13.3
Impact Factor
Theranostics 2022; 12(9):4310-4329. doi:10.7150/thno.71086 This issue Cite
Review
Pyroptosis in inflammatory diseases and cancer
1. Engineering Research Center of Molecular & Neuroimaging, Ministry of Education, School of Life Science and Technology, Xidian University, Xi'an, Shaanxi, 710126, P. R. China
2. Academy of Advanced Interdisciplinary Research, Xidian University, Xi'an, Shaanxi, 710071, P. R. China
3. Department of Radiotherapy, Chinese PLA General Hospital, Beijing, 100071, P. R. China.
* These authors contributed equally to this work.
Received 2022-1-15; Accepted 2022-5-7; Published 2022-5-16
Abstract
Pyroptosis is a lytic and inflammatory type of programmed cell death that is usually triggered by inflammasomes and executed by gasdermin proteins. The main characteristics of pyroptosis are cell swelling, membrane perforation, and the release of cell contents. In normal physiology, pyroptosis plays a critical role in host defense against pathogen infection. However, excessive pyroptosis may cause immoderate and continuous inflammatory responses that involves in the occurrence of inflammatory diseases. Attractively, as immunogenic cell death, pyroptosis can serve as a new strategy for cancer elimination by inducing pyroptotic cell death and activating intensely antitumor immunity. To make good use of this double-edged sword, the molecular mechanisms, and therapeutic implications of pyroptosis in related diseases need to be fully elucidated. In this review, we first systematically summarize the signaling pathways of pyroptosis and then present the available evidences indicating the role of pyroptosis in inflammatory diseases and cancer. Based on this, we focus on the recent progress in strategies that inhibit pyroptosis for treatment of inflammatory diseases, and those that induce pyroptosis for cancer therapy. Overall, this should shed light on future directions and provide novel ideas for using pyroptosis as a powerful tool to fight inflammatory diseases and cancer.
Keywords: Pyroptosis, signaling pathway, gasdermin, inflammatory diseases, cancer
Introduction
The body maintains a dynamic balance between cell proliferation and cell death, which plays a significant role in the physiopathological processes of multicellular organisms. Cell death is usually categorized as non-programmed cell death and programmed cell death (PCD). Pyroptosis is a type of inflammatory PCD. In 1992, researchers discovered that mouse macrophages infected with Shigella flexneri eventually underwent cell death [1]. Later, researchers revealed that inflammatory caspase-1 was activated during Shigella flexneri- or Salmonella-induced cell death [2, 3]. So, this type of cell death was originally considered as caspase-dependent apoptosis. However, in 2001, Cookson et al. found that Salmonella-induced cell death displayed completely different characteristics from those of apoptosis. Apoptotic cells have intact membranes accompanied by cell shrinkage, while the membrane integrity of Salmonella-infected macrophages is destroyed by cell swelling [4, 5]. Hence, a new term, pyroptosis, was proposed to describe this type of cell death [5], which is characterized by cell membrane pore formation, membrane rupture, cell swelling, and release of cell contents. The factors released during cell death, such as interleukin-1β (IL-1β) and interleukin-18 (IL-18), amplify the inflammatory effects and activate immune responses [6, 7].
Although pyroptosis has been proposed for a long time, the underlying mechanism was only uncovered in 2015 upon the discovery and identification of gasdermin D (GSDMD) protein. It was found that cleavage of GSDMD by caspase-1 results in the release of its N-terminal domain (GSDMD-NT), which then forms pores in the cell membrane, thus demonstrating that GSDMD is the central executor of pyroptosis [8]. In addition to GSDMD, gasdermin family includes five other members. The human gasdermin family comprises of GSDMA, GSDMB, GSDMC, GSDMD, GSDME/DFNA5, and PVJK/DFNB59. In mice, there are five gasdermin members, including GSDMA, GSDMC, GSDMD, GSDME, and PJVK/DFNB59, but no GSDMB [9, 10]. All gasdermins except DFNB59 have two conserved domains, an N-terminal effector domain and a C-terminal inhibitory domain [11]. In general, binding of the C-terminal inhibits the pore-forming activity of the N-terminal. In the presence of numerous microbes or other stimulations, gasdermin is cleaved by active caspases or granzymes to liberate the N-terminal domain, which forms large pores in the membrane to release cell contents and execute pyroptosis [12]. The Ragulator-Rag-mTORC1 pathway is required for GSDMD oligomerization and pore formation in macrophages [13]. Cell-surface protein NINJ1 has an essential role in the induction of plasma membrane rupture, which is responsible for releasing intracellular molecules that propagate the inflammatory response [14]. However, the membrane pore can be repaired by endosomal sorting complex required for transport machinery, which initiates by calcium influx through GSDMD pores [15]. The membrane repair can allow cells to restrict pyroptosis and provide insight into cellular survival mechanisms during pyroptosis.
The occurrence of pyroptosis often crosstalk with a variety of cell death such as apoptosis and necroptosis. Although these different types of cell death induced by distinct mechanisms, they share some similarities and could be activated alone or simultaneously under different conditions. During apoptosis, cleavage of GSDME by caspase-3 mediates progression to pyroptotic cell death [16]. Apoptotic caspase-8, generally correlated to apoptosis, was shown to cleave GSDMD and induce pyroptosis [17]. In turn, the inflammatory caspase-1 could activate apoptosis in the absence of GSDMD. This caspase-1-induced apoptosis depends on caspase-3 and involves caspase-9 [18]. Crosstalk between necroptosis and pyroptosis was also discovered recently. Mixed-lineage kinase domain-like protein, the executioner of necroptosis, can also activate NLRP3 inflammasome to promote the maturation of IL-18 and IL-1β [19]. However, the maturation and release of cytokines are independent of GSDMD from necroptotic cells [20].
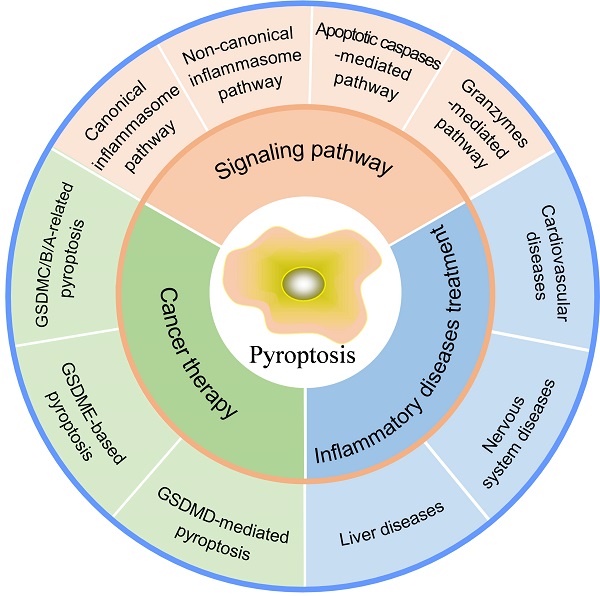
Normally, moderate pyroptosis contributes to host defense against pathogen infection, but excessive pyroptosis leads to intemperate inflammatory responses, massive cell death, and serious tissue damage, causing inflammatory or autoimmune diseases. Meanwhile, as a pro-inflammatory type of cell death, pyroptosis paves a new way for cancer elimination by activating antitumor immune response. Here, we first demonstrate different signaling pathways of pyroptosis to gain deep insight into molecular mechanisms. Next, the functions and therapeutic applications of pyroptosis in inflammatory diseases are discussed. Finally, we summarize the roles of pyroptosis in cancer and recent progress in strategies that induce pyroptosis for cancer therapy (Figure 1), which will point out the direction for future research.
Pyroptosis in inflammatory diseases and cancer.
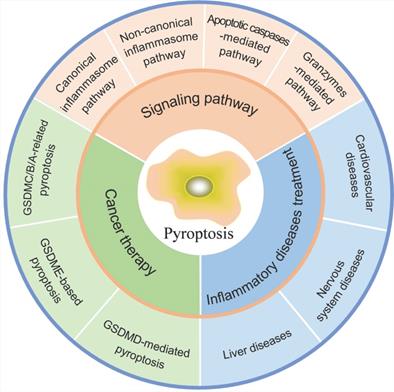
Signaling pathways of pyroptosis
At present, there are mainly four distinct signaling pathways that have been identified to induce pyroptosis, including canonical and non-canonical inflammasome pathways, apoptotic caspases-mediated pathway, and granzymes-based pathway (Figure 2). In these signaling pathways, gasdermin proteins are the final executioners, which need to be cleaved by upstream caspases or granzymes. Caspases can be categorized into inflammatory and apoptotic caspases based on function [21]. Commonly, caspases-1/4/5/11 belong to inflammatory caspases, which play key roles in the innate immune response by inducing pyroptosis to interrupt replication of invading pathogens, and by processing pro-inflammatory cytokines to maturation and release [22]. Activation of inflammatory caspase provides the first line of defense against infectious pathogens. Caspase-1 is activated in a multiprotein complex called the inflammasome in the canonical pyroptosis pathway. The inflammatory caspase-4/5/11 do not need such molecular complex for their activation, which were shown to bind lipopolysaccharide (LPS) directly. Apoptotic caspases function predominantly to initiate and execute apoptosis. Recent studies have shown that they can serve as the proteases to cleave gasdermins for pyroptosis induction [16]. The details of each signaling pathway of pyroptosis are discussed below.
Schematic illustration of the different pyroptosis pathways. (A) In the canonical inflammasome pathway, pathogen-associated molecular patterns or damage-associated molecular patterns like viruses, bacteria, toxins, ATP, or ROS stimulates inflammasome, which then activates caspase-1 to cleave GSDMD for pore formation. (B) LPS from Gram-negative bacteria activates caspase-4/5/11 directly, followed by GSDMD cleavage to execute pyroptosis in the non-canonical inflammasome pathway. (C) Apoptotic caspases-mediated pyroptosis pathway can be engaged through mechanisms such as caspase-3/GSDME, caspase-8/GSDMC, caspase-6/GSDMB, and so on. (D) In the granzymes-mediated pathway, GZMA or GZMB derived from cytotoxic lymphocytes can cleave GSDMB or GSDME respectively for pore formation and pyroptosis.
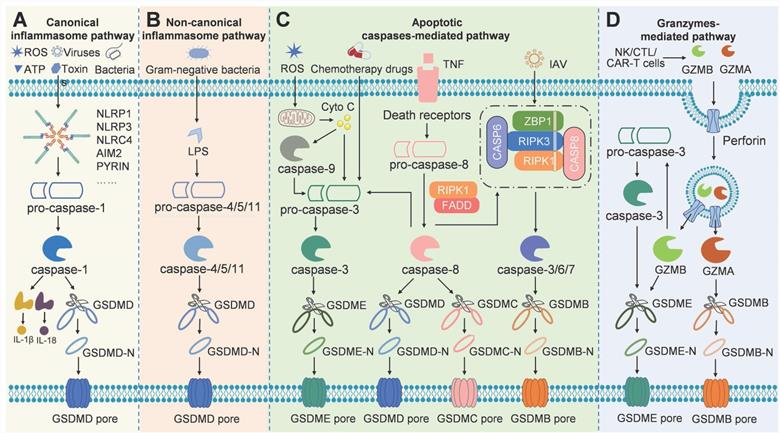
Canonical inflammasome pathway
The canonical inflammasome pathway was the first to be discovered. Inflammasomes are multi-protein complexes assembled in response to pathogen-associated molecular patterns or non-pathogen-related damage-associated molecular patterns. Generally, inflammasomes are comprised of intracellular pattern recognition receptors (PRRs), apoptosis-associated speck-like protein containing a caspase-recruitment domain (ASC), and inflammatory caspases [23]. The most common PRRs include nucleotide-binding oligomerization domain-like receptors (NLRs, including NLRP1, NLRP3, and NLRC4), absent in melanoma 2 (AIM2), and pyrin [24, 25] (Figure 2A). NLRP1 is composed of an N-terminal pyrin domain (PYD), a nucleotide-binding oligomerization domain (NOD), a leucine-rich repeats (LRR), and a C-terminal caspase recruitment domain (CARD) [26]. The PYD is required for combining with ASC. NOD involves in adenosine triphosphate (ATP)-dependent activation of the signal. LRR is responsible for ligand recognition and auto-inhibition. CARD takes part in pro-caspase-1 recruitment. Anthrax lethal toxin, muramyl dipeptide, and components of Toxoplasma gondii can activate NLRP1 [27]. NLRP3 consists of an N-terminal PYD, a NOD, and an LRR, without C-terminal CRAD. The NLRP3 is activated by various factors, including bacteria, viruses, fungi, uric acid, reactive oxygen species (ROS), adenosine triphosphoric (ATP), and endogenous damage signals [28]. Extracellular ATP induces IL-1β secretion and caspase-1 activation by activating the P2X purinoreceptor 7 (P2X7) and inducing K+ efflux [29]. NLRC4 has an N-terminal CARD domain, a central NBD domain, and a C-terminal LRR domain. NLRC4 responds to type III secretory system proteins and flagellin [30]. AIM2 holds a PYD domain and a DNA-binding HIN-200 domain that can sense bacteria- or viruses-derived double-stranded DNA [31]. Pyrin has a PYD domain, two B-boxes, and a C-terminal SPRY/PRY domain. Pyrin mainly recognizes the inactivating modifications of host Rho guanosine triphosphatases mediated by various bacterial toxins or effectors [25]. Upon stimulation of PRRs, pro-caspase-1 is recruited directly by CARD-carrying PRRs or indirectly via ASC to assemble caspase-1-dependent inflammasomes, which is followed by caspase-1 activation through self-cleavage. Active caspase-1 not only cleaves inactive IL-1β and IL-18 precursors, but also cleaves GSDMD to release GSDMD-NT for pore-formation, eventually leading to inflammatory responses and pyroptosis [32]. The canonical inflammasome pathway-mediated pyroptosis mainly occurs in immune cells and serves as a host defense mechanism against pathogen infection.
Non-canonical inflammasome pathway
The non-canonical inflammasome pathway is independent of the classical inflammasome complex. Most Gram-negative bacteria activate the non-canonical inflammasome pathway. Extracellular LPS can induce the expression of type I interferon, which then forms a feedback loop and activates type I interferon receptor to induce caspase-11 expression [33, 34]. Vacuolar Gram-negative bacteria release their LPS into the cytosol through vacuolar rupture triggered by interferon-inducible guanylate-binding proteins. The released LPS can directly bind to and activate caspase-11, which then cleaves GSDMD to promote pyroptosis [35, 36] (Figure 2B). In human, caspase-4/5 can be activated by intracellular LPS. Caspase-4/5/11 cannot cleave pro-IL-18 and pro-IL-1β directly, but K+ efflux caused by GSDMD-NT pores can activate NLRP3 and caspase-1, eventually leading to maturation and release of IL-18 and IL-1β [37]. In addition, Yang et al. demonstrated that cleavage of the pannexin-1 channel and ATP release occur in a caspase-11-dependent manner upon LPS stimulation, which then activate ATP-gated ion channel P2X7, ultimately resulting in K+ efflux and subsequent NLRP3/caspase-1 activation in bone marrow-derived macrophages [38]. Therefore, the activation of NLRP3 inflammasome induced by the active caspase-11 is required for IL-1β processing in the non-canonical inflammasome pathway.
Apoptotic caspases-mediated pathway
In addition to inflammatory caspase-1/4/5/11, some apoptotic caspases can also trigger pyroptosis (Figure 2C). Chemotherapy drugs can induce caspase-3-mediated apoptosis, if the target cells express GSDME, the activated caspase-3 can cleave GSDME to induce pyroptosis, which switches the mode of cell death. Wang et al. found that cisplatin and other conventional chemotherapy drugs can induce pyroptosis through caspase-3-mediated cleavage of GSDME [39]. Another apoptotic caspase that can trigger pyroptosis is caspase-8, which can induce the cleavage of GSDMD to elicit pyroptosis during Yersinia infection [17, 40]. When transforming growth factor-β-activated kinase 1 is inhibited by the Yersinia effector YopJ, lysosome Rag-Ragulator served as a platform for activating a Fas-associated death domain/receptor-interacting serine-threonine protein kinase 1/caspase-8 complex to trigger pyroptosis [41]. Besides, caspase-8 can also cleave GSDMC, liberating the N-terminus of GSDMC to form pores in the cancer cell membrane [42]. In addition, Chao et al. showed that apoptosis-related caspase-3/6/7 cleaves GSDMB, thus removing the C-terminal repressor domain, to cause the release of the N-terminal effector domain, which perforates the cell membrane and ultimately evokes cell pyroptosis [43].
Granzymes-mediated pathway
Recently, studies have shown that natural killer cells, cytotoxic T lymphocytes, or chimeric antigen receptor T cells derived granzymes, which are delivered by perforin into target cells, can cleave specific gasdermin family members to induce cancer cell pyroptosis (Figure 2D). Granzyme A (GZMA) is the most abundant serine protease of the granzyme family, which has traditionally been recognized as a mediator of cell death. However, there are many reports have shown that GZMA fails to kill target cells in vitro unless very high concentrations are used [44-46]. Accumulating evidence now suggests the role of GZMA in modulating inflammation, such as inducing the maturation and release of pro-inflammatory cytokines [47-49]. Pyroptosis, one type of cell death that is accompanied by pro-inflammatory cytokines release, may be associated with GZMA. Recently, Zhou et al found that GZMA derived from cytotoxic T lymphocytes cleaves GSDMB to form pores in the membrane, resulting in pyroptosis of GSDMB-expressing cancer cells [50]. So, whether the GZMA can kill cancer cells through pyroptosis also depends on the expression of GSDMB, which do not express in some human tissue and is absent in mouse. Natural killer cell-derived granzyme B (GZMB) can directly cleave GSDME at the same site that is cleaved by caspase-3, leading to the release of the effector N-terminal, which perforates the cell membrane [51]. GZMB induces GSDME-dependent pyroptosis in tumor targets both directly by cleaving GSDME and indirectly by activating caspase-3. The direct cleavage of GSDME by GZMB provides a simple mechanism and pathway for triggering inflammatory death. Caspase-resistant cancer cells should be susceptible to this direct pathway, provided that the cancer cells express GSDME. Granzymes-mediated cancer cell pyroptosis may amplify the inflammatory response in the tumor microenvironment (TME), thereby recruiting more immune cells for antitumor immunity.
Inhibiting pyroptosis to treat inflammatory diseases
In normal physiology, moderate pyroptosis plays an important role in the host defense against pathogenic microorganisms [52, 53]. However, dysregulated inflammatory response and cell death caused by overactivated pyroptosis may be involved in the pathological progression of many diseases [54, 55], especially inflammatory diseases. Herein, we mainly discuss the role and therapeutic potential of pyroptosis in inflammatory diseases, like cardiovascular diseases, liver diseases, and nervous system diseases.
Cardiovascular diseases
Cardiovascular diseases are the primary cause of patient suffering and high mortality worldwide. Recently, many studies have shown that pyroptosis is closely related to the occurrence and development of cardiovascular diseases, such as atherosclerosis, ischemia-reperfusion injury (IRI), and myocardial infarction (MI).
The pathogenesis of atherosclerosis involves smooth muscle cell proliferation and migration, endothelial cell dysfunction, pro-inflammatory cytokine secretion, and cell death [56]. Previous studies have illustrated that pyroptosis in macrophages, endothelial cells, and smooth muscle cells are related to the progression of atherosclerosis [57]. Duewell et al. showed that cholesterol crystals can activate caspase-1 through the NLRP3 inflammasome, which cleaves pro-IL-18 and pro-IL-1β to produce their mature forms, resulting in inflammation and atherosclerosis formation [58].
IRI involves different types of cell death, among which pyroptosis is one of the commonly observed cell death modes. Lou et al. illustrated that microRNA (miR)-424 is markedly upregulated in IRI conditions, which reduces the expression of cysteine-rich secretory protein LCCL domain-containing 2 and results in the upregulation of caspase-1, IL-18, and IL-1β in cardiac pyroptosis under IRI [59].
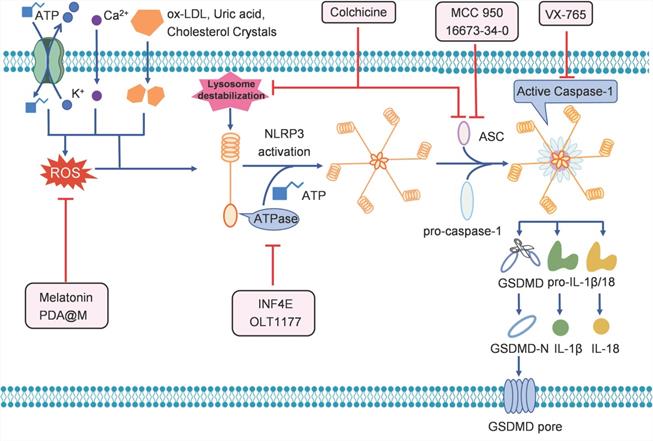
Since pyroptosis is involved in the occurrence and progression of cardiovascular diseases, many strategies have been developed to target pyroptosis for the treatment of these diseases (Table 1, Figure 3). There are numerous inhibitors of NLRP3 inflammasome, such as INF4E and OLT1177, when given in mouse model of IRI, the inhibitors significantly reduce infarct size by inhibiting the ATPase activity of NLRP3 [60, 61]. The small molecule 16673-34-0 prevents NLRP3 oligomerization in cardiomyocytes and limits myocardial injury after myocardial ischemia-reperfusion in the mouse model [62]. MCC950 is shown to inhibit NLRP3-induced ASC oligomerization, by which reducing infarct size, improving cardiac remodeling, and preventing left ventricular dysfunction in a pig model of MI [63]. Colchicine acts upstream of NLRP3 to block the opening of P2X7 channel and interfere with ASC polymerization [64, 65]. Treatment with colchicine successfully attenuates NLRP3 inflammasome activity, improves cardiac function, and prolongs survival after MI [66].
Potential strategies targeting pyroptosis to treat cardiovascular diseases
| Targets | Agents | Disease model | Findings | Ref. |
|---|---|---|---|---|
| NLRP3 | INF4E | IRI in mouse | Reduces infarct size at 60 min | [60] |
| OLT1177 | IRI in mouse | Limits infarct size and preserves left ventricular contractile function | [61] | |
| 6673-34-0 | IRI in mouse | Limits the infarct size | [62] | |
| MCC950 | MI in pig | Reduces infarct size and preserves cardiac function | [63] | |
| Colchicine | MI in mouse | Improves chronic cardiac function and survival | [66] | |
| Melatonin | Atherosclerosis in mouse | Reduces the atherosclerotic plaque in aorta | [67] | |
| PDA@M | IRI in rat | Decreases the infarct size and improves the cardiac function | [68] | |
| Caspase-1 | VX-765 | IRI in rat | Reduces infarction and preserves ventricular function | [69] |
Besides the inhibitors that directly affect NLRP3, there are agents that can suppress the activity of NLRP3 indirectly. Zhang et al. showed that the anti-inflammatory agent melatonin can prevent endothelial cell pyroptosis by regulating the signaling pathway of maternally expressed gene 3/miR-223/NLRP3 in atherosclerosis [67]. Wang et al. showed that melatonin reduced cigarette smoke extract-induced pyroptosis by inhibiting the ROS/NLRP3 axis in atherosclerosis [70]. Liraglutide alleviates NLRP3 inflammasome-mediated pyroptosis in H9c2 cells, by regulating the sirtuin 1 (SIRT1)/NAPDH oxidase 4/ROS pathway [71]. Wei et al. reported that a polydopamine-based biomimetic nanoplatform (PDA@M) can inhibit pyroptosis to protect the myocardium against IRI. PDA@M consists of a polydopamine core and a macrophage membrane shell, to achieve site-specific antioxidative efficacy [68]. The results demonstrated that PDA@M targets the infarcted myocardium to suppress the NLRP3/caspase-1 pathway, thus exerting antioxidative and antipyroptosis functions, suggesting that it may serve as a potential therapeutic agent for IRI.
Caspase-1 inhibitors can also inhibit pyroptosis, and thus, serve to be useful in the treatment of cardiovascular diseases. For example, the caspase-1 inhibitor VX-765 has been shown to produce a sustained reduction in myocardial infarct size and facilitate preservation of ventricular function in a pre-clinical model of IRI treated with a P2Y12 receptor antagonist [69]. Moreover, VX-765 was able to reduce myocardial infarction in a model of IRI, demonstrating that caspase-1 inhibition is an effective method for treating pyroptosis-triggered cardiovascular diseases [72].
Liver diseases
Liver diseases are serious problems that endanger human health worldwide. Recently, studies have demonstrated that pyroptosis is responsible for the progression of liver diseases. When the intestinal flora is out of balance, the gut microflora can enter the liver through the intestine-liver axis, which then triggers pyroptosis in liver cells [73].
Non-alcoholic fatty liver disease (NAFLD) has become a serious health problem owing to its high incidence and high risk of cirrhosis. The roles of cell necrosis and apoptosis in NAFLD have been emphasized, but it has only recently been recognized that pyroptosis may also play an important role in this condition. NAFLD is further categorized into non-alcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis (NASH). NAFL is characterized by the accumulation of triglycerides in hepatocytes, while NASH involves massive cell damage, inflammatory cell infiltration, and hepatocyte expansion [74, 75]. After sensing lipotoxicity-associated ceramide, the NLRP3 inflammasome induces caspase-1 cleavage, and ultimately leads to pyroptosis at the NAFL stage [76]. The inflammation or fibrosis induced by pyroptosis is more serious at the stage of NASH [74, 75]. A study proved that GSDMD-NT was upregulated in NAFL and showed higher levels in NASH [77]. GSDMD knockout mice fed with methionine-choline deficiency showed milder steatosis and inflammation compared with WT mice. These results indicated that pyroptosis executor GSDMD-NT is responsible for the pathogenesis of NAFLD by regulating adipogenesis and secreting inflammatory cytokines [77].
Due to the serious inflammatory response and liver damage caused by the excessive intake of alcohol, alcoholic liver disease presents a very high mortality worldwide. However, owing to our poor understanding of the molecular mechanisms underlying the condition, currently, there is still no effective treatment strategy for it. It is well established that excessive uptake of alcohol is often related to different forms of cell death, including pyroptosis. Heo et al. discovered that alcohol can decrease the expression of miR-148a through forkhead box O1 (FoxO1) in hepatocytes, which leads to overexpression of thioredoxin-interacting protein and activation of NLRP3 inflammasome, eventually inducing pyroptosis in hepatocytes [78]. By reducing caspase-1-induced pyroptosis, selenium-enriched S. platensis displays a protective role in chronic alcohol-induced liver injury [79].
Potential strategies targeting pyroptosis for the treatment of cardiovascular diseases.
Targeting the signaling pathways of pyroptosis to treat liver diseases
| Targets | Agents | Disease | Mechanism | Ref. |
|---|---|---|---|---|
| NLRP3 | MCC550 | Liver fibrosis | Reduces expression of IL-1β and IL-18, and suppresses neutrophil infiltration and hepatic cell death | [82] |
| P2X7 inhibitor | Liver disease | Prevents ATP-mediated activation of NLRP3 | [83] | |
| Silybin | NAFLD | Inhibits assembly of NLRP3 inflammasome | [84] | |
| Dihydroquercetin | Alcoholic liver disease | Decreases expression of P2X7and NLRP3, and suppresses cleavage of caspase-1 | [85] | |
| Liraglutide | NAFLD | Inhibits the NLRP3 inflammasome and pyroptosis activation | [86] | |
| Downstream of NLRP3 | Caspase-1 inhibitor | Liver disease | Prevents caspase-1-dependent cell death | [87] |
| Rosiglitazone | NAFLD | Inhibits production of hepatic IL-18 | [88] | |
| IL-1β receptor antagonist | Liver fibrosis | Block IL-1-mediated inflammation in selective liver fibrotic disease | [89] |
Additionally, liver inflammation has been shown to be related to pyroptosis during the development of liver fibrosis. The fibrosis-related proteins are mainly derived from hepatic stellate cells, which get activated and produce collagen through pyroptosis [80]. In addition to hepatic stellate cells, infiltrated eosinophils have been shown to induce secretion of pro-inflammatory cytokines IL-18 and IL-1β, or even pyroptotic cell death of hepatocytes, leading to liver fibrosis. The caspase-1 inhibitors significantly suppress this process, further suggesting that pyroptosis plays a crucial role in eosinophil-induced hepatic fibrosis [81].
The above studies indicate that inhibiting pyroptosis might be a potential therapeutic strategy for liver diseases. So, there are many researches targeting pyroptosis for the treatment of liver diseases, mainly involving two strategies: direct inhibition of NLRP3 inflammasome and restraining of downstream signaling pathways of the NLRP3 inflammasome (Table 2). Qu et al. [82] demonstrated that MCC950, which is known as an NLRP3 inhibitor, significantly alleviates bile duct ligation-induced liver fibrosis by reducing IL-18 and IL-1β expression, and suppressing neutrophil infiltration and hepatic cell death. P2X7 inhibitors prevent ATP-mediated activation of NLRP3 [83]. In addition to these inhibitors, some herbal extracts and ingredients can inhibit signaling pathways of pyroptosis and reduce liver damage. Zhang et al. showed that silybin significantly inhibits the assembly of NLRP3 inflammasome in mice with NAFLD [84]. A study has demonstrated that dihydroquercetin can decrease the expression of P2X7 and NLRP3, and subsequently suppress cleavage of caspase-1 in an animal model of alcoholic liver steatosis [85]. Liraglutide, an analog of glucagon-like peptide-1, has been shown to inhibit NLRP3 inflammasome-mediated pyroptosis and attenuate mitochondrial dysfunction, which significantly ameliorates NASH [86].
The potential effects of caspase-1 [87], IL-18 [88], and IL-1β [89] inhibitors have been studied to target the downstream signaling pathways of the NLRP3 inflammasome. GSDMD is the final executor, but there are very few studies targeting it. The potential effect of GSDMD inhibitors requires further investigation for the treatment of various liver diseases in the future.
Nervous system diseases
Emerging studies imply that pyroptosis may be involved in the pathology of nervous system diseases such as ischemic stroke, Parkinson's disease (PD), and Alzheimer's disease (AD). AD is a common neurodegenerative disease that is characterized by dementia and cognitive decline. The main pathological features of AD are β-amyloid protein (Aβ) deposition in the extracellular neuritic plaque, neurofibrillary tangles due to aggregation of abnormally phosphorylated tau protein, vascular amyloidosis, and neuronal death in the brain. Aβ or hyperphosphorylated tau can activate NLRP1, AIM2, and NLRP3 inflammasome, eventually resulting in pyroptosis of neurons both in vitro and in vivo [90, 91].
PD is another neurodegenerative disorder characterized by the loss of dopaminergic neurons in the midbrain. Accumulating evidence demonstrates the involvement of pyroptosis in PD. miR-135b alleviates 1-methyl-4-phenylpyridinium-induced PD in an in vitro model by suppressing FoxO1-induced NLRP3 inflammasome activation and pyroptosis, which suggests that pyroptosis contributes to PD progression [92]. Moreover, the long non-coding RNA HOTAIR facilitates NLRP3-mediated pyroptosis to aggravate neuronal damage in PD [93]. Taken together, these studies indicate that inhibiting pyroptosis might be a novel therapeutic strategy for PD.
In addition to AD and PD, recent studies have demonstrated that pyroptosis of microglia or neurons participates in ischemic stroke. Yan et al. showed that neuronal pyroptosis is conducive to early ischemic injury through the SIRT1-ROS-tumor necrosis factor (TNF) receptor-associated factor 6 signaling pathway [94]. Moreover, neuron pyroptosis may cause mitochondrial dysfunction, eventually leading to increased ROS levels and aggravated ischemic injuries. In addition, the diffusion of intracellular inflammatory factors is facilitated by GSDMD-mediated pyroptosis in astrocytes, microglia, and infiltrating macrophages, which promotes ischemic brain injury [95-97].
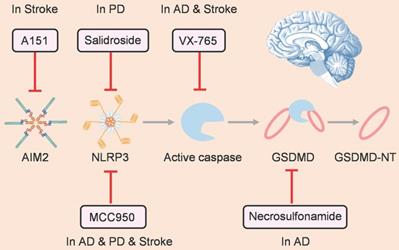
As pyroptosis plays a prominent role in pathological process of nervous system diseases, many small-molecule inhibitors have been developed to target pyroptosis-related signaling pathways for treating these diseases (Figure 4). Inflammasome family members have attracted the most attention as the starting point of pyroptosis. MCC950 is a well-known selective inhibitor of NLRP3, which can alleviate the pathological progression of various nervous system diseases, such as AD [98], PD [99], and ischemic stroke [94]. Furthermore, salidroside can suppress NLRP3-dependent pyroptosis to ameliorate PD [100]. In addition, as an antagonist of cyclic GMP-AMP synthase and AIM2 inflammasome, A151 prevents pyroptosis of microglia and reduces infarct volume, ultimately relieving neurodeficits after ischemic stroke [96].
Downstream of the inflammasome, active caspase can cleave gasdermin protein and drive pyroptosis. Hence, caspase is another attractive target for inhibiting pyroptosis. For instance, as a caspase-1 inhibitor, VX-765 can reduce pyroptosis to alleviate injury after AD [101] and stroke [102]. Gasdermin proteins are the final executors of pyroptosis, and there are drugs that target gasdermins directly. Han et al. showed that necrosulfonamide, which can inhibit GSDMD oligomerization by binding to the amino acid of C191, suppresses Aβ-triggered neuronal pyroptosis in vivo [91].
Therapeutic strategies for treating nervous system diseases by targeting pyroptosis.
Inducing cancer cell pyroptosis for cancer therapy
The mechanisms of pyroptosis in cancer cells and immune cells are different. In cancer cells, inflammasome is not necessary for pyroptosis induction and other active proteases except caspases are able to cleave gasdermins [103], in which almost all gasdermins can serve as the executors. Pyroptosis plays a vital role in tumor development and antitumor immunity, by acting as a double-edged sword that can show both tumor-promoting and tumor-suppressing effects. On the one hand, long-term chronic pyroptosis of cancer cells triggered by the adverse TME is more likely to promote cancer progression. Chronic pyroptosis triggers pro-inflammatory cytokines that facilitate the formation and maintenance of an inflammatory microenvironment for tumor growth. It has been reported that GSDME-mediated pyroptosis promotes the development of colitis-associated colorectal cancer by releasing high-mobility group box protein 1, which induces tumor cell proliferation and the expression of proliferating nuclear antigen through the ERK1/2 pathway [104]. Chronic inflammation and pyroptosis also involves the development of asbestos-associated mesothelioma [105]. On the other hand, acute and immense activation of pyroptosis results in numerous immune cells infiltration, which not only induces massive cancer cell death but also activates antitumor immunity to repress tumor growth [106]. The antitumor immunity of pyroptosis involves many respects, which starts with the release of damage-associated molecular patterns and inflammatory cytokines that directly modulates the innate immune response, to enhance the recruitment of adaptive immune cells along with increased antigen presentation, resulting in extensive immune activation. The released inflammatory cytokines IL-1β can induce dendritic cell (DC) maturation, activate CD8+ T cells, and inhibit the differentiation of immunosuppressive T regulatory cells [107]. IL-18 plays critical role in natural killer (NK) cell recruitment and activation, as well as Th-1 polarization [108]. All of these alter the immunosuppressive microenvironment and increase tumor-infiltrating lymphocytes. Thus, inducing acute and massive cancer cell pyroptosis is a potential strategy for tumor treatment. Herein, we summarize the latest progress in pyroptosis-based cancer therapy (Table 3), and the related immune methods are also summarized (Table 4).
GSDMD-mediated pyroptosis for cancer therapy
GSDMD was the first gasdermin discovered to be associated with pyroptosis. Shi and Kayagaki et al. showed that GSDMD participates in both canonical and non-canonical pyroptosis [8, 36]. To date, it has been found that both inflammatory caspase-1/4/5/11 and apoptotic caspase-8 can cleave GSDMD to induce pyroptosis.
Inducing cancer cell pyroptosis for cancer therapy
| Strategy | Cancer types | Mechanism | Ref. |
|---|---|---|---|
| GSDMD-mediated pyroptosis for cancer therapy | |||
| Simvastatin | NSCLC | NLRP3/caspase-1/GSDMD | [109] |
| a-NETA | Ovarian cancer | Caspase-4/GSDMD | [110] |
| Lip-MOF | Cervical cancer | Caspase/GSDMD | [111] |
| TBD-R | Breast cancer, cervix carcinoma, and glioblastoma | ROS/caspase-1/GSDMD | [112] |
| VTPA | Breast cancer | Lysosomal rupture and ROS/NLRP3/caspase-1/GSDMD | [113] |
| AMPCP | Melanoma | ATP/NLRP3/caspase-1/GSDMD | [114] |
| GSDME-mediated pyroptosis for cancer therapy | |||
| Paclitaxel, cisplatin | Lung cancer | Caspase-3/GSDME | [115] |
| Lobaplatin | Colon cancer | ROS and pJNK/ Bax/ Cytochrome c/Caspase-3/9/GSDME | [116] |
| As2O3-NPs | Hepatocellular carcinoma | Caspase-3/GSDME | [117] |
| DAC+LipoDDP | Breast cancer | Caspase-3/GSDME | [118] |
| DOX/JQ1-IBRN | Breast cancer | Caspase-3/GSDME | [119] |
| BNP | Breast cancer | Ca2+/Cytochrome c/ Caspase-3/GSDME | [120] |
| NCyNP | Breast, lung, and cervical cancers. | CyNH2/Cytochrome c/ Caspase-3/GSDME | [121] |
| MCPP | Colon cancer | ROS/ Caspase-3/GSDME | [122] |
| GSDMC/B/A-mediated pyroptosis for cancer therapy | |||
| α-KG | Cervical cancer and melanoma | ROS/DR6/Caspase-8/GSDMC | [123] |
| GSDMB | Colon cancer | GZMA/GSDMB | [50] |
| Phe-BF3+NP-GA3 | Cervical and breast cancer | Phe-BF3/GSDMA3 | [124] |
Summary of the strategies that induce pyroptosis for cancer therapy related to immune methods
| Strategy | Cancer types | Immune response | Ref. |
|---|---|---|---|
| AMPCP | Melanoma | Remodels ITME and sensitizes tumors to anti-PD-L1 therapy | [114] |
| DAC+LipoDDP | Breast cancer | Secrets IL-1β and HMGB1, induces the DCs maturation, and increases presence of CTLs | [118] |
| DOX/JQ1-IBRN | Breast cancer | Modulates ITME, JQ1 blocks PD-L1 mediated immune evasion, and reduces Tregs | [119] |
| BNP | Breast cancer | Secrets pro-inflammatory factors to induce DC maturation and T cell activation in TDLNs | [120] |
| NCyNH2, NCyNP | Breast, lung, and cervical cancers. | Promotes CTLs infiltration in TME and DCs maturation in TDLNs, synergizes with αPD-1 to induce antitumor immunity and generates an immune memory effect | [121] |
| MCPP | Colon cancer | Initiates adaptive immunity, boosts the PD-1 blockade efficiency, generates immunological memory, and prevents tumor recurrence. | [122] |
| GSDMB | Colon cancer | Promotes CTL-mediated tumor clearance when combined with αPD-1 | [50] |
| Phe-BF3+NP-GA3 | Cervical and breast cancer | Increases CD4+, CD8+, and NK cell populations, decreases Tregs and myeloid-derived suppressor cell populations | [124] |
Simvastatin is a well-established anti-hyperlipidemic drug that inhibits 3-hydroxy-3-methylglutaryl-coenzyme A reductase to reduce cholesterol levels. Recently, Wang et al. demonstrated that simvastatin can activate NLRP3-caspase-1 pathway to induce pyroptosis in non-small cell lung cancer (NSCLC) cell lines and mouse models [109]. Inhibition of pyroptosis reduced the effects of simvastatin on cancer cell viability and mobility. These data suggest that the anti-hyperlipidemic drug simvastatin may serve as a novel therapeutic agent for NSCLC via pyroptosis. Qiao et al. reported that 2-(anaphthoyl) ethyl-trimethylammonium iodide (a-NETA) induces pyroptosis of epithelial ovarian cancer cells via the caspase-4/GSDMD pathway [110]. The cytotoxic effect of a-NETA was strongly blocked by knockdown of either GSDMD or caspase-4 in ovarian cancer cells. Treatment with a-NETA significantly decreased the size of the epithelial ovarian tumors in vivo. These results imply that a-NETA may be a promising antitumor molecule for epithelial ovarian cancer therapy through pyroptosis.
Owing to their high self-renewal and clonogenic capacity, cancer stem cells (CSCs) are regarded as the root of tumors. However, due to drug resistance, the current therapies fail to eradicate colorectal CSCs effectively. The phenotype of CSCs and their resistance to chemotherapy drugs are related to C-X-C motif chemokine receptor 4 (CXCR4) overexpression in colorectal cancer. Based on this fact, Serna et al. constructed a self-assembling toxin nanoparticle, in which the CXCR4 ligand T22 was fused with the therapeutic material diphtheria toxin (DITOX) (T22-DITOX-H6) [125]. T22 endows the specificity of toxin nanoparticles to target and kill CXCR4+-CSCs. Protein synthesis was hindered by DITOX, which eventually led to pyroptotic cell death. T22-DITOX-H6 also showed greater inhibition of tumor growth compared to that in the control group in vivo. Thus, owing to the specific CXCR4+ targeting and effective cytotoxicity of DITOX, this nanoparticle can efficiently eliminate apoptotic-resistant CXCR4+ colorectal CSCs through pyroptosis, demonstrating a promising method for colorectal cancer therapy.
To a certain degree, cellular survival depends on ion homeostasis. Altering the concentration of a specific ion is usually used as a strategy to trigger different forms of cell death. Nevertheless, because the ion balance is tightly regulated by cells, the investigation of certain ions influence on cells in a controlled manner has been obstructed. Specific hybrid metal-organic framework (MOF) nanoparticles serve as a promising candidate for transporting ions stealthily into cells and releasing an overdose of ions in a controlled manner. Ploetz et al. designed a lipid-coated MIL-100 consisting of ferric ions and trimesic acid (Lip-MOFs), to transport high amounts of iron ions into cells [111]. The coated lipid not only prevents cellular recognition of the ions on the MOF surface, but also facilitates cellular uptake via endocytosis. After uptake, the Lip-MOFs transfer to lysosomes and then degrade into trimesic acids and Fe3+ ions by means of pH-dependent and cysteine-involved reduction. Lysosomal rupture and subsequent pyroptosis are triggered by large amounts of Fe3+ ions. The reduced expression of full-length GSDMD and increased release of IL-1β observed in this study demonstrated that pyroptosis was the dominant cell death mode. This protective ion delivery and controlled release to cells may pave the way for future applications of similar nanostructures that may be used to eliminate tumor cells in the acidic tumor environment by means of pyroptosis and elicit an immune response simultaneously. In addition, iron was also reported to induce a GSDME-dependent pyroptosis [126]. Iron has been shown to trigger oxidative stress by elevating ROS. On the one hand, ROS can activate the NLRP3 inflammasome and then induce GSDMD-dependent canonical pyroptosis; on the other hand, iron enhanced ROS can cause the oxidation of the mitochondrial outer membrane protein Tom20. Oxidized Tom20 recruits Bax to mitochondria, which promotes the release of cytochrome c to activate caspase-3, eventually triggering pyroptosis by inducing GSDME cleavage. Hence, iron can induce GSDMD- or GSDME-mediated pyroptosis depending on the cell context.
As an inflammatory form of PCD, pyroptosis is a promising strategy for fighting against cancer. In an attempt to reduce side effects and achieve non-invasiveness, Wu et al. designed a series of membrane-anchoring photosensitizers to induce pyroptosis for cancer cell ablation [112]. 1,1,2,2-tetraphe-nylethene-benzo[c] [1,2,5] thiadiazole-2- (diphenyl methylene) malononitrile (TBD) and phenyl rings (TBD-R) were conjugated with cationic chains to obtain aggregation-induced emission photosensitizers. Upon light irradiation, the produced ROS led to direct damage to the cell membrane and ablation of cancer cells. Along with the increase of the membrane-anchoring capability of TBD-R, pyroptosis gradually became the dominant cell death mode. To uncover the mechanism of TBD-R-initiated pyroptotic cell death, the study also evaluated the protein expression of caspase-1 and GSDMD in 4T1 cells after different treatments. The results showed that caspase-1 activation and GSDMD cleavage were enhanced upon photodynamic therapy with TBD-R. Together, this study provides a pyroptosis-based and photo-activated powerful approach for cancer cell removal.
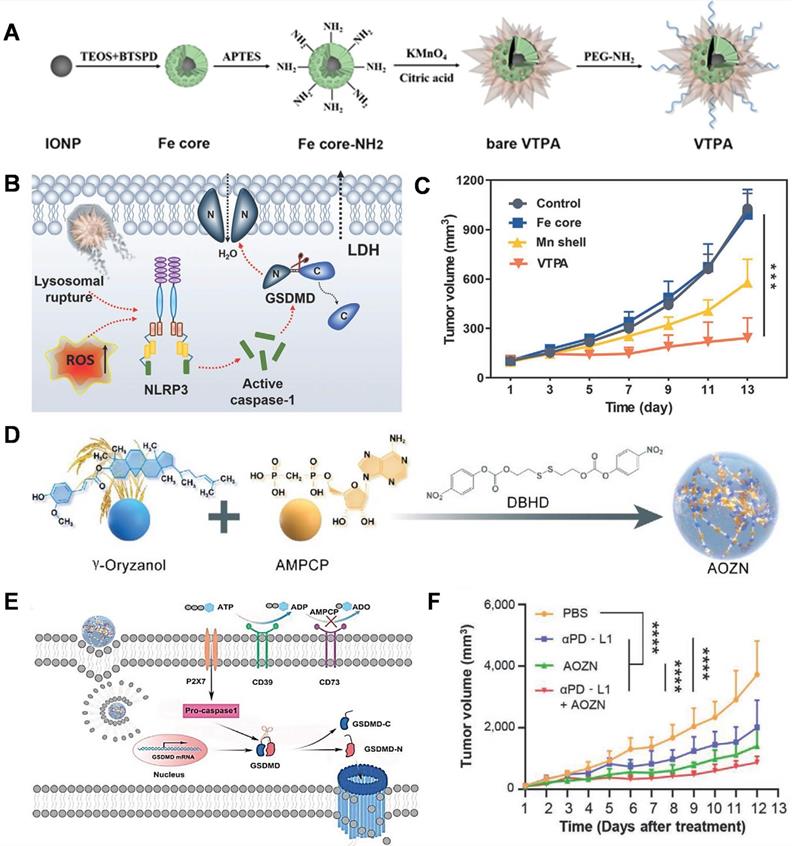
The lack of tumor-specific pyroptotic agents in vivo impedes the actual applications of pyroptosis-based cancer therapy. Nadeem et al. reported the development of a virus-spike tumor-activatable pyroptotic agent (VTPA) for cancer-specific therapy [113]. The VTPA consists of a manganese dioxide spiky structure and an organosilica-coated iron oxide nanoparticle (IONP) core (Figure 5A). Protrusions facilitate lysosomal rupture, following which the tumor overexpressed glutathione (GSH) triggers the degradation of VPTA to release Mn ions and IONPs for rapid and persistent ROS generation, which synergistically activates the NLRP3/caspase-1/GSDMD signaling pathway for pyroptosis (Figure 5B). Moreover, VTPA showed excellent tumor growth inhibition via pyroptosis in vivo (Figure 5C). This study provides a tumor-activatable and nanostructure-dependent pyroptotic agent, highlighting a novel direction for the development of next-generation cancer-specific pyroptotic nanomedicine in the future.
With limited T-cell responses, it is challenging to overcome innate or adaptive resistance to immune checkpoint inhibitor therapy in solid tumors. As an inflammatory form of PCD, pyroptosis is a promising strategy for enhancing cancer immunotherapy. Xiong et al. designed a GSH-responsive nanomicelles prodrug, composed of the adenosine inhibitor α, β-methylene adenosine 5' diphosphate (AMPCP) and the epigenetic modulator γ-oryzanol (Orz) for tumor therapy, which they termed as AOZN (Figure 5D) [114]. When AOZN reaches the tumor site, high GSH in the TME triggers AMPCP and Orz release. The DNA methyltransferase inhibitor Orz can upregulate the expression of GSDMD, AMPCP acts as an ecto-5′-nucleotidase inhibitor to reduce adenosine levels and increase ATP accumulation, subsequently initiating NLRP3 inflammasome assembly and caspase-1 activation. Active caspase-1 directly cleaves GSDMD and induces pyroptosis in tumor cells (Figure 5E). Moreover, Orz and AMPCP synergistically combat the immunosuppressive TME (ITME). After treatment with AOZN, a more marked increase in CD8- and CD4-positive T cells was observed in the tumor tissue, while there is a significant decrease in the frequencies of regulatory T cells and CD8 T cell exhaustion in the AOZN group, as compared to those in the control group. Additionally, Orz can sensitize tumors to anti-programmed death-ligand 1 (PD-L1) therapy by increasing the expression of PD-L1 (Figure 5F). In summary, this work proposes a promising strategy to enhance cancer immunotherapy and overcome the resistance to immune checkpoint blockers.
GSDMD-mediated pyroptosis for cancer therapy. (A) Schematic presentation of the designed virus-spike tumor-activatable pyroptotic agent (VTPA). (B) The molecular mechanism of VTPA triggered pyroptosis in tumor cells. (C) Changes in the tumor volume after different treatments. (D) Schematic illustration of designed nanomicelles loaded with AMPCP and Orz (AOZN) for cancer immunotherapy. (E) The mechanism of pyroptosis induced by AOZN. (F) Tumor growth curves after different treatments. Adapted with permission from [113], copyright 2021 John Wiley and Sons, and [114], copyright 2021 John Wiley and Sons.
GSDME-based pyroptosis for cancer therapy
The expression of GSDME varies in different cancers and is mainly activated by apoptotic caspase-3 and caspase-8. Chemotherapy can activate caspase-3 to trigger pyroptosis in GSDME-expressing cancer cells [16, 39]. Zhang et al. showed that the chemotherapeutic drug paclitaxel can trigger pyroptosis in A549 cells, which is closely related to the levels of activated caspase-3 and GSDME-NT [115]. Compared to paclitaxel, cisplatin induced more severe pyroptosis in NSCLC cells, indicating that cisplatin may have more advantages than other drugs for the treatment of tumors with high GSDME expression. In addition to cisplatin, lobaplatin is one of the third-generation antitumor platinum that has stronger antitumor effects but fewer side effects. However, the inflammatory characteristics of lobaplatin in tumor treatment have not been reported. Yu et al. showed that lobaplatin induced ROS elevation and c-Jun N-terminal kinase phosphorylation in HT-29 and HCT116 cells, which further recruit Bax to the mitochondria, and thereby, stimulate the release of cytochrome c, followed by caspase-3/9 activation and GSDME cleavage, eventually triggering pyroptosis [116]. This study showed that GSDME-mediated pyroptosis is a novel mechanism for eradicating cancer cells using lobaplatin, which is of great significance for clinical applications.
In addition to classical chemotherapy drugs, arsenic trioxide (As2O3) can accelerate the differentiation of viable cancer cells and reduce the risk of metastasis, which partly achieves better treatment responses with lower recurrence rates than traditional drugs. However, it is challenging to realize effective As2O3 accumulation inside a solid tumor with few systemic toxicities. To address this issue, Hu et al. designed a triblock copolymer monomethoxy (polyethylene glycol)-poly (d, l-lactide-co-glycolide)-poly (l-lysine) (mPEG-b-PLGA-b-PLL) nano-drug system to deliver As2O3 (As2O3-NPs). After the As2O3-NPs are internalized by tumor cells, the As2O3 is released into the cytoplasm and GSDME is cleaved following caspase-3 activation. The cleaved GSDME N-domains form membrane pores, eventually leading to pyroptosis. In vivo antitumor study showed that As2O3 moderately inhibited tumor growth, while As2O3-NPs substantially reduced tumor growth. As2O3-NPs treatment resulted in an increase in the protein levels of cleaved caspase-3 and GSDME-NT, with a decrease in those of Dnmt1, Dnmt3a, and Dnmt3b, thus uncovering the mechanism of the antitumor activity of As2O3-NPs [117]. These data provide a new vision and strategy for future hepatocellular carcinoma therapy based on pyroptosis mediated by As2O3.
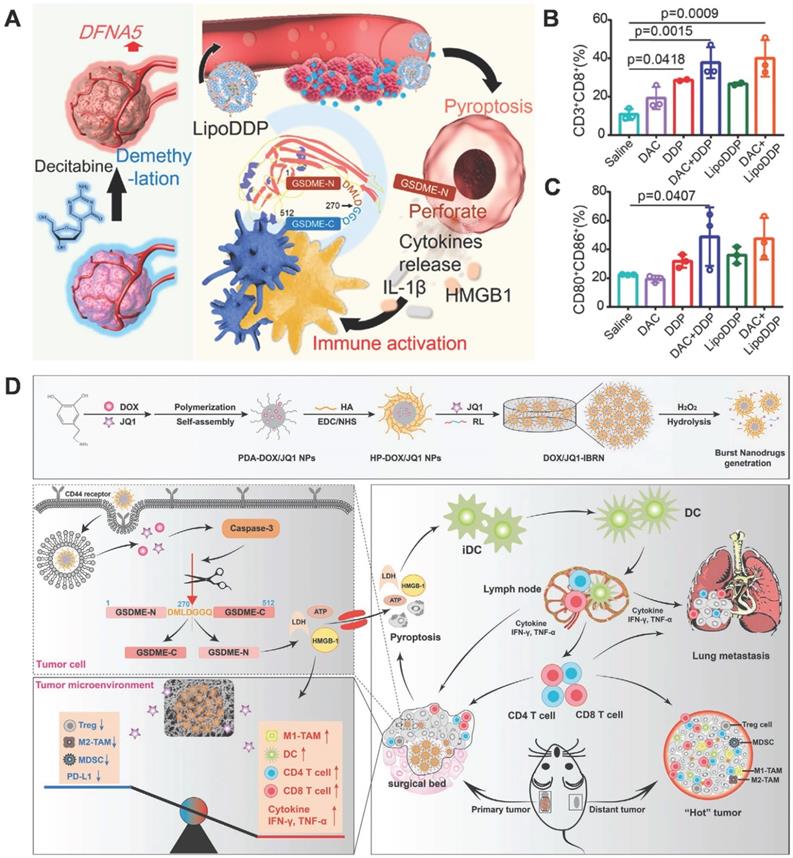
As mentioned above, the expression of GSDME in cancer cells varies. It is silenced in some types of tumors due to the hypermethylation of the GSDME/DFN59 gene; owing to this, GSDME-mediated pyroptosis is absent in these tumors. Fan et al. developed a strategy of combining chemotherapy with DNA demethylation to trigger cancer cell pyroptosis, which amplifies the immune effect to further eliminate tumors via immune therapy [118]. Pretreatment of tumor cells with decitabine (DAC), a commonly used DNA methyltransferase inhibitor, resulted in the up-regulation of DFNA5 expression. Subsequently, cisplatin-loaded nanoliposome (LipoDDP) was used to activate caspase-3 and induce pyroptosis in DAC-treated tumor cells (Figure 6A). Based on its performance in terms of antitumor activities and metastasis inhibition, this combined strategy triggered the immunological effects of chemotherapy and provided a novel insight into tumor immunotherapy (Figure 6B-C).
Residual microscopic lesions after surgery and the ITME contribute to a high rate of post-operative tumor recurrence and metastasis (TRM). Drug-loaded scaffolds have the potential to inhibit TRM, but the actual therapeutic effects are limited by the ITME and untargeted toxicity from non-selective drug release. Zhao et al. constructed an implantable bio-responsive nanoarray (IBRN) to reprogram the ITME and achieve accurate tumor targeting in a controlled manner, for effective post-operative tumor therapy and TRM prevention. The chemotherapeutic DOX and epigenetic modulator JQ1 are packaged into hyaluronic acid-modified polydopamine nanoparticles, which are then linked by a ROS-responsive linker to obtain a tumor-targeted nanoarray loaded with another part of JQ1 (DOX/JQ1-IBRN) (Figure 6D). Upon reaching the tumor site, high H2O2 triggers the release of JQ1 and DOX, which realize ITME modulation and induce GSDME-dependent pyroptosis, further eliciting antitumor immunity and wiping out the residual tumor completely [119]. The results showed that DOX/JQ1-IBRN inhibited post-surgical TRM and prolonged survival in tumor models with low toxicity. In summary, IBRN realizes accurate tumor pyroptosis and ITME conversion to activate antitumor immunity, for effective and safe prevention of TRM, thus providing novel insights for post-operative treatment.
Pyroptosis is considered an excellent choice to promote the immune response for cancer therapy, because of its pro-inflammatory characteristics. Zhao et al. designed a biomimetic nanoparticle (BNP) by fusing a breast cancer membrane shell onto a PLGA polymeric core loaded with indocyanine green and DAC, for photo-activated cancer cell pyroptosis and cancer immunotherapy. Due to the homing capability of the cancer cell membrane, BNP can effectively accumulate in the tumor site with low immunogenicity. The loaded indocyanine green can perforate the tumor cell membrane and induce a sudden increase in cytoplasmic Ca2+ through near-infrared (NIR) irradiation, which activates caspase-3 by promoting the release of cytochrome c. Meanwhile, DAC inhibits DNA methylation, which is followed by upregulation of GSDME, eventually causing pyroptosis. In vivo, the primary and distant tumor growth was significantly repressed within 28 days of using this strategy. After BNP treatment plus photo-activation, a high percentage of CD8+ T cells and CD4+ T cells were detected in distant tumors and spleens, and a high rate of mature DCs were detected in the primary tumor and tumor-draining lymph nodes, compared to those upon carrying out other treatments, indicating that photo-activated pyroptosis further induces inspiring antitumor immunity for cancer therapy [120]. Together, BNP provides a novel strategy for photo-activated cancer cell pyroptosis and robust solid tumor immunotherapy with high compatibility.
GSDME-mediated pyroptosis for cancer therapy. (A) Schematic illustration of the demethylation and immune activation process mediated by decitabine and LipoDDP via pyroptosis. (B) Quantification of CD4+ and CD8+ T cell-gating on CD3+ cells in the tumors. (C) Statistical analysis of CD80+CD86+ cell-gating on CD11c+ cells within tumor-draining lymph nodes. (D) Illustration of the DOX/JQ1-IBRN for post-surgical tumor treatment, involving pyroptosis of tumor cells, conversion of the ITME, and cascade activation of immunity. Adapted with permission from [118], copyright 2019 ACS, and [119], copyright 2020 John Wiley and Sons.
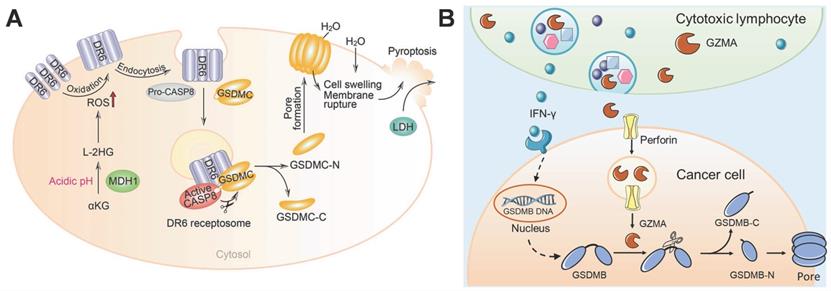
GSDMC/B-based pyroptosis for cancer therapy. (A) The working model of α-KG-induced pyroptosis via GSDMC. Adapted with permission from [123], copyright 2021 Springer Nature. (B) Cytotoxic lymphocyte-derived GZMA cleaves GSDMB in cancer cells to perforate the cell membrane and induce pyroptosis.
Pyroptosis can effectively eradicate cancer cells by boosting anticancer immunity; however, due to its non-selectivity, most of the current pyroptosis inducers may cause severe side effects in cancer therapy. Wang et al. first reported that the NIR fluorophore-based hemicyanine CyNH2 can kill cancer cells and boost antitumor immunity by inducing pyrolysis. To realize the tumor-specificity of CyNH2, cancer cells high expressing NAD(P)H: quinone oxidoreductase isozyme 1 (NQO1)-responsive CyNH2 (NCyNH2) were designed to trigger pyroptosis and further activate systemic antitumor immunity. In addition, NCyNH2 was further encapsulated in PEG-b-PLGA as a theranostic nanocarrier (NCyNP) for systemic administration and fluorescence imaging in vivo. NCyNPs combined with anti-programmed death-1 (PD-1) enhance the antitumor effect and prevent tumor recurrence by producing powerful memory efficacy [120]. Hence, the NIR fluorophore-based CyNH2 may represent a novel theranostic agent for initiating tumor pyroptosis selectively and triggering immunotherapy efficiently. To achieve higher tumor specificity, Xiao et al. designed a TME reactive ROS/GSH dual-responsive nano-prodrug loaded with photosensitizer purpurin 18 and paclitaxel (MCPP) to induce GSDME-mediated pyroptosis in cancer cells specifically. Upon laser irradiation, ROS produced by photosensitizer purpurin 18 realizes controlled release and triggers cancer cell pyroptosis with paclitaxel via chemo-photodynamic therapy. Pyroptotic cancer cells further initiate adaptive immunity and boost immune checkpoint blockade efficiency to prevent tumor growth and recurrence [122]. This study not only provides a highly efficient strategy to induce pyroptosis in tumor cells specifically, but also resolves a challenge in immune checkpoint blockade via pyroptosis.
GSDMC/B/A-related pyroptosis for cancer therapy
In addition to GSDME and GSDMD, the N-terminal domain of GSDMC/B/A also has the capacity to form pores in the cell membrane, to execute pyroptosis. Hou et al. showed that nuclear PD-L1 (nPD-L1) switches TNF-α-induced apoptosis to GSDMC-mediated pyroptosis in cancer cells [42]. Under hypoxic stress, p-Stat3 interacts with PD-L1 and promotes its nuclear translocation, which transcriptionally activates GSDMC expression. After TNF-α treatment, active caspase-8 cleaves GSDMC to release its N-terminal domain, which forms pores on the cell membrane, eventually inducing pyroptosis. In addition, nPD-L1-switched pyroptosis is required for tumor necrosis in vivo. In brief, this study found a novel function of PD-L1 and identified that caspase-8/GSDMC mediates the pyroptosis pathway in cancer cells, which facilitates tumor necrosis.
Metabolic homeostasis and metabolites affect cell fate. α-ketoglutarate (α-KG) is an essential metabolite in the tricarboxylic acid cycle that plays important roles in many physiological processes, such as oxidative stress reduction and cell death. Nevertheless, the role of α-KG in pyroptosis remains unknown. Zhang et al. demonstrated that dimethyl α-KG can pass through the cell membrane and induce the oxidation and endocytosis of death receptor 6 (DR6) by elevating ROS levels [123]. After DR6 internalization, both pro-caspase-8 and GSDMC are recruited to the DR6 receptosome, where active caspase-8 cleaves the GSDMC, finally resulting in pyroptosis (Figure 7A). α-KG-induced pyroptosis has been shown to be sufficient for inhibiting tumor growth and metastasis in vivo. Fascinatingly, in an acidic environment, α-KG can be reduced to L-2-hydroxyglutarate (L-2HG) by the metabolic enzyme malate dehydrogenase 1, which further boosts ROS and promotes pyroptosis. Treatment with lactic acid produces more L-2HG because of the improved acidic environment, which turns pyroptosis-resistant cancer cells into pyroptosis-sensitive cancer cells. Collectively, this study links metabolites with the pyroptosis pathway and illustrates how α-KG triggers DR6 endocytosis to bring about caspase-8/GSDMC-mediated pyroptosis, and thus, has application in cancer therapy.
During the period when apoptosis was considered the dominant form of PCD, granzymes were thought to kill target cells by means of apoptosis. However, the discovery of pyroptosis has updated our understanding of PCD. It is important to explore whether gasdermin proteins respond to granzymes and induce pyroptosis. Zhou et al. demonstrated that cytotoxic T lymphocyte- or NK cell-derived GZMA can cleave GSDMB to release its pore-forming N-terminal domain for pyroptosis induction [50]. Moreover, interferon-γ increased the expression of GSDMB to further promote pyroptosis (Figure 7B). Heterologous overexpression of GZMA-cleavable human GSDMB in mouse cancer cells accelerates the elimination of tumors in vivo. This study identified a novel killing mechanism of cytotoxic lymphocytes through gasdermin-mediated pyroptosis, which ensures sufficient antitumor immunity.
Bioorthogonal chemistry presents a wonderful strategy for investigating many biological processes, such as immunity and cell death in live animals. Wang et al. constructed a nano-bioorthogonal chemical system, in which gasdermin A3 (GA3) was linked to nanoparticles via the trimethylsilyl ether linker (NP-GA3), and this linker could be cleaved by a cancer-imaging probe phenylalanine trifluoroborate (Phe-BF3) [124]. When HeLa and EMT6 cells were treated with NP-GA3 and Phe-BF3, the cells showed obvious pyroptotic morphology. It should be noted that only a small fraction of the tumor cells undergoing pyroptosis could erase the entire tumor, which implies the role of the immune system. Furthermore, only one round of injection of NP-GA3 and Phe-BF3 could not prevent tumor growth. In contrast, tumor growth was markedly inhibited by one round of injection plus anti-PD-1 therapy, which demonstrates that inflammation induced by pyroptosis can trigger robust antitumor immunity and synergize with immune checkpoint blockade for tumor immunotherapy.
Conclusion
Pyroptosis is a form of inflammatory PCD mediated by gasdermin proteins, which are often activated by caspases. Initially, researchers focused on the role of inflammatory caspases (caspases-1/4/5/11) in pyroptosis. Afterwards, researchers have found that apoptotic caspases (caspases-3/6/8) are also involved in the process of pyroptosis. Moreover, recent studies have shown that GZMA/GZMB can trigger pyroptosis as well as caspases. These studies renovate our understanding of pyroptosis. Future research will continue to update the novel and precise activation modes of pyroptosis, for instance, which caspases or other factors mediate the cleavage of the GSDMA.
Generally, GSDMD cleavage by caspase-1 via the canonical inflammasome pathway plays an important role in host defense against pathogen infections. However, excessive inflammatory responses and cell death caused by pyroptosis may be involved in various diseases, such as cardiovascular diseases, nervous system diseases, and liver diseases. Hence, many studies have focused on inhibiting pyroptosis to treat these diseases by targeting NLRP3, caspase-1, or GSDMD. VX-765 is a safe and effective inhibitor of caspase-1 that has been proved to be well tolerated in phase II clinical trial in patients with partial epilepsy. Therefore, VX-765 is a clinical-grade drug that could potentially be used in other pyroptosis-related diseases. Another anti-pyroptotic drug currently in clinical application is lncRNA NBR2, which regulates endothelial pyroptosis by targeting GSDMD in sepsis. Nevertheless, due to non-specificity, many current inhibitors may result in unexpected side effects. Further research is needed to improve the specificity of pyroptotic inhibitors. In addition, current therapeutic targets for the treatment of these diseases mainly focus on canonical inflammasome signaling, that is, the caspase-1/GSDMD pathway. It should be further investigated whether the non-canonical inflammasome pathway or apoptotic caspases-mediated pathway of pyroptosis is implicated in these inflammatory diseases and can serve as therapeutic targets.
Although pyroptosis produces pathogenic effects on inflammatory diseases, as a pro-inflammatory cell death, it also paves a new way for cancer clearance by deliberate induction of pyroptotic cell death and intense antitumor immunity. In cancer, pyroptosis has been shown to be triggered by almost all signaling pathways in which GSDME, GSDMD, GSDMC, or GSDMB serve as the executors. Induction of pyroptosis to eliminate tumor cells has become a promising strategy for the treatment of tumor by boosting antitumor immunity. However, gasdermins are also expressed in normal tissues. Extensive pyroptosis may cause severe damage to the normal tissues. Hence, there are several points to be noted for pyroptosis-based cancer therapy. Firstly, how to specifically induce pyroptosis in cancer cells but not in normal cells for cancer therapy. Some studies have designed TME responsive nanodrug to induce pyroptosis in cancer cells specifically. Secondly, how to visualize pyroptosis in vivo, to further improve its accuracy. Future studies are urgently needed to develop more precise and tumor-specific pyroptotic treatments, and more clinical trials are needed to explore the potential application of pyroptosis-based cancer therapy.
Abbreviations
ASC: apoptosis-associated speck-like protein containing a caspase recruitment domain; AD: alzheimer's disease; AIM2: absent in melanoma 2; AMPCP: α, β-methylene adenosine 5' diphosphate; AOZN: AMPCP and Orz loaded nanomicelles; ATP: adenosine triphosphoric acid; Aβ: β-amyloid protein; BNP: biomimetic nanoparticle; CARD: caspase recruitment domain-containing; CSCs: cancer stem cells; CXCR4: C-X-C motif chemokine receptor 4; DAC: decitabine; DC: dendritic cell; DITOX: diphtheria toxin; DR6: death receptor 6; FoxO1: forkhead box O1; GA3: gasdermin A3; GSDMD: gasdermin D; GSDMD-NT: gasdermin D N-terminal domain; GSH: glutathione; GZMA: granzyme A; GZMB: granzyme B; IBRN: implantable bio-responsive nanoarray; IL-18: interleukin-18; IL-1β: interleukin-1β; IONPs: iron oxide nanoparticles; IRI: ischemia-reperfusion injury; ITME: immunosuppressive tumor microenvironment; LipoDDP: cisplatin loaded nanoliposome; LPS: lipopolysaccharide; LRR: leucine-rich repeats; MCPP: dual-responsive nano-prodrug loaded with photosensitizer purpurin 18 and paclitaxel; MOF: metal-organic framework; NAFL: non-alcoholic fatty liver; NAFLD: Nonalcoholic-fatty liver disease; NASH: non-alcoholic steatohepatitis; NIR: near-infrared; NK: natural killer; NLRC4: nucleotide-binding oligomerization domain-like receptor caspase recruitment domain-containing 4; NLRP1/3: nucleotide-binding oligomerization domain-like receptor pyrin domain-containing1/3; NOD: nucleotide-binding oligomerization domain; NSCLC: non-small cell lung cancer; Orz: γ-oryzanol; P2X7: P2X purinoreceptor 7; PCD: programmed cell death; PD: parkinson's disease; PDA@M: polydopamine-based biomimetic nanoplatform; PD-L1: programmed death-ligand 1; Phe-BF3: phenylalanine tri‑uoroborate; PRRs: pattern recognition receptors; PYD: pyrin domain; ROS: reactive oxygen species; SIRT1: silent information regulator factor 2 related enzyme 1; TBD: 1,1,2,2-tetraphe-nylethene-benzo[c][1,2,5] thiadiazole-2-(diphenyl methylene) malononitrile; TME: tumor microenvironment; TNF: tumor necrosis factor; TRM: tumor recurrence and metastasis; VTPA: virus-spike tumor-activatable pyroptotic agent; α-KG: α-ketoglutarate; α-NETA: 2-(anaphthoyl) ethyl-trimethylammonium iodide.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (Nos. 2017YFC1309100 and 2017YFA0205200), National Natural Science Foundation of China (Nos. 21804104, 91959124, 81671753, 32101147 and 32071406), Innovation Capability Support Program of Shaanxi (Program No. 2022TD-52), Natural Science Foundation of Shaanxi Province of China (No. 2020PT-020), The Youth Innovation Team of Shaanxi Universities, Natural Science Basic Research Plan in Shaanxi Province of China (Nos. 2021JM-147 and 2021JQ-212), and the Fundamental Research Funds for the Central Universities (Nos. JB211201, JB211202, JB211204).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167-9
2. Hilbi H, Chen Y, Thirumalai K, Zychlinsky A. The interleukin 1beta-converting enzyme, caspase 1, is activated during Shigella flexneri-induced apoptosis in human monocyte-derived macrophages. Infect Immun. 1997;65:5165-70
3. Hersh D, Monack DM, Smith MR, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci U S A. 1999;96:2396-401
4. Boise LH, Collins CM. Salmonella-induced cell death: apoptosis, necrosis or programmed cell death? Trends Microbiol. 2001;9:64-7
5. Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113-4
6. Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8:1812-25
7. Fink SL, Cookson BT. Pyroptosis and host cell death responses during Salmonella infection. Cell Microbiol. 2007;9:2562-70
8. Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660-5
9. Feng S, Fox D, Man SM. Mechanisms of gasdermin family members in inflammasome signaling and cell death. J Mol Biol. 2018;430:3068-80
10. Broz P, Pelegrin P, Shao F. The gasdermins, a protein family executing cell death and inflammation. Nat Rev Immunol. 2020;20:143-57
11. Ding J, Wang K, Liu W, She Y, Sun Q, Shi J. et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111-6
12. Aglietti RA, Dueber EC. Recent insights into the molecular mechanisms underlying pyroptosis and gasdermin family functions. Trends Immunol. 2017;38:261-71
13. Evavold CL, Hafner-Bratkovic I, Devant P, D'Andrea JM, Ngwa EM, Borsic E. et al. Control of gasdermin D oligomerization and pyroptosis by the Ragulator-Rag-mTORC1 pathway. Cell. 2021;184:4495-511 e19
14. Kayagaki N, Kornfeld OS, Lee BL, Stowe IB, O'Rourke K, Li Q. et al. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature. 2021;591:131-6
15. Rühl S, Shkarina K, Demarco B, Heilig R, Santos JC, Broz P. ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science. 2018;362:956-60
16. Rogers C, Fernandes-Alnemri T, Mayes L, Alnemri D, Cingolani G, Alnemri ES. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun. 2017;8:14128
17. Sarhan J, Liu BC, Muendlein HI, Li P, Nilson R, Tang AY. et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc Natl Acad Sci U S A. 2018;115:E10888-E97
18. Tsuchiya K, Nakajima S, Hosojima S, Thi Nguyen D, Hattori T, Manh Le T. et al. Caspase-1 initiates apoptosis in the absence of gasdermin D. Nat Commun. 2019;10:2091
19. Conos SA, Chen KW, De Nardo D, Hara H, Whitehead L, Nunez G. et al. Active MLKL triggers the NLRP3 inflammasome in a cell-intrinsic manner. Proc Natl Acad Sci U S A. 2017;114:E961-E9
20. Gutierrez KD, Davis MA, Daniels BP, Olsen TM, Ralli-Jain P, Tait SW. et al. MLKL activation triggers NLRP3-mediated processing and release of IL-1beta independently of gasdermin-D. J Immunol. 2017;198:2156-64
21. Kesavardhana S, Malireddi RKS, Kanneganti TD. Caspases in cell death, inflammation, and pyroptosis. Annu Rev Immunol. 2020;38:567-95
22. Jimenez Fernandez D, Lamkanfi M. Inflammatory caspases: key regulators of inflammation and cell death. Biol Chem. 2015;396:193-203
23. Zheng D, Liwinski T, Elinav E. Inflammasome activation and regulation: toward a better understanding of complex mechanisms. Cell Discov. 2020;6:36
24. Wu J, Fernandes-Alnemri T, Alnemri ES. Involvement of the AIM2, NLRC4, and NLRP3 inflammasomes in caspase-1 activation by Listeria monocytogenes. J Clin Immunol. 2010;30:693-702
25. Xu H, Yang J, Gao W, Li L, Li P, Zhang L. et al. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature. 2014;513:237-41
26. Ting JP, Lovering RC, Alnemri ES, Bertin J, Boss JM, Davis BK. et al. The NLR gene family: a standard nomenclature. Immunity. 2008;28:285-7
27. Mitchell PS, Sandstrom A, Vance RE. The NLRP1 inflammasome: new mechanistic insights and unresolved mysteries. Curr Opin Immunol. 2019;60:37-45
28. Elliott EI, Sutterwala FS. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol Rev. 2015;265:35-52
29. Kahlenberg JM, Dubyak GR. Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am J Physiol Cell Physiol. 2004;286:C1100-8
30. Zhao Y, Yang J, Shi J, Gong YN, Lu Q, Xu H. et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477:596-600
31. Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR. et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514-8
32. Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A. et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136-42
33. Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J. et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117-21
34. Man SM, Kanneganti TD. Regulation of inflammasome activation. Immunol Rev. 2015;265:6-21
35. Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P. et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187-92
36. Kayagaki N, Stowe IB, Lee BL, O'Rourke K, Anderson K, Warming S. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666-71
37. Ruhl S, Broz P. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K(+) efflux. Eur J Immunol. 2015;45:2927-36
38. Yang D, He Y, Munoz-Planillo R, Liu Q, Nunez G. Caspase-11 requires the pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity. 2015;43:923-32
39. Wang Y, Gao W, Shi X, Ding J, Liu W, He H. et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99-103
40. Orning P, Weng D, Starheim K, Ratner D, Best Z, Lee B. et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science. 2018;362:1064-9
41. Zheng Z, Deng W, Bai Y, Miao R, Mei S, Zhang Z. et al. The lysosomal Rag-Ragulator complex licenses RIPK1 and caspase-8-mediated pyroptosis by Yersinia. Science. 2021;372:eabg0269
42. Hou J, Zhao R, Xia W, Chang CW, You Y, Hsu JM. et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat Cell Biol. 2020;22:1264-75
43. Chao KL, Kulakova L, Herzberg O. Gene polymorphism linked to increased asthma and IBD risk alters gasdermin-B structure, a sulfatide and phosphoinositide binding protein. Proc Natl Acad Sci U S A. 2017;114:E1128-E37
44. Chowdhury D, Lieberman J. Death by a thousand cuts: granzyme pathways of programmed cell death. Annu Rev Immunol. 2008;26:389-420
45. Metkar SS, Menaa C, Pardo J, Wang B, Wallich R, Freudenberg M. et al. Human and mouse granzyme A induce a proinflammatory cytokine response. Immunity. 2008;29:720-33
46. Joeckel LT, Bird PI. Are all granzymes cytotoxic in vivo? Biol Chem. 2014;395:181-202
47. Wensink AC, Hack CE, Bovenschen N. Granzymes regulate proinflammatory cytokine responses. J Immunol. 2015;194:491-7
48. Campbell RA, Franks Z, Bhatnagar A, Rowley JW, Manne BK, Supiano MA. et al. Granzyme A in human platelets regulates the synthesis of proinflammatory cytokines by monocytes in aging. J Immunol. 2018;200:295-304
49. van Daalen KR, Reijneveld JF, Bovenschen N. Modulation of inflammation by extracellular granzyme A. Front Immunol. 2020;11:931
50. Zhou Z, He H, Wang K, Shi X, Wang Y, Su Y. et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science. 2020;368:eaaz7548
51. Zhang Z, Zhang Y, Xia S, Kong Q, Li S, Liu X. et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature. 2020;579:415-20
52. Demarco B, Chen KW, Broz P. Cross talk between intracellular pathogens and cell death. Immunol Rev. 2020;297:174-93
53. Aachoui Y, Sagulenko V, Miao EA, Stacey KJ. Inflammasome-mediated pyroptotic and apoptotic cell death, and defense against infection. Curr Opin Microbiol. 2013;16:319-26
54. Gong W, Shi Y, Ren J. Research progresses of molecular mechanism of pyroptosis and its related diseases. Immunobiology. 2019;225:151884
55. Liang F, Zhang F, Zhang L, Wei W. The advances in pyroptosis initiated by inflammasome in inflammatory and immune diseases. Inflamm Res. 2020;69:159-66
56. Hoseini Z, Sepahvand F, Rashidi B, Sahebkar A, Masoudifar A, Mirzaei H. NLRP3 inflammasome: its regulation and involvement in atherosclerosis. J Cell Physiol. 2018;233:2116-32
57. He B, Nie Q, Wang F, Han Y, Yang B, Sun M. et al. Role of pyroptosis in atherosclerosis and its therapeutic implications. J Cell Physiol. 2021;236:7159-75
58. Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG. et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357-61
59. Lou Y, Wang S, Qu J, Zheng J, Jiang W, Lin Z. et al. miR-424 promotes cardiac ischemia/reperfusion injury by direct targeting of CRISPLD2 and regulating cardiomyocyte pyroptosis. Int J Clin Exp Pathol. 2018;11:3222-35
60. Mastrocola R, Penna C, Tullio F, Femmino S, Nigro D, Chiazza F. et al. Pharmacological inhibition of NLRP3 inflammasome attenuates myocardial ischemia/reperfusion injury by activation of RISK and mitochondrial pathways. Oxid Med Cell Longev. 2016;2016:5271251
61. Toldo S, Mauro AG, Cutter Z, Van Tassell BW, Mezzaroma E, Del Buono MG. et al. The NLRP3 inflammasome inhibitor, OLT1177 (dapansutrile), reduces infarct size and preserves contractile function after ischemia reperfusion injury in the mouse. J Cardiovasc Pharmacol. 2019;73:215-22
62. Marchetti C, Chojnacki J, Toldo S, Mezzaroma E, Tranchida N, Rose SW. et al. A novel pharmacologic inhibitor of the NLRP3 inflammasome limits myocardial injury after ischemia-reperfusion in the mouse. J Cardiovasc Pharmacol. 2014;63:316-22
63. van Hout GP, Bosch L, Ellenbroek GH, de Haan JJ, van Solinge WW, Cooper MA. et al. The selective NLRP3-inflammasome inhibitor MCC950 reduces infarct size and preserves cardiac function in a pig model of myocardial infarction. Eur Heart J. 2017;38:828-36
64. Toldo S, Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol. 2018;15:203-14
65. Toldo S, Mauro AG, Cutter Z, Abbate A. Inflammasome, pyroptosis, and cytokines in myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2018;315:H1553-H68
66. Fujisue K, Sugamura K, Kurokawa H, Matsubara J, Ishii M, Izumiya Y. et al. Colchicine improves survival, left ventricular remodeling, and chronic cardiac function after acute myocardial infarction. Circ J. 2017;81:1174-82
67. Zhang Y, Liu X, Bai X, Lin Y, Li Z, Fu J. et al. Melatonin prevents endothelial cell pyroptosis via regulation of long noncoding RNA MEG3/miR-223/NLRP3 axis. J Pineal Res. 2018;64:e12449
68. Wei Y, Zhu M, Li S, Hong T, Guo X, Li Y. et al. Engineered biomimetic nanoplatform protects the myocardium against ischemia/reperfusion injury by inhibiting pyroptosis. ACS Appl Mater Interfaces. 2021;13:33756-66
69. Audia JP, Yang XM, Crockett ES, Housley N, Haq EU, O'Donnell K. et al. Caspase-1 inhibition by VX-765 administered at reperfusion in P2Y12 receptor antagonist-treated rats provides long-term reduction in myocardial infarct size and preservation of ventricular function. Basic Res Cardiol. 2018;113:32
70. Wang X, Bian Y, Zhang R, Liu X, Ni L, Ma B. et al. Melatonin alleviates cigarette smoke-induced endothelial cell pyroptosis through inhibiting ROS/NLRP3 axis. Biochem Biophys Res Commun. 2019;519:402-8
71. Chen A, Chen Z, Xia Y, Lu D, Yang X, Sun A. et al. Liraglutide attenuates NLRP3 inflammasome-dependent pyroptosis via regulating SIRT1/NOX4/ROS pathway in H9c2 cells. Biochem Biophys Res Commun. 2018;499:267-72
72. Do Carmo H, Arjun S, Petrucci O, Yellon DM, Davidson SM. The caspase 1 inhibitor VX-765 protects the isolated rat heart via the RISK pathway. Cardiovasc Drugs Ther. 2018;32:165-8
73. Rosadini CV, Kagan JC. Early innate immune responses to bacterial LPS. Curr Opin Immunol. 2017;44:14-9
74. Beier JI, Banales JM. Pyroptosis: An inflammatory link between NAFLD and NASH with potential therapeutic implications. J Hepatol. 2018;68:643-5
75. Bence KK, Birnbaum MJ. Metabolic drivers of non-alcoholic fatty liver disease. Mol Metab. 2021;50:101143
76. Svegliati-Baroni G, Pierantonelli I, Torquato P, Marinelli R, Ferreri C, Chatgilialoglu C. et al. Lipidomic biomarkers and mechanisms of lipotoxicity in non-alcoholic fatty liver disease. Free Radic Biol Med. 2019;144:293-309
77. Xu B, Jiang M, Chu Y, Wang W, Chen D, Li X. et al. Gasdermin D plays a key role as a pyroptosis executor of non-alcoholic steatohepatitis in humans and mice. J Hepatol. 2018;68:773-82
78. Heo MJ, Kim TH, You JS, Blaya D, Sancho-Bru P, Kim SG. Alcohol dysregulates miR-148a in hepatocytes through FoxO1, facilitating pyroptosis via TXNIP overexpression. Gut. 2019;68:708-20
79. Fu X, Zhong Z, Hu F, Zhang Y, Li C, Yan P. et al. The protective effects of selenium-enriched Spirulina platensis on chronic alcohol-induced liver injury in mice. Food Funct. 2018;9:3155-65
80. Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev. 2017;121:27-42
81. Palacios-Macapagal D, Connor J, Mustelin T, Ramalingam TR, Wynn TA, Davidson TS. Cutting edge: eosinophils undergo caspase-1-mediated pyroptosis in response to necrotic liver cells. J Immunol. 2017;199:847-53
82. Qu J, Yuan Z, Wang G, Wang X, Li K. The selective NLRP3 inflammasome inhibitor MCC950 alleviates cholestatic liver injury and fibrosis in mice. Int Immunopharmacol. 2019;70:147-55
83. Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov. 2018;17:588-606
84. Zhang B, Xu D, She L, Wang Z, Yang N, Sun R. et al. Silybin inhibits NLRP3 inflammasome assembly through the NAD(+)/SIRT2 pathway in mice with nonalcoholic fatty liver disease. FASEB J. 2018;32:757-67
85. Zhang Y, Jin Q, Li X, Jiang M, Cui BW, Xia KL. et al. Amelioration of alcoholic liver steatosis by dihydroquercetin through the modulation of AMPK-dependent lipogenesis mediated by P2X7R-NLRP3-inflammasome activation. J Agric Food Chem. 2018;66:4862-71
86. Yu X, Hao M, Liu Y, Ma X, Lin W, Xu Q. et al. Liraglutide ameliorates non-alcoholic steatohepatitis by inhibiting NLRP3 inflammasome and pyroptosis activation via mitophagy. Eur J Pharmacol. 2019;864:172715
87. Woolbright BL, Ding WX, Jaeschke H. Caspase inhibitors for the treatment of liver disease: friend or foe? Expert Rev Gastroenterol Hepatol. 2017;11:397-9
88. Wang HN, Wang YR, Liu GQ, Liu Z, Wu PX, Wei XL. et al. Inhibition of hepatic interleukin-18 production by rosiglitazone in a rat model of nonalcoholic fatty liver disease. World J Gastroenterol. 2008;14:7240-6
89. Meier RPH, Meyer J, Montanari E, Lacotte S, Balaphas A, Muller YD. et al. Interleukin-1 receptor antagonist modulates liver inflammation and fibrosis in mice in a model-dependent manner. Int J Mol Sci. 2019;20:1295
90. Tan MS, Tan L, Jiang T, Zhu XC, Wang HF, Jia CD. et al. Amyloid-beta induces NLRP1-dependent neuronal pyroptosis in models of Alzheimer's disease. Cell Death Dis. 2014;5:e1382
91. Han C, Yang Y, Guan Q, Zhang X, Shen H, Sheng Y. et al. New mechanism of nerve injury in Alzheimer's disease: beta-amyloid-induced neuronal pyroptosis. J Cell Mol Med. 2020;24:8078-90
92. Zeng R, Luo DX, Li HP, Zhang QS, Lei SS, Chen JH. MicroRNA-135b alleviates MPP(+)-mediated Parkinson's disease in in vitro model through suppressing FoxO1-induced NLRP3 inflammasome and pyroptosis. J Clin Neurosci. 2019;65:125-33
93. Zhang Q, Huang XM, Liao JX, Dong YK, Zhu JL, He CC. et al. LncRNA HOTAIR promotes neuronal damage through facilitating NLRP3 mediated-pyroptosis activation in Parkinson's disease via regulation of miR-326/ELAVL1 axis. Cell Mol Neurobiol. 2020;41:1773-86
94. Wang H, Chen H, Jin J, Liu Q, Zhong D, Li G. Inhibition of the NLRP3 inflammasome reduces brain edema and regulates the distribution of aquaporin-4 after cerebral ischaemia-reperfusion. Life Sci. 2020;251:117638
95. Xu P, Zhang X, Liu Q, Xie Y, Shi X, Chen J. et al. Microglial TREM-1 receptor mediates neuroinflammatory injury via interaction with SYK in experimental ischemic stroke. Cell Death Dis. 2019;10:555
96. Li Q, Cao Y, Dang C, Han B, Han R, Ma H. et al. Inhibition of double-strand DNA-sensing cGAS ameliorates brain injury after ischemic stroke. EMBO Mol Med. 2020;12:e11002
97. Wang K, Sun Z, Ru J, Wang S, Huang L, Ruan L. et al. Ablation of GSDMD improves outcome of ischemic stroke through blocking canonical and non-canonical inflammasomes dependent pyroptosis in microglia. Front Neurol. 2020;11:577927
98. Dempsey C, Rubio Araiz A, Bryson KJ, Finucane O, Larkin C, Mills EL. et al. Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-beta and cognitive function in APP/PS1 mice. Brain Behav Immun. 2017;61:306-16
99. Gordon R, Albornoz EA, Christie DC, Langley MR, Kumar V, Mantovani S. et al. Inflammasome inhibition prevents alpha-synuclein pathology and dopaminergic neurodegeneration in mice. Sci Transl Med. 2018;10:eaah4066
100. Zhang X, Zhang Y, Li R, Zhu L, Fu B, Yan T. Salidroside ameliorates Parkinson's disease by inhibiting NLRP3-dependent pyroptosis. Aging (Albany NY). 2020;12:9405-26
101. Flores J, Noel A, Foveau B, Beauchet O, LeBlanc AC. Pre-symptomatic caspase-1 inhibitor delays cognitive decline in a mouse model of Alzheimer disease and aging. Nat Commun. 2020;11:4571
102. Li J, Hao JH, Yao D, Li R, Li XF, Yu ZY. et al. Caspase-1 inhibition prevents neuronal death by targeting the canonical inflammasome pathway of pyroptosis in a murine model of cerebral ischemia. CNS Neurosci Ther. 2020;26:925-39
103. Hou J, Hsu JM, Hung MC. Molecular mechanisms and functions of pyroptosis in inflammation and antitumor immunity. Mol Cell. 2021;81:4579-90
104. Tan G, Huang C, Chen J, Zhi F. HMGB1 released from GSDME-mediated pyroptotic epithelial cells participates in the tumorigenesis of colitis-associated colorectal cancer through the ERK1/2 pathway. J Hematol Oncol. 2020;13:149
105. Zolondick AA, Gaudino G, Xue J, Pass HI, Carbone M, Yang H. Asbestos-induced chronic inflammation in malignant pleural mesothelioma and related therapeutic approaches-a narrative review. Precis Cancer Med. 2021;4:27
106. Du T, Gao J, Li P, Wang Y, Qi Q, Liu X. et al. Pyroptosis, metabolism, and tumor immune microenvironment. Clin Transl Med. 2021;11:e492
107. Ben-Sasson SZ, Hogg A, Hu-Li J, Wingfield P, Chen X, Crank M. et al. IL-1 enhances expansion, effector function, tissue localization, and memory response of antigen-specific CD8 T cells. J Exp Med. 2013;210:491-502
108. Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev. 2018;281:8-27
109. Wang F, Liu W, Ning J, Wang J, Lang Y, Jin X. et al. Simvastatin suppresses proliferation and migration in non-small cell lung cancer via pyroptosis. Int J Biol Sci. 2018;14:406-17
110. Qiao L, Wu X, Zhang J, Liu L, Sui X, Zhang R. et al. alpha-NETA induces pyroptosis of epithelial ovarian cancer cells through the GSDMD/caspase-4 pathway. FASEB J. 2019;33:12760-7
111. Ploetz E, Zimpel A, Cauda V, Bauer D, Lamb DC, Haisch C. et al. Metal-organic framework nanoparticles induce pyroptosis in cells controlled by the extracellular pH. Adv Mater. 2020;32:e1907267
112. Wu M, Liu X, Chen H, Duan Y, Liu J, Pan Y. et al. Activation of pyroptosis by membrane-anchoring AIE photosensitizer design: new prospect for photodynamic cancer cell ablation. Angew Chem Int Ed Engl. 2021;60:9093-8
113. Nadeem S, Yang C, Du Y, Li F, Chen Z, Zhou Y. et al. A virus-spike tumor-activatable pyroptotic agent. Small. 2021;17:e2006599
114. Xiong H, Ma X, Wang X, Su W, Wu L, Zhang T. et al. Inspired epigenetic modulation synergy with adenosine inhibition elicits pyroptosis and potentiates cancer immunotherapy. Adv Funct Mater. 2021;31:2100007
115. Zhang CC, Li CG, Wang YF, Xu LH, He XH, Zeng QZ. et al. Chemotherapeutic paclitaxel and cisplatin differentially induce pyroptosis in A549 lung cancer cells via caspase-3/GSDME activation. Apoptosis. 2019;24:312-25
116. Yu J, Li S, Qi J, Chen Z, Wu Y, Guo J. et al. Cleavage of GSDME by caspase-3 determines lobaplatin-induced pyroptosis in colon cancer cells. Cell Death Dis. 2019;10:193
117. Hu J, Dong Y, Ding L, Dong Y, Wu Z, Wang W. et al. Local delivery of arsenic trioxide nanoparticles for hepatocellular carcinoma treatment. Signal Transduct Target Ther. 2019;4:28
118. Fan JX, Deng RH, Wang H, Liu XH, Wang XN, Qin R. et al. Epigenetics-based tumor cells pyroptosis for enhancing the immunological effect of chemotherapeutic nanocarriers. Nano Lett. 2019;19:8049-58
119. Zhao H, Song Q, Zheng C, Zhao B, Wu L, Feng Q. et al. Implantable bioresponsive nanoarray enhances postsurgical immunotherapy by activating pyroptosis and remodeling tumor microenvironment. Adv Funct Mater. 2020;30:2005747
120. Zhao P, Wang M, Chen M, Chen Z, Peng X, Zhou F. et al. Programming cell pyroptosis with biomimetic nanoparticles for solid tumor immunotherapy. Biomaterials. 2020;254:120142
121. Wang K, Xiao X, Jiang M, Li J, Zhou J, Yuan Y. An NIR-fluorophore-based theranostic for selective initiation of tumor pyroptosis-induced immunotherapy. Small. 2021: e2102610.
122. Xiao Y, Zhang T, Ma X, Yang QC, Yang LL, Yang SC. et al. Microenvironment-responsive prodrug-induced pyroptosis boosts cancer immunotherapy. Adv Sci (Weinh). 2021;8:e2101840
123. Zhang JY, Zhou B, Sun RY, Ai YL, Cheng K, Li FN. et al. The metabolite alpha-KG induces GSDMC-dependent pyroptosis through death receptor 6-activated caspase-8. Cell Res. 2021;31:980-97
124. Wang Q, Wang Y, Ding J, Wang C, Zhou X, Gao W. et al. A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature. 2020;579:421-6
125. Serna N, Alamo P, Ramesh P, Vinokurova D, Sanchez-Garcia L, Unzueta U. et al. Nanostructured toxins for the selective destruction of drug-resistant human CXCR4(+) colorectal cancer stem cells. J Control Release. 2020;320:96-104
126. Zhou B, Zhang JY, Liu XS, Chen HZ, Ai YL, Cheng K. et al. Tom20 senses iron-activated ROS signaling to promote melanoma cell pyroptosis. Cell Res. 2018;28:1171-85
Author Biographies
 Dr. Zhiping Rao received her Ph.D. from the Institute of Neuroscience, State Key Laboratory of Neuroscience, CAS center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences in 2018. She then joined the School of Life and Technology, Xidian University as a lecturer and her research focuses on the programmed cell death and disease treatment, especially ferroptosis and pyroptosis-based cancer therapy.
Dr. Zhiping Rao received her Ph.D. from the Institute of Neuroscience, State Key Laboratory of Neuroscience, CAS center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences in 2018. She then joined the School of Life and Technology, Xidian University as a lecturer and her research focuses on the programmed cell death and disease treatment, especially ferroptosis and pyroptosis-based cancer therapy.
 Prof. Jianxiong Li received his doctor's degree from the PLA Medical College. Currently, he serves as deputy director of the Radiotherapy Department of Oncology Department of PLA General Hospital. His research interests are specialized in precision radiotherapy and cyberknife therapy for common malignancies including breast, lung, liver, gastrointestinal tumors, head and neck cancer, lymphoma, gynecological tumor, brain primary tumors and metastases, bone metastases, skin cancer, and hematologic malignancies, and involves radiotherapy for benign diseases such as scarring, lupus erythematosus, hemangioma, and so on.
Prof. Jianxiong Li received his doctor's degree from the PLA Medical College. Currently, he serves as deputy director of the Radiotherapy Department of Oncology Department of PLA General Hospital. His research interests are specialized in precision radiotherapy and cyberknife therapy for common malignancies including breast, lung, liver, gastrointestinal tumors, head and neck cancer, lymphoma, gynecological tumor, brain primary tumors and metastases, bone metastases, skin cancer, and hematologic malignancies, and involves radiotherapy for benign diseases such as scarring, lupus erythematosus, hemangioma, and so on.
 Dr. Pengbo Ning received his PhD from Northwest A&F University in 2013. He worked at the College of Veterinary Medicine, Northwest A&F University from 2007 to 2015, and the field of study was genome-wide analysis in interactions between viruses and macrophages. He then moved to the School of Life and Technology, Xidian University, as an Associate Professor, and his research focuses on gene-modified macrophages and their potential application for cancer immunotherapy and drug delivery.
Dr. Pengbo Ning received his PhD from Northwest A&F University in 2013. He worked at the College of Veterinary Medicine, Northwest A&F University from 2007 to 2015, and the field of study was genome-wide analysis in interactions between viruses and macrophages. He then moved to the School of Life and Technology, Xidian University, as an Associate Professor, and his research focuses on gene-modified macrophages and their potential application for cancer immunotherapy and drug delivery.
 Prof. Zhongliang Wang received his PhD from Institute of chemistry, Chinese Academy of Sciences in 2009. He then moved to the University of Florida as a postdoc fellow working with Professor Y. Charles Cao. In 2013, he joined the Laboratory of Molecular Imaging and Nanomedicine (LOMIN) at the National Institutes of Health (NIH) as a postdoctoral fellow under the supervision of Prof. Xiaoyuan (Shawn) Chen. In 2014, he joined the School of Life and Technology, Xidian University as a Professor and his research focuses on the development of smart and biomimetic materials for diagnostics and therapeutics of various diseases.
Prof. Zhongliang Wang received his PhD from Institute of chemistry, Chinese Academy of Sciences in 2009. He then moved to the University of Florida as a postdoc fellow working with Professor Y. Charles Cao. In 2013, he joined the Laboratory of Molecular Imaging and Nanomedicine (LOMIN) at the National Institutes of Health (NIH) as a postdoctoral fellow under the supervision of Prof. Xiaoyuan (Shawn) Chen. In 2014, he joined the School of Life and Technology, Xidian University as a Professor and his research focuses on the development of smart and biomimetic materials for diagnostics and therapeutics of various diseases.
![]() Corresponding authors: 301301ljxcom; pbningedu.cn; wangzledu.cn
Corresponding authors: 301301ljxcom; pbningedu.cn; wangzledu.cn
 Global reach, higher impact
Global reach, higher impact