brain barrier; BTB: blood tumor barrier; IV: intravenous.
Iron oxide nanoparticles typically have a solid iron oxide core with a polymer coating (Figure 3A). They are unique and have been shown to have on-target effects due to their magnetic guidance and ligand targeting [45]. External magnetic fields can also be applied to metal-based nanoparticles to direct the nanoparticles loaded with therapeutics into the desired area of the brain (Figure 3A). Additionally, the radiofrequency field allows for magnetic nanoparticles to exert heat and temporarily open the BBB in a localized area [72]. Magnetic nanoparticles typically have an iron oxide core, mainly magnetite Fe3O4, and are divided into paramagnetic nanoparticles (PMNPs) that are 100 nm in diameter and superparamagnetic iron oxide nanoparticles (SPIONs) that are less than 100 nm. SPIONs are favorable due to their small size, susceptibility to magnetism, prolonged circulation and retention in tissues, and low toxicity [71, 73]. However, not much is known about iron's potential side effects and toxicities on genetic material, nervous system functioning, and development. Careful consideration of surface coating materials and size are paramount in developing a nanoparticle and are major determinants of effects on cellular structures as well as toxicities.
Preclinical and clinical studies using metal-based nanoparticles and their functionalizations for the treatment of brain tumors are summarized in Table 2.
Polymer-based nanoparticles
Polymer-based nanoparticles are a form of organic nanotechnology produced by polymerizing various monomers and loaded with the desired agent (Figure 3A). The characteristics of polymeric nanoparticles are also tunable such as charge, size, shape, and surface ligands, to suit specific applications, thus targeting the BBB. Synthetic polymers, such as poly (alkyl cyanoacrylate), poly(lactic-co-glycolic acid), poly-ε‐caprolactone, and polyamidoamine dendrimers have been developed but are not without limitations. Drawbacks include low biodegradation rate due to hydrophobicity, toxicity, high cost, and purity [90]. Although synthetic polymeric nanoparticles have been investigated and developed over time, clinical success is still limited. Hence further study has been focused on the kinetics of drug loading, targeting, and release. Natural polymeric nanoparticles have also been investigated and seem to solve the issues that synthetic polymers have, such as being less toxic, more sustainable, biodegradable and less costly [90]. Naturally occurring polymers include, but not limited to, chitosan, a commonly used polysaccharide, and alginate, a polysaccharide from brown seaweed.
Polymeric nanoparticles for CNS delivery are still under investigation, with some showing promise in the preclinical setting. Other studies show that nanoparticles, combined with mechanical BBB disruption methods such as focused ultrasound and convection-enhanced delivery, are more beneficial [91]. A detailed review on this area has been published recently by Zhang and colleagues [90].
A summary of preclinical studies for polymer-based nanoparticle delivery for brain tumor treatment is listed below in Table 3.
Blood-brain barrier modulation
Osmotic opening
Hyperosmolar BBB disruption is the shrinkage of endothelial cells caused by hyperosmolar plasma conditions, which cause the vasodilation of capillaries and opening of tight junctions [100]. This loss of water from brain tissue into the circulation is caused by a higher osmotic pressure, where this calcium-mediated process causes changes in the endothelial cytoskeleton and junctional proteins, modifying the junctional width [100]. The influx of water into the brain assists in drawing chemotherapeutic drugs in, thereby enhancing therapeutic efficacy. In 2004, Brown et al. discovered a regional variability in mannitol BBB opening effectiveness, where sucrose permeability post mannitol infusion was higher in cortex and midbrain compared to cerebellum and brainstem [100]. In a preclinical rat model, infusion of hyperosmolar compound (1.6M mannitol) was tested, and permeability was analysed by 86Rb+ (a marker for K+ transport), 14C sucrose and Evans blue albumin. Only sucrose and Rb had increased permeability, and sucrose showed regional differences - cortex and midbrain higher permeability than brainstem and cerebellum [100].
To date, mannitol has shown promising results in PCNSL. Intraarterial hyperosmolar mannitol infusion is considered safe and has been used to transiently open the BBB to increase the penetration of macromolecule chemotherapies by 100-fold and thereby improve the response to treatment [101]. A safety and efficacy multicentre study on PCNSL showed that 40 out of 43 patients had a complete response to intra-arterial Methotrexate (MTX) before hypertonic mannitol in combination with IV cyclophosphamide and etoposide [102]. This same study also investigated neuroectodermal tumors, germ cell tumors and GBM treated with intraarterial carboplatin after hypertonic mannitol with IV cyclophosphamide 10 min before mannitol, as well as IV etoposide for several days, which resulted in patients with stable disease and minimal complications [102]. A long term follow up study in 2000, in patients with PCNSL treated with MTX and mannitol BBB disruption without radiation therapy, showed 36 out of 48 patients exhibited complete response with a 5-year survival rate of 42% and median survival of 40 months and no cognitive loss [103]. This demonstrates the safety and feasibility of this approach, but Phase 3 trials are needed to determine whether the addition of mannitol improved outcomes.
BBB disruption with hypertonic mannitol is reversible with minimal permanent brain changes. The maximum osmotic effect on the BBB lasts for 10 min but can be enhanced by Na+/Ca2+ channel blockers for 30 min. This is beneficial as the brains receive longer exposure to the drug and less systemic exposure. A prolonged opening may also be beneficial for drugs that are only active in specific phases of the tumor cell cycle, such as MTX. However, limitations for this approach include repeated hospitalisation, associated with a need for sedation, and toxicities include neurological deficits, strokes and seizures [104].
Despite being developed in the 1970s, mannitol is still being explored to help improve chemotherapy penetration to brain tumors. A Phase I/II clinical trial (NCT00303849) is currently recruiting patients to undergo mannitol BBB disruption with chemotherapies carboplatin, melphalan, etoposide, and sodium thiosulfate for the treatment of previously treated brain tumors. In addition, intra-arterial infusion of cetuximab is being investigated with mannitol BBB disruption for newly diagnosed GBM (NCT02861898).
There is currently a lack of completed studies using mannitol to treat other primary brain tumors other than GBM. The safety and feasibility demonstrated in PCNSL suggests that application of mannitol warrants further research.
Inhibiting drug efflux
ATP-binding cassette (ABC) transporters are a superfamily of integral membrane proteins that transport solutes across cellular membranes by ATP. Pgp, MRP and breast cancer resistance protein (BCRP) are ABC transporters expressed in the BBB and play a role in limiting the entry of therapeutics into the brain and creating multi-drug resistance. This poses a significant problem in delivering therapeutics into the brain, especially in brain tumor treatment.
Polymer drug delivery preclinical studies in brain tumors
| Polymer Drug Delivery | Nanoparticle Size | Functionalization | Drug | Nanoparticle Status | In vivo results | Outcomes |
|---|---|---|---|---|---|---|
| Polymer-based nanoparticles | 1-1000 nm | PLGA [92] | Docetaxel [92], PTX [93], | Investigational compound in pre-clinical studies | PLGA docetaxel injected peritoneally were able to penetrate the BBB in C57BL/6 mice [92] | Not yet reported |
| PBCA [94] | antibiotics, cytostatics, DOX [95], CNS-active drugs [94] | Investigational compound in pre-clinical studies | PBCA DOX produced high antitumor effect against intracranial GBM in rats [95] | Not yet reported | ||
| PBAE [96] | anticancer plasmid DNA | Investigational compound in pre-clinical studies | PBAE anticancer plasmid DNA were able to penetrate orthotopic brain tumor tissue in rats [96] | Not yet reported | ||
| polysorbate 80-coated PBCA [95, 97, 98] | DOX[95], hexapeptide dalargin [97] | Investigational compound in pre-clinical studies | Increased accumulation of NPs in intracranial glioma tumor-bearing mice [99] | Not yet reported |
Abbreviations: PLGA: PEGylated-poly (L-lactic co-glycolic acid); PBCA: polybutylcyanoacrylate; PBAE: poly(β-amino ester); DOX: doxorubicin; PTX: paclitaxel; CNS: central nervous system; GBM: glioblastoma; DNA: deoxyribonucleic acid.
Tournier, et al. showed that ABCB1 (Pgp) and ABCG2 (BCRP) efflux transporters at the BBB and work concurrently to restrict access to tyrosine kinase inhibitors. Elacridar is a well-known ABCB1 and ABCG2 inhibitor and has been studied in CNS metastases models. In mice, elacridar successfully increased brain uptake of erlotinib, however, preclinical data in humans did not show the same success [105]. Elacridar however has undergone significant preclinical and clinical study and has been shown to significantly increase brain penetration of dasatinib, gefitinib, and sorafenib [106-108]. Novel molecules have been investigated and continue to be developed to provide potent Pgp inhibition. A recent study by Fallacara and colleagues developed a dual Src/Pgp inhibitor, Si306, and found in in vitro studies that it inhibited cell growth 2-fold that of dasatinib and had dose-dependent suppression of Pgp activity [109]. They also found that administration of the prodrug form of Si306 could increase median survival in mice harboring orthotopic GBM tumors [110].
Drugs have also been modified to have a lower affinity for efflux transporters. A study by Becker, et al. investigated PI3K/mTOR inhibitors for the treatment of GBM in mouse models where they modified GDC-0980, an inhibitor with high affinity for efflux transporters, and its analog GNE-317. Both inhibitors displayed decreased efflux and 3-fold higher drug penetration into the tumor core, as well as decreased histological staining for effector proteins [111].
Currently, research of efflux transporter inhibition is still progressing toward finding potent dual ABCB1/ABCG2 inhibitors. A study by Strope, et al. in 2019 investigated a more specific ABCG2 inhibitor, botryllamide G, and found that it could significantly increase brain concentrations of lapatinib. However, ABCB1 would also need to be blocked for a complete efflux transporter inhibition [112]. In addition, off-target effects of Pgp inhibition are a significant pitfall since ABCB1 and ABCG2 are expressed in the gut, liver, and kidney.
A phase 1 clinical trial with tariquidar (XR9576) for inhibition of Pgp, in combination with Dox, vinorelbine or docetaxel was conducted in pediatric patients with a range of solid tumors, including brain tumors (NCT00011414) [113]. It found that the Pgp substrate fluorescent dye 99mTc-sestamibi had increased tumor accumulation by 22% with administration of tariquidar. Out of 29 enrolled subjects, one patient had a complete objective response, and 2 had partial responses. Toxicities from tariquidar were minimal, including transient hypotension, loss of taste and nausea. However, when administered in combination with chemotherapeutic agents' docetaxel and vinorelbine, systemic clearance was reduced thereby increasing drug exposure causing drug associated toxicities [113].
Thus, the large availability of preclinical studies into Pgp inhibitors has not translated well into a clinical setting, and the search for more highly potent, selective, and efficacious Pgp inhibitors is still ongoing.
Chemical modification of blood-brain barrier and blood-tumor barrier using TNFα
Inflammatory cytokine tumor necrosis factor α (TNFα) has been shown to disrupt the BBB by decreasing levels of tight junction proteins, causing changes in endothelial cell adhesion, and compromising barrier function and integrity [114]. Human TNF conjugated with a cyclic peptide Cys-Asn-Gly-Arg-Cys NGR (NGR-hTNF) targets CD13+ vessels has been tested at low doses and has been found to increase vascular permeability and assist in drug penetration in central nervous system (CNS) lymphoma and melanoma in animal models [114, 115]. NGR-hTNF is also in phase II and III clinical trials for different tumor types with and without chemotherapy and has shown safety and activity. A high-dose NGR-hTNF phase I trial was completed and showed that the dose could be safely escalated while maintaining tolerability with chills being the only toxicity observed [116]. R-CHOP is a commonly used chemotherapy for primary CNS lymphoma (PCNSL) comprising rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine, and prednisolone and has been tested in combination with NGR-hTNF. In a clinical trial with 12 PCNSL patients, 9 displayed rapid tumor regression after therapy, with a complete response in 8 patients (NCT03536039) [114]. There were 2 severe adverse events and grade 4 toxicities that arose in this study, but both were resolved and treated. While it is not possible to evaluate the clinical efficacy of TNF-α with this small number of patients, the response rate and lack of toxicity is encouraging. This is the only clinical application of TNF-α for the disruption of the BBB that has been studied to date.
Mechanical disruption of the Blood-brain barrier/Blood-tumor barrier
Focused ultrasound
Focused ultrasound (FUS) can focus acoustic energy into a focal spot to deliver selective BBB disruption and enhanced permeability [117]. FUS can provide reversible BBB disruption to enhance permeability by concentrating acoustic energy to a focal point. FUS can be used in conjunction with clinically available drugs and is a relatively benign procedure that can be modified to match chemoschedule. Microbubbles have been integrated into the FUS approach, which has helped focus the effect of FUS to the blood vessel walls, thereby minimizing the damage to the surrounding healthy brain tissues. In MB-facilitated FUS, circulating microbubbles interact closely with the low-intensity FUS, causing a temporary disassembly of tight junctions and increased permeability of the BBB [118]. Research has shown that repeated application of FUS and microbubbles to open the BBB over a long term (4-20 months) did not result in oedema in the majority of the cases [119]. There was however, a significant increase of reaction time during a neurotoxicity test task on the day of FUS and microbubble application which returned to baseline within 4-5 days, demonstrating the safety of this method [119].
Microbubbles and FUS have been successfully combined with multiple drugs, including Dox, TMZ, BCNU (1,3-bis(2- chloroethyl)-1-nitrosourea) bevacizumab and MTX in preclinical models [120-126]. It has even evolved further to be used with macro-agents such as magnetic nanoparticles, small interfering siRNA, and even stem cells [127-130]. In addition, focused ultrasound is currently in clinical trials for brain tumors, including low-grade glioma and GBM, as well as in Alzheimer's Disease, Parkinson's disease dementia and breast cancer with brain metastasis [131, 132]. These studies provide preliminary evidence that FUS can transiently increase BBB permeability and enhance local concentrations of antitumor drugs to further inhibit tumor development. One of the main limitations of this technology is that FUS is usually only targeted to small brain volumes or areas and therefore only small regions of the BBB are opened.
Stereotactic radiation therapy
Radiation therapy plays a prominent role in treating many types of cancer, with almost 50% of patients receiving ionizing radiation at part of their treatment [133]. There are different types of radiation treatments that are used based on tumor type, location and the radiosensitivity of the cancer cells. Radiation therapy works by depositing its energy in the tumor site to damage the DNA of the tumor cells thereby inducing apoptosis of the cells. Unfortunately, due to the nature of ionizing radiation, it can also cause DNA damage to surrounding tissues, thereby damaging glial, neuronal, and vascular cells of the brain, which is often considered an adverse consequence of ionizing radiation. However, because both endothelial cells and oligodendrocytes are radiation responsive, ionizing radiation can be used in a targeted and controlled manner to purposefully damage tissue and increase the BBB permeability [134].
Previous studies have shown at both the in vitro and in vivo level that high doses of radiation have been shown to induce BBB permeability, tight junction morphology changes, reductions in cell density, and the formation of actin stress fibers in cerebral endothelial cells in healthy regions of the brain [135, 136]. Although these studies show promise, optimal dosing remains undetermined (with BBB permeability seen at doses ranging from 0.1 Gy to 20 Gy), the therapeutic window remains undetermined and the side effects associated with this method, including oedema, remain concerning. Radiation therapy has gained interest with its potential to be combined with nanoparticle technology for targeted therapy delivery to the tumor site and immunotherapy [137]. However, very few clinical trials have been completed using this method (NCT00019578) with some still in the active or recruiting phase (NCT03561896, NCT02974803).
Electric field modulation
Electric field modulation has been used in other fields of medicine and has recently been used as a novel treatment method in oncology. Electric field modulation has recently been re-termed tumor-treating fields and has been used to open the BBB for chemotherapeutic drug delivery in a method known as electrochemotherapy [138]. Tumor-treating fields therapy delivers low‐intensity (1-3 V/cm), intermediate‐frequency (100-300 kHz), alternating electric fields to the tumor using transducer arrays placed on the skin around the region of the body containing the tumor [138]. This method causes irreversible electroporation through the needle electrode delivery of electric pulses inducing nonthermal focal ablation [139]. This causes cell death due disrupted membrane integrity.
Preclinically tumor treating fields have been used successfully to inhibit cell proliferation in cancer cell lines, including human glioma, non-small-cell lung carcinoma, breast carcinoma as well as animal models of glioma and melanoma and in combination with chemotherapeutics [140-142]. There are currently numerous clinical trials in the recruiting phase testing tumor treating fields for the treatment of brain metastases in small cell lung cancer, and ependymoma. Clinical trials on brain metastasis from primary lung cancer, brain cancers and GBM have been completed with some evidence that tumor-treating fields can increase the permeability of the BBB [143, 144]. Unfortunately, clinical effects have been small and the practicality of applying electric field modulation is burdensome with devices needing to be worn for 20 h a day.
Laser induced thermotherapy
Laser induced thermotherapy (also known as interstitial laser thermotherapy) (LITT) was first described in 1990 by Kiesslin, et al. who introduced the hypothesis that laser light could be used to cause a concentrated disruption by focally applying a neodymium-doped yttrium aluminium garnet (Nd:YAG) laser pulse [145]. The procedure involves the use of a stereotactic device to introduce optical fibers to deliver laser light interstitially. This laser light is deposited at low power and over a long exposure time to increase the target area temperature. When the thermal threshold temperature between 50 °C and 80 °C is reached, protein denaturation and irreversible tissue coagulation occurs resulting in permanent tissue damage [89]. Lower temperatures of 43 °C to 45 °C for more than 10 min will sensitize cancer cells to chemotherapy and radiation therapy. Laser therapy has been used in combination with tumor-localizing photosensitizers such as prodrug 5-aminolevulinic acid (5-ALA). 5-ALA has been employed for the treatment of glioma due to its tumor specificity and rapid systemic clearance. 5-ALA-laser combination treatment disrupts the BBB rapidly via developing and enlarging of endothelial gaps. LITT, much like FUS, can be used in conjunction with nanotechnologies to target drug delivery. In 2011, Choi, et al. proved that near-infrared ultrashort pulsed laser in combination with large molecules such as nanoparticles and genetically engineered viruses, could penetrate the BBB [146]. LITT has since been shown preclinically to enhance survival in a GBM model [147].
Unfortunately, at the clinical trial level there is little to no reported information on LITT in brain cancers even though some clinical trials have run to completion (NCT01651078, NCT02451215, NCT00207350, NCT01851733) or are currently still in the recruitment phase (NCT00787982, NCT02389855, NCT02392078, NCT02311582, NCT04181684) [148, 149]. Clinical trials showed that LITT may have a role in the management of radiation necrosis and can allow a timeframe for additional therapeutic intervention such as further local radiation or systemic therapies [150].
Approaches that bypass BBB/BTB
Intrathecal and intraventricular administration
Intrathecal administration is the delivery of therapeutics directly into the cerebrospinal fluid (CSF) that surrounds the spinal cord, termed the intrathecal space or subarachnoid space, bypassing the BBB and achieving higher therapeutic concentrations due to the proximity to the target, thereby limiting potential systemic toxicities [151]. There may also be lower levels of enzymatic breakdown of drugs in the CSF compared to plasma which can lead to an increase in target drug concentrations [151]. Intrathecal administration was firstly used for the delivery of analgesics in the 1980s for acute and chronic pain but has been further developed for use with chemotherapeutics or other biological macromolecules.
Intrathecal therapeutics can be administered by an external pump or implantable devices. Implantable infusion pumps, however, are costly, invasive and cause secondary issues such as formation of granulomas and infection which may lead to catheter dysfunctions, compression of the spinal cord and paralysis [152]. Single injection strategies are favored over implantable devices or repeated intrathecal administrations but need to have robust stability to reside in tissues for therapeutic effect and combat the physiological characteristic of bulk flow [151]. Administered molecules must also be stabilized and have good penetrative properties to infiltrate the parenchyma [151]. However, there are limitations that have hindered this technique from further development. Discrepancies between CSF concentration and parenchymal concentrations suggest a physiological CSF-brain barrier that could limit delivery into brain tissue, along with high rates of clearance from CSF [151].
Various in vivo studies have been conducted using intrathecal delivered agents for leptomeningeal dissemination. A study using intrathecal MX2, an anthracycline inhibiting topoisomerase II, in a rat model of dissemination showed only a modest increase in survival, which could be explained by inadequate infiltration into parenchyma [153]. A patient study investigating intrathecal triothriethylenephosphoramide (thio-TEPA) for ependymal or leptomeningeal dissemination from anaplastic astrocytoma or GBM was conducted and found a modest improvement in survival at 10 months [154].
Intraventricular administration is the placement of a subcutaneous device, an Ommaya reservoir, with a catheter in the lateral ventricle, allowing therapeutics to enter directly into the ventricular CSF. The benefit of this technique is allowing for a more consistent drug distribution with higher concentrations achieved in comparison to intralumbar injections [155]. This technique is regularly used in pediatric tumors, such as AT/RT and infant medulloblastoma with conventional cytotoxics, however it is used in this setting for the treatment or prevention of leptomeningeal spread rather than intraparenchymal disease.
Intranasal delivery
Intranasal delivery has been investigated in the context of neurological diseases such as neurodegenerative conditions, migraines, schizophrenia, etc [155]. The intranasal route can bypass the BBB via the olfactory and trigeminal nerve pathways. This method is safe and non-invasive with an increased rate of absorption and capable of achieving higher concentrations due to the high vascularization of the nasal epithelium [156]. The use of intranasal delivery is limited for brain tumors, but early data is promising. Studies in rats show accumulation of MTX, raltitrexed and 5-fluorouracil in the brain after intranasal administration [157-159]. The intranasal delivery of GRN163, a telomerase inhibitor, in a human GBM xenograft in rats showed good uptake and distribution in the tumor 4 h after delivery and a significant increase in survival at median 75.5 days compared to 35 days in control groups [160]. Intranasal administration of natural compounds, curcumin and anthranoid 4,5-dihydroxyanthraquinone-2-carboxylic acid or rhein, have been shown to be efficacious in GBM mouse models by exhibiting tumor regression and extensions in survival [161, 162]. MTX, commonly used for a wide range of cancers but a poor penetrator of the BBB, was studied for intranasal delivery in rats. It was found that CSF concentration of MTX 15 min after intranasal delivery was higher than in plasma, indicating rapid absorption and transport into CSF [163]. Chitosan microspheres encapsulated MTX were shown to be more effective than MTX solution and intravenous MTX by higher concentrations of MTX found in rat brain tissue [164]. TMZ, despite its good BBB penetrability, is ineffective in prolonging survival in patients with GBM. A study comparing oral, intravenous or intranasal administration routes of TMZ in a rat glioma model showed that intranasal delivery was the most effective at reducing tumor volume and significantly increasing survival [165].
Perillyl alcohol, a natural compound, was investigated via the intranasal route for the treatment of GBM where 37 recurrent glioma patients were enrolled and treated with the agent by inhalation and resulted in 14 patients showing either partial response or stable disease [166]. Subsequently, a larger cohort study consisting of 198 patients with primary GBM, grade III astrocytoma or anaplastic oligodendroglioma showed intranasal perillyl alcohol could significantly prolong survival compared to control group, with 19% of patients under remission after 4 years [167]. Another study with 198 recurrent GBM patients treated with perillyl alochol inhalation was conducted over 4 years and showed significant survival increase as well as 19% of the cohort showing clinical remission over 4 years [168]. Following these promising results, a safety and efficacy study for perillyl alcohol in recurrent grade IV glioma was initiated in 2016 and is currently undergoing recruitment (NCT02704858).
However, limitations of the intranasal technique includes small volumes of administration, limited retention in the nasal cavity for absorption, potential mucociliary clearance or enzymatic drug degradation [169]. Despite the downfalls of this administration route, studies have been conducted and shown to be successful in the treatment of brain tumors and is currently still under investigation.
Intratumoral and intracavitary delivery
Intracavitary chemotherapy is administered in the form of implantable biodegradable polymer wafers in the brain or in a tumor cavity during surgery, allowing for a continuous release of chemotherapeutics locally at the tumor site and limiting systemic exposure. Direct injection of chemotherapeutics was the earliest method for intracavitary delivery, by injecting directly into the resection cavity, surrounding parenchyma or the ventricle, using a needle or implantable catheter with reservoir. This method has been used for various therapeutics such as chemotherapies, radioactive compounds, and antibodies [170]. Despite the relative simplicity of this approach, intracavitary or intraparenchymal injections involves surgical intervention, increasing the risk of complications. Additionally, this technique requires an optimal concentration gradient and distribution which is difficult to achieve and could result in toxic concentrations in the surrounding areas [170].
Bortezomib, a proteasome inhibitor, is ineffective for the treatment of GBM, however implantation of a mini-osmotic pump to intratumorally deliver the drug was shown to be successful and significantly increased survival in an orthotopic GBM mouse model [171].
To overcome systemic toxicity of chemotherapies, Gliadel® wafer was developed, which is composed of polyanhydride polymer impregnated with BCNU for the treatment of malignant brain tumors. Development of a BCNU implantable polymer that releases the drug directly into the CNS has made it possible to achieve high local drug concentrations while minimizing systemic toxicity and circumventing the need for a drug to cross the BBB. BCNU has been successfully used preclinically and has since shown clinical success in patients with primary malignant gliomas. However, some adverse side effects have been reported, such as increased cerebrospinal fluid leaks and intracranial hypertension [172]. More recently, Gliadel® has been used in combination with TMZ and carmustine preclinically to treat GBM demonstrating a significant survival benefit in vivo [173, 174]. Gliadel wafers are Food and Drug Administration (FDA) approved and used in adult GBM. A pediatric study with Gliadel wafers combined with low-dose etoposide for recurrent anaplastic ependymoma but was found to be ineffective as relapse occurred 4 months after implantation [175]. Often, Gliadel wafers are used in conjunction with other therapeutics to improve efficacy. In a Phase II trial (NCT00362921), Gliadel was combined with O6-benzylguanine infusions in recurrent GBM patients. The overall survival of the fifty-two patients enrolled was 82%, with 1-year and 2-year overall survival of only 47% and 10%, respectively. Toxicities documented were hydrocephalus, CSF leak and infection [176].
Another biodegradable polymer, n-butylidenephthalide, suppressed growth of subcutaneous rat and human brain tumors, reduced GBM tumor volumes and significantly prolonged the survival rate in a preclinical model and has since been incorporated into the 'Cerebraca wafer', which is the first human-use drug product and is a biodegradable implant comprised of (Z)-n-butylidenephthalide ((Z)-BP) and Carboxyphenoxypropane-Sebacic Acid Copolymer (NCT03234595) [177].
A summary of the preclinical investigations and clinical trials for the use of biodegradable polymer wafers are summarized in Table 4.
Intratumoral delivery can be a carried out in a range of techniques, such as local injection, surgical insertion of a catheter with a reservoir, or convection enhanced delivery (CED). Intratumoral delivery of bleomycin in a phase 1 clinical trial was done via an Ommaya reservoir, an implantable device with a delivery tube directed to the center of the tumor and used to treat patients with recurrent GBM. The maximum tolerated dose and recommended Phase II dose was determined and no dose limiting systemic toxicity was documented [178]. Intratumoral delivery of DNX-2401, an oncolytic adenovirus, was investigated in a phase 1 clinical trial (NCT00805376), where recurrent malignant glioma patients received the virus by either a single intratumoral injection or single intratumoral injection followed by surgical resection and further virus injection into the cavity via an implanted catheter. The 3-year overall survival of the first group was 20% and 3 out of 25 patients exhibited a 95% decrease is tumor size [21]. Various other viruses are currently in clinical investigation for high-grade gliomas, such as Delta 24 RGD (NCT00805376, NCT01582516), PVSRIPO with lomustine (NCT02986178), Reovirus with sargramostim (NCT02444546), and seems to be a promising avenue utilizing intratumoral delivery.
Monoclonal antibodies may be used as vectors for delivery of local adjuvant radiotherapy. A phase II study by Reardon, et al. in 2002 detailed the use of 131I-labeled antitenascin monoclonal antibody 81C6 in post-surgical resection cavities in 33 patients with malignant gliomas. Median survival was greatly increased to 86.7% compared to historical controls, and 79.4 weeks for specifically GBM patients. At 93 weeks follow up, 11 patients survived. Side effects included treatment-related hematologic toxicity that was treatable in 9 patients, neurologic toxicity in 5 patients, and radionecrosis in 1 patient [179]. Intratumoral and intracavitary based delivery are emerging as a valuable technique for delivering viral vectors, antibodies, and radiation. Although promising, none have been approved by the Food and Drug Administration.
Convection enhanced delivery
Convection enhanced delivery (CED) is a drug delivery technique developed in the early 1990s. This method relies on a continual hydraulic pressure to distribute a homogenous supply of any sized therapeutic molecule directly the site. Although this technique demonstrates a direct route for administration, this method is highly invasive as a catheter is inserted through burr holes into the interstitial spaces of the brain [181]. Careful placement of the catheter in the interstitial space of the brain allows molecules, independent of size to enter, thus allowing a wide range of cancer therapeutics to reach the brain. In vivo studies were conducted in DIPG and pediatric HGG mouse models, where liposomal Dox was delivered by CED into the pons or the thalamus. However, excessive toxicity was seen with injection of Dox into the pons, indicating a potential issue with using CED in certain anatomical locations [182]. Efforts have been focused on the use of recombinant toxins delivered by CED for the treatment of brain tumors, where a variety have reached the stage of clinical testing. An early phase II trial showed the use of transferrin-CRM107 in malignant gliomas, which resulted in 35% of the patients having complete or partial responses, however some toxicity to normal tissue was observed, as well as increased cerebral edema, impeding further investigation [183]. CED delivery of interleukin-13 with bacterial toxin PE38QQR showed promise in early phase clinical trials for malignant gliomas (NCT00024570, NCT00024557). The largest multicenter Phase III trial for CED was the PRECISE trial, which was commenced for patients with recurrent GBM. Significant improvements in median survival and progression-free survival were seen with CED of IL13-PE38QQR when compared to a Gliadel control arm (NCT00076986) [184]. This agent has also been explored in DIPG, where a Phase I trial in 2018 recruited 5 DIPG patients for CED delivery of IL13-PE38QQR, which resulted in temporary cessation of disease progression in 2 of the patients. However, all 5 patients exhibited progression by 12 weeks after first infusion, and 2 patients reported side effects of cranial nerve deficits and lethargy after infusion (NCT0088061) [185].
Chemotherapy drugs with low BBB penetrability, such as topotecan and PTX, have been investigated with CED delivery in patients with malignant gliomas, showing tumor regression in 69% of patients and 73% response rate, respectively. However, side effects were observed: dose-dependent meningitis and infusate leakage into the CSF [186, 187].
Clinical investigation of CED for the delivery of topotecan into two patients harboring DIPG tumors, has demonstrated feasibility and safety of the technique at low infusions rates, however no prolongation of survival was observed (NCT00324844) [188].
Several Phase I clinical trials are still underway for an investigation into CED for malignant gliomas and DIPG. A more in-depth review by Mehta, et al. was published in 2017 that details ongoing clinical trials [181].
Anatomical differences still pose a major problem for implementing CED. Brainstem tumors such as DIPG involve delicate structures that are sensitive to invasive procedures. Further developments into improving catheter design as well as precise placement to prevent reflux and enhance distribution are currently being investigated. Additionally, imaging should also be used during CED infusions to monitor drug distribution and retention.
Biodegradable polymer wafers for the treatment of brain tumors
| Biodegradable polymers | Polymer type | Drug | Status | Clinical Use | Clinical Trial | Outcomes |
|---|---|---|---|---|---|---|
| 150-190nm | PLGA wafer | 5-fluorouracil | Investigational compound in pre-clinical studies | Not yet reported | ||
| Gliadel® wafer | Carmustine with 5-ALA followed by RT and TMZ | Phase II (completed) | Primary GBM | NCT01310868 | Gliadel® and 5-ALA increased cerebrospinal fluid leaks, intracranial hypertension, no survival data published | |
| Gliadel® wafer | carmustine | Phase II (recruiting) | Metastatic brain tumor | NCT04222062 | Not yet reported | |
| Gliadel® wafer | Carmustine and systemic O6-benzylguanine | Phase II (completed) | Recurrent GBM | NCT00362921 | Significant improvement in OS with increase in adverse events of hydrocephalus, CSF leak and CSF/brain infection [180]. | |
| Cerebraca wafer | (Z)-n-butylidenephthalide ((Z)-BP; and Carboxyphenoxypropane-Sebacic Acid Copolymer with adjuvant TMZ | Cerebraca wafer (Phase I & IIa) (recruiting) | Recurrent HGG | NCT03234595 | Not yet reported |
Abbreviations: PLGA: PEGylated-poly (L-lactic co-glycolic acid); GBM: glioblastoma; TMZ: temozolomide; HGG: high-grade glioma; CSF: cerebrospinal fluid; 5-ALA: 5-aminolevulinic acid.
Conclusion
The BBB is a unique and complex structure that is a significant impediment to effective drug delivery to the brain for the treatment of brain tumors. This review detailed the established and emerging techniques investigated to bypass the BBB, from modulation of drugs to modulation or mechanical disruption of the BBB. The future directions of improving drug penetrability into the brain for treating brain tumors greatly rely on technological advancements. Furthermore, clinical trials for adults and children are pivotal for the further development of these techniques. The discovery of a safe and efficacious approach to bypassing the BBB for clinical use for the treatment of brain tumors is urgently needed and has the potential to change the course of brain tumor therapy greatly.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Pardridge WM. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32:1959-72
2. Yokel RA. Nanoparticle brain delivery: a guide to verification methods. Nanomedicine. 2020;15:409-32
3. Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C. et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557-61
4. Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41-53
5. Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7:a020412-a
6. Pardridge WM. The blood-brain barrier: Bottleneck in brain drug development. NeuroRX. 2005;2:3-14
7. Arvanitis CD, Ferraro GB, Jain RK. The blood-brain barrier and blood-tumor barrier in brain tumors and metastases. Nat Rev Cancer. 2020;20:26-41
8. Lin F, de Gooijer MC, Roig EM, Buil LC, Christner SM, Beumer JH. et al. ABCB1, ABCG2, and PTEN determine the response of glioblastoma to temozolomide and ABT-888 therapy. Clin Cancer Res. 2014;20:2703-13
9. Ningaraj NS, Rao M, Hashizume K, Asotra K, Black KL. Regulation of Blood-Brain Tumor Barrier Permeability by Calcium-Activated Potassium Channels. J Pharmacol Exp Ther. 2002;301:838
10. CC K. Integrin alpha v beta 3 as a Therapeutic Target for Blocking Tumor-Induced Angiogenesis. Curr Drug Targets. 2003;4:123-31
11. Ruan H, Chen X, Xie C, Li B, Ying M, Liu Y. et al. Stapled RGD Peptide Enables Glioma-Targeted Drug Delivery by Overcoming Multiple Barriers. ACS Appl Mater Interfaces. 2017;9:17745-56
12. Li X-T, Tang W, Xie H-J, Liu S, Song X-L, Xiao Y. et al. The efficacy of RGD modified liposomes loaded with vinorelbine plus tetrandrine in treating resistant brain glioma. J Liposome Res. 2019;29:21-34
13. Chen L, Zeng D, Xu N, Li C, Zhang W, Zhu X. et al. Blood-Brain Barrier- and Blood-Brain Tumor Barrier-Penetrating Peptide-Derived Targeted Therapeutics for Glioma and Malignant Tumor Brain Metastases. ACS Applied Materials & Interfaces. 2019;11:41889-97
14. Liu Y, Mei L, Xu C, Yu Q, Shi K, Zhang L. et al. Dual Receptor Recognizing Cell Penetrating Peptide for Selective Targeting, Efficient Intratumoral Diffusion and Synthesized Anti-Glioma Therapy. Theranostics. 2016;6:177-91
15. Ullah I, Chung K, Bae S, Li Y, Kim C, Choi B. et al. Nose-to-Brain Delivery of Cancer-Targeting Paclitaxel-Loaded Nanoparticles Potentiates Antitumor Effects in Malignant Glioblastoma. Mol Pharm. 2020;17:1193-204
16. Hou J, Diao Y, Li W, Yang Z, Zhang L, Chen Z. et al. RGD peptide conjugation results in enhanced antitumor activity of PD0325901 against glioblastoma by both tumor-targeting delivery and combination therapy. Int J Pharm. 2016;505:329-40
17. Gilbert MR, Kuhn J, Lamborn KR, Lieberman F, Wen PY, Mehta M. et al. Cilengitide in patients with recurrent glioblastoma: the results of NABTC 03-02, a phase II trial with measures of treatment delivery. J Neurooncol. 2012;106:147-53
18. Nabors LB, Mikkelsen T, Hegi ME, Ye X, Batchelor T, Lesser G. et al. A safety run-in and randomized phase 2 study of cilengitide combined with chemoradiation for newly diagnosed glioblastoma (NABTT 0306). Cancer. 2012;118:5601-7
19. Stupp R, Hegi ME, Gorlia T, Erridge SC, Perry J, Hong YK. et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1100-8
20. Nabors LB, Fink KL, Mikkelsen T, Grujicic D, Tarnawski R, Nam DH. et al. Two cilengitide regimens in combination with standard treatment for patients with newly diagnosed glioblastoma and unmethylated MGMT gene promoter: results of the open-label, controlled, randomized phase II CORE study. Neuro Oncol. 2015;17:708-17
21. Lang FF, Conrad C, Gomez-Manzano C, Yung WKA, Sawaya R, Weinberg JS. et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J Clin Oncol. 2018;36:1419-27
22. Barbieri F, Bajetto A, Pattarozzi A, Gatti M, Wurth R, Thellung S. et al. Peptide receptor targeting in cancer: the somatostatin paradigm. Int J Pept. 2013;2013:926295
23. Fricker G, Nobmann S, Miller DS. Permeability of porcine blood brain barrier to somatostatin analogues. Br J Pharmacol. 2002;135:1308-14
24. Mawrin C, Schulz S, Pauli SU, Treuheit T, Diete S, Dietzmann K. et al. Differential Expression of sst1, sst2A, and sst3 Somatostatin Receptor Proteins in Low-Grade and High-Grade Astrocytomas. J Neuropathol Exp Neurol. 2004;63:13-9
25. Pozsgai E, Schally AV, Halmos G, Rick F, Bellyei S. The Inhibitory Effect of a Novel Cytotoxic Somatostatin Analogue AN-162 on Experimental Glioblastoma. Horm Metab Res. 2010;42:781-6
26. Bhowmik A, Chakravarti S, Ghosh A, Shaw R, Bhandary S, Bhattacharyya S. et al. Anti-SSTR2 peptide based targeted delivery of potent PLGA encapsulated 3,3'-diindolylmethane nanoparticles through blood brain barrier prevents glioma progression. Oncotarget. 2017;8:65339-58
27. Kim JA, Casalini T, Brambilla D, Leroux J-C. Presumed LRP1-targeting transport peptide delivers β-secretase inhibitor to neurons in vitro with limited efficiency. Sci Rep. 2016;6:1-12
28. Wee P, Wang Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers (Basel). 2017;9:52
29. Barth RF, Wu G, Meisen WH, Nakkula RJ, Yang W, Huo T. et al. Design, synthesis, and evaluation of cisplatin-containing EGFR targeting bioconjugates as potential therapeutic agents for brain tumors. Onco Targets Ther. 2016;9:2769-81
30. Hamblett KJ, Kozlosky CJ, Siu S, Chang WS, Liu H, Foltz IN. et al. AMG 595, an anti-EGFRvIII antibody-drug conjugate, induces potent antitumor activity against EGFRvIII-expressing glioblastoma. Mol Cancer Ther. 2015;14:1614-24
31. Rosenthal M, Curry R, Reardon DA, Rasmussen E, Upreti VV, Damore MA. et al. Safety, tolerability, and pharmacokinetics of anti-EGFRvIII antibody-drug conjugate AMG 595 in patients with recurrent malignant glioma expressing EGFRvIII. Cancer Chemother Pharmacol. 2019;84:327-36
32. Guo Q, Zhu Q, Miao T, Tao J, Ju X, Sun Z. et al. LRP1-upregulated nanoparticles for efficiently conquering the blood-brain barrier and targetedly suppressing multifocal and infiltrative brain metastases. J Control Release. 2019;303:117-29
33. Khan NU, Ni J, Ju X, Miao T, Chen H, Han L. Escape from abluminal LRP1-mediated clearance for boosted nanoparticle brain delivery and brain metastasis treatment. Acta Pharm Sin B. 2021;11:1341-54
34. Kumthekar P, Tang S-C, Brenner AJ, Kesari S, Piccioni DE, Anders C. et al. ANG1005, a Brain-Penetrating Peptide-Drug Conjugate, Shows Activity in Patients with Breast Cancer with Leptomeningeal Carcinomatosis and Recurrent Brain Metastases. Clin Cancer Res. 2020;26:2789-99
35. Drappatz J, Brenner A, Wong ET, Eichler A, Schiff D, Groves MD. et al. Phase I study of GRN1005 in recurrent malignant glioma. Clin Cancer Res. 2013;19:1567-76
36. Ju X, Miao T, Chen H, Ni J, Han L. Overcoming Mfsd2a-Mediated Low Transcytosis to Boost Nanoparticle Delivery to Brain for Chemotherapy of Brain Metastases. Adv Healthc Mater. 2021;10:2001997
37. Miao T, Ju X, Zhu Q, Wang Y, Guo Q, Sun T. et al. Nanoparticles Surmounting Blood-Brain Tumor Barrier Through Both Transcellular and Paracellular Pathways to Target Brain Metastases. Adv Funct Mater. 2019;29:1900259
38. Han L. Modulation of the Blood-Brain Barrier for Drug Delivery to Brain. Pharmaceutics. 2021;13:2024
39. Jain A, Cheng K. The principles and applications of avidin-based nanoparticles in drug delivery and diagnosis. J Control Release. 2017;245:27-40
40. Pulkkinen M, Pikkarainen J, Wirth T, Tarvainen T, Haapa-aho V, Korhonen H. et al. Three-step tumor targeting of paclitaxel using biotinylated PLA-PEG nanoparticles and avidin-biotin technology: Formulation development and in vitro anticancer activity. Eur J Pharm Biopharm. 2008;70:66-74
41. Xia CF, Zhang Y, Zhang Y, Boado RJ, Pardridge WM. Intravenous siRNA of brain cancer with receptor targeting and avidin-biotin technology. Pharm Res. 2007;24:2309-16
42. Grana CM, Chinol M, De Cicco C, Bartolomei M, Cremonesi M, Bodei L. et al. Eleven-year experience with the avidin-biotin pretargeting system in glioblastoma: Toxicity, Efficacy and survival. Open Nucl Med J. 2012:14-20
43. Rahman M, Hoh B, Kohler N, Dunbar EM, Murad GJ. The future of glioma treatment: stem cells, nanotechnology and personalized medicine. Future Oncol. 2012;8:1149-56
44. Bhujbal SV, de Vos P, Niclou SP. Drug and cell encapsulation: alternative delivery options for the treatment of malignant brain tumors. Adv Drug Deliv Rev. 2014;67-68:142-53
45. Bredlau AL, Dixit S, Chen C, Broome AM. Nanotechnology Applications for Diffuse Intrinsic Pontine Glioma. Curr Neuropharmacol. 2017;15:104-15
46. Kempe K, Nicolazzo JA. Biodegradable Polymeric Nanoparticles for Brain-Targeted Drug Delivery. Nanomedicines for Brain Drug Delivery. New York, NY: Springer US. 2021 p. 1-27
47. Noble GT, Stefanick JF, Ashley JD, Kiziltepe T, Bilgicer B. Ligand-targeted liposome design: challenges and fundamental considerations. Trends Biotechnol. 2014;32:32-45
48. Vieira DB, Gamarra LF. Getting into the brain: liposome-based strategies for effective drug delivery across the blood-brain barrier. Int J Nanomedicine. 2016;11:5381-414
49. Ju R-J, Zeng F, Liu L, Mu L-M, Xie H-J, Zhao Y. et al. Destruction of vasculogenic mimicry channels by targeting epirubicin plus celecoxib liposomes in treatment of brain glioma. Int J Nanomedicine. 2016;11:1131
50. Tian W, Ying X, Du J, Guo J, Men Y, Zhang Y. et al. Enhanced efficacy of functionalized epirubicin liposomes in treating brain glioma-bearing rats. Eur J Pharm Sci. 2010;41:232-43
51. Charest G, Sanche L, Fortin D, Mathieu D, Paquette B. Optimization of the route of platinum drugs administration to optimize the concomitant treatment with radiotherapy for glioblastoma implanted in the Fischer rat brain. J Neurooncol. 2013;115:365-73
52. Huo T, Barth RF, Yang W, Nakkula RJ, Koynova R, Tenchov B. et al. Preparation, biodistribution and neurotoxicity of liposomal cisplatin following convection enhanced delivery in normal and F98 glioma bearing rats. PloS one. 2012;7:e48752-e
53. Kakinuma K, Tanaka R, Takahashi H, Watanabe M, Nakagawa T, Kuroki M. Targeting chemotherapy for malignant brain tumor using thermosensitive liposome and localized hyperthermia. J Neurosurg. 1996;84:180-4
54. Shibata S, Ochi A, Mori K. Liposomes as carriers of cisplatin into the central nervous system-experiments with 9L gliomas in rats. Neurol Med Chir (Tokyo). 1990;30:242-5
55. Firth GB, Firth M, McKeran RO, Rees J, Walter P, Uttley D. et al. Application of radioimmunoassay to monitor treatment of human cerebral gliomas with bleomycin entrapped within liposomes. J Clin Pathol. 1988;41:38-43
56. McKeran RO, Firth G, Oliver S, Uttley D, O'Laoire S. A potential application for the intracerebral injection of drugs entrapped within liposomes in the treatment of human cerebral gliomas. J Neurol Neurosurg Psychiatry. 1985;48:1213-9
57. Ashrafzadeh MS, Akbarzadeh A, Heydarinasab A, Ardjmand M. In vivo Glioblastoma Therapy Using Targeted Liposomal Cisplatin. Int J Nanomedicine. 2020;15:7035-49
58. Estella-Hermoso de Mendoza A, Préat V, Mollinedo F, Blanco-Prieto MJ. In vitro and in vivo efficacy of edelfosine-loaded lipid nanoparticles against glioma. J Control Release. 2011;156:421-6
59. Zhang J, Xiao X, Zhu J, Gao Z, Lai X, Zhu X. et al. Lactoferrin- and RGD-comodified, temozolomide and vincristine-coloaded nanostructured lipid carriers for gliomatosis cerebri combination therapy. Int J Nanomedicine. 2018;13:3039-51
60. Song S, Mao G, Du J, Zhu X. Novel RGD containing, temozolomide-loading nanostructured lipid carriers for glioblastoma multiforme chemotherapy. Drug Deliv. 2016;23:1404-8
61. Garcia-Pinel B, Porras-Alcala C, Ortega-Rodriguez A, Sarabia F, Prados J, Melguizo C. et al. Lipid-Based Nanoparticles: Application and Recent Advances in Cancer Treatment. Nanomaterials (Basel). 2019 9
62. Li XT, Ju RJ, Li XY, Zeng F, Shi JF, Liu L. et al. Multifunctional targeting daunorubicin plus quinacrine liposomes, modified by wheat germ agglutinin and tamoxifen, for treating brain glioma and glioma stem cells. Oncotarget. 2014;5:6497-511
63. Albrecht KW, de Witt Hamer PC, Leenstra S, Bakker PJ, Beijnen JH, Troost D. et al. High concentration of Daunorubicin and Daunorubicinol in human malignant astrocytomas after systemic administration of liposomal Daunorubicin. J Neurooncol. 2001;53:267-71
64. Fiorillo A, Maggi G, Greco N, Migliorati R, D'Amico A, De Caro MD. et al. Second-line chemotherapy with the association of liposomal daunorubicin, carboplatin and etoposide in children with recurrent malignant brain tumors. J Neurooncol. 2004;66:179-85
65. Ying X, Wen H, Lu WL, Du J, Guo J, Tian W. et al. Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals. J Control Release. 2010;141:183-92
66. Zucchetti M, Boiardi A, Silvani A, Parisi I, Piccolrovazzi S, D'Incalci M. Distribution of daunorubicin and daunorubicinol in human glioma tumors after administration of liposomal daunorubicin. Cancer Chemother Pharmacol. 1999;44:173-6
67. Chastagner P, Devictor B, Geoerger B, Aerts I, Leblond P, Frappaz D. et al. Phase I study of non-pegylated liposomal doxorubicin in children with recurrent/refractory high-grade glioma. Cancer Chemother Pharmacol. 2015;76:425-32
68. Chastagner P, Labussiere M, Aarnink A, Pinel S, Fouyssac F, Bernier V. Comparison of doxorubicin and a liposomal form, doxorubicine chlorhydrate, as radiosensitizer in high grade glioma and rhabdomyosarcoma xenografts; 2006
69. Chirio D, Gallarate M, Peira E, Battaglia L, Muntoni E, Riganti C. et al. Positive-charged solid lipid nanoparticles as paclitaxel drug delivery system in glioblastoma treatment. Eur J Pharm Biopharm. 2014;88:746-58
70. Guo Z, Zhang P, Chakraborty S, Chetwynd AJ, Abdolahpur Monikh F, Stark C. et al. Biotransformation modulates the penetration of metallic nanomaterials across an artificial blood-brain barrier model. Proc Natl Acad Sci U S A. 2021 118
71. Thomsen LB, Thomsen MS, Moos T. Targeted drug delivery to the brain using magnetic nanoparticles. Ther Deliv. 2015;6:1145-55
72. Busquets MA, Espargaró A, Sabaté R, Estelrich J. Magnetic Nanoparticles Cross the Blood-Brain Barrier: When Physics Rises to a Challenge. Nanomaterials (Basel). 2015;5:2231-48
73. Naqvi S, Samim M, Abdin M, Ahmed FJ, Maitra A, Prashant C. et al. Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. Int J Nanomedicine. 2010;5:983-9
74. Coluccia D, Figueiredo CA, Wu MY, Riemenschneider AN, Diaz R, Luck A. et al. Enhancing glioblastoma treatment using cisplatin-gold-nanoparticle conjugates and targeted delivery with magnetic resonance-guided focused ultrasound. Nanomedicine. 2018;14:1137-48
75. Cheng Y, Dai Q, Morshed RA, Fan X, Wegscheid ML, Wainwright DA. et al. Blood-brain barrier permeable gold nanoparticles: An efficient delivery platform for enhanced malignant glioma therapy and imaging. Small. 2014;10:5137-50
76. Ung C, Tsoli M, Liu J, Cassano D, Pocovi-Martinez S, Upton DH. et al. Doxorubicin-Loaded Gold Nanoarchitectures as a Therapeutic Strategy against Diffuse Intrinsic Pontine Glioma. Cancers (Basel). 2021 13
77. Orza A, Soriţău O, Tomuleasa C, Olenic L, Florea A, Pana O. et al. Reversing chemoresistance of malignant glioma stem cells using gold nanoparticles. Int J Nanomedicine. 2013;8:689-702
78. Dhar S, Reddy EM, Shiras A, Pokharkar V, Prasad BL. Natural gum reduced/stabilized gold nanoparticles for drug delivery formulations. Chemistry. 2008;14:10244-50
79. Jensen SA, Day ES, Ko CH, Hurley LA, Luciano JP, Kouri FM. et al. Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Sci Transl Med. 2013;5:209ra152
80. Norouzi M, Yathindranath V, Thliveris JA, Kopec BM, Siahaan TJ, Miller DW. Doxorubicin-loaded iron oxide nanoparticles for glioblastoma therapy: a combinational approach for enhanced delivery of nanoparticles. Sci Rep. 2020;10:11292
81. Liu H, Zhang J, Chen X, Du XS, Zhang JL, Liu G. et al. Application of iron oxide nanoparticles in glioma imaging and therapy: from bench to bedside. Nanoscale. 2016;8:7808-26
82. Mu Q, Lin G, Patton VK, Wang K, Press OW, Zhang M. Gemcitabine and Chlorotoxin Conjugated Iron Oxide Nanoparticles for Glioblastoma Therapy. J Mater Chem B. 2016;4:32-6
83. Chang Y, Li Y, Meng X, Liu N, Sun D, Liu H. et al. Dendrimer functionalized water soluble magnetic iron oxide conjugates as dual imaging probe for tumor targeting and drug delivery. Polym Chem. 2013;4:789-94
84. Shen B, Ma Y, Yu S, Ji C. Smart Multifunctional Magnetic Nanoparticle-Based Drug Delivery System for Cancer Thermo-Chemotherapy and Intracellular Imaging. ACS Appl Mater Interfaces. 2016;8:24502-8
85. Huang WC, Lu IL, Chiang WH, Lin YW, Tsai YC, Chen HH. et al. Tumortropic adipose-derived stem cells carrying smart nanotherapeutics for targeted delivery and dual-modality therapy of orthotopic glioblastoma. J Control Release. 2017;254:119-30
86. Zhao M, Liang C, Li A, Chang J, Wang H, Yan R. et al. Magnetic paclitaxel nanoparticles inhibit glioma growth and improve the survival of rats bearing glioma xenografts. Anticancer Res. 2010;30:2217-23
87. Thomsen LB, Linemann T, Pondman KM, Lichota J, Kim KS, Pieters RJ. et al. Uptake and transport of superparamagnetic iron oxide nanoparticles through human brain capillary endothelial cells. ACS Chem Neurosci. 2013;4:1352-60
88. Shi D, Mi G, Bhattacharya S, Nayar S, Webster TJ. Optimizing superparamagnetic iron oxide nanoparticles as drug carriers using an in vitro blood-brain barrier model. Int J Nanomedicine. 2016;11:5371-9
89. Cabada TF, de Pablo CSL, Serrano AM, del Pozo Guerrero F, Olmedo JJS, Gomez MR. Induction of cell death in a glioblastoma line by hyperthermic therapy based on gold nanorods. Int J Nanomedicine. 2012;7:1511
90. Zhang W, Mehta A, Tong Z, Esser L, Voelcker NH. Development of Polymeric Nanoparticles for Blood-Brain Barrier Transfer-Strategies and Challenges. Adv Sci (Weinh). 2021;8:2003937 -
91. Fisher DG, Price RJ. Recent Advances in the Use of Focused Ultrasound for Magnetic Resonance Image-Guided Therapeutic Nanoparticle Delivery to the Central Nervous System. Front Pharmacol. 2019;10:1348
92. Grover A, Hirani A, Pathak Y, Sutariya V. Brain-targeted delivery of docetaxel by glutathione-coated nanoparticles for brain cancer. AAPS PharmSciTech. 2014;15:1562-8
93. Nance E, Zhang C, Shih TY, Xu Q, Schuster BS, Hanes J. Brain-penetrating nanoparticles improve paclitaxel efficacy in malignant glioma following local administration. ACS Nano. 2014;8:10655-64
94. Wilson B. Brain targeting PBCA nanoparticles and the blood-brain barrier. Nanomedicine. 2009;4:499-502
95. Steiniger SC, Kreuter J, Khalansky AS, Skidan IN, Bobruskin AI, Smirnova ZS. et al. Chemotherapy of glioblastoma in rats using doxorubicin-loaded nanoparticles. Int J Cancer. 2004;109:759-67
96. Mastorakos P, Zhang C, Song E, Kim YE, Park HW, Berry S. et al. Biodegradable brain-penetrating DNA nanocomplexes and their use to treat malignant brain tumors. J Control Release. 2017;262:37-46
97. Kreuter J, Alyautdin RN, Kharkevich DA, Ivanov AA. Passage of peptides through the blood-brain barrier with colloidal polymer particles (nanoparticles). Brain Res. 1995;674:171-4
98. Gulyaev AE, Gelperina SE, Skidan IN, Antropov AS, Kivman GY, Kreuter J. Significant transport of doxorubicin into the brain with polysorbate 80-coated nanoparticles. Pharm Res. 1999;16:1564-9
99. Xin H, Jiang X, Gu J, Sha X, Chen L, Law K. et al. Angiopep-conjugated poly(ethylene glycol)-co-poly(ε-caprolactone) nanoparticles as dual-targeting drug delivery system for brain glioma. Biomaterials. 2011;32:4293-305
100. Brown RC, Egleton RD, Davis TP. Mannitol opening of the blood-brain barrier: regional variation in the permeability of sucrose, but not 86Rb+ or albumin. Brain Res. 2004;1014:221-7
101. Kiviniemi V, Korhonen V, Kortelainen J, Rytky S, Keinänen T, Tuovinen T. et al. Real-time monitoring of human blood-brain barrier disruption. PLoS One. 2017;12:e0174072
102. Doolittle ND, Miner ME, Hall WA, Siegal T, Jerome E, Osztie E. et al. Safety and efficacy of a multicenter study using intraarterial chemotherapy in conjunction with osmotic opening of the blood-brain barrier for the treatment of patients with malignant brain tumors. Cancer. 2000;88:637-47
103. McAllister LD, Doolittle ND, Guastadisegni PE, Kraemer DF, Lacy CA, Crossen JR. et al. Cognitive Outcomes and Long-term Follow-up Results after Enhanced Chemotherapy Delivery for Primary Central Nervous System Lymphoma. Neurosurgery. 2000;46:51-61
104. Parodi A, Rudzińska M, Deviatkin AA, Soond SM, Baldin AV, Zamyatnin AA. Established and Emerging Strategies for Drug Delivery Across the Blood-Brain Barrier in Brain Cancer. Pharmaceutics. 2019;11:245
105. Tournier N, Goutal S, Auvity S, Traxl A, Mairinger S, Wanek T. et al. Strategies to Inhibit ABCB1- and ABCG2-Mediated Efflux Transport of Erlotinib at the Blood-Brain Barrier: A PET Study on Nonhuman Primates. J Nucl Med. 2017;58:117-22
106. Chen Y, Agarwal S, Shaik NM, Chen C, Yang Z, Elmquist WF. P-glycoprotein and breast cancer resistance protein influence brain distribution of dasatinib. J Pharmacol Exp Ther. 2009;330:956-63
107. Agarwal S, Sane R, Gallardo JL, Ohlfest JR, Elmquist WF. Distribution of gefitinib to the brain is limited by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2)-mediated active efflux. J Pharmacol Exp Ther. 2010;334:147-55
108. Agarwal S, Sane R, Ohlfest JR, Elmquist WF. The role of the breast cancer resistance protein (ABCG2) in the distribution of sorafenib to the brain. J Pharmacol Exp Ther. 2011;336:223-33
109. Fallacara AL, Zamperini C, Podolski-Renić A, Dinić J, Stanković T, Stepanović M. et al. A New Strategy for Glioblastoma Treatment: In Vitro and In Vivo Preclinical Characterization of Si306, a Pyrazolo[3,4-d]Pyrimidine Dual Src/P-Glycoprotein Inhibitor. Cancers (Basel). 2019;11:848
110. Vignaroli G, Iovenitti G, Zamperini C, Coniglio F, Calandro P, Molinari A. et al. Prodrugs of Pyrazolo[3,4-d]pyrimidines: From Library Synthesis to Evaluation as Potential Anticancer Agents in an Orthotopic Glioblastoma Model. J Med Chem. 2017;60:6305-20
111. Becker CM, Oberoi RK, McFarren SJ, Muldoon DM, Pafundi DH, Pokorny JL. et al. Decreased affinity for efflux transporters increases brain penetrance and molecular targeting of a PI3K/mTOR inhibitor in a mouse model of glioblastoma. Neuro Oncol. 2015;17:1210-9
112. Strope JD, Peer CJ, Sissung TM, Hall OM, Huang PA, Harris EM. et al. Botryllamide G is an ABCG2 inhibitor that improves lapatinib delivery in mouse brain. Cancer Biol Ther. 2020;21:223-30
113. Fox E, Widemann BC, Pastakia D, Chen CC, Yang SX, Cole D. et al. Pharmacokinetic and pharmacodynamic study of tariquidar (XR9576), a P-glycoprotein inhibitor, in combination with doxorubicin, vinorelbine, or docetaxel in children and adolescents with refractory solid tumors. Cancer Chemother Pharmacol. 2015;76:1273-83
114. Ferreri AJM, Calimeri T, Conte GM, Cattaneo D, Fallanca F, Ponzoni M. et al. R-CHOP preceded by blood-brain barrier permeabilization with engineered tumor necrosis factor-alpha in primary CNS lymphoma. Blood. 2019
115. Sacchi A, Gasparri A, Gallo-Stampino C, Toma S, Curnis F, Corti A. Synergistic antitumor activity of cisplatin, paclitaxel, and gemcitabine with tumor vasculature-targeted tumor necrosis factor-alpha. Clin Cancer Res. 2006;12:175-82
116. Corti A, Curnis F, Rossoni G, Marcucci F, Gregorc V. Peptide-mediated targeting of cytokines to tumor vasculature: the NGR-hTNF example. BioDrugs. 2013;27:591-603
117. Yang F-Y, Wang H-E, Lin G-L, Lin H-H, Wong T-T. Evaluation of the increase in permeability of the blood-brain barrier during tumor progression after pulsed focused ultrasound. Int J Nanomedicine. 2012;7:723
118. Xie F, Boska MD, Lof J, Uberti MG, Tsutsui JM, Porter TR. Effects of transcranial ultrasound and intravenous microbubbles on blood brain barrier permeability in a large animal model. Ultrasound Med Biol. 2008;34:2028-34
119. Chen H, Chen CC, Acosta C, Wu S-Y, Sun T, Konofagou EE. A New Brain Drug Delivery Strategy: Focused Ultrasound-Enhanced Intranasal Drug Delivery. PLoS One. 2014;9:e108880
120. Treat LH, McDannold N, Zhang Y, Vykhodtseva N, Hynynen K. Improved anti-tumor effect of liposomal doxorubicin after targeted blood-brain barrier disruption by MRI-guided focused ultrasound in rat glioma. Ultrasound Med Biol. 2012;38:1716-25
121. Aryal M, Vykhodtseva N, Zhang YZ, Park J, McDannold N. Multiple treatments with liposomal doxorubicin and ultrasound-induced disruption of blood-tumor and blood-brain barriers improve outcomes in a rat glioma model. J Control Release. 2013;169:103-11
122. Wei K-C, Chu P-C, Wang H-YJ, Huang C-Y, Chen P-Y, Tsai H-C. et al. Focused ultrasound-induced blood-brain barrier opening to enhance temozolomide delivery for glioblastoma treatment: a preclinical study. PloS One. 2013 8
123. Liu H-L, Huang C-Y, Chen J-Y, Wang H-YJ, Chen P-Y, Wei K-C. Pharmacodynamic and therapeutic investigation of focused ultrasound-induced blood-brain barrier opening for enhanced temozolomide delivery in glioma treatment. PloS one. 2014 9
124. Liu H-L, Hua M-Y, Chen P-Y, Chu P-C, Pan C-H, Yang H-W. et al. Blood-brain barrier disruption with focused ultrasound enhances delivery of chemotherapeutic drugs for glioblastoma treatment. Radiology. 2010;255:415-25
125. Liu H-L, Hsu P-H, Lin C-Y, Huang C-W, Chai W-Y, Chu P-C. et al. Focused ultrasound enhances central nervous system delivery of bevacizumab for malignant glioma treatment. Radiology. 2016;281:99-108
126. Mei J, Cheng Y, Song Y, Yang Y, Wang F, Liu Y. et al. Experimental study on targeted methotrexate delivery to the rabbit brain via magnetic resonance imaging-guided focused ultrasound. J Ultrasound Med. 2009;28:871-80
127. Liu HL, Chen PY, Yang HW, Wu JS, Tseng IC, Ma YJ. et al. In vivo MR quantification of superparamagnetic iron oxide nanoparticle leakage during low-frequency-ultrasound-induced blood-brain barrier opening in swine. J Magn Reson Imaging. 2011;34:1313-24
128. Liu H-L, Hua M-Y, Yang H-W, Huang C-Y, Chu P-C, Wu J-S. et al. Magnetic resonance monitoring of focused ultrasound/magnetic nanoparticle targeting delivery of therapeutic agents to the brain. Proc Natl Acad Sci U S A. 2010;107:15205
129. Burgess A, Huang Y, Querbes W, Sah DW, Hynynen K. Focused ultrasound for targeted delivery of siRNA and efficient knockdown of Htt expression. J Control Release. 2012;163:125-9
130. Alkins R, Burgess A, Ganguly M, Francia G, Kerbel R, Wels WS. et al. Focused ultrasound delivers targeted immune cells to metastatic brain tumors. Cancer Res. 2013;73:1892-9
131. Carpentier A, Canney M, Vignot A, Reina V, Beccaria K, Horodyckid C. et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci Transl Med. 2016;8:343re2
132. Idbaih A, Canney M, Belin L, Desseaux C, Vignot A, Bouchoux G. et al. Safety and Feasibility of Repeated and Transient Blood-Brain Barrier Disruption by Pulsed Ultrasound in Patients with Recurrent Glioblastoma. Clin Cancer Res. 2019: clincanres.3643. 2018
133. Moding EJ, Kastan MB, Kirsch DG. Strategies for optimizing the response of cancer and normal tissues to radiation. Nat Rev Drug Discov. 2013;12:526-42
134. Appelboom G, Detappe A, LoPresti M, Kunjachan S, Mitrasinovic S, Goldman S. et al. Stereotactic modulation of blood-brain barrier permeability to enhance drug delivery. Neuro Oncol. 2016;18:1601-9
135. Fauquette W, Amourette C, Dehouck M-P, Diserbo M. Radiation-induced blood-brain barrier damages: An in vitro study. Brain Res. 2012;1433:114-26
136. Sprowls SA, Arsiwala TA, Kielkowski BN, Pizzuti V, Siochi RAC, Lockman PR. Radiation increases BTB permeability in a preclinical model of breast cancer brain metastasis. 2020.
137. Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J. et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86:343-9
138. Davies AM, Weinberg U, Palti Y. Tumor treating fields: a new frontier in cancer therapy. Ann N Y Acad Sci. 2013;1291:86-95
139. Neal R, Rossmeisl J, D'alfonso V, Robertson J, Garcia P, Elankumaran S. et al. In vitro and numerical support for combinatorial irreversible electroporation and electrochemotherapy glioma treatment. Ann Biomed Eng. 2014;42:475-87
140. Kirson ED, Dbalý V, Tovaryš F, Vymazal J, Soustiel JF, Itzhaki A. et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci U S A. 2007;104:10152-7
141. Kirson ED, Schneiderman RS, Dbalý V, Tovaryš F, Vymazal J, Itzhaki A. et al. Chemotherapeutic treatment efficacy and sensitivity are increased by adjuvant alternating electric fields (TTFields). BMC Med Phys. 2009;9:1-13
142. Schneiderman R, Kirson E, Vymazal J, Vlasek T, Burianova Z, Itzhaki A. et al. Synergism between chemotherapy and alternating electric fields (TTFields) in cancer cell proliferation inhibition and solid tumor treatment. AACR. 2008
143. Rodrigues G, Yartsev S, Tay KY, Pond GR, Lagerwaard F, Bauman G. A phase II multi-institutional study assessing simultaneous in-field boost helical tomotherapy for 1-3 brain metastases. Radiat Oncol. 2012;7:42
144. Ansstas G, Tran DD. Treatment with Tumor-Treating Fields Therapy and Pulse Dose Bevacizumab in Patients with Bevacizumab-Refractory Recurrent Glioblastoma: A Case Series. Case Rep Neurol. 2016;8:1-9
145. Kiessling M, Herchenhan E, Eggert HR. Cerebrovascular and metabolic effects on the rat brain of focal Nd: YAG laser irradiation. J Neurosurg. 1990;73:909-17
146. Choi M, Ku T, Chong K, Yoon J, Choi C. Minimally invasive molecular delivery into the brain using optical modulation of vascular permeability. Proc Natl Acad Sci U S A. 2011;108:9256-61
147. Salehi A, Paturu M, Caine M, Klein R, Kim AH. Laser Therapy Enhances Blood-Brain Barrier and Blood-Tumor Barrier Permeability in a Mouse Model of Glioblastoma. Neurosurgery. 2019 66
148. Ahluwalia M, Barnett GH, Deng D, Tatter SB, Laxton AW, Mohammadi AM. et al. Laser ablation after stereotactic radiosurgery: a multicenter prospective study in patients with metastatic brain tumors and radiation necrosis. J Neurosurg. 2018;130:804-11
149. Leuthardt EC, Duan C, Kim MJ, Campian JL, Kim AH, Miller-Thomas MM. et al. Hyperthermic Laser Ablation of Recurrent Glioblastoma Leads to Temporary Disruption of the Peritumoral Blood Brain Barrier. PLoS One. 2016;11:e0148613
150. Ahluwalia M, Barnett GH, Deng D, Tatter SB, Laxton AW, Mohammadi AM. et al. Laser ablation after stereotactic radiosurgery: a multicenter prospective study in patients with metastatic brain tumors and radiation necrosis. J Neurosurg. 2019;130:804
151. Soderquist RG, Mahoney MJ. Central nervous system delivery of large molecules: challenges and new frontiers for intrathecally administered therapeutics. Expert Opin Drug Deliv. 2010;7:285-93
152. Smith HS, Deer TR, Staats PS, Singh V, Sehgal N, Cordner H. Intrathecal drug delivery. Pain Physician. 2008;11:S89-s104
153. Mizumatsu S, Matsumoto K, Ono Y, Tamiya T, Furuta T, Ohmoto T. Intrathecal Chemotherapy with MX2 for Treating Glioma Dissemination in vivo. J Neurooncol. 2000;49:41-7
154. Witham TF, Fukui MB, Meltzer CC, Burns R, Kondziolka D, Bozik ME. Survival of patients with high grade glioma treated with intrathecal thiotriethylenephosphoramide for ependymal or leptomeningeal gliomatosis. Cancer. 1999;86:1347-53
155. Triarico S, Maurizi P, Mastrangelo S, Attinà G, Capozza MA, Ruggiero A. Improving the Brain Delivery of Chemotherapeutic Drugs in Childhood Brain Tumors. Cancers (Basel). 2019;11:824
156. Pardeshi CV, Belgamwar VS. Direct nose to brain drug delivery via integrated nerve pathways bypassing the blood-brain barrier: an excellent platform for brain targeting. Expert Opin Drug Deliv. 2013;10:957-72
157. Wang F, Jiang X, Lu W. Profiles of methotrexate in blood and CSF following intranasal and intravenous administration to rats. Int J Pharm. 2003;263:1-7
158. Wang D, Gao Y, Yun L. Study on brain targeting of raltitrexed following intranasal administration in rats. Cancer Chemother Pharmacol. 2006;57:97-104
159. Shingaki T, Hidalgo IJ, Furubayashi T, Katsumi H, Sakane T, Yamamoto A. et al. The transnasal delivery of 5-fluorouracil to the rat brain is enhanced by acetazolamide (the inhibitor of the secretion of cerebrospinal fluid). Int J Pharm. 2009;377:85-91
160. Hashizume R, Ozawa T, Gryaznov SM, Bollen AW, Lamborn KR, Frey WH, II. et al. New therapeutic approach for brain tumors: Intranasal delivery of telomerase inhibitor GRN163. Neuro Oncol. 2008;10:112-20
161. Mukherjee S, Baidoo J, Fried A, Atwi D, Dolai S, Boockvar J. et al. Curcumin changes the polarity of tumor-associated microglia and eliminates glioblastoma. Int J Cancer. 2016;139:2838-49
162. Blacher E, Ben Baruch B, Levy A, Geva N, Green KD, Garneau-Tsodikova S. et al. Inhibition of glioma progression by a newly discovered CD38 inhibitor. Int J Cancer. 2015;136:1422-33
163. Shingaki T, Inoue D, Furubayashi T, Sakane T, Katsumi H, Yamamoto A. et al. Transnasal delivery of methotrexate to brain tumors in rats: a new strategy for brain tumor chemotherapy. Mol Pharm. 2010;7:1561-8
164. Sun Y, Shi K, Wan F, Cui F-d. Methotrexate-loaded microspheres for nose to brain delivery: in vitro/in vivo evaluation. J Drug Deliv Sci Technol. 2012;22:167-74
165. Li Y, Gao Y, Liu G, Zhou X, Wang Y, Wang Y. et al. Intranasal administration of temozolomide for brain-targeting delivery: therapeutic effect on glioma in rats. Nan Fang Yi Ke Da Xue Xue Bao. 2014;34:631-5
166. da Fonseca CO, Schwartsmann G, Fischer J, Nagel J, Futuro D, Quirico-Santos T. et al. Preliminary results from a phase I/II study of perillyl alcohol intranasal administration in adults with recurrent malignant gliomas. Surg Neurol. 2008;70:259-66 discussion 66-7
167. CO DAF, Teixeira RM, Silva JC, J DESDGF, Meirelles OC, Landeiro JA. et al. Long-term outcome in patients with recurrent malignant glioma treated with Perillyl alcohol inhalation. Anticancer Res. 2013;33:5625-31
168. da Fonseca CO, Simão M, Lins IR, Caetano RO, Futuro D, Quirico-Santos T. Efficacy of monoterpene perillyl alcohol upon survival rate of patients with recurrent glioblastoma. J Cancer Res Clin Oncol. 2011;137:287-93
169. Erdő F, Bors LA, Farkas D, Bajza Á, Gizurarson S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res Bull. 2018;143:155-70
170. Chaichana KL, Pinheiro L, Brem H. Delivery of local therapeutics to the brain: working toward advancing treatment for malignant gliomas. Ther Deliv. 2015;6:353-69
171. Wang W, Cho HY, Rosenstein-Sisson R, Marín Ramos NI, Price R, Hurth K. et al. Intratumoral delivery of bortezomib: impact on survival in an intracranial glioma tumor model. J Neurosurg. 2018;128:695-700
172. Nagpal S. The role of BCNU polymer wafers (Gliadel) in the treatment of malignant glioma. Neurosurg Clin N Am. 2012;23:289-95 ix
173. Shapira-Furman T, Serra R, Gorelick N, Doglioli M, Tagliaferri V, Cecia A. et al. Biodegradable wafers releasing Temozolomide and Carmustine for the treatment of brain cancer. J Control Release. 2019;295:93-101
174. Ashby LS, Smith KA, Stea B. Gliadel wafer implantation combined with standard radiotherapy and concurrent followed by adjuvant temozolomide for treatment of newly diagnosed high-grade glioma: a systematic literature review. World J Surg Oncol. 2016;14:225
175. Sardi I, Sanzo M, Giordano F, Sandri A, Mussa F, Donati PA. et al. Intracavitary chemotherapy (Gliadel) and oral low-dose etoposide for recurrent anaplastic ependymoma. Oncol Rep. 2008;19:1219-23
176. Quinn JA, Jiang SX, Carter J, Reardon DA, Desjardins A, Vredenburgh JJ. et al. Phase II trial of Gliadel plus O6-benzylguanine in adults with recurrent glioblastoma multiforme. Clin Cancer Res. 2009;15:1064-8
177. Tsai N-M, Chen Y-L, Lee C-C, Lin P-C, Cheng Y-L, Chang W-L. et al. The natural compound n-butylidenephthalide derived from Angelica sinensis inhibits malignant brain tumor growth in vitro and in vivo. J Neurochem. 2006;99:1251-62
178. Patchell RA, Regine WF, Ashton P, Tibbs PA, Wilson D, Shappley D. et al. A Phase I Trial of Continuously Infused Intratumoral Bleomycin for the Treatment of Recurrent Glioblastoma Multiforme. J Neurooncol. 2002;60:37-42
179. Reardon DA, Akabani G, Edward Coleman R, Friedman AH, Friedman HS, Herndon JE. et al. Phase II Trial of Murine 131I-Labeled Antitenascin Monoclonal Antibody 81C6 Administered Into Surgically Created Resection Cavities of Patients With Newly Diagnosed Malignant Gliomas. J Clin Oncol. 2002;20:1389-97
180. Quinn JA, Jiang SX, Carter J, Reardon DA, Desjardins A, Vredenburgh JJ. et al. Phase II trial of Gliadel plus O6-benzylguanine in adults with recurrent glioblastoma multiforme. Clin Cancer Res. 2009;15:1064-8
181. Mehta AM, Sonabend AM, Bruce JN. Convection-Enhanced Delivery. Neurotherapeutics. 2017;14:358-71
182. Sewing ACP, Lagerweij T, van Vuurden DG, Meel MH, Veringa SJE, Carcaboso AM. et al. Preclinical evaluation of convection-enhanced delivery of liposomal doxorubicin to treat pediatric diffuse intrinsic pontine glioma and thalamic high-grade glioma. J Neurosurg Pediatr. 2017;19:518-30
183. Weaver M, Laske DW. Transferrin receptor ligand-targeted toxin conjugate (Tf-CRM107) for therapy of malignant gliomas. J Neurooncol. 2003;65:3-13
184. Kunwar S, Chang S, Westphal M, Vogelbaum M, Sampson J, Barnett G. et al. Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro Oncol. 2010;12:871-81
185. Heiss JD, Jamshidi A, Shah S, Martin S, Wolters PL, Argersinger DP. et al. Phase I trial of convection-enhanced delivery of IL13-Pseudomonas toxin in children with diffuse intrinsic pontine glioma. Journal of neurosurgery Pediatrics. 2018;23:333-42
186. Lidar Z, Mardor Y, Jonas T, Pfeffer R, Faibel M, Nass D. et al. Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: a phase I/II clinical study. J Neurosurg. 2004;100:472-9
187. Bruce JN, Fine RL, Canoll P, Yun J, Kennedy BC, Rosenfeld SS. et al. Regression of recurrent malignant gliomas with convection-enhanced delivery of topotecan. Neurosurgery. 2011;69:1272-80
188. Anderson RC, Kennedy B, Yanes CL, Garvin J, Needle M, Canoll P. et al. Convection-enhanced delivery of topotecan into diffuse intrinsic brainstem tumors in children. J Neurosurg Pediatr. 2013;11:289-95
Author contact
![]() Corresponding author: E-mail: d.ziegleredu.au.
Corresponding author: E-mail: d.ziegleredu.au.
 Global reach, higher impact
Global reach, higher impact