13.3
Impact Factor
Theranostics 2022; 12(10):4564-4580. doi:10.7150/thno.68972 This issue Cite
Research Paper
FGFR blockade boosts T cell infiltration into triple-negative breast cancer by regulating cancer-associated fibroblasts
1. Chongqing Key Laboratory of Molecular Oncology and Epigenetics, The First Affiliated Hospital of Chongqing Medical University; Chongqing, 400016, China.
2. Department of Endocrine and Breast Surgery, The First Affiliated Hospital of Chongqing Medical University; Chongqing, 400016, China.
3. Department of Breast and Thyroid Surgery, Chongqing General Hospital, Chongqing, 401147, China.
4. Department of Pharmacology, Chongqing Medical University; Chongqing, 400016, China.
5. Department of Pathophysiology, Chongqing Medical University; Chongqing, 400016, China
6. Department of Respiratory, The First Affiliated Hospital of Chongqing Medical University; Chongqing, 400016, China.
7. Department of Oncology, The First Affiliated Hospital of Chongqing Medical University; Chongqing, 400016, China.
8. Department of Oncology, Beijing Friendship Hospital, Capital Medical University; Beijing, 100050, China.
9. Department of Dermatology, Shenzhen People's Hospital (The Second Clinical Medical College, Jinan University; The First Affiliated Hospital, Southern University of Science and Technology); Shenzhen, 518020, China.
# These authors contributed equally.
Abstract
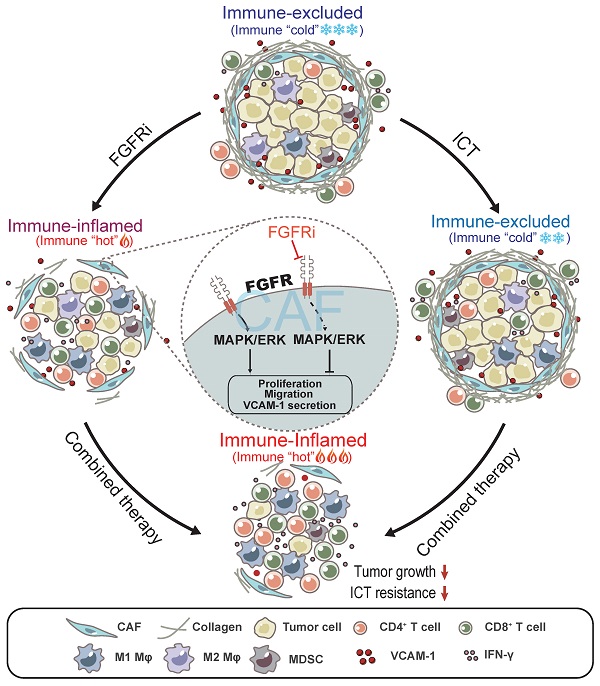
Background: Since T cell exclusion contributes to tumor immune evasion and immunotherapy resistance, how to improve T cell infiltration into solid tumors becomes an urgent challenge.
Methods: We employed deep learning to profile the tumor immune microenvironment (TIME) in triple negative breast cancer (TNBC) samples from TCGA datasets and noticed that fibroblast growth factor receptor (FGFR) signaling pathways were enriched in the immune-excluded phenotype of TNBC. Erdafitinib, a selective FGFR inhibitor, was then used to investigate the effect of FGFR blockade on TIME landscape of TNBC syngeneic mouse models by flow cytometry, mass cytometry (CyTOF) and RNA sequencing. Cell Counting Kit-8 (CCK-8) assay and transwell migration assay were carried out to detect the effect of FGFR blockade on cell proliferation and migration, respectively. Cytokine array, western blot, enzyme-linked immunosorbent assay (ELISA) and immunofluorescence (IF) were employed to investigate the potential mechanism by which FGFR inhibition enhanced T cell infiltration.
Results: Blocking FGFR pathway by Erdafitinib markedly suppressed tumor growth with increased T cell infiltration in immunocompetent mouse models of TNBC. Mechanistically, FGFR blockade inhibited cancer-associated fibroblasts (CAFs) proliferation, migration and secretion of vascular cell adhesion molecule 1 (VCAM-1) by down-regulating MAPK/ERK pathway in CAFs, thus promoting T cell infiltration by breaking physical and chemical barriers built by CAFs in TIME. Furthermore, we observed that FGFR inhibition combined with immune checkpoint blockade therapy (ICT) greatly improved the therapeutic response of TNBC tumor models.
Conclusions: FGFR blockade enhanced ICT response by turning immune “cold” tumor into “hot” tumor, providing remarkable implications of FGFR inhibitors as adjuvant agents for combinatorial immunotherapy.
Keywords: FGFR, breast cancer, fibroblast, VCAM-1, immunotherapy
 Global reach, higher impact
Global reach, higher impact