13.3
Impact Factor
Theranostics 2022; 12(10):4477-4497. doi:10.7150/thno.72167 This issue Cite
Research Paper
Selective brain entry of lipid nanoparticles in haemorrhagic stroke is linked to biphasic blood-brain barrier disruption
1. Pharmacology Department, School of Science and Technology, Nottingham Trent University, Nottingham, NG11 8NS, United Kingdom.
2. Nanomedicine Lab, Division of Pharmacy and Optometry, Faculty of Biology, Medicine and Health, AV Hill Building, The University of Manchester, Manchester M13 9PT, United Kingdom.
3. Division of Neuroscience and Experimental Psychology, School of Biological Sciences, Faculty of Biology, Medicine and Health, University of Manchester, Manchester, M13 9PT, United Kingdom.
4. Division of Cardiovascular Sciences, Lydia Becker Institute of Immunology and Inflammation, School of Medical Sciences, Faculty of Biology, Medicine and Health, Manchester Academic Health Science Centre, The University of Manchester, Manchester, United Kingdom.
5. Manchester Centre for Clinical Neurosciences, Salford Royal NHS Foundation Trust, Manchester Academic Health Science Centre, Salford, United Kingdom.
6. Nanomedicine Lab, Catalan Institute of Nanoscience and Nanotechnology (ICN2), Bellaterra UAB Campus, Barcelona, Spain.
7. Geoffrey Jefferson Brain Research Centre, The Manchester Academic Health Science Centre, Northern Care Alliance NHS Group, University of Manchester, United Kingdom.
# Shared co-first authorship
Abstract
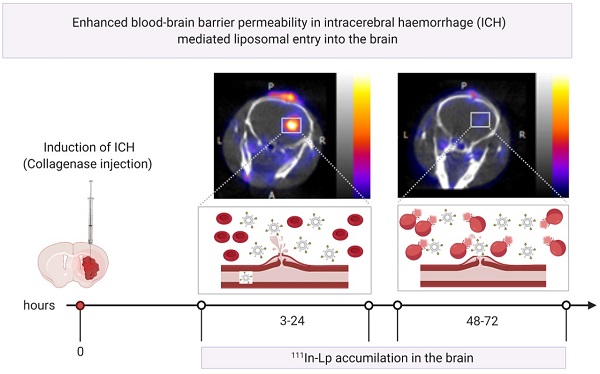
Haemorrhagic stroke represents a significant public health burden, yet our knowledge and ability to treat this type of stroke are lacking. Previously we showed that we can target ischaemic-stroke lesions by selective translocation of lipid nanoparticles through the site of blood-brain barrier (BBB) disruption. The data we presented in this study provide compelling evidence that haemorrhagic stroke in mice induces BBB injury that mimics key features of the human pathology and, more importantly, provides a gate for entry of lipid nanoparticles-based therapeutics selectively to the bleeding site.
Methods: Haemorrhagic stroke was induced in mice by intra-striatal collagenase injection. lipid nanoparticles were injected intravenously at 3 h, 24 h & 48 h post-haemorrhagic stroke and accumulation in the brain studied using in-vivo optical imaging and histology. BBB integrity, brain water content and iron accumulation were characterised using dynamic contrast-enhanced MRI, quantitative T1 mapping, and gradient echo MRI.
Results: Using in-vivo SPECT/CT imaging and optical imaging revealed biphasic lipid nanoparticles entry into the bleeding site, with an early phase of increased uptake at 3-24 h post-haemorrhagic stroke, followed by a second phase at 48-72 h. Lipid nanoparticles entry into the brain post-haemorrhage showed an identical entry pattern to the trans-BBB leakage rate (Ktrans [min-1]) of Gd-DOTA, a biomarker for BBB disruption, measured using dynamic contrast-enhanced MRI.
Discussion: Our findings suggest that selective accumulation of liposomes into the lesion site is linked to a biphasic pattern of BBB hyper-permeability. This approach provides a unique opportunity to selectively and efficiently deliver therapeutic molecules across the BBB, an approach that has not been utilised for haemorrhagic stroke therapy and is not achievable using free small drug molecules.
Keywords: Liposomes, blood-brain barrier, drug delivery, haemorrhagic stroke, lipid nanoparticles
 Global reach, higher impact
Global reach, higher impact