13.3
Impact Factor
Theranostics 2022; 12(9):4415-4430. doi:10.7150/thno.70821 This issue Cite
Research Paper
Diabetes impairs cardioprotective function of endothelial progenitor cell-derived extracellular vesicles via H3K9Ac inhibition
1. Center for Translational Medicine, Lewis Katz School of Medicine, Temple University, Philadelphia, PA, 19140
2. Department of Emergency Medicine, The Ohio State University Wexner Medical Center, Columbus, OH 43210
3. Dorothy M Davis Heart and Lung Research Institute, The Ohio State University Wexner Medical Center, Columbus, OH 43210
4. Department of Cardiovascular Sciences, Lewis Katz School of Medicine, Temple University, Philadelphia, PA 19140
Abstract
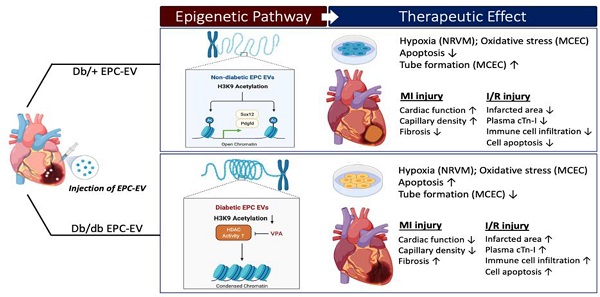
Background and Purpose: Myocardial infarction (MI) in diabetic patients results in higher mortality and morbidity. We and others have previously shown that bone marrow-endothelial progenitor cells (EPCs) promote cardiac neovascularization and attenuate ischemic injury. Lately, small extracellular vesicles (EVs) have emerged as major paracrine effectors mediating the benefits of stem cell therapy. Modest clinical outcomes of autologous cell-based therapies suggest diabetes-induced EPC dysfunction and may also reflect their EV derivatives. Moreover, studies suggest that post-translational histone modifications promote diabetes-induced vascular dysfunctions. Therefore, we tested the hypothesis that diabetic EPC-EVs may lose their post-injury cardiac reparative function by modulating histone modification in endothelial cells (ECs).
Methods: We collected EVs from the culture medium of EPCs isolated from non-diabetic (db/+) and diabetic (db/db) mice and examined their effects on recipient ECs and cardiomyocytes in vitro, and their reparative function in permanent ligation of left anterior descending (LAD) coronary artery and ischemia/reperfusion (I/R) myocardial ischemic injuries in vivo.
Results: Compared to db/+ EPC-EVs, db/db EPC-EVs promoted EC and cardiomyocyte apoptosis and repressed tube-forming capacity of ECs. In vivo, db/db EPC-EVs depressed cardiac function, reduced capillary density, and increased fibrosis compared to db/+ EPC-EV treatments after MI. Moreover, in the I/R MI model, db/+ EPC-EV-mediated acute cardio-protection was lost with db/db EPC-EVs, and db/db EPC-EVs increased immune cell infiltration, infarct area, and plasma cardiac troponin-I. Mechanistically, histone 3 lysine 9 acetylation (H3K9Ac) was significantly decreased in cardiac ECs treated with db/db EPC-EVs compared to db/+ EPC-EVs. The H3K9Ac chromatin immunoprecipitation sequencing (ChIP-Seq) results further revealed that db/db EPC-EVs reduced H3K9Ac level on angiogenic, cell survival, and proliferative genes in cardiac ECs. We found that the histone deacetylase (HDAC) inhibitor, valproic acid (VPA), partly restored diabetic EPC-EV-impaired H3K9Ac levels, tube formation and viability of ECs, and enhanced cell survival and proliferative genes, Pdgfd and Sox12, expression. Moreover, we observed that VPA treatment improved db/db EPC-mediated post-MI cardiac repair and functions.
Conclusions: Our findings unravel that diabetes impairs EPC-EV reparative function in the ischemic heart, at least partially, through HDACs-mediated H3K9Ac downregulation leading to transcriptional suppression of angiogenic, proliferative and cell survival genes in recipient cardiac ECs. Thus, HDAC inhibitors may potentially be used to restore the function of diabetic EPC and other stem cells for autologous cell therapy applications.
Keywords: Cardiac injury, Endothelial progenitors, Extracellular vesicle, Histone acetylation, Angiogenesis
 Global reach, higher impact
Global reach, higher impact