13.3
Impact Factor
Theranostics 2022; 12(10):4629-4655. doi:10.7150/thno.72465 This issue Cite
Review
How to improve photodynamic therapy-induced antitumor immunity for cancer treatment?
1. Department of Pharmaceutics, School of Pharmacy, Qingdao University, Qingdao 266021, China
2. Institute of Biomedical Materials and Engineering, College of Materials Sciences and Engineering, Qingdao University, Qingdao 266071, China
# These authors contributed equally to this review
Received 2022-2-28; Accepted 2022-4-27; Published 2022-5-29
Abstract
Photodynamic therapy (PDT) is a promising method of tumor ablation and function-preserving oncological intervention, which is minimally invasive, repeatable, and has excellent function and cosmetic effect, with no cumulative toxicity. More importantly, PDT can induce immunogenic cell death and local inflammation, thus stimulating the body's immune response. However, the weak immunity induced by PDT alone is insufficient to trigger a systemic immune response towards cancer cells. To overcome this obstacle, multiple strategies have been investigated, including tumor microenvironment remodeling, tumor vaccines, subcellular-targeted PDT, and synergistic therapies. This review summarizes the latest progress in the development of strategies to improve the PDT-induced immune effect for enhanced cancer treatment.
Keywords: Photodynamic therapy, Antitumor immunity, Tumor microenvironment, Tumor vaccine, Synergistic therapies
1. Introduction
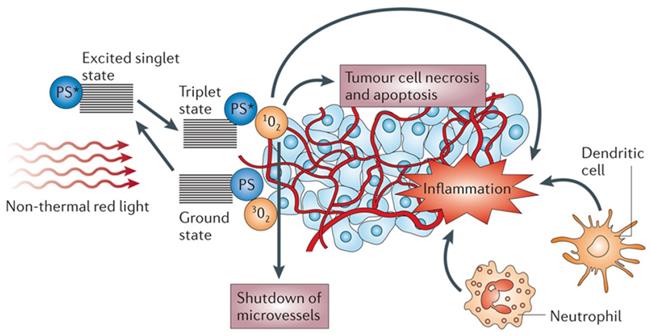
Photodynamic therapy (PDT) is minimally invasive, with excellent function and no cumulative toxicity. PDT has been approved by the US Food and Drug Administration for the clinical treatment of various malignant tumors, including breast cancer, bladder cancer, and esophageal cancer [1-3]. PDT has attracted increasing interest since Dougherty et al. [4] first demonstrated its application in 1975. PDT involves the local injection of a photosensitizer (PS) and local irradiation of the tumor with a specific wavelength of light to activate the PS. Subsequently, the excited PS transfers its energy to O2, resulting in cytotoxic reactive oxygen species (ROS), such as singlet oxygen (1O2), which can oxidize key cellular macromolecules and lead to tumor cell ablation [5, 6]. PDT not only uses ROS to kill tumor cells and target tumor blood vessels but also activates the immune system to induce inflammation and immune response to tumor cells [7-10] (Figure 1).
It has been widely accepted that PDT can effectively stimulate both innate and adaptive immunity by immunogenic cell death (ICD). This form of cell death releases damage-associated molecular patterns (DAMPs), including calreticulin (CRT), high mobility group box 1 protein (HMGB1), adenosine triphosphate (ATP), and heat shock proteins (HSPs) [11, 12], leading to activation of the immune system, particularly by inducing the maturation of antigen presenting cells (APCs), which eventually migrate to lymph nodes, where cross-presentation of antigens to immature T cells triggers differentiation into cytotoxic CD8+ T cells. In addition, cytokines, such as interferon-γ (INF-γ), tumor necrosis factor-α (TNF-α), and interleukins (such as IL-2, IL-6, and IL-12), have also been reported to increase and contribute to immune stimulation [13]. Therefore, photodynamically-induced ICD can activate specific immunogenicity and improve the therapeutic effect.
The mechanism of action on tumors in photodynamic therapy. Adapted with permission from [9], copyright 2006 Springer Nature.
However, in most cases, PDT alone is insufficient to induce an adequate immune response as tumor cells also secrete immunosuppressive cytokines or other tumor-promoting molecules through non-immunogenic pathways, resulting in an immune-suppressive microenvironment, which inhibits the anticancer immune response. Hence, many studies have focused on improving the PDT-induced immune response, and have made significant progress. For instance, PDT combined with angiogenesis inhibitors can overcome the immunosuppression caused by hypoxia [14], while PDT combined with drug-targeted immunosuppression directly destroys the structure of the tumor microenvironment (TME) to improve PDT-induced anti-cancer immunity [15]. Furthermore, in situ vaccines can be used to induce repeated cycles of immune initiation-immune effect-tumor cell death-antigen release, which can lead to an immune restart-immune effect in the local tumor to maximize the effect of anti-tumor immunity [16].
Recently, with the development of tumor immunology, some scholars have put forward the concepts of “cold” and “hot” tumors, which have gained attention in the context of anti-tumor immune research [17-19]. The terms “hot” and “cold” have been commonly used to refer to T-cell infiltrating, inflamed but uninfiltrating, and uninflamed tumors. In the simplest terms, the so-called “cold” tumor is a non-immunogenic tumor with immunosuppression and insufficient T cell infiltration, while the “hot” tumor is the opposite. “Cold” tumors are good at camouflaging themselves from the immune system, thereby reducing the effectiveness of the immune response, which brings great obstacles to anti-tumor immunotherapy. Regulating the immune process to transform “cold” tumors into “hot” tumors has become a research hotspot in anti-tumor immunotherapy. Since researchers first reported that ICD can stimulate anti-tumor immunity, PDT-induced ICD has represented an attractive method to treat “cold” tumors. It is worth mentioning that the immune checkpoints have been shown to have a great prospect in cancer treatment, and their combination with PDT improves the prognosis of cancer and provides better control and effect for anti-tumor immunity [20]. Additionally, PDT combined with other methods (such as photothermal therapy (PTT) [6, 21], chemotherapy [22-24], and radiotherapy [25]) can better increase the recruitment of dendritic cells (DCs), promote the development of CD8+ T cells, regulate the TME, and activate host immune function.
Therefore, a comprehensive and in-depth depiction of the recent development of PDT-induced immune response is desirable. Compared to other current reviews, which mainly focus on nanoparticles [26], or a single aspect (such as TME [27], mitochondria [28]), and with the further study of anti-tumor immune mechanisms, tumor vaccines have become the focus of immunotherapy research. However, to the best of our knowledge, there are few studies on the relationship between PDT and tumor vaccines, and no related review has been published. Thus, in this review, we comprehensively summarize the recent research on improving PDT-induced anti-tumor immunity, including reprogramming the TME, constructing tumor vaccines, and synergistic therapies (Figure 2, Table 1).
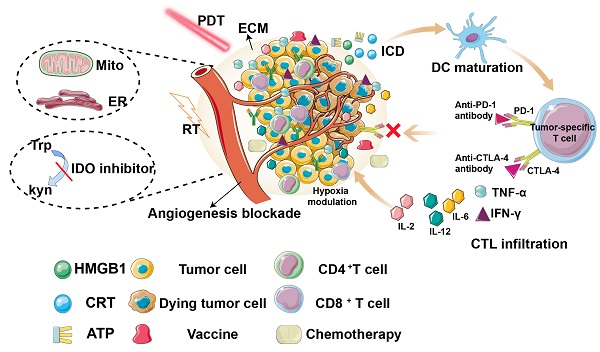
Overview of the strategies for improving photodynamic therapy-induced antitumor immunity for cancer treatment, including hypoxia modulation, ECM destruction, angiogenesis blockade, subcellular targeted, tumor vaccine construction, synergistic therapies.
Summary of different strategies for PDT-induced antitumor immunity.
| Category | Strategies | Ref |
|---|---|---|
| Reprogramming tumor microenvironment | Disrupting the ECM | 37 |
| Angiogenesis blockade | 43 | |
| Delivering O2 to hypoxic tumors | 22, 52 | |
| Producing O2 at the hypoxic tumor | 54, 56, 59 | |
| Subcellular targeted photodynamic therapy | Endoplasmic reticulum targeted PDT | 64, 65 |
| Mitochondrial-targeted PDT | 75, 76 | |
| PDT combine with immunotherapy | Adjuvant | 85, 86 |
| DC vaccine | 82,90 | |
| PDT-motivated autologous tumor cell vaccine (P-ATV) | 89 | |
| PDT combined with ICB | 97, 99 | |
| PDT combined with IDO | 104, 105 | |
| Other combination therapies | PDT combined with chemotherapy | 107 |
| PDT combined with PTT | 6, 114 | |
| PDT combined with radiotherapy | 25 |
2. Reprogramming the TME
The host immune system determines the fate of tumor progression. Therefore, manipulating the immune system to activate the host immune response represents a promising strategy to develop an effective cancer treatment. Understanding the TME is critical to enhance the response of patients to immunotherapy. The TME is composed of cancer cells, various stromal cells (including fibroblasts, immune cells, endothelial cells, bone marrow-derived immature cells), cytokines (i.e., TNF, VEGF, IL-1), and chemokines (i.e., CXCL12, CCL27, CCL21) [29, 30]. Cancer cells do not “work” alone, but interact closely with the extracellular matrix, stromal cells, and immune cells to promote chronic inflammation and immunosuppression.
Theoretically, treatment methods that act directly on tumor cells have many shortcomings, such as heterogeneity between tumors and tumor cells within tumor tissues, and instability of the biological or genetic characteristics of tumor cells. Therapeutic strategies targeting the TME have advantages, such as the fact that tumor interstitial cells have a stable genetic background and are not prone to mutation and drug resistance, the heterogeneity of the TME is reduced, and the curative effect is relatively stable. It is possible to predict the response of tumor tissue to treatment, which can play a critical role in controlling tumor metastasis [31].
As the importance of the TME in tumor progression and treatment is recognized, research into the improvement of PDT-mediated anti-tumor immunity by remodeling the TME is increasing [32-34] (Table 2).
Summary of different formulations for PDT-induced antitumor immunity by reprogramming TME. (↑= upregulation, ↓= downregulation)
| Formulation | Therapeutic agents | Immune expression | Ref |
|---|---|---|---|
| DEX-HAase | Ce6, anti-PDL1 | TAAs, IFN-γ, TNF-α, CD8+, mDC↑ HIF-1α, M2 macrophage↓ | 37 |
| TOOKAD | WST11, anti-PD-1/anti-PD-L1 | TAAs, CD4+, CD8+ ↑ Treg, PD-L1↓ | 43 |
| FS@PMPt | Ce6, PFC | TAAs, mDC, CD4+, CD8+, TGF-β, IFN-γ↑ HIF-1α, Treg↓ | 22 |
| PF11DG | DiD | TAAs, mDC, NK, CD8+↑ MDSC, HIF-1α↓ | 52 |
| FA-L@MD@CAT | MBDP, CAT | TAAs, mDC, CTL, M1 macrophage↑, M2, Treg, Hypoxia↓ | 54 |
| MnO2@chitosan-CyI | MnO2, CyI | TAAs, CTL, IFN-γ, TNF-α↑ M2 macrophage, Hypoxia↓ | 56 |
| CeO2@MSNs@IR780/Met | CeO2, IR780 | TAAs, mDC, CD4+, CD8+↑ HIF-1α, PD-L1↓ | 59 |
2.1 Disrupting the tumor extracellular matrix (ECM)
The ECM is the “soil” of cells, and its topology and composition affect cell proliferation and differentiation. The ECM is a dense protein network surrounding normal and cancer cells, which forms an obstacle to drug delivery and seriously limits the penetration of immunosuppressants and other antineoplastic agents and the infiltration ability of immune cells to tumor sites [35]. Therefore, the destruction of the ECM may represent an ideal way in which to improve PDT-induced anti-cancer immunity.
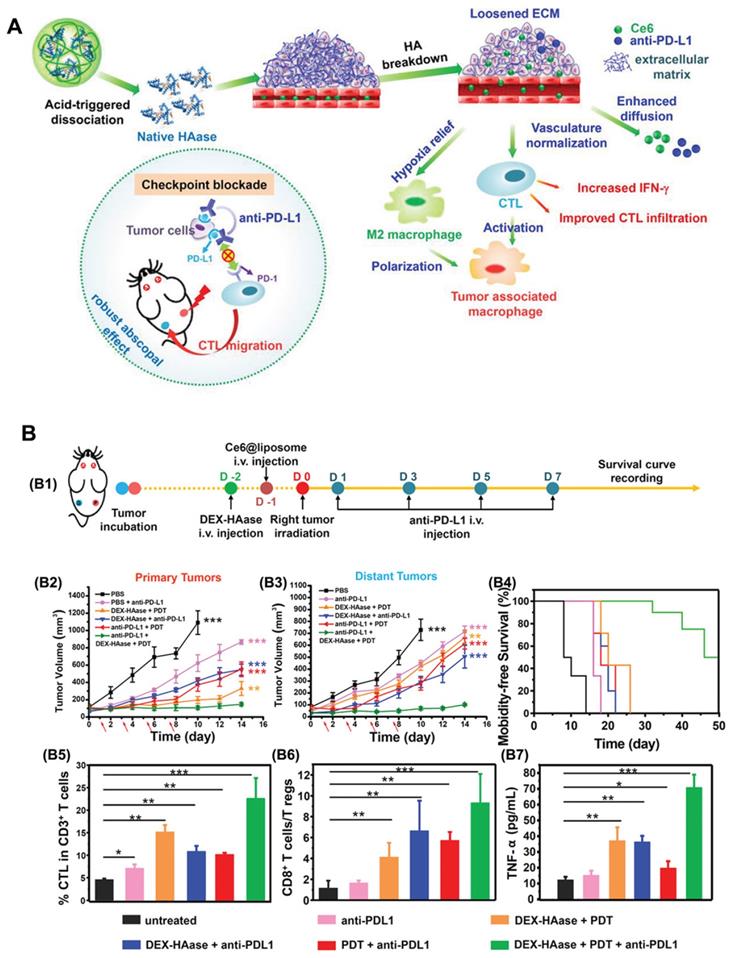
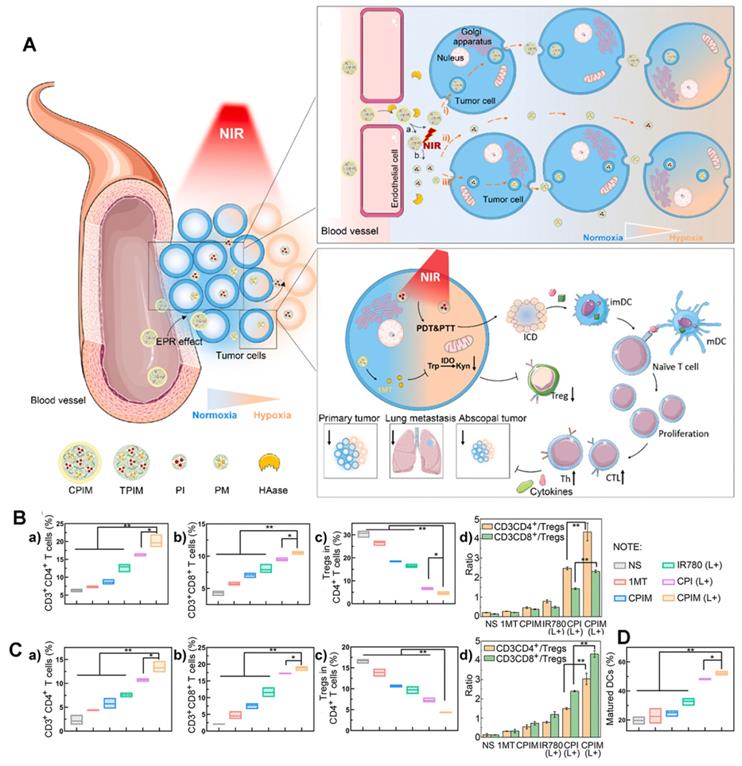
Hyaluronic acid (HA) is a major component of the tumor ECM. Hyaluronidase (HAase) can rapidly degrade the ECM of tumors, loosening the skeleton of the ECM, thus enhancing drug penetration and T lymphocyte infiltration at the tumor site, which helps improve the effect of tumor immunotherapy [36]. In light of this, Wang et al. [37] developed a stimulus-responsive HAase release system by using the natural macromolecular dextran (DEX) as a carrier. In this research, HAase was bonded with DEX through acidic cleavable groups under mild reaction conditions to obtain DEX-HAase nanoparticles that are stable in the physiological environment and rapidly release HAase in the acidic tumor environment (Figure 3). Specifically, in the acidic TME, the released HAase can decompose HA and thus destroy the ECM, increasing the content of effective blood vessels and improving the hypoxic conditions. Additionally, the increased oxygen in the tumor not only improves the efficacy of subsequent PDT but also increases the infiltration of cytotoxic T lymphocytes after PDT, thus achieving enhanced anti-tumor immunity. Compared to the large area of hypoxia observed in the untreated group, the hypoxia-associated fluorescence and the positive signals of hypoxia-inducible factor (HIF-1α) in the DEX-HAase group were significantly decreased. Moreover, the results of in vivo administration demonstrated significantly increased expression of IFN-γ, as well as increased infiltration of macrophages and CD8+ T cells in the DEX-HAase group, suggesting that DEX-HAase+PDT combined therapy can enhance cellular immunity. A bilateral tumor mouse model study further confirmed that PDT could enhance the release of tumor-associated antigens and the activation of DCs induced by ICD, which served to promote the infiltration of mature T cells to the TME.
2.2 Angiogenesis blockade
The local overgrowth of tumor blood vessels causes destruction of the tumor vascular structure, and the rich matrix components, such as collagen and HA, in the tumor constitute the physical barrier of intra-tumoral infiltration and jointly limit the infiltration of immune cells. Furthermore, the TME prevents immune cells, such as CD4+ and CD8+ T cells, from infiltrating the tumor site. Indeed, blocking tumor blood vessels can effectively inhibit tumor growth and prevent metastasis [38-40].
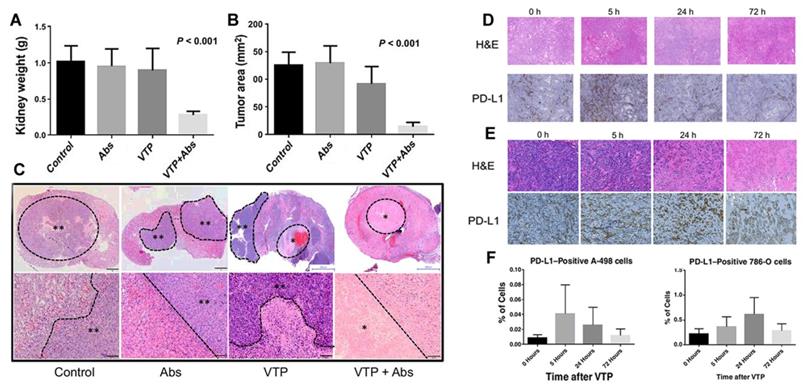
Vascular targeting PDT with the photosensitizer WST11 (TOOKAD-VTP) has been used clinically for treating early antecedent adenocarcinoma [41, 42]. The results of phase II clinical trials of vascular targeting PDT (VTP) hemi ablation in patients with prostate cancer showed that compared to the untreated population, patients receiving VTP semi-ablation therapy had a high rate of no tumors after several years of treatment. O'Shaughnessy et al. [43] further studied the immunological potential of systematic anti-tumor treatment with TOOKAD-VTP in a mouse model of lung metastatic renal cell carcinoma. Primary tumors were treated with VTP, PD-1/PD-L1 antagonistic antibody (Abs), or combined therapy (VTP + Abs). The treatment response was observed, including immune infiltration of the primary and metastatic sites. As proposed in Figures 4A-C, compared to VTP or Abs alone, VTP combined with systemic PD-1/PD-L1 pathway block reduced the size of the primary tumor. Subsequently, histological analysis of the kidneys of mice receiving combined treatment showed no surviving tumor or necrosis. Moreover, comparing 17.5 days of VTP treatment and 19 days of Abs alone, the combined treatment group showed a prolonged survival time (27 days). The analysis of tumor-infiltrating lymphocytes showed that the therapeutic effect was related to the ratio of CD8+ Treg cells to CD4+FoxP3-Treg in the primary renal tumor, proving that VTP with the photosensitizer WST11 can induce a local immune response. More importantly, they found that VTP could induce PD-L1 expression in a human renal cell carcinoma xenograft model. As shown in Figures 4D-F, the proportion of cells expressing PD-L1 increased significantly after VTP treatment.
2.3 Response to tumor hypoxia
Hypoxia directly regulates the expression of various cytokines and affects the function of various immune cells, such as macrophages, APCs, and myeloid derived suppressive cells (MDSCs) [44]. The heterogeneity of hypoxia can promote tumor invasion, metastasis, angiogenesis, and the increase in multidrug resistance proteins, all of which reduce the efficacy of anti-cancer drugs [45]. Recently, various therapeutic strategies have been proposed for photodynamic hypoxia, including the delivery of oxygen to hypoxic tumors and the production of oxygen in hypoxic tumors [46-49].
The mechanism of (A) DEX-HAase adjuvant and PD-L1 checkpoint enhancing PDT and anti-tumor immune response. (B) Evaluation of anti-tumor immunity in vivo. (B1) Schematic illustration of enhanced PDT and anti-PD-L1 combination therapy. In vivo results of (B2) primary and (B3) distant tumors growth curves of different groups of mice after various treatments. (B4) Morbidity-free survival of different groups of mice. (B5) CTL infiltration in tumors and (B6) the ratio of CD8+ T cells to regulatory T cells of mice post various treatments. B7) The production of TNF-α in serum of mice post various treatments. Adapted with permission from [37], copyright 2019 John Wiley and Sons.
VTP and PD-1/PD-L1 blockade synergize to reject orthotopic renal tumors and prolong survival. On the 21st day, renal tumor growth was evaluated by renal weight (A) and maximum cross-sectional tumor area (B); (C) The kidneys were collected on the 21st day and stained with H&E. PD-L1 expression induced by VTP in (D) A-498 tumors and (E) 786-O tumors in the flanks of nude mice; (F) The ratio of PD-L1 positive cells in Amur498 tumor and 786muro tumor was evaluated. Adapted with permission from [43], copyright 2018 American Association for Cancer Research.
2.2.1 Delivering oxygen to hypoxic tumors
Using a suitable carrier to deliver O2 directly to the tumor is a commonly used method to overcome tumor hypoxia during PDT. Compared to the low loading efficiency of hemoglobin, the solubility of O2 in perfluorocarbon (PFC) is very high (approximately 40-50 mL O2/100 mL liquid, which is equivalent to the O2 solubility of 200 mL blood at 25°C and 1 atm). As a result, these substances have been used as key components in oxygen delivery systems [50, 51]. For instance, Li et al. [22] synthesized a fluorinated cisplatin carrier (FS-PAMAM-Pt) and fluorinated photosensitizer Ce6 copolymer (F-Ce6-PEG), which could be assembled into nanoclusters FS@PMPt by interacting fluorine-fluorinated (FF) with PFC. Under laser irradiation, the oxygen in PFC ensured sufficient production of ROS to promote ICD-induced immune activation. Moreover, in the mouse breast 4T1 tumor model, the tumor growth treated with FS-PAMAM-Pt was inhibited, the tumor volume was decreased, the morphology was altered, and high rates of necrosis were observed. Flow cytometry detection of CD40 and CD86 on CD11c+ DCs showed that after FS@PMPt treatment, CD8+ T cell infiltration was increased, while the proportion of Tregs was decreased. Furthermore, the activation of the anti-tumor immune response was accompanied by the production of IFN-γ. Following treatment with the FS@PMPt laser, the production of IFN- γ was increased by 4.4%, while the expression of TGF-β decreased by approximately 53%, which further proved the transformation from an immunosuppressive “cold” tumor to a “hot” tumor and enhanced photodynamic immunotherapy.
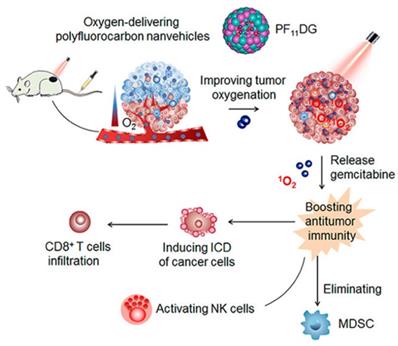
However, the FS@PMPt particle size of 165 nm may be associated with problems such as limited penetration of the tumor anoxic zone. Alternatively, fluorocarbon chains can be chemically linked or polymerized into polyhedral amphiphilic polymers and further assembled into nanocarriers, which have been used to promote cell membrane penetration or tumor-targeted biomedical imaging. Therefore, Wang et al. [52] reported the ability of an amphiphilic oxygen-supplied polyfluorocarbon nanocarrier loaded with the photosensitizer DiIC18 (5) (DiD) and the gemcitabine prodrug (named as PF11DG) to relieve tumor hypoxia and enhance anti-tumor immunotherapy. DiD as a photosensitizer can effectively produce ROS in tumor cells and induce the occurrence of ICD, while gemcitabine can enhance anti-tumor immunity through activating the cytotoxicity of natural killer cells and eliminating immunosuppressive MDSCs in the tumor. (Figure 5). In tumors, PF11DG showed flexible intratumoral infiltration under laser irradiation, which enhanced the strong anti-tumor immune response. It is worth noting that PF11DG plus laser irradiation (PF11DG+L) significantly delayed tumor growth, with an inhibition rate of 82.96% in the 4T1 breast cancer model and 93.6% in the PANC02 pancreatic cancer model.
2.2.2 Producing oxygen in a hypoxic tumor
Due to metabolic abnormalities, cancer cells produce higher levels of hydrogen peroxide than normal cells (concentrations ranging from 10-4 to 10-3 M) [53]. Therefore, the design of compounds that can degrade endogenous H2O2 to produce O2 in tumors is one way to alleviate the level of hypoxia.
Schematic illustration of mechanism of PF11DG to enhance tumor oxygenation and elicit antitumor immune responses for cancer therapy. Adapted with permission from [52], copyright 2021 American Chemical Society.
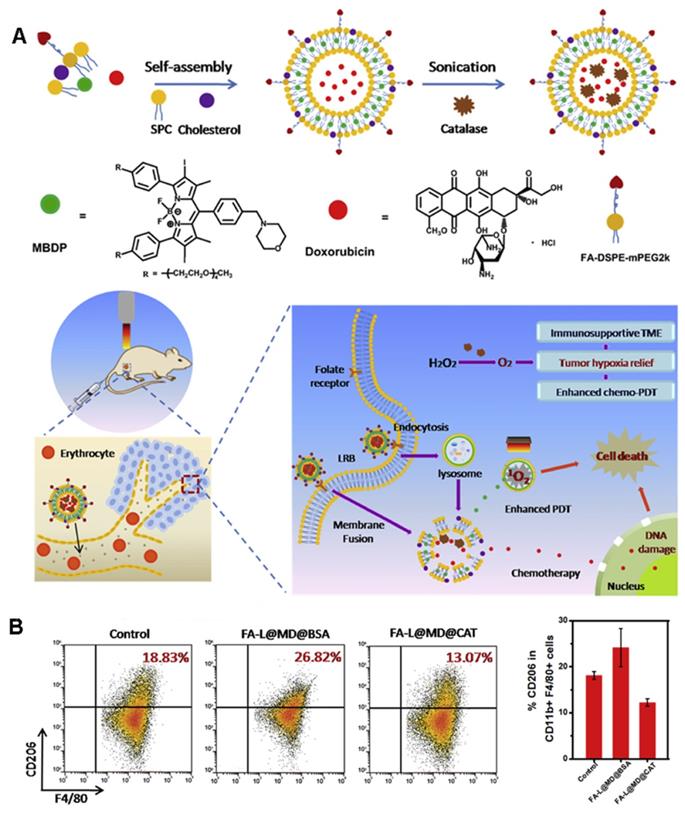
Shi et al. [54] developed a unique liposome FA-L@MD@CAT to encapsulate catalase (CAT), a photosensitizer (MBDP), and adriamycin to catalyze the overexpression of H2O2 in tumors and increase tumor oxygenation, thereby reversing the immunosuppressive TME (Figure 6A). Under 660-nm irradiation, FA-L@MD@CAT can greatly promote the production of 1O2. Most importantly, under the condition of hypoxia, the polarization of macrophages changes from the anti-tumor M1 phenotype to the tumor-promoting M2 phenotype [55]. As shown in Figure 6B, the proportion of M2-type macrophages in the FA-L@MD@CAT treatment group was significantly lower than that in the control group. Similarly, a significant increase in the proportion of cytotoxic T lymphocyte (CTLs) was detected by flow cytometry. Therefore, the enhancement of tumor oxygenation significantly induces tumor cell death by regulating the expression of immune cytokines, reversing the immunosuppressive TME, and promoting the anti-tumor immune response.
In addition to enzymes that can catalyze H2O2, metal-based oxygen generators such as manganese dioxide (MnO2), calcium oxide, cerium oxide (CeO2), and titanium oxide (TiO2) can catalyze H2O2 to generate O2, thereby relieving tumor hypoxia, enhancing PDT, and further enhancing anti-tumor immunity.
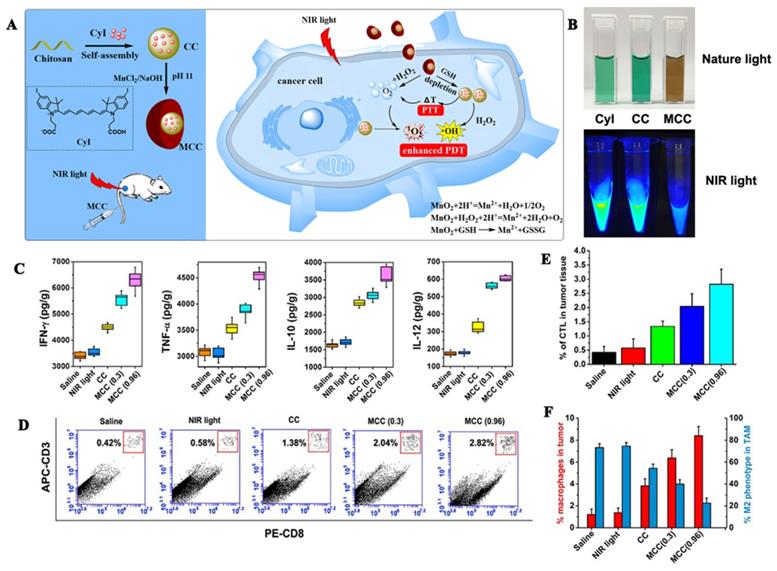
MnO2 catalyzes the decomposition of H2O2 to O2 under neutral conditions and reacts with H+ to O2 under acidic conditions. An advantage of MnO2 is that it can adsorb different types of small molecules by physical adsorption or Mn-N coordination bonds. Our research group [56] used manganese dioxide to design a MnO2@chitosan-CyI (MCC) dual oxygen production nanosystem to enhance the combined therapy of tumors (Figures 7A, B). In this experiment, self-assembly of the iodinated indocyanine green (ICG) derivative CyI and chitosan was conducted to prepare a TME-sensitive nanosystem, following which, a shell of MnO2 nanoparticles was constructed on the surface through electrostatic interactions, and Mn-N coordination bonds. Under the irradiation of near-infrared light, MCC can enhance the production of ROS and heat. Additionally, once the material is internalized by the cells, MnO2 can be used as an efficient in situ oxygen generator to alleviate hypoxia in the TME. Meanwhile, the heat generated by the nanosystem can also increase the temperature and accelerate the blood flow in the body, thereby further alleviating hypoxia. Additionally, as shown in Figures 7C-D, enhanced PDT can also trigger an acute immune response, and the resulting combination of PDT-photothermal-immune therapy can effectively eliminate the primary tumor and suppress tumor metastasis.
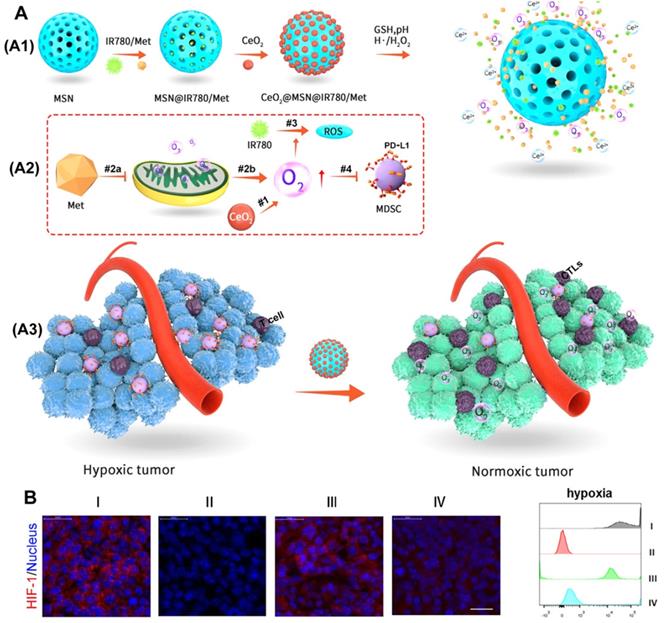
CeO2 has a similar chemical catalytic activity to peroxidase and oxidase and can convert H2O2 into highly toxic hydroxyl radicals under acidic tumor conditions [57, 58]. Therefore, as proposed in Figure 8A, Zuo et al. [59] used the catalytic oxygen production ability of CeO2 to wrap it on mesoporous silica nanoparticles (MSNs) and load metformin (Met) and photosensitizer (IR780) inside to prepare an oxygen-replenished photodynamic nanoplatform (CeO2@MSNs@IR780/Met). CeO2 nanoparticles can catalyze the overexpression of H2O2 in tumor tissues to produce oxygen, while the inhibition of mitochondrial respiration by Met can cause the accumulation of O2 in the tumor and further reduce tumor hypoxia. Thus, this nanoplatform can be used to effectively modify the hypoxia of the TME (Figure 8B). More importantly, the nanoplatform can effectively recruit CTLs and significantly reduce the expression of PD-L1 on MDSCs, which together, greatly destroyed the immunosuppressive function of MDSCs and significantly enhanced the anti-tumor immune response of PDT.
3. Subcellular-targeted PDT
Recently, an increasing number of studies have shown that selective delivery of drugs to specific subcellular organelles can significantly influence the immunotherapeutic effects. Among them, the endoplasmic reticulum (ER) and mitochondria play an important role in regulating the apoptosis and metabolism of cancer cells and are more vulnerable to hyperthermia and oxidative damage. Therapeutic strategies targeting the endoplasmic reticulum (ER) and mitochondria have broad prospects in the field of PDT-induced immunotherapy.
(A) The preparation scheme and mechanism of FA-L@MD@CAT to Enhance Antitumor Immune Responses for Cancer Therapy. (B) Representative flow cytometer plots and the corresponding quantification of M2-type macrophages (CD206+). Adapted with permission from [54], copyright 2020 Elsevier.
3.1 Endoplasmic reticulum-targeted PDT
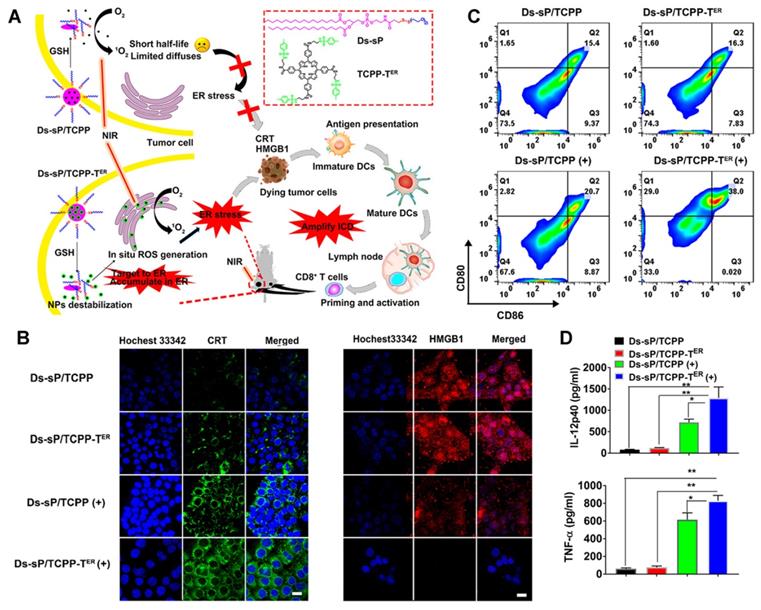
As a unique multifunctional organelle, ER plays a crucial role in maintaining intracellular signal transduction, calcium homeostasis, protein synthesis, and processing [60, 61]. ROS production induces endoplasmic reticulum stress, which is considered a major cause of ICD [62, 63]. Therefore, exploring the use of PDT for targeting the ER is an optimal scheme to effectively induce photodynamic immunity. For example, Deng et al. [64] synthesized reduction-sensitive nanoparticles, Ds-sP NPs, and uploaded an efficient ER targeting photosensitizer, TCPP-TER (Figure 9A). As proposed in Figures 9B-D, under NIR irradiation, Ds-sP/TCPP-TER NPs can selectively accumulate in the ER and produce ROS locally, which can induce ER stress, magnify ICD, and activate immune cells, thus enhancing the effect of immunotherapy. Both in vitro and in vivo studies have shown that the ER-targeting PDT strategy can enhance the ICD effect and promote the release of DMAPs. Additionally, the secretion of cytokines in the tumor site increased, as did the infiltration of CD8+ T cells, suggesting that this strategy can improve the efficiency of immunotherapy.
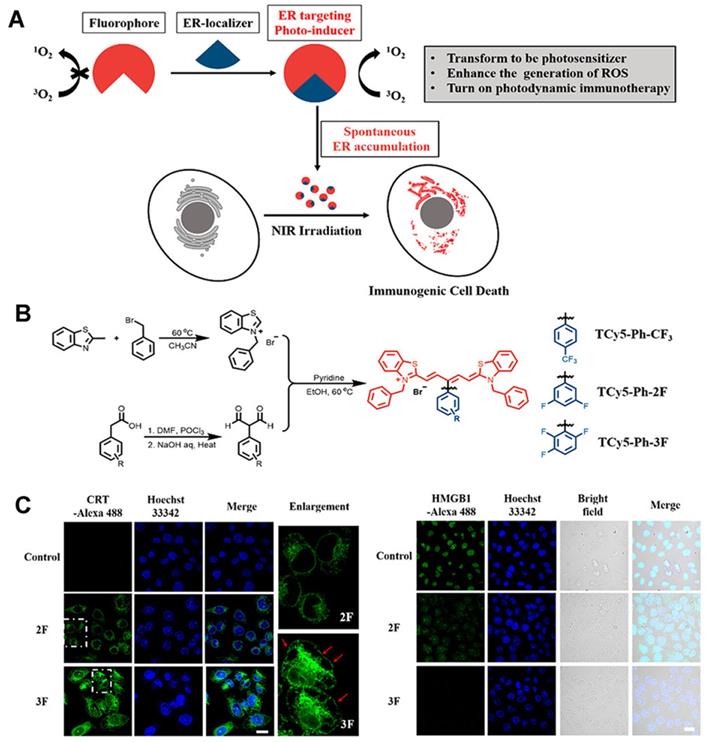
The use of an efficient ROS generator targeting the ER is the best scheme to effectively induce endoplasmic reticulum stress. Thus, Ma et al. [65] developed the first thio-pentamethyne cyanine photosensitizer, TCy5-Ph-3F, which can be used as an ICD photoinducer (Figures 10A-B). Irrespective of normoxic or hypoxic conditions, TCy5-Ph-3F has a selective tendency toward ER accumulation and excellent ROS production ability (the singlet oxygen quantum yield [ΦΔ] is 39%). With the help of targeting ligands, the photosensitizer can be localized to the ER, and, under near-infrared radiation, exert a photodynamic effect, generate a large amount of ROS, and cause ER stress, triggering an immune response. Moreover, TCy5-Ph-3F could induce the production of many DMAPs, including up-regulation of glucose-regulated protein-78 (GRP-78), HMGB1 efflux, and secretion of ATP, CRT, and HSP70 (Figure 10C). The maturation of DCs and the activation of CD8+ T cells were also detected in vivo, indicating that TCy5-Ph-3F can activate the systemic immune response. These results can guide the design of an efficient ICD photoinducer and expand the application prospect of organic molecules in cancer immunotherapy.
3.2 Mitochondrial-targeted PDT
Mitochondria play an important role in determining cell fate and regulating cell physiological changes, signal transduction pathways, and metabolism [66, 67]. More importantly, many studies have emphasized that mitochondria are essential for the metabolism and activation of immune cells [68-70]. Indeed, increased mitochondrial biomass and reserve respiratory capacity have been shown to enhance T cell persistence and provide the necessary bioenergy advantages to eradicate cancer cells and prevent recurrence [71, 72]. As the most sensitive subcellular organelle to ROS, mitochondrial-targeted PDT has been considered an effective strategy for anti-tumor immunotherapy.
(A) Activation mechanism of MCC nanosystems for highly efficient phototherapy and acute immune response; (B) Photos of CyI, CC, and MCC in water solution under ambient light and NIR light; (C)The immune factor concentrations of IFN-γ, TNF-α, IL-10, IL-12; (D) Flow cytometry data of cytotoxic T lymphocyte (CTL) infiltration in tumors. CD3+ and CD8+ T cells were defined as CTLs; (E) CTL quantification of flow cytometry results; (F) Macrophage infiltration and polarization within tumors after various treatment. Adapted with permission from [56], copyright 2020 Ivyspring.
(A) Working principle of CeO2@MSNs@IR780/Met NPs. (A1) CeO2@MSNs@IR780/Met NPs were synthesized by loading IR780 and Met into MSNs with CeO2 as the gatekeepers. (A2) Schematic diagram of the CeO2@MSNs@IR780/Met NPs for enhanced PDT. (A3) Application of CeO2@MSNs@IR780/Met NPs markedly activated and strengthened the immune response; (B) Immunofluorescence images and FCM showing the HIF-1 expression in B16F10 cells after different treatments. Adapted with permission from [59], copyright 2020 American Chemical Society.
Based on the principle that the inner mitochondrial membrane is highly negatively charged, the lipophilic cationic triphenylphosphine (TPP) targeting of mitochondria has been widely used. As the mitochondrial membrane potential of tumor cells is higher than that of normal cells, lipophilic cationic TPP as the mitochondrial targeting ligand can penetrate the mitochondrial membrane only by electrostatic adsorption, which greatly increases the aggregation of particles in the mitochondria of tumor cells [73-75].
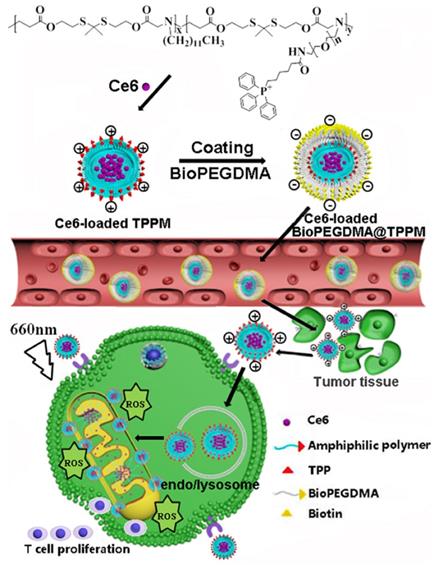
As illustrated in Figure 11, Peng et al. [75] successfully constructed a mitochondrial targeted delivery system. In this study, positively charged micelles loaded with amphiphilic copolymer Ce6 were used as the core and the biotin-PEG4000-NH2 modified by anionic 2, 3-dimethylmaleic anhydride (DMA) was used as the shell to form BioPEGDMA@TPPM. First, the biotin in the shell led the particles to target the tumor cells and accumulated effectively in the tumor tissue. Secondly, the shell falls off and the exposed TPP targets the mitochondria. Consequently, Ce6 induces ICD, which promotes the production of immune cytokines, thereby enhancing the immune response. The photodynamic antitumor activity and immune response of mitochondria in Kunming mice and BALB/C nude mice bearing CT26 cells were detected in vivo. Compared to other treatment groups, the Ce6-loaded BioPEGDMA@TPPM system showed a good inhibitory effect on primary tumors, with a tumor inhibition rate of 93.2% and 84.1% in Kunming mice and BALB/c mice, respectively. On the 14th day after PDT treatment, various immune signal molecules were detected in tumor and lymphoid tissues, including IFN-γ, TNF-α, and CD4+T cells, CD8+ T cells, and DCs. The results showed that the fluorescence intensity and activation percentage of CD4+ T cells, CD8+ T cells, and DCs were significantly increased in tumor tissues following treatment with the Ce6-loaded BioPEGDMA@TPPM system. Following accumulation in the mitochondria, this system effectively boosted the cellular immune response and assisted PDT to exert an anti-tumor immune response.
The concept of combining PDT with ICD has been proposed to overcome the problems of high tumor recurrence rates and tumor drug resistance, which cannot be solved by a single therapy. However, most previous studies focused on the ER-stress injury-related DAMPs, while ignoring the secretion and function of mitochondria-related DAMPs.
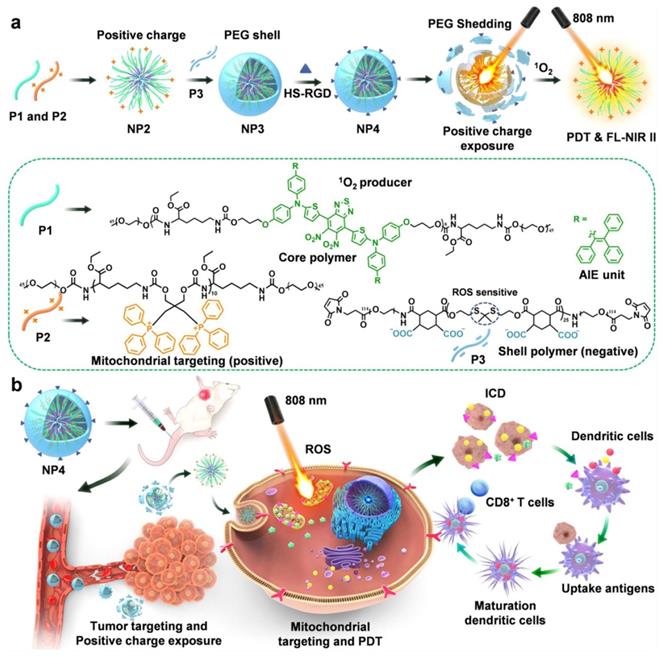
As illustrated in Figure 12A, Wei et al. [76] developed a nanodelivery system that can be controlled by NIR to achieve cascade targeting of cancer cells and immune cells. In this study, they synthesized three types of polymers by polycondensation, namely, degradable infrared two-zone fluorescent polymer P1, triphenylphosphine polymer P2 with mitochondrial targeting, and polymer P3, whose thione structure can be destroyed in the presence of ROS. The positively charged NP2 nanoparticles were prepared by co-assembly of P1 and P2, then NP2 and P3 were used to form NP3 by electrostatic adsorption. Finally, NP3 was combined with the targeting group HS-RGD to obtain NP4 nanoparticles with cascade targeting ability. To verify the biological effects of the nanoparticles, they constructed an in situ tumor model of triple-negative breast cancer 4T1 cells. NP4 injected into mice through the tail vein was shown to pass through the blood circulation before accumulating at the tumor site. At the tumor site, NP4s produce ROS under the excitation of an 808-nm laser, which makes the PEG on the surface of NP4 fall off to generate NP2 nanoparticles, thus realizing charge reversal. NP2 can further penetrate the tumor tissue and target the mitochondria in the tumor cells. As shown in Figure 12B, under continuous light, NP2s produce a large amount of ROS in mitochondria, which can kill cancer cells. Simultaneously, DAMPs induced by PDT promote the maturation of DCs, and mature DCs present antigens to T cells, thus inducing CD8+ T cell differentiation and activating adaptive immunity, thereby realizing the combined therapy of PDT and host immunity.
(A) Schematic diagram of the Ds-sP/TCPP-TER for enhanced PDT. (B) CLSM images showing surface translocation of CRT and HMGB1 release with Ds-sP/TCPP or Ds-sP/TCPP-TER with or without 670 nm laser irradiation. (C) 4T1 cells were treated with Ds-sP/TCPP or Ds-sP/TCPP-TER with or without 670 nm laser irradiation, followed by coculture with BMDCs. The BMDCs were stained with anti-CD86 and anti-CD80 antibodies and analyzed using flow cytometry. (D) Quantification of secretion of IL-12P40 and TNF-α in DC suspensions. Adapted with permission from [64], copyright 2020 American Chemical Society.
(A) Schematic of the ER localizer mediating precise construction of the photosensitizer for the ER. B) Schematic illustration of the synthesis of TCy5 derivatives. (C) Immunofluorescence staining images of calreticulin proteins, HMGB1-positive and extracellular release of ATP after different treatments. Adapted with permission from [65], copyright 2022 American Chemical Society.
4. PDT combined with Immunotherapy
Immunotherapy enhances the ability of the immune system to target cancer cells and has shown efficacy against various tumors. The local stimulation induced by PDT is insufficient to induce an effective immune response. Therefore, the combination of PDT with immunotherapy has become a promising choice in recent years [20, 77, 78]. In this section, we will summarize the PDT combination immunotherapy (Table 3).
4.1 Tumor vaccines
As a promising method of cancer immunotherapy, therapeutic tumor vaccines have become a powerful weapon for treating cancer. Tumor vaccines refer to the use of a tumor antigen, through active immunity, to induce the body to produce specific anti-tumor effects stimulate the body's immune protection mechanism, treat tumors or prevent recurrence. PDT can release tumor-associated antigens (TAAs) through local selective photochemistry, induce ICD, enhance antigen presentation, and activate in situ immune cells (such as T cells) to form an in situ tumor vaccine. However, the residual tumor after PDT may be insufficient to independently induce an effective anti-tumor response. Consequently, additional immune stimulation is often needed to induce the immune system to respond to residual tumor fragments or metastatic tumor cells, including immune adjuvants [79, 80] and immune cells [81, 82], [20, 83].
Summary of different formulations for PDT-induced antitumor immunity by combination therapy. (↑= upregulation, ↓= downregulation)
| Formulation | Therapeutic agents | Immune expression | Ref |
|---|---|---|---|
| PCN-ACF-CpG@HA | CpG, H2TCPP | TAAs, IFN-γ, TNF-α, CTL, mDC↑ | 85 |
| Semiconducting polymer nano-immunomodulator (SPNI) | R837, PCPDTBT | TAAs, CD4+, CD8+, mDC, central memory T cell↑ | 86 |
| DC vaccine | OR414 | TAAs, CD4+, CD8+, mDC↑ | 82 |
| ALA-PDT-DC cancer vaccine | ALA | TAAs, IFN-γ, TNF-α, CTL, mDC↑ IL-10↓ | 90 |
| PC-Cell@gel | PEI-CE6, FK-PBA | TAAs, IFN-γ, TNF-α, CTL, mDC↑ | 89 |
| MB@MSP | MB, anti-PD-L1 polypeptide | TAAs, IFN-γ, TNF-α, CTL, mDC, IL-6↑ Treg, IL-10↓ | 97 |
| ZnPc/α-CTLA4@Ac-DEX | ZnPc, α-CTLA4 | TAAs, CD4+, CD8+, mDC↑ Treg, CTLA4↓ | 99 |
| PpIX-NLG@ Lipo | NLG919, PPIX | TAAs, CD8+, Trp↑ Kyn↓ | 104 |
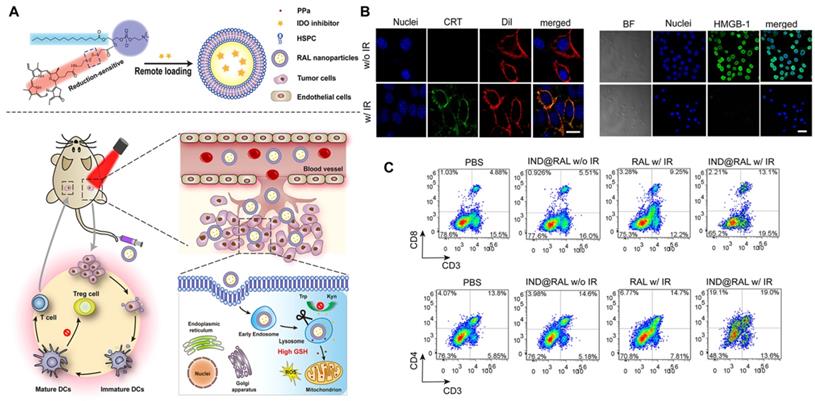
| IND@RAL | NLG8189, RAL | TAAs, mDC, CD4+, CD8+↑ Kyn, Treg↓ | 105 |
4.1.1 Adjuvant
An adjuvant, also known as an immune potentiator, is a vaccine additive that, when injected into the body before or mixed with antigen, can enhance the immune response to antigen or change the type of immune response. Adjuvants serve as non-specific immune enhancers but have no antigenicity [84].
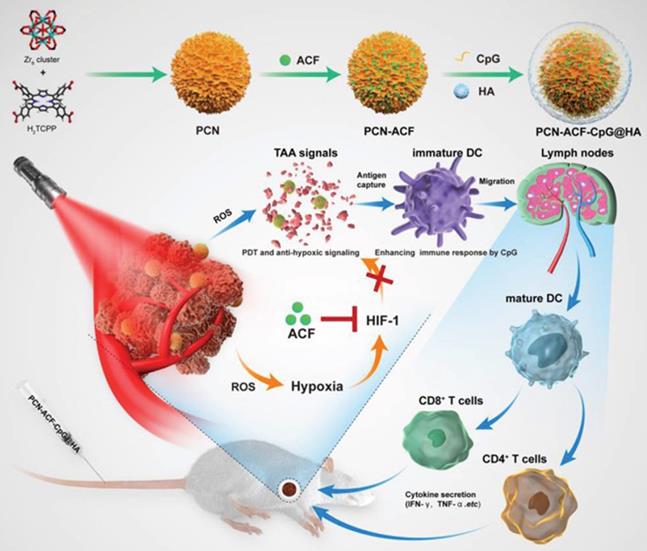
For instance, the adjuvant CpG can directly activate B cells and DCs to produce an environment rich in pro-inflammatory factors and Th1 cells. CpG can promote the development of CTL through IFN-c induced T cells and increase the production of IL-6 and IL-12 to promote antibody secretion [84]. Accordingly, Cai et al. [85] designed and synthesized nanoparticles based on a metal-organic framework, which combined PDT and antioxidant signal transduction with a CpG adjuvant as an in situ tumor vaccine to enhance the PDT-induced anticancer response (Figure 13). The results showed a strong ROS signal after PDT of cancer cells, specifically targeting the CD44 receptor overexpressed by HA, while Acriflavine blocked the expression of HIF-1α. With the aid of CpG, TAAs produced by PDT-induced tumor cell damage can enhance the presentation ability of DCs, which promotes the maturation of CD4+T cells and CD8+ T cells and the release of cytokines (including INF-γ, TNF-α). Taken together, these novel in situ immunostimulatory strategies can enhance the anti-tumor effect of PDT by activating the host anti-tumor immune response in vivo and in vitro.
Schematic diagram of the composition and transportation process of BioPEGDMA@TPPM for enhanced PDT. Adapted with permission from [75], copyright 2020 Elsevier.
(A) Design of core shell nanoparticles and illustration with dual-cascade targeting (DCT) performance triggered by NIR light for maximizing the efficacy of photodynamic therapy (PDT) and cancer immunotherapy. Adapted with permission from [76], copyright 2021 Elsevier.
Nanodrugs have great potential to provide safer and more effective cancer immunotherapies by improving the pharmacokinetics and biodistribution of immunotherapeutic drugs and enhancing their interaction with immune cells. Additionally, nanomedicine can provide multiple treatments in one preparation and work in conjunction with immunotherapy to develop more effective anti-tumor combination therapies. Therefore, endogenous trigger-sensitive nanodrugs show great potential in the context of anti-tumor immunity in a controlled manner for the accurate delivery of immunotherapeutic agents.
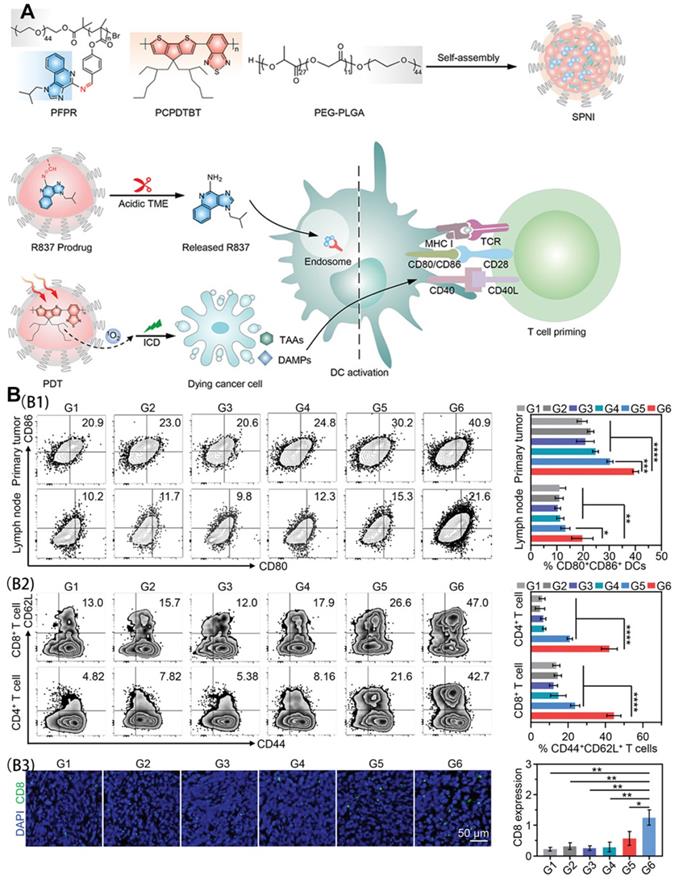
Liu et al. [86] synthesized an intelligent semiconducting polymer nanoimmunomodulator (SPNI) for acid TME-activated precision photodynamic immunotherapy using endogenous and exogenous triggers. Through the coupling of an acid-labile Schiff-based linker with Toll-like receptor 7 (TLR7) agonists, R837 was introduced to trigger TLR7 junctions located in the endomembrane to promote DC maturation and pro-inflammatory cytokine secretion. After accumulation in the tumor site, SPNI is hydrolyzed and limits the specific release of R837 in response to the acidic environment in the tumor tissue. When exposed to near-infrared light, SPNI exerts a photodynamic effect to mediate direct tumor ablation and ICD production. The released immunogenic factors and the precise activation of the tumor TLR7 pathway trigger adaptive anti-tumor responses through an in situ vaccine-like function.
As shown in Figure 14A, SPNI not only mediates the production of a large amount of ROS but also significantly up-regulates CRT and HMGB1 in SPNI-treated cells under light radiation. The activation of DCs in tumor-draining lymph nodes was consistent with the results of DC activation in primary tumors. Analysis of the proportion of CD4+ T, CD8+ T, and central memory T cells (CD44+ CD62L+) showed that SPNI-mediated photodynamic immunotherapy can be used as a vaccine for cancer to stimulate strong anti-tumor T cell immunity, which helps to inhibit local and surrounding tumor growth (Figure 14B).
4.1.2 DC vaccines
DCs are powerful APCs that effectively enhance the immune response. DCs are considered the central cell of the immune system because they provide a bridge between innate and acquired immune responses [87, 88]. Recently, the progress of anti-cancer based on the combination of DC immunotherapy and PDT is in full swing [81, 82, 89]. Rempolec et al. [82] constructed a PDT-based DC vaccine with a new photosensitizer OR141 (which can also be used as an ICD inducer) to enhance the priming effect of DCs to promote anti-tumor effects. The increased expression of costimulatory molecules, CD40, CD80, and CD86, indicated that the PDT-based DC vaccine successfully activates DC maturation. The prepared PDT-based DC vaccine conferred a higher survival rate to mice compared to those treated with CTLA-4 antibodies. This is further supported by the enhanced expression of CD8+ T cells, CD4+T cells, and INF-γ. Additionally, Zhang et al. [90] demonstrated that ALA (5-aminolevulinic acid)-PDT-induced tumor fragments could enhance the antigen presentation ability of DCs. Furthermore, in 2018, it was shown that ALA-PDT combined with a DC vaccine could induce an anti-tumor immune response [81]. In this experiment, the DC vaccine based on PDT was shown to increase the number and activity of CD4+ T cells and CD8+ T cells in tumor tissue. In addition, in the PDT-DC vaccine group, the levels of INF-γ and IL-12 increased significantly in the peripheral blood, while the level of the immunosuppressive IL-10 decreased.
Schematic illustration of the preparation procedure and the working principle of PCN‐ACF‐CpG@HA to integrate PDT, antihypoxic signaling, and CpG adjuvant as in situ tumor vaccine. Adapted with permission from [85], copyright 2020 John Wiley and Sons.
(A) Schematic illustration of semiconducting polymer nano-immunomodulator for NIR-induced photodynamic immunotherapy of cancer; (B) In vivo immune responses after NIR photodynamic immunotherapy. (B1) Representative flow cytometric plots and quantitative analysis of mature DCs (CD80+CD86+) in primary tumors and draining lymph nodes at day 3 after photoirradiation. (B2) Representative flow cytometric plots and quantitative analysis of central memory T cells (CD44+CD62L+) among CD4+ T cell subsets and CD8+ T cell subsets in primary tumors at day 24 after photoirradiation. (B3) Immunofluorescence staining images and quantitative analysis of CD8 of primary tumor sections from mice following various treatments. G1, PBS; G2, R837; G3, SPNC (-); G4, SPNI (-); G5, SPNC (+); G6, SPNI (+). Adapted with permission from [86], copyright 2022 John Wiley and Sons.
4.1.3 PDT-motivated autologous tumor cell vaccine
In addition to immune cells, tumor cells can be used in combination with PDT for effective tumor vaccines. Fang et al. [89] prepared a PDT-motivated autologous tumor cell vaccine (P-ATV) by coating the copolymer of porphin E6 (PEI-CE6) on the surface of autologous tumor cells through electrostatic interaction. Subsequently, Fmoc-KCRGDK-phenylboronic acid (FK-PBA) solution and PC-cells dispersed in an alkaline solution were successively injected into the surgical site. FK-PBA can achieve enrichment in postoperative residual tumor sites by targeting sialic acid on the surface of tumor cells and gelation on demand, effectively encapsulating PEI-Ce6-coated autologous tumor cells (PC-Cell) to form PC-Cell@gel. The results showed that PC-Cell@gel combined with PDT could promote the maturation of APCs (mainly DCs) and inhibit regulatory T cells. More importantly, P-ATV was shown to effectively enhance CD8+ T cells specific for the new epitope, thus activating personalized immunity and significantly inhibiting tumor recurrence in melanoma and colon cancer mouse models.
4.2 PDT combined with immune checkpoint blockade
Although the current anti-tumor immunotherapy dominated by immune checkpoint blockades (ICBs) has achieved great clinical success, there remain many patients who do not respond well to ICB treatment [91]. In-depth studies have shown that there is a lack of effective cytotoxic T cell infiltration in the TME of these patients, which is replaced by many immunosuppressive cells [92, 93]. Photosensitizers can exert a photodynamic effect, generate ROS to induce tumor inflammation, improve tumor immunogenicity and promote intratumoral infiltration of cytotoxic T lymphocytes. Moreover, recent animal experiments have shown that PDT combined with ICB can produce synergistic therapy and activate a collective immune response [94-96]. Therefore, the combination of PDT and ICB to trigger the anti-cancer immune response has been extensively studied.
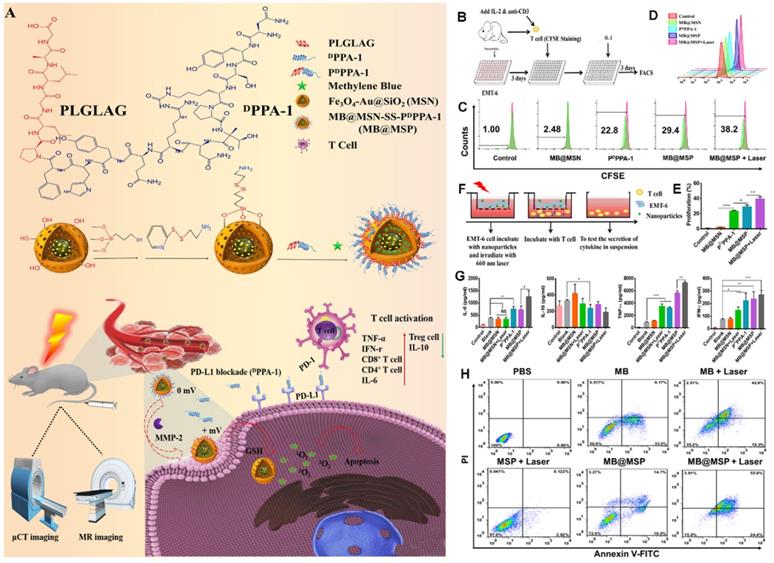
PD-1 is an important immunosuppressive transmembrane protein expressed on the surface of T cells. PD-L1 is the ligand of PD-1 and is induced by proinflammatory cytokines. In the TME, tumor cells can express PD-L1, which binds to PD-1 to inhibit T cell activation and cytokine production; thus, inhibition of the PD-1 pathway will enhance autoimmunity. As proposed in Figure 15A, Feng et al. [97] developed a novel immunomodulatory multifunctional nanoplatform (MB@MSP) based on a Fe3O4-Au core for photodynamic and ICB therapy guided by magnetic resonance (MR) and micro-computed tomography (μCT) imaging. In this nanoplatform, mesoporous silica nanoparticles with an Fe3O4-Au core can be used in nuclear MRI and μCT imaging to realize a non-invasive diagnosis of solid tumors and real-time dynamic monitoring of the drug delivery process. An anti-PD-L1 polypeptide (PDPPA-1) with matrix metalloproteinase-2 (MMP-2) activity was covalently linked to the surface of mesoporous silicon to prevent the leakage of the photosensitizer methylene blue (MB) during blood circulation. The cleavage of the PDPPA-1 peptide by highly expressed MMP-2 in the tumor matrix and the further destruction of the disulfide bond by a high concentration of glutathione in tumor cells eventually reduce the size of MB@MSP and reverse the charge. These changes promote the penetration and absorption of nanocarriers to tumors. The released PDPPA-1 peptide can block the immune checkpoint, create an environment conducive to the activation of cytotoxic T lymphocytes, and enhance tumor cell immunogenic death and tumor cell apoptosis caused by PDT, thus significantly improving the therapeutic effect (Figure 15A). In vitro cell experiments (Figures 15B-H) and in vivo results indicated that the proportion of T cells in the MB@MSP treatment group was significantly higher than that in the other groups. In other words, MB-mediated photodynamic and PDPPA-1 peptide-mediated PD-L1 blocking eliminate the systemic anti-tumor immune response by recruiting tumor-infiltrating CTLs. Additionally, the percentage of Treg cells in the MB@MSP treatment group decreased significantly, indicating that MB@MSP can regulate the immunosuppressive TME and further inhibit tumor metastasis and distal tumor development.
Another frequently exploited checkpoint is CTLA-4, which is expressed in Treg cells. The application of anti-CTLA-4 antibody combined with PDT has proven efficacy as an anti-cancer treatment that can restore immunity [98]. Chen et al. [99] explored the photosensitizer zinc phthalocyanine (ZnPc) and α-CTLA-4 co-encapsulated dextran nanoparticles, namely, ZnPc/α-CTLA4@Ac-DEX NPs (Figure 16). The acidic TME can trigger ZnPc and the release of antibodies released during the degradation of Ac-Dexan by Ac-DEX nanoparticles. These nanoparticles with self-dissociation behavior were formed by an acid-degradable polymer matrix during the process of emulsion. In the 4T1 model of BALB/c mice, PS could produce cytotoxic ROS, damage tumor cells under 660 nm light irradiation, and combined with α-CTLA-4 to induce immunotherapy, but did not cause the systemic immune disorder.
Indoleamine 2,3-dioxygenase (IDO) is an intracellular enzyme overexpressed in tumor cells, which converts tryptophan (Trp) to kynurenine (Kyn), resulting in T cell anergy and promoting the differentiation of Tregs [100], severely impacting immune responses. Therefore, the combination of PDT and IDO inhibitors is considered a potential therapeutic strategy to improve anti-tumor immunity [101-104].
Recently, Huang et al. [104] reported a treatment method by coupling the IDO inhibitor NLG919 with the photosensitizer PPIX into liposomes to form PPIX-NLG@Lipo. After intravenous injection, nanoscaled PPIX-NLG@Lipo can preferentially gather in the tumor site and produce ROS, directly damaging primary tumor cells under light and leading to ICD, enhancing tumor immunogenicity. Meanwhile, PPIX-NLG@Lipo can interfere with IDO's regulation of Trp/Kyn metabolism, and ultimately reverse the immunosuppressive effect of the TME, turning “cold” tumors into “hot” tumors. Therefore, the combination of PDT and IDO blockers leads to the proliferation and infiltration of magnified CD8+ T cells, which inhibits not only primary and treated tumors but also distant tumors.
Liu et al. [105] further investigated the systemic immune effect induced by the combination of PDT and IDO to understand how to reverse the immunosuppressive TME. The researchers developed a redox-activated liposome called IND@RAL (Figure 17A), which could induce ICD and reverse the tumor suppressor microenvironment simultaneously by porphyrin-phospholipid coupling self-assembly and remote loading of IDO inhibitors (NLG8189) in the cavity. After intravenous injection, the IND@RAL prolonged the blood circulation of 4T1 tumor-bearing mice and promoted tumor accumulation. Following endocytosis of tumor cells, the nanovesicles activate fluorescence signaling and PDT activity exponentially in response to high intracellular glutathione levels, thus effectively inhibiting tumor growth. More importantly, redox-activated PDT induces intratumoral infiltration of CTLs by inducing the ICD of tumor cells, activating the innate immune system, and promoting antigen presentation (Figure 17B). Combined with IDO inhibitors, the anti-tumor immune response of the system will be further enhanced. As shown in Figure 17C, the percentage of CD3+ CD8+ T cells in the tumor tissue increased significantly after treatment, while the percentage of Treg cells decreased significantly, indicating that the combination of PDT and IDO inhibitors can systematically inhibit IDO activity and reverse the immunosuppressive TME. It activates innate immune cells to recognize necrotic tumor cells, deliver tumor-derived antigens to T cells, stimulates the infiltration of tumor-specific T cells, and thus has a strong killing effect on primary and distal tumors. In all, this strategy is expected to be used in PDT synergistic immunotherapy of metastatic cancer.
(A) Schematic illustration of the therapeutic mechanism of MB@MSP for PDT and immune therapy. (B) Schematic diagram of T cells and EMT-6 tumor cells co-culture system. (C, D) The proliferation rate of T cells was assessed after various treatments by flow cytometry analysis. (E) Quantification of the proliferation rate of T cells. (F) Schematic illustration of the in vitro Transwell co-cultured system, in which EMT- 6 tumor cells were pre-treated and then incubated with T cells. Tumor cells were placed in the upper chamber, and T cells were cultured in the lower chamber. Finally, cytokine secretion by T cells cultured system, measured by ELISA (G). (H) Flow cytometry data for Annexin V-FITC/PI-stained EMT-6 cells with different treatments, the lower left, lower right, upper right, and upper left quadrants represent the viable, early apoptotic, late apoptotic, and dead cells, respectively. Adapted with permission from [97], copyright 2021 Elsevier.
Scheme of the MN-assisted co-delivery system and possible mechanism of antitumor immune responses induced by MN-assisted PDT in combination with immune checkpoint blockade. Adapted with permission from [99], copyright 2020 Elsevier.
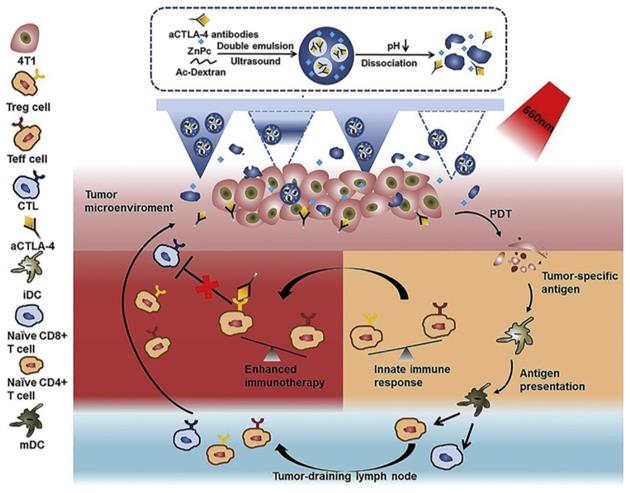
(A) Schematic Illustration of Combined PDT and Immunotherapy by IND@RAL for Combating Cancer. (B) Immunofluorescence imaging of CRT expression and HMGB1 release treated with RAL in the presence or absence of laser irradiation. (C) Flow cytometric analysis of CD3 and CD8, CD3 and CD4 positive cytotoxic T lymphocyte in spleen. Adapted with permission from [105], copyright 2019 American Chemical Society.
5. PDT combined with other therapies
Traditional cancer treatments, including chemotherapy and radiotherapy, as well as emerging cancer immunotherapies, including photothermal therapy, have potential and advantages in the field of cancer treatment. Therefore, a combination of multiple treatments is more effective than any single treatment in producing improved anti-cancer activity. In this section, the advantages of PDT-mediated immunization along with other treatments are summarized.
5.1 PDT combined with chemotherapy
Recent studies have found that some chemotherapeutic drugs, such as oxaliplatin [106], doxorubicin (DOX) [107, 108], and mitoxantrone (MTX) [109], can not only kill tumor cells but also cause ICD. Therefore, some researchers have combined chemotherapy with PDT to activate the T-cell immune response in the TME [107, 110-113].
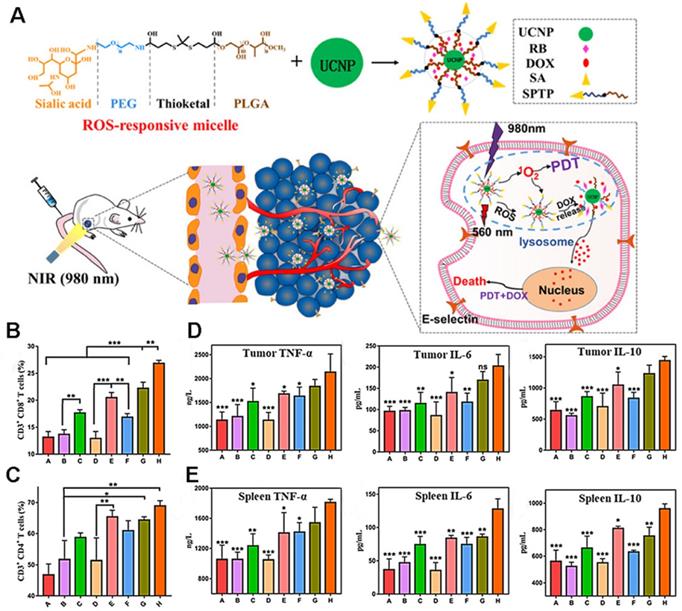
As shown in Figure 18A, Jin et al. [107] co-encapsulated DOX and the photosensitizer rose bengal (RB) in ROS-responsive micelles based on upconversion nanoparticles (UCNP). The sialic acid (SA) on the surface of the micelle can specifically recognize the overexpressed E-selectin on the surface of tumor cells, leading to efficient targeting. After reaching the tumor, under the irradiation of near-infrared light, the photosensitizer RB receives energy to produce 1O2, which functions to kill tumor cells. Simultaneously, ROS production promotes the rupture of the micelle, releasing DOX, thereby demonstrating the synergistic effect of PDT and chemotherapy. The results showed that the prepared micellar particles initiated a large amount of 4T1 cell death (73.1%), and exhibited deep permeability and tumor accumulation. Moreover, the increase in CRT, ATP, and HMGB1 secretion levels indicated that PDT and DOX successfully induced the ICD and improved the anti-tumor immune response. The results of flow cytometry showed that the ratio of activated CD8+ T and CD4+ T cells was significantly increased in the micelle group, as were the levels of TNF-a, IL-10, and IL-6, suggesting that PDT combined with chemotherapy can activate the immune response in vivo (Figures 18B-E).
(A) Illustration of the anticancer application of SPTP@UCNP-Rb-DOX in TNBC murine model by cascade amplification of chemo-PDT with systematic anti-tumor immunity. Analysis of CD4+ T cells (B) and CD8+ T cells and (C) proliferation in spleen tissues collected from all mice groups. Quantitative analysis of TNF-α, IL-6, and IL-10 from tumor lysis solution (D) and (E) in spleen lysis solution by the Elisa kit. Adapted with permission from [107], copyright 2020 American Chemical Society.
Schematic representation of the synthesis and phototherapeutic functions of the theranostic HPC. Adapted with permission from [6], copyright 2020, Royal Society of Chemistry.
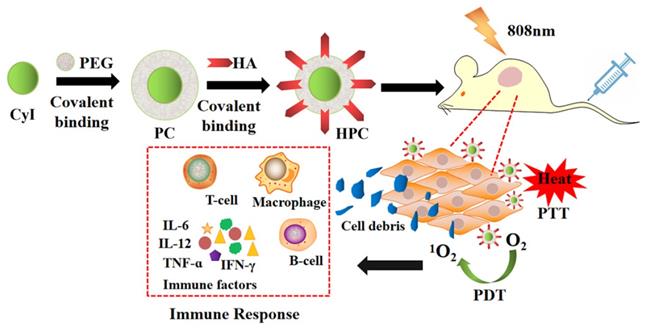
5.2 PDT combined with photothermal therapy
Compared to PDT or PTT alone, synergistic phototherapy may conquer the extreme heterogeneity and complexity of refractory tumors and achieve better therapeutic effects. Recently, we synthesized a new iodinated-cyanine dye, named CyI, with stronger singlet oxygen (1O2) generating ability while maintaining excellent photothermal conversion efficiency and near-infrared fluorescence imaging compared to traditional cyanine dyes [6]. Based on this, a novel targeted nanocarrier (HA-PEG-CyI) was designed by inducing the self-assembly of PEGylated CyI (iodinated-cyanine) by attaching a HA ligand on the surface [6] (Figure 19). HA is a biocompatible polysaccharide molecule that specifically binds to CD44 receptors overexpressed on the surface of tumor cells. Modifying HA with PEG-CyI is expected to increase the targeting ability of CyI. In vivo studies have shown that the novel targeted nanocarrier could effectively ablate tumors with a synergistic PDT/PTT effect, and further induce the release of tumor-related pro-inflammatory mediators (TNF-α, IFN-γ, IL-12, CTL), resulting in systemic anti-tumor immunity and secondary death of tumor cells.
The lack of deep infiltration of tumor tissue is a major limitation of drug therapy. In addition to active targeting, Yang et al. [114] for the first-time combined size-transforming and transcytosis strategies, which effectively enhanced passive diffusion and active transport to construct anti-tumor immune nanodrugs with efficient delivery efficiency (named CPIM) (Figure 20A). In terms of passive diffusion, cluster-bomb-like nanoplatforms (135 nm) release small drug-loaded “particles” (PAMAM loaded with IR780/1- methyltryptophan (1-MT), particle size < 10 nm) under the action of high concentrations of ROS in the TME, and promote the permeation and diffusion of IR780 and 1-MT in tumor tissue. Regarding active transport, the nanopreparation can be delivered by transcytosis, and in terms of anti-tumor activity, as shown in Figures 20B-D, CPIM significantly increased the penetration of CTL, with the highest proportion of CTL/Tregs and a significant increase in the DC maturity ratio. Moreover, the secretion of cytokines (including INF- γ, TNF- α, IL-6, IL-2) increased significantly. It was also confirmed that the nanopreparation can induce ICD, turn a “cold” immunosuppressive TME into a “hot” immunogenic TME, and inspire immunotherapy in vivo.
5.3 PDT combined with radiotherapy
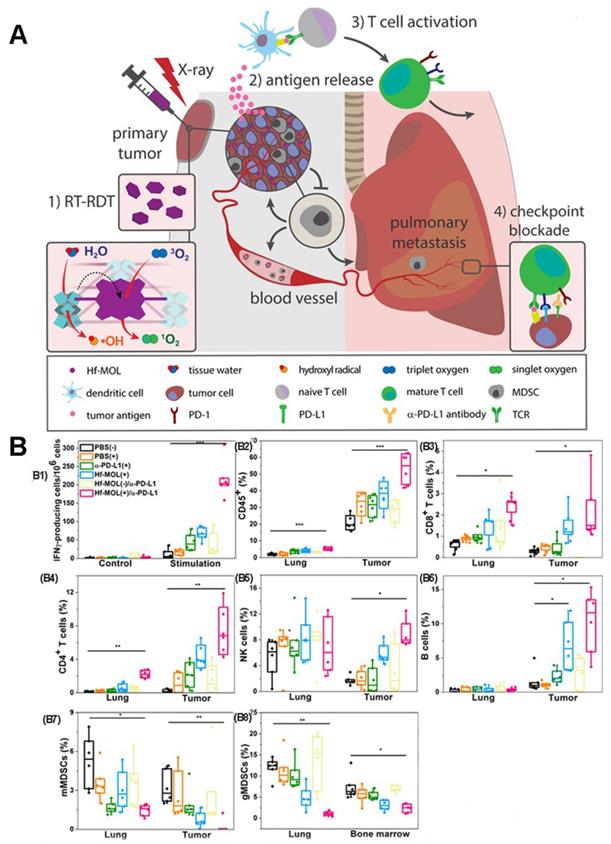
Previous studies have shown that Radiotherapy (RT) can induce systemic immune response [115, 116]. Therefore, the combination of PDT and radiotherapy (known as Radio therapy-Radiodynamic therapy, RT-RDT) is also a method to improve the anti-tumor immune response [25, 117, 118]. As proposed in Figure 21A, Lin et al [25] reported a new nanoscale metal-organic layer (nMOL), Hf-MOL, to effectively treated local tumors via radiotherapy-radiodynamic therapy (RT-RDT) with low-dose X-rays. When Hf-MOL is used in combination with immune checkpoint blockers, it can reverse metastatic tumor, improve tumor inflammation and activate systemic immunity by inducing immunogenic cell death (ICD).
The researchers evaluated the immunogenicity of Hf-MOL-mediated RT-RDT. By detecting the ATP, HMGB1 and CRT at the cellular level, the immunogenicity of Hf-MOL-mediated RT-RDT was significantly higher than that of nMOF-mediated radiotherapy. It was found that Hf-MOL mediated RT-RDT alone can effectively inhibit in situ tumor, but cannot achieve the inhibition of distal metastasis; while the combination of Hf-MOL mediated RT-RDT and α-PD-L1 mediated can not only greatly eliminate in situ tumor, but also effectively inhibit distal metastasis. After treatment, they analyzed the changes of tumor-infiltrating immune cells in the TME. Flow cytometry analysis showed that the expression of tumor-specific effector T cells, helper T cells, natural killer (NK) cells, and B cells up-regulated in tumors. When using ELISpot to detect IFN- γ in the spleen, the researchers found that the untreated tumor-bearing mice showed severe spleen enlargement compared with other non-lung-metastatic breast tumor models. Spleen size is generally regarded as a symbol of the immune response, and splenomegaly is considered a sign of acute immune toxicity. They described the splenocytes and detected significant upregulation of granulocytic MDSCs (gMDSCs) in the splenomegaly group. Only the gMDSC of HF-MOL(+)/α-PD-L1 treatment group decreased significantly, indicating that MOL(+)/α-PD-L1 could systematically consume gMDSC, and prevent abnormal splenic enlargement, which is a typical symptom of high metastatic tumor mice (Figure 21B).
(A) Schematic illustration of the in vivo performance; (B) Analysis of percentages of Ths (CD3+ CD4+), CTLs (CD3+ CD8+), Tregs (CD4+ FOXP3+) and the ratio of Ths/Tregs and CTLs/Tregs in abscopal tumors (n=3); (C) Analysis of percentages of Ths (CD3+CD4+), CTLs (CD3+ CD8+), Tregs (CD4+ FOXP3+) and the ratio of Ths/Tregs and CTLs/Tregs in spleens (n=3); (D) Analysis of percentages of matured DCs (CD80+ CD86+) in tumor-draining lymph nodes (n=3). Adapted with permission from [114], copyright 2021 Elsevier.
(A) Schematic illustration of combination therapy of PDT and X-rays. (B) Evaluation of anti-metastatic immunity in vivo. (B1) ELISPOT assay was performed to detect tumor-specific IFN-γ producing T cells. CD45+ (B2), CD8+ T cells(B3), CD4+ T cells(B4), NK cells(B5), B cells(B6), mMDSCs(B7) and gMDSCs (B8) with respect to the total tumor cells. Adapted with permission from [25], copyright 2019 Elsevier.
6. Summary and prospects
Increasing evidence suggests that malignant tumors are not just a local disease, but instead represent a component of a complex adaptive system that involves inflammation, immunity, metabolism, and genetic disorders. Immunotherapy has been proven to have a great prospect in cancer treatment, but its low response rate and potential side effects remain major obstacles to its wide application in the clinic. Various combination therapies overcome these shortcomings while improving treatment outcomes. PDT, with minimal invasiveness, repeatability, no cumulative toxicity, excellent function, and cosmetic effect, is one such popular strategy, which can reduce the long-term incidence and improve the quality of life of patients. Most importantly, after the action of PDT, TAAs and related molecular damage patterns will be released, resulting in the death of immunogenic cells and the stimulation of the immune response. However, the weak immunity induced by PDT alone is insufficient to trigger a systemic immune response to cancer cells. Hence, in this study, we summarized and discussed recent advances and developments in the design of PDT-induced antitumor immunity in this review.
First, the complexity of the TME and the residue of cytokines and immune cells made it difficult to maximize the anti-tumor effect. Therefore, increasing attention has been paid to the role of PDT-mediated anti-tumor immunity in remodeling the immune TME, for example, using hyaluronidase targeting to destroy the ECM, VTP to break the physical barrier, or various oxygen supplement strategies to alleviate tumor hypoxia to improve the TME. Second, it has recently been found that the endoplasmic reticulum and mitochondria have immune potential as targets. Therefore, subcellular organelle and mitochondrial targeted PDT are also options for anti-tumor immunity. Additionally, PDT can be used as an in situ vaccine to promote anti-tumor effects. PDT can be combined with various additional immune stimuli (such as immune adjuvants and immune cells) to build a more powerful tumor vaccine. Finally, combination therapy—including immunotherapy, photothermal therapy, chemotherapy, and radiotherapy—is an effective strategy to improve the anti-tumor immune effect of PDT and transform “cold” tumors into “hot” tumors.
Although progress has been made, more efforts are needed to study photodynamic mediated anti-tumor immunity. For example, nanophotodynamic may be recognized and cleared by the reticuloendothelial system (RES) [119] or the mononuclear system (MPS) [120], thereby reducing the drug concentration at the tumor site; accordingly, appropriate biomaterials that mediate phototherapy should be developed. Although nanoparticles can be modified with PEG, PEG-modified nanoparticles can produce antibodies [121] and “accelerate blood clearance” [122] after the first injection (that is, PEG-encapsulated nanoparticles are quickly cleared by the liver at subsequent doses).
Modern immunotherapy brings new hope for tumor types with limited treatment methods and poor efficacy. From monotherapy to combination therapy, and onward to the discovery of new indications for drugs. In solid tumors, the greatest successes are obtained using ICB, with different mechanisms of action and clinical applications continuing to emerge. The shortcomings of immunotherapy also continue to emerge. Indeed, tumor cells can escape the killing effect of the innate and adaptive immune system, which makes ICB therapy ineffective. The inherent mechanisms of tumor immune escape include affecting the formation, presentation, and processing of new antigens through genetic and epigenetic changes, as well as altering signal transduction pathways that damage the role of cytotoxic T cells. External mechanisms include non-cancerous stromal or immune cells or other systemic effects that can cooperate with cancer cells to promote cancer cell growth and ICB resistance, such as host microbiota [93, 123]. Importantly, several experimental studies have shown that the combination of phototherapy and ICB can promote the release of TAAs, thus triggering the tumor immune response and further promoting the blocking effect of ICB. However, there remains a lack of relevant clinical trials. Moreover, the differences in cytotoxicity and efficacy or treatment resistance caused by ICB are different from those of traditional chemotherapy or targeted therapy. At present, most clinical trials are limited to several tumor types, as well as lack of specific and effective targets [124].
Immunologic tolerance is a state of specific unresponsiveness when immunoreactive cells come into contact with antigenic substances. If T and B cells that respond specifically to antigens cannot be activated by antigen stimulation, they cannot produce specific immune effector cells to remember specific antibodies and thus cannot mount an effective immune response. There are many kinds of tumor-promoting immune cells in the TME, such as MDSCs, Tregs, and tumor-associated macrophages (TAMs). MDSCs secrete ROS, NO to inactivate T cells, and TGF β and IL-10 to activate Tregs [125, 126]. Moreover, ROS can induce the accumulation of SUMO-specific protease 3 (SENP3), which is related to the anti-tumor immunosuppression of Treg to T cells [127]. Additionally, ROS can stimulate the transformation of TAMs [128] and induce Tregs [129] to inhibit the expression of CTLs. Therefore, ROS are not only a metabolic by-product of biological cells but also an important intracellular signal molecule involved in tumor immune response and tolerance, although the specific mechanism requires further study.
Finally, due to the inconsistent results of different models of primary tumors and the difficulty of combining biomarkers, the effect and efficiency of the comprehensive treatment are not always comparable. As such, more comprehensive treatment research could be conducive to the development of successful photo-immunotherapy drugs.
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (No. 32171362) and Natural Science Foundation of Shandong Province (No. ZR2021MH087).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Li X, Lovell JF, Yoon J, Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol. 2020;17:657-74
2. Ostanska E, Aebisher D, Bartusik-Aebisher D. The potential of photodynamic therapy in current breast cancer treatment methodologies. Biomed Pharmacother. 2021;137:111302
3. Railkar R, Agarwal PK. Photodynamic therapy in the treatment of bladder cancer: past challenges and current innovations. Eur Urol Focus. 2018;4:509-11
4. Dougherty TJ, Grindey GB, Fiel R, Weishaupt KR, Boyle DG. Photoradiation therapy. II. cure of animal tumors with hematoporphyrin and light. J Natl Cancer Inst. 1975;55:115-21
5. Donohoe C, Senge MO, Arnaut LG, Gomes-da-Silva LC. Cell death in photodynamic therapy: from oxidative stress to anti-tumor immunity. Biochim Biophys Acta Rev Cancer. 2019;1872:188308
6. Chi J, Ma Q, Shen Z, Ma C, Zhu W, Han S. et al. Targeted nanocarriers based on iodinated-cyanine dyes as immunomodulators for synergistic phototherapy. Nanoscale. 2020;12:11008-25
7. van Straten D, Mashayekhi V, de Bruijn HS, Oliveira S, Robinson DJ. Oncologic photodynamic therapy: basic principles, current clinical status and future directions. Cancers (Basel). 2017;9:19
8. Dong Y, Cao W, Cao J. Treatment of rheumatoid arthritis by phototherapy: advances and perspectives. Nanoscale. 2021;13:14591-608
9. Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer. 2006;6:535-45
10. Alzeibak R, Mishchenko TA, Shilyagina NY, Balalaeva IV, Vedunova MV, Krysko DV. Targeting immunogenic cancer cell death by photodynamic therapy: past, present and future. J Immunother Cancer. 2021;9:e001926
11. Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860-75
12. Cramer GM, Cengel KA, Busch TM. Forging forward in photodynamic therapy. Cancer Res. 2022;82:534-6
13. Fucikova J, Kepp O, Kasikova L, Petroni G, Yamazaki T, Liu P. et al. Detection of immunogenic cell death and its relevance for cancer therapy. Cell Death Dis. 2020;11:1013
14. Min H, Wang J, Qi Y, Zhang Y, Han X, Xu Y. et al. Biomimetic metal-organic framework nanoparticles for cooperative combination of antiangiogenesis and photodynamic therapy for enhanced efficacy. Adv Mater. 2019;31:e1808200
15. Zhang D, Feng F, Li Q, Wang X, Yao L. Nanopurpurin-based photodynamic therapy destructs extracellular matrix against intractable tumor metastasis. Biomaterials. 2018;173:22-33
16. Korbelik M. Cancer vaccines generated by photodynamic therapy. Photochem Photobiol Sci. 2011;10:664-9
17. Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18:197-218
18. Martin JD, Cabral H, Stylianopoulos T, Jain RK. Improving cancer immunotherapy using nanomedicines: progress, opportunities and challenges. Nat Rev Clin Oncol. 2020;17:251-66
19. Liu YT, Sun ZJ. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics. 2021;11:5365-86
20. Kim S, Kim SA, Nam GH, Hong Y, Kim GB, Choi Y. et al. In situ immunogenic clearance induced by a combination of photodynamic therapy and rho-kinase inhibition sensitizes immune checkpoint blockade response to elicit systemic antitumor immunity against intraocular melanoma and its metastasis. J Immunother Cancer. 2021;9:e00148
21. Yan S, Zeng X, Tang Y, Liu BF, Wang Y, Liu X. Activating antitumor immunity and antimetastatic effect through polydopamine-encapsulated core-shell upconversion nanoparticles. Adv Mater. 2019;31:e1905825
22. Li Z, Zhu L, Sun H, Shen Y, Hu D, Wu W. et al. Fluorine assembly nanocluster breaks the shackles of immunosuppression to turn the cold tumor hot. Proc Natl Acad Sci U S A. 2020;117:32962-9
23. Huang LL, Wan JQ, Wu HH, Chen XN, Bian Q, Shi L L. et al. Quantitative self-assembly of photoactivatable small molecular prodrug cocktails for safe and potent cancer chemo-photodynamic therapy. Nano Today. 2021;36:101030
24. Chen XN, Xie BB, Huang LL, Wan JQ, Wang YC, Shi XW. et al. Quantitative self-assembly of pure drug cocktails as injectable nanomedicines for synergistic drug delivery and cancer therapy. Theranostics. 2021;11:5713-27
25. Ni K, Lan G, Chan C, Duan X, Guo N, Veroneau SS. et al. Ultrathin metal-organic layer-mediated radiotherapy-radiodynamic therapy enhances immunotherapy of metastatic cancers. Matter. 2019;1:1331-53
26. Hou X, Tao Y, Pang Y, Li X, Jiang G, Liu Y. Nanoparticle-based photothermal and photodynamic immunotherapy for tumor treatment. Int J Cancer. 2018;143:3050-60
27. Yang M, Li J, Gu P, Fan X. The application of nanoparticles in cancer immunotherapy: Targeting tumor microenvironment. Bioact Mater. 2021;6:1973-87
28. Li X, Zhao Y, Zhang T, Xing D. Mitochondria-specific agents for photodynamic cancer therapy: a key determinant to boost the efficacy. Adv Healthc Mater. 2021;10:e2001240
29. Anderson NM, Simon MC. The tumor microenvironment. Curr Biol. 2020;30:R921-5
30. DeBerardinis RJ. Tumor microenvironment, metabolism, and immunotherapy. N Engl J Med. 2020;382:869-71
31. Murciano-Goroff YR, Warner AB, Wolchok JD. The future of cancer immunotherapy: microenvironment-targeting combinations. Cell Res. 2020;30:507-19
32. Zhu H, Liu Q, Miao L, Musetti S, Huo M, Huang L. Remodeling the fibrotic tumor microenvironment of desmoplastic melanoma to facilitate vaccine immunotherapy. Nanoscale. 2020;12:3400-10
33. Chon HJ, Lee WS, Yang H, Kong SJ, Lee NK, Moon ES. et al. Tumor microenvironment remodeling by intratumoral oncolytic vaccinia virus enhances the efficacy of immune-checkpoint blockade. Clin Cancer Res. 2019;25:1612-23
34. Pan Y, Lu F, Fei Q, Yu X, Xiong P, Yu X. et al. Single-cell RNA sequencing reveals compartmental remodeling of tumor-infiltrating immune cells induced by anti-CD47 targeting in pancreatic cancer. J Hematol Oncol. 2019;12:124
35. Chen Q, Liu G, Liu S, Su H, Wang Y, Li J. et al. Remodeling the tumor microenvironment with emerging nanotherapeutics. Trends in Pharmacological Sciences. 2018;39:59-74
36. Gong H, Chao Y, Xiang J, Han X, Song G, Feng L. et al. Hyaluronidase to enhance nanoparticle-based photodynamic tumor therapy. Nano Lett. 2016;16:2512-21
37. Wang HR, Han X, Dong ZL, Xu J, Wang J, Liu Z. Hyaluronidase with pH-responsive dextran modification as an adjuvant nanomedicine for enhanced photodynamic-immunotherapy of cancer. Adv Funct Mater. 2019;29:1902440
38. Zhang C, Ni D, Liu Y, Yao H, Bu W, Shi J. Magnesium silicide nanoparticles as a deoxygenation agent for cancer starvation therapy. Nat Nanotechnol. 2017;12:378-86
39. Song Y, Tang C, Yin C. Combination antitumor immunotherapy with VEGF and PIGF siRNA via systemic delivery of multi-functionalized nanoparticles to tumor-associated macrophages and breast cancer cells. Biomaterials. 2018;185:117-32
40. Jahanban-Esfahlan R, Seidi K, Banimohamad-Shotorbani B, Jahanban-Esfahlan A, Yousefi B. Combination of nanotechnology with vascular targeting agents for effective cancer therapy. J Cell Physiol. 2018;233:2982-92
41. Azzouzi A R, Barret E, Bennet J, Moore C, Taneja S, Muir G. et al. TOOKAD(R) soluble focal therapy: pooled analysis of three phase II studies assessing the minimally invasive ablation of localized prostate cancer. World J Urol. 2015;33:945-53
42. Azzouzi AR, Vincendeau S, Barret E, Cicco A, Kleinclauss F, van der Poel HG. et al. Padeliporfin vascular-targeted photodynamic therapy versus active surveillance in men with low-risk prostate cancer (CLIN1001 PCM301): an open-label, phase 3, randomised controlled trial. Lancet Oncol. 2017;18:181-91
43. O'Shaughnessy MJ, Murray KS, La Rosa SP, Budhu S, Merghoub T, Somma A. et al. Systemic antitumor immunity by PD-1/PD-L1 inhibition is potentiated by vascular-targeted photodynamic therapy of primary tumors. Clin Cancer Res. 2018;24:592-9
44. Riera-Domingo C, Audige A, Granja S, Cheng WC, Ho PC, Baltazar F. et al. Immunity, hypoxia, and metabolism-the menage a trois of cancer: implications for immunotherapy. Physiol Rev. 2020;100:1-102
45. Sharma A, Arambula JF, Koo S, Kumar R, Singh H, Sessler JL. et al. Hypoxia-targeted drug delivery. Chem Soc Rev. 2019;48:771-813
46. Li X, Kwon N, Guo T, Liu Z, Yoon J. Innovative strategies for hypoxic-tumor photodynamic therapy. Angew Chem Int Ed Engl. 2018;57:11522-31
47. Day RA, Estabrook DA, Logan JK, Sletten EM. Fluorous photosensitizers enhance photodynamic therapy with perfluorocarbon nanoemulsions. Chem Commun (Camb). 2017;53:13043-6
48. Liu Z, Xue Y, Wu M, Yang G, Lan M, Zhang W. Sensitization of hypoxic tumor to photodynamic therapy via oxygen self-supply of fluorinated photosensitizers. Biomacromolecules. 2019;20:4563-73
49. Zhang Y, Bo S, Feng T, Qin X, Wan Y, Jiang S. et al. A versatile theranostic nanoemulsion for architecture-dependent multimodal imaging and dually augmented photodynamic therapy. Adv Mater. 2019;31:e1806444
50. Liang X, Chen M, Bhattarai P, Hameed S, Dai Z. Perfluorocarbon@porphyrin nanoparticles for tumor hypoxia relief to enhance photodynamic therapy against liver metastasis of colon cancer. ACS Nano. 2020;14:13569-83
51. Zhang Y, Liao Y, Tang Q, Lin J, Huang P. Biomimetic nanoemulsion for synergistic photodynamic-immunotherapy against hypoxic breast tumor. Angew Chem Int Ed Engl. 2021;60:10647-53
52. Wang Z, Gong X, Li J, Wang H, Xu X, Li Y. et al. Oxygen-delivering polyfluorocarbon nanovehicles improve tumor oxygenation and potentiate photodynamic-mediated antitumor immunity. ACS Nano. 2021;15:5405-19
53. Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794-8
54. Shi C, Li M, Zhang Z, Yao Q, Shao K, Xu F. et al. Catalase-based liposomal for reversing immunosuppressive tumor microenvironment and enhanced cancer chemo-photodynamic therapy. Biomaterials. 2020;233:119755
55. Pathria P, Louis TL, Varner JA. Targeting tumor-associated macrophages in cancer. Trends Immunol. 2019;40:310-27
56. Shen Z, Xia J, Ma Q, Zhu W, Gao Z, Han S. et al. Tumor microenvironment-triggered nanosystems as dual-relief tumor hypoxia immunomodulators for enhanced phototherapy. Theranostics. 2020;10:9132-52
57. Wang ZZ, Shen XM, Gao XF. Density functional theory mechanistic insight into the peroxidase- and oxidase-like activities of nanoceria. J Phys Chem C. 2021;125:23098-104
58. Xu KQ, Cheng Y, Yan J, Feng YL, Zheng RX, Wu XQ. et al. Polydopamine and ammonium bicarbonate coated and doxorubicin loaded hollow cerium oxide nanoparticles for synergistic tumor therapy. Nano Res. 2019;12:2947-53
59. Zuo HQ, Hou YC, Yu YJ, Li ZQ, Liu HX, Liu C. et al. Circumventing myeloid-derived suppressor cell-mediated immunosuppression using an oxygen-generated and -economized nanoplatform. Acs Appl Mater Interfaces. 2020;12:55723-36
60. Csordas G, Weaver D, Hajnoczky G. Endoplasmic reticulum-mitochondrial contactology: structure and signaling functions. Trends Cell Biol. 2018;28:523-40
61. Chen X, Cubillos-Ruiz JR. Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nat Rev Cancer. 2021;21:71-88
62. Kepp O, Menger L, Vacchelli E, Locher C, Adjemian S, Yamazaki T. et al. Crosstalk between ER stress and immunogenic cell death. Cytokine Growth Factor Rev. 2013;24:311-8
63. Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97-111
64. Deng H, Zhou Z, Yang W, Lin LS, Wang S, Niu G. et al. Endoplasmic reticulum targeting to amplify immunogenic cell death for cancer immunotherapy. Nano Lett. 2020;20:1928-33
65. Ma H, Lu Y, Huang Z, Long S, Cao J, Zhang Z. et al. ER-targeting cyanine dye as an NIR photoinducer to efficiently trigger photoimmunogenic cancer cell death. J Am Chem Soc. 2022;144:3477-86
66. Yan J, Chen J, Zhang N, Yang Y, Zhu W, Li L. et al. Mitochondria-targeted tetrahedral DNA nanostructures for doxorubicin delivery and enhancement of apoptosis. J Mater Chem B. 2020;8:492-503
67. Kim S, Jana B, Go EM, Lee JE, Jin S, An EK. et al. Intramitochondrial disulfide polymerization controls cancer cell fate. ACS Nano. 2021;15:14492-508
68. Mills EL, Kelly B, O'Neill L AJ. Mitochondria are the powerhouses of immunity. Nat Immunol. 2017;18:488-98
69. Zhao J, Schank M, Wang L, Li Z, Nguyen LN, Dang X. et al. Mitochondrial functions are compromised in CD4 T cells from ART-controlled PLHIV. Front Immunol. 2021;12:658420
70. Saha T, Dash C, Jayabalan R, Khiste S, Kulkarni A, Kurmi K. et al. Intercellular nanotubes mediate mitochondrial trafficking between cancer and immune cells. Nat Nanotechnol. 2022;17:98-106
71. Bailis W, Shyer JA, Zhao J, Canaveras JCG, Al Khazal FJ, Qu R. et al. Distinct modes of mitochondrial metabolism uncouple T cell differentiation and function. Nature. 2019;571:403-7
72. Lisci M, Barton PR, Randzavola LO, Ma CY, Marchingo JM, Cantrell DA. et al. Mitochondrial translation is required for sustained killing by cytotoxic T cells. Science. 2021;374:eabe9977
73. Zielonka J, Joseph J, Sikora A, Hardy M, Ouari O, Vasquez-Vivar J. et al. Mitochondria-targeted triphenylphosphonium-based compounds: syntheses, mechanisms of action, and therapeutic and diagnostic applications. Chem Rev. 2017;117:10043-120
74. Lee YH, Park HI, Chang WS, Choi JS. Triphenylphosphonium-conjugated glycol chitosan microspheres for mitochondria-targeted drug delivery. Int J Biol Macromol. 2021;167:35-45
75. Peng N, Yu H, Yu W, Yang M, Chen H, Zou T. et al. Sequential-targeting nanocarriers with pH-controlled charge reversal for enhanced mitochondria-located photodynamic-immunotherapy of cancer. Acta Biomater. 2020;105:223-38
76. Wei DS, Chen Y B, Huang Y, Li P, Zhao Y, Zhang XH. et al. NIR-light triggered dual-cascade targeting core-shell nanoparticles enhanced photodynamic therapy and immunotherapy. Nano Today. 2021;41:101288
77. He C, Duan X, Guo N, Chan C, Poon C, Weichselbaum RR. et al. Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nat Commun. 2016;7:12499
78. Jin L, Shen S, Huang Y, Li D, Yang X. Corn-like Au/Ag nanorod-mediated NIR-II photothermal/photodynamic therapy potentiates immune checkpoint antibody efficacy by reprogramming the cold tumor microenvironment. Biomaterials. 2021;268:120582
79. Hwang HS, Cherukula K, Bang YJ, Vijayan V, Moon MJ, Thiruppathi J. et al. Combination of photodynamic therapy and a flagellin-adjuvanted cancer vaccine potentiated the anti-PD-1-mediated melanoma suppression. Cells. 2020;9:2432
80. Ding B, Shao S, Yu C, Teng B, Wang M, Cheng Z. et al. Large-pore mesoporous-silica-coated upconversion nanoparticles as multifunctional immunoadjuvants with ultrahigh photosensitizer and antigen loading efficiency for improved cancer photodynamic immunotherapy. Adv Mater. 2018;30:e1802479
81. Zhang H, Wang P, Wang X, Shi L, Fan Z, Zhang G. et al. Antitumor effects of DC vaccine with ALA-PDT-induced immunogenic apoptotic cells for skin squamous cell carcinoma in mice. Technol Cancer Res Treat. 2018;17:1533033818785275
82. Trempolec N, Doix B, Degavre C, Brusa D, Bouzin C, Riant O. et al. Photodynamic therapy-based dendritic cell vaccination suited to treat peritoneal mesothelioma. Cancers (Basel). 2020;12:545
83. Zhang Y, Liao Y, Tang Q, Lin J, Huang P. Biomimetic nanoemulsion for synergistic photodynamic-immunotherapy against hypoxic breast tumor. Angew Chem Int Ed Engl. 2021;60:10647-53
84. Shi S, Zhu H, Xia X, Liang Z, Ma X, Sun B. Vaccine adjuvants: understanding the structure and mechanism of adjuvanticity. Vaccine. 2019;37:3167-78
85. Cai Z, Xin F, Wei Z, Wu M, Lin X, Du X. et al. Photodynamic therapy combined with antihypoxic signaling and CpG adjuvant as an in situ tumor vaccine based on metal-organic framework nanoparticles to boost cancer immunotherapy. Adv Healthc Mater. 2020;9:e1900996
86. Liu J, He S, Luo Y, Zhang Y, Du X, Xu C. et al. Tumor-microenvironment-activatable polymer nano-immunomodulator for precision cancer photoimmunotherapy. Adv Mater. 2022;34:e2106654
87. Garg AD, Coulie PG, Van den Eynde BJ, Agostinis P. Integrating next-generation dendritic cell vaccines into the current cancer immunotherapy landscape. Trends Immunol. 2017;38:577-93
88. Sabado RL, Balan S, Bhardwaj N. Dendritic cell-based immunotherapy. Cell Res. 2017;27:74-95
89. Fang L, Zhao Z, Wang J, Zhang P, Ding Y, Jiang Y. et al. Engineering autologous tumor cell vaccine to locally mobilize antitumor immunity in tumor surgical bed. Sci Adv. 2020;6:eaba4024
90. Ji J, Fan Z, Zhou F, Wang X, Shi L, Zhang H. et al. Improvement of DC vaccine with ALA-PDT induced immunogenic apoptotic cells for skin squamous cell carcinoma. Oncotarget. 2015;6:17135-46
91. Sharma P, Allison J P. The future of immune checkpoint therapy. Science. 2015;348:56-61
92. Ventola CL. Cancer immunotherapy, Part 3: challenges and future trends. P T. 2017;42:514-21
93. Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer. 2018;118:9-16
94. Wang Z, Zhang F, Shao D, Chang Z, Wang L, Hu H. et al. Janus nanobullets combine photodynamic therapy and magnetic hyperthermia to potentiate synergetic anti-metastatic immunotherapy. Adv Sci (Weinh). 2019;6:1901690
95. Zhang R, Zhu Z, Lv H, Li F, Sun S, Li J. et al. Immune checkpoint blockade mediated by a small-molecule nanoinhibitor targeting the PD-1/PD-L1 pathway synergizes with photodynamic therapy to elicit antitumor immunity and antimetastatic effects on breast cancer. Small. 2019;15:e1903881
96. Theodoraki MN, Lorenz K, Lotfi R, Furst D, Tsamadou C, Jaekle S. et al. Influence of photodynamic therapy on peripheral immune cell populations and cytokine concentrations in head and neck cancer. Photodiagnosis Photodyn Ther. 2017;19:194-201
97. Feng Y, Xie X, Zhang H, Su Q, Yang G, Wei X. et al. Multistage-responsive nanovehicle to improve tumor penetration for dual-modality imaging-guided photodynamic-immunotherapy. Biomaterials. 2021;275:120990
98. Cramer GM, Moon EK, Cengel KA, Busch TM. Photodynamic therapy and immune checkpoint blockade (dagger). Photochem and Photobiol. 2020;96:954-61
99. Chen S X, Ma M, Xue F, Shen S, Chen Q, Kuang Y. et al. Construction of microneedle-assisted co-delivery platform and its combining photodynamic/immunotherapy. J Control Release. 2020;324:218-27
100. Liu M, Wang X, Wang L, Ma X, Gong Z, Zhang S. et al. Targeting the IDO1 pathway in cancer: from bench to bedside. J Hematol Oncol. 2018;11:100
101. Xing L, Gong JH, Wang Y, Zhu Y, Huang ZJ, Zhao J. et al. Hypoxia alleviation-triggered enhanced photodynamic therapy in combination with IDO inhibitor for preferable cancer therapy. Biomaterials. 2019;206:170-82
102. Zhao L P, Zheng R R, Huang J Q, Chen X Y, Deng F A, Liu Y B. et al. Self-delivery photo-immune stimulators for photodynamic sensitized tumor immunotherapy. ACS Nano. 2020;14:17100-13
103. Song W, Kuang J, Li C X, Zhang M, Zheng D, Zeng X. et al. Enhanced immunotherapy based on photodynamic therapy for both primary and lung metastasis tumor eradication. ACS Nano. 2018;12:1978-89
104. Huang Z, Wei G, Zeng Z, Huang Y, Huang L, Shen Y. et al. Enhanced cancer therapy through synergetic photodynamic/immune checkpoint blockade mediated by a liposomal conjugate comprised of porphyrin and IDO inhibitor. Theranostics. 2019;9:5542-57
105. Liu D, Chen B, Mo Y, Wang Z, Qi T, Zhang Q. et al. Redox-activated porphyrin-based liposome remote-loaded with indoleamine 2,3-Dioxygenase (IDO) inhibitor for synergistic photoimmunotherapy through induction of immunogenic cell death and blockage of IDO pathway. Nano Lett. 2019;19:6964-76
106. Lu J, Liu X, Liao YP, Salazar F, Sun B, Jiang W. et al. Nano-enabled pancreas cancer immunotherapy using immunogenic cell death and reversing immunosuppression. Nat Commun. 2017;8:1811
107. Jin F, Qi J, Zhu M, Liu D, You Y, Shu G. et al. NIR-triggered sequentially responsive nanocarriers amplified cascade synergistic effect of chemo-photodynamic therapy with inspired antitumor immunity. ACS Appl Mater Interfaces. 2020;12:32372-87
108. Yang Y, Chen F, Xu N, Yao Q, Wang R, Xie X. et al. Red-light-triggered self-destructive mesoporous silica nanoparticles for cascade-amplifying chemo-photodynamic therapy favoring antitumor immune responses. Biomaterials. 2022;281:121368
109. Li C, Sun H, Wei W, Liu Q, Wang Y, Zhang Y. et al. Mitoxantrone triggers immunogenic prostate cancer cell death via p53-dependent PERK expression. Cell Oncol (Dordr). 2020;43:1099-116
110. Kuai R, Yuan W, Son S, Nam J, Xu Y, Fan Y. et al. Elimination of established tumors with nanodisc-based combination chemoimmunotherapy. Sci Adv. 2018;4:eaao1736
111. Chen Q, He Y, Wang Y, Li C, Zhang Y, Guo Q. et al. Penetrable nanoplatform for "cold" tumor immune microenvironment reeducation. Adv Sci (Weinh). 2020;7:2000411
112. Huang L, Chen X, Bian Q, Zhang F, Wu H, Wang H. et al. Photosensitizer-stabilized self-assembling nanoparticles potentiate chemo/photodynamic efficacy of patient-derived melanoma. J Control Release. 2020;328:325-338
113. Xie B, Wan J, Chen X, Han W, Wang H. Preclinical evaluation of a cabazitaxel prodrug using nanoparticle delivery for the treatment of taxane-resistant malignancies. Mol Cancer Ther. 2020;19:822-34
114. Zhang Y, Du X, Liu S, Yan H, Ji J, Xi Y. et al. NIR-triggerable ROS-responsive cluster-bomb-like nanoplatform for enhanced tumor penetration, phototherapy efficiency and antitumor immunity. Biomaterials. 2021;278:121135
115. Gameiro SR, Jammeh ML, Wattenberg MM, Tsang KY, Ferrone S, Hodge JW. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget. 2014;5:403-16
116. Weichselbaum RR, Liang H, Deng L, Fu YX. Radiotherapy and immunotherapy: a beneficial liaison? Nat Rev Clin Oncol. 2017;14:365-79
117. Ni K, Lan G, Song Y, Hao Z, Lin W. Biomimetic nanoscale metal-organic framework harnesses hypoxia for effective cancer radiotherapy and immunotherapy. Chem Sci. 2020;11:7641-53
118. Sang W, Xie L, Wang G, Li J, Zhang Z, Li B. et al. Oxygen-enriched metal-phenolic X-ray nanoprocessor for cancer radio-radiodynamic therapy in combination with checkpoint blockade immunotherapy. Adv Sci (Weinh). 2021;8:2003338
119. Li S, Liu J, Sun M, Wang J, Wang C, Sun Y. Cell Membrane-camouflaged nanocarriers for cancer diagnostic and therapeutic. Front Pharmacol. 2020;11:24
120. Zhen X, Cheng P, Pu K. Recent advances in cell membrane-camouflaged nanoparticles for cancer phototherapy. Small. 2019;15:e1804105
121. Zhang P, Sun F, Liu S, Jiang S. Anti-PEG antibodies in the clinic: current issues and beyond PEGylation. J Control Release. 2016;244:184-93
122. Shiraishi K, Hamano M, Ma H, Kawano K, Maitani Y, Aoshi T. et al. Hydrophobic blocks of PEG-conjugates play a significant role in the accelerated blood clearance (ABC) phenomenon. J Control Release. 2013;165:183-90
123. Li X, Shao C, Shi Y, Han W. Lessons learned from the blockade of immune checkpoints in cancer immunotherapy. J Hematol Oncol. 2018;11:31
124. de Miguel M, Calvo E. Clinical challenges of immune checkpoint inhibitors. Cancer Cell. 2020;38:326-33
125. Ohl K, Tenbrock K. Reactive oxygen species as regulators of MDSC-mediated immune suppression. Front Immunol. 2018;9:2499
126. Kirtonia A, Sethi G, Garg M. The multifaceted role of reactive oxygen species in tumorigenesis. Cell Mol Life Sci. 2020;77:4459-83
127. Yu X, Lao Y, Teng X L, Li S, Zhou Y, Wang F. et al. SENP3 maintains the stability and function of regulatory T cells via BACH2 deSUMOylation. Nat Commun. 2018;9:3157
128. Chen LM, Tseng HY, Chen YA, Al Haq AT, Hwang PA, Hsu HL. Oligo-fucoidan prevents M2 macrophage differentiation and HCT116 tumor progression. Cancers (Basel). 2020;12:421
129. Kraaij MD, Savage ND, van der Kooij SW, Koekkoek K, Wang J, van den Berg JM. et al. Induction of regulatory T cells by macrophages is dependent on production of reactive oxygen species. Proc Natl Acad Sci U S A. 2010;107:17686-91
Author contact
![]() Corresponding author: Dr. Jie Cao (caojie0829edu.cn)
Corresponding author: Dr. Jie Cao (caojie0829edu.cn)
 Global reach, higher impact
Global reach, higher impact