13.3
Impact Factor
Theranostics 2022; 12(3):1342-1372. doi:10.7150/thno.65778 This issue Cite
Review
Integrative biology of extracellular vesicles in diabetes mellitus and diabetic complications
1. Division of Endocrinology and Metabolism, National Clinical Research Center for Geriatrics, State Key Laboratory of Biotherapy and Cancer Center, West China Medical School, West China Hospital, Sichuan University and Collaborative Innovation Center of Biotherapy, Chengdu, China.
2. Department of Geriatric Medicine, Lanzhou University Secondary Hospital, Lanzhou, Gansu, China.
3. Department and Laboratory of Integrated Traditional Chinese and Western Medicine, Sichuan Provincial Pancreatitis Centre and West China-Liverpool Biomedical Research Centre, West China Hospital, Sichuan University, Chengdu, China.
4. Division of Endocrinology and Metabolism, Center for Diabetes and Metabolism Research, Laboratory of Diabetes and Islet Transplantation Research, West China Medical School, West China Hospital, Sichuan University, Chengdu, China.
#Co-first author.
Received 2021-8-6; Accepted 2021-12-11; Published 2022-1-1
Abstract
Diabetes mellitus (DM) is a chronic systemic disease with increasing prevalence globally. An important aspect of diabetic pathogenesis is cellular crosstalk and information exchange between multiple metabolic organs and tissues. In the past decade, increasing evidence suggested that extracellular vesicles (EVs), a class of cell-derived membrane vesicles that transmit information and perform inter-cellular and inter-organ communication, are involved in the pathological changes of insulin resistance (IR), inflammation, and endothelial injury, and implicated in the development of DM and its complications. The biogenesis and cargo sorting machinery dysregulation of EVs may mediate their pathogenic roles under diabetic conditions. Moreover, the biogenesis of EVs, their ubiquitous production by different cells, their function as mediators of inter-organ communication, and their biological features in body fluids have generated great promise as biomarkers and clinical treatments. In this review, we summarize the components of EV generation and sorting machinery and highlight their role in the pathogenesis of DM and associated complications. Furthermore, we discuss the emerging clinical implications of EVs as potential biomarkers and therapeutic strategies for DM and diabetic complications. A better understanding of EVs will deepen our knowledge of the pathophysiology of DM and its complications and offer attractive approaches to improve the prevention, diagnosis, treatment, and prognosis of these disorders.
Keywords: diabetes mellitus, extracellular vesicles, biogenesis, sorting, adipocytes, macrophages, islet, endothelial cells
Introduction
Diabetes mellitus (DM), a systemic disease with an alarming increase in incidence worldwide, is characterized by chronic hyperglycemia resulting from insulin resistance (IR) and/or insulin secretion deficiency. The International Diabetes Federation estimates that 9.3% of adults aged 20-79 years are currently living with DM and this prevalence is projected to rise to 10.2% by 2030 and to 10.9% by 2045 [1]. DM and associated complications account for 11.3% of global deaths from all causes [1]. Moreover, DM is an independent risk factor for various diseases, including coronary heart disease, stroke, cancer, chronic kidney disease, blindness, and lower limb amputation, placing a heavy burden on global health [2-6]. There are no efficacious pharmacological treatments for DM, primarily due to its complicated pathophysiologic mechanisms and unclear etiopathogenesis.
Generally, DM can be mainly divided into type 1 diabetes (T1D) and type 2 diabetes (T2D). T2D is the most common type of DM, accounting for about 90% of cases worldwide, whereas T1D constitutes more than 10% [7, 8]. Although the etiologies of T1D and T2D are distinct, the progression of both diseases is primarily due to dysregulated intercellular and inter-organ communication. In T1D, the interaction between immune cells and pancreatic islets contributes to the dysfunction and death of β cells. More recently, the involvement of gut microbiota in the auto-immune response against β cells has added another layer of complexity to T1D pathogenesis [9]. In T2D, intricate inter-organ communication among the pancreas, adipose tissue (AT), liver, muscle, intestine, hypothalamus, and other tissues plays an essential role in hyperglycemia and IR, two hallmarks of T2D [10]. For example, increased inflammatory cytokines and free fatty acids (FFAs) derived from obese AT can induce lipotoxicity and IR in the liver and skeletal muscle, and also impair the glucoregulatory function of the central nervous system (CNS) and gut, in turn, perturbing the AT secretome and reinforce its IR [11]. Furthermore, systemic IR triggers a rise in insulin demand, overstressing β cells and eventually resulting in islet dysfunction and relative insulin insufficiency. Therefore, dysregulated inter-organ communication plays a key role in initiating and amplifying the deleterious vicious cycle of IR and hyperglycemia in T2D. Under these circumstances, signaling molecules mediating inter-organ conversation are likely key pathogenic factors for T1D and T2D. Indeed, several classes of signaling mediators, including adipokines, hepatokines, peptides from CNS, and hormones from the pancreas and intestine, are crucial in the initiation and progression of both T1D and T2D [12-18], and therapeutic strategies targeting these molecules have been partially applied in the clinic benefiting patients.
Recently, extracellular vesicles (EVs) have emerged as a novel class of signaling molecules mediating intercellular and inter-organ communication. Released by various cell types, EVs are widely distributed in diverse tissues and body fluids. Moreover, bioactive contents loaded in EVs including proteins, DNAs, RNAs, lipids, and metabolites, are protected by the lipid bilayer membrane against harsh environments and prevented from degradation and digestion. The size, quantity, morphology, cargoes, and other characteristics of EVs are highly variable and influenced by the parental cell type. Because of these fundamental features, EVs are well-suited to serve as versatile carriers and transporters transmitting signals from parental cells to recipient cells. Correspondingly, EVs have been shown to regulate various biological and physiological processes and are implicated in multiple human diseases, such as cancer, cardiovascular diseases, metabolic disorders, and neurodegenerative diseases [19]. In particular, understanding the role of EVs in the crosstalk among multiple metabolic tissues would provide a new perspective to understand the pathogenesis of DM and diabetic complications and develop therapeutic strategies.
Here we outline the current knowledge of diabetic pathogenesis, focusing on the potential mechanisms underlying the altered EV biogenesis in DM and the role of EVs originating from different cells in regulating systemic metabolism. Finally, we summarize studies of EV-RNAs as markers and discuss potential applications of EVs derived from native cells to treat DM and diabetic complications.
Pathology of DM and its complications
IR, also known as low insulin sensitivity, is an essential mechanism underlying T2D occurrence and a critical driver of associated complications [20]. Although there is no consensus on the molecular mechanism(s) triggering IR, inter-organ communication has been widely recognized as a key contributor [21]. During obesity, massive expansion of AT, often accompanied by inadequate vascularization, induces hypoxic response and inflammation, leading to increased infiltration of pro-inflammatory macrophages and inflammatory cytokine release [22]. Inflammation can further disturb insulin signaling in AT, resulting in enhanced lipolysis and increased release of FFAs and adipokines into circulation. Subsequently, elevated circulating FFAs elicit lipotoxicity and impair insulin action in the liver and skeletal muscle [23, 24]. Also, downstream pathways aroused by IR cooperatively induce reactive oxygen species (ROS) production and systemic inflammation, further worsening IR. Consequently, IR suppresses plasma membrane translocation of the glucose transporter (GLUT) and glucose uptake, leading to elevated blood glucose levels and systemic energy metabolism disturbance. In addition to classic metabolic tissues, other organs such as the gut, vascular endothelium, and brain, have recently been shown to participate in the development of IR and T2D [25-29]. For example, vascular endothelium can function as an adjustable barrier to control the transport of metabolic macromolecules such as FFAs, lipoproteins, and glucose to metabolic organs, including the skeletal muscle and AT. Bioactive molecules secreted by endothelial cells (ECs), for e.g., nitric oxide and growth factors, may modulate systemic metabolism by modulating insulin sensitivity, maintaining pancreatic islet structure, and insulin secretion [30, 31].
Pancreatic β cell failure, another hallmark of T2D, is also associated with inter-organ communication. Increased demand for insulin, typically due to peripheral IR, leads to excess insulin secretion and elevated islet amyloid polypeptide (IAPP) production. Simultaneously expressed and secreted with insulin, IAPP is a membrane-permeant toxic agent, and its accumulation forms amyloid deposits, causing pancreatic damage [32]. Meanwhile, chronic elevated FFAs and glucose elicit endoplasmic reticulum (ER) stress and inflammatory response in islets, which aggravate the pancreatic injury and compromise insulin secretion [33, 34]. In this circumstance, the combination of IR and β cell decompensation contributes to overt T2D.
Insulin deficiency, primarily resulting from reduced β cell function and mass, is the major driver of T1D. Insulitis induced by autoimmune response results in β cell death and chronic autoantigen exposure, reamplifying the immune attack [35]. The uncontrollable autoimmune response against β cells accounts for T1D pathology. Besides the crosstalk between the islet and immune cells, gut and immune system communication has also been implicated in T1D pathogenesis. For example, loss of gut integrity and changes in metabolites caused by enteric dysbacteriosis can significantly promote innate and adaptive autoimmune response against islet cells, thereby participating in T1D development [36-39].
The development of diabetic complications shares multiple pathological processes with DM, such as glucose variability, lipotoxicity, and activation of advanced glycation end products (AGEs) and receptors for AGE (RAGEs) signaling, and consequent mitochondrial dysfunction, oxidative stress, epigenetic changes, and inflammation response [40-47]. Besides the in-depth understanding of underlying molecular mechanisms, the significance of inter-organ crosstalk in the pathogenesis of diabetic complications has recently been emphasized. For instance, there is increased lipoprotein secretion by insulin-resistant hepatocytes that can be glycated and oxidated, leading to renal lipid metabolism disorder and promoting the development of diabetic nephropathy (DN) [48-51].
Furthermore, inter-organ and intercellular communication are crucial in the pathogenesis of DM and diabetic complications, and our current understanding can only be considered as the tip of the iceberg. In this context, EVs, an emerging mediator of intra- and inter-organ crosstalk, have been shown to play a critical role in various pathological pathways of DM and its complications (Figure 1), providing a novel paradigm in pathological mechanisms and therapeutic interventions. Future investigation is required to delineate the molecular mechanisms of EV-mediated signaling under diabetic conditions and further explore their implications in treating these disorders.
EVs
Based on their biogenesis, EVs can be divided into two major groups, exosomes (30-100 nm) and microvesicles (MVs, 50-1000 nm) (also known as ectosomes, microparticles) [52]. MVs are generated directly by budding and shedding from the plasma membrane, while exosome generation involves intraluminal vesicle (ILV) budding and shedding, intracellular multivesicular endosome (MVE) trafficking, and ILV release [53, 54]. In this section, we mainly introduce EVs from two perspectives: (i) generative processes and (ii) mechanisms of cargo sorting.
EV generation
Both exosomes and MVs are vesicles formed by membrane budding away from the cytosol, and their generation requires an integrative cytoskeleton and membrane reorganization (Figure 2).
Exosome generation
The generation of exosomes principally consists of biogenesis, transport, and release. ILV formation is the first step of exosome generation, which depends on the endosomal sorting complex required for transport (ESCRT). Several ESCRT-independent ILV formation pathways mediated by ceramide-, CD63-, Rab31, and others have been detected [55-58]. As the best known ESCRT-independent mode, cone-shaped lipid ceramide enriched in specific microdomains of the endosomal membrane can effectively lead to membrane curvature alteration and budding of ILVs [55]. Tetraspanin CD63, the specific surface marker of exosomes, can favor the budding of ILVs by interacting with a cluster of other tetraspanins and proteins [56, 57]. Although several distinct exosome generation cellular pathways have been reported, the regulatory mechanisms within cells have not yet been elucidated.
The formation of ILVs follows the transport of MVEs toward the plasma membrane. Various intracellular trafficking molecules have been shown to participate in this process, including the cytoskeleton, molecular motors, and Rab GTPases. The final step is the fusion of MVEs with the plasma membrane for which the soluble N-ethylmaleimide-sensitive fusion attachment protein receptor (SNARE) complex is believed to be essential [59-63]. The interaction between vesicle-membrane SNAREs (v-SNAREs) and target-membrane SNAREs (t-SNAREs) initiates the SNARE complex assembly, presumably allowing the fusion of MVEs with the plasma membrane and leading to exosome secretion.
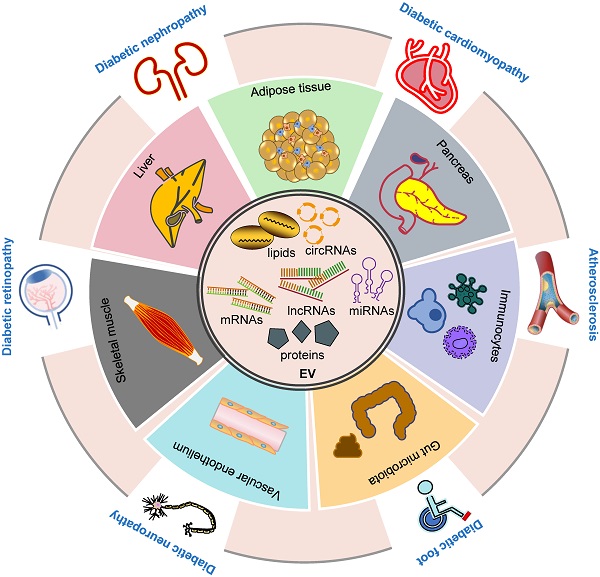
Inter-organ crosstalk mediated by EVs in the pathogenesis of DM and diabetic complications. EVs contain different proteins, RNAs, DNAs and lipids (inner circle). EVs participate in the development of DM and its complications via multiple ways. EVs derived from various tissues, including adipose, liver, pancreas, skeletal muscle, immunocytes, vascular endothelium and gut microbiota, play a role in the development and progression of DM (inner ring). Moreover, these EVs are involved in the pathogenesis of diabetic complications including diabetic foot, cardiomyopathy, nephropathy, retinopathy, neuropathy and atherosclerosis (outer ring). Abbreviations: circRNAs: circular RNAs; DM: diabetes mellitus; EV: extracellular vesicle; lncRNAs: long noncoding RNAs; miRNAs: microRNAs.
MV generation
MV generation consists of two crucial steps: plasma membrane blebbing and scissoring. Plasma membrane rearrangement involving lipid and protein composition remodeling is the first essential step for membrane budding, and is believed to be a calcium (Ca2+)-dependent process [64]. A group of Ca2+dependent enzymes, including flippases, floppases, and lipid scramblases, are involved in the rearrangement of membrane phospholipids [65]. Mechanistically, phospholipid redistribution and maintenance can induce membrane lipid asymmetry and alter membranous curvature [66, 67]. Besides lipid redistribution, unlocking the plasma membrane-cytoskeletal anchorage is necessary for membrane blebbing and vesiculation. In this respect, calpain, a Ca2+activated cysteine protease, can disrupt the attachment between the plasma membrane and cytoskeleton by cleaving several cytoskeletal components under the plasma membrane, such as actin and filamin [68, 69]. However, our understanding of MV generation is limited and further mechanistic investigation is required.
In addition to the EV biogenesis machinery, recent studies suggest that several types of cell death, such as apoptosis, necroptosis, pyroptosis, and neutrophil extracellular trap formation (NETosis), are associated with EV generation, indicating the involvement of additional sophisticated mechanisms modulating EV biogenesis [70-76]. EVs secreted by necroptotic cells mediate MLKL release, which can, in turn, serve as a self-control mechanism of necroptosis [77]. These findings collectively indicate that EVs can function as a specialized intra- and inter-cellular messaging system, highlighting the importance of illustrating EV generation mechanisms.
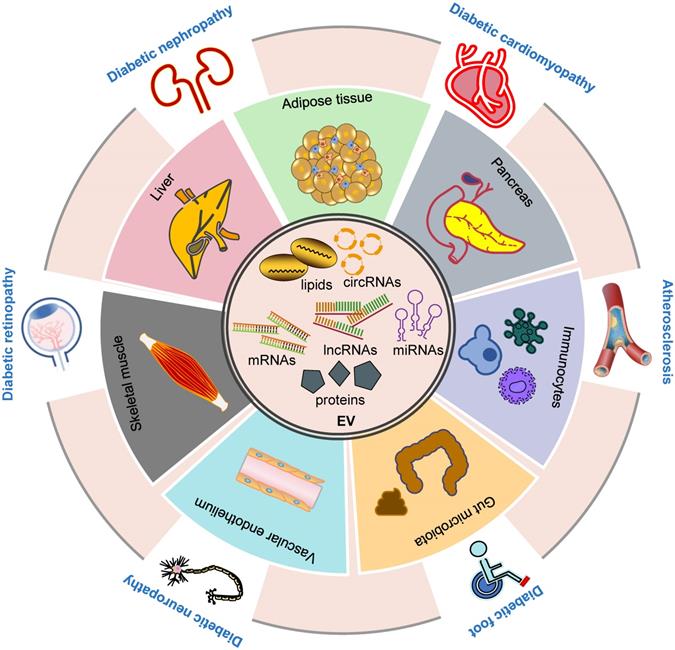
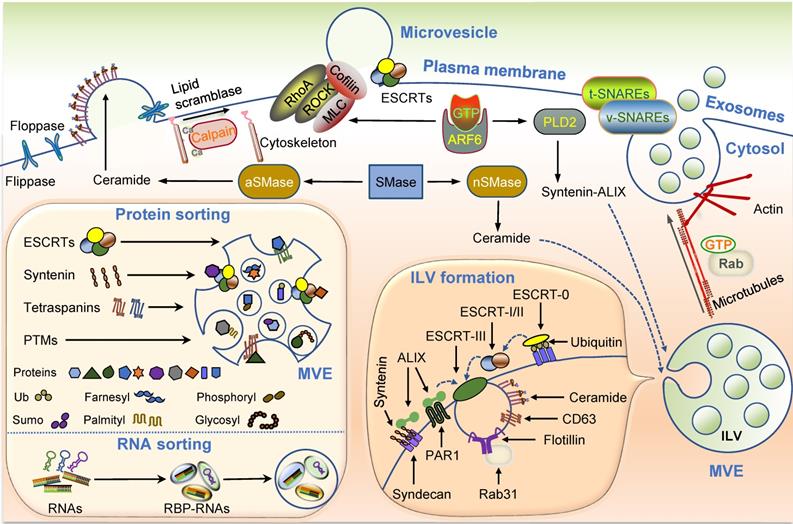
EV biogenesis and cargo sorting. Microvesicles and exosomes are two major categories of EVs. Microvesicles are released directly from plasma membrane budding and shedding. Exosomes are generated by inward budding of endosomes, known as MVEs, which fuse to plasma membrane, and are followed by the release of exosomes. Multiple molecules are implicated in the biogenesis of microvesicles and exosomes, such as ESCRT complexes and related proteins, ceramide, SMase, syntenin, syndecan, calpain, Rab GTPases, and so on (see text). Exosomes contain different types of proteins and RNAs, whose sorting are modulated by several molecules, including ESCRT complexes, syntenin, tetraspanins, and RBPs. PTMs on certain proteins also have a role in the sorting of exosomal cargos. Abbreviations: aSMase: acid sphingomyelinase; ESCRT: endosomal sorting complex required for transport; EVs: extracellular vesicles; ILV: intraluminal vesicle; MVE: multivesicular endosome; nSMase: neutral sphingomyelinase; PTMs: post-translational modifications; RBP: RNA binding protein; SMase: sphingomyelinase, SNARE: soluble N-ethylmaleimide-sensitive fusion attachment protein receptor; t-SNAREs: target-membrane SNAREs; v-SNAREs: vesicle-membrane SNAREs.
Sorting mechanism of EVs
Bioactive molecules, including proteins, DNAs, mRNAs, non-coding RNAs (ncRNAs), and metabolites, are encapsulated in EVs. Accumulating evidence suggests that cargoes are not randomly packaged into EVs or simply replicate the composition of their parental cells [78]. Because of the significance of cargoes in signal communication, the mechanisms of cargo sorting of EVs are central to shed light on the physiological and pathological functions of EVs and their therapeutic implications. Although these mechanisms are far from being fully elucidated, recent advances provide exciting insights into this topic.
Recent studies have provided some clues on the sorting of proteins. First, ESCRT components and their related proteins can recruit exosomal cargoes through direct molecular interactions. For example, the ESCRT-I component TSG101 can recruit BAG6 into EVs, possibly playing a key role in directing EV proteins [79]. In addition, the noncanonical ESCRT-dependent syntenin pathway also contributes to the sorting of specific exosomal cargoes, including LMP1 and KRS [80, 81]. Second, common protein markers of EVs, particularly tetraspanins, have been suggested to account for sorting a great proportion of the exosomal proteins [82-85]. High-throughput proteomic analysis of potential proteins interacting with tetraspanin-enriched microdomains revealed a significant overlap between the tetraspanin interactome and exosomal proteome, highlighting tetraspanins as important sorting machinery for protein inclusion into exosomes [82]. Third, specific post-translational modifications (PTMs) seem to be emerging determinants for protein sorting in EVs, such as ubiquitylation [86-91], sumoylation [92], palmitoylation [93-96], farnesylation [97], phosphorylation [98-100], glycosylation [101-103], and lipidation [103]. For instance, there was a 60% reduction of total protein levels in EVs derived from ubiquitin-like 3 (UBL3)-knockout mice, and UBL3 could function as a PTM factor by directly interacting with more than 1,200 proteins [86]. Also, ESCRT components HRS, STAM, and TSG101 with their ubiquitin-binding domains might participate in ubiquitination [104]. These observations emphasize the significance of ubiquitination, one of the most common PTMs, in sorting EV protein cargoes. Interestingly, ubiquitinated proteins have also been detected in the EVs secreted by insulin-secreting β cells, indicating a potential involvement in EV-associated islet cell dysfunction and T2D pathogenesis [105].
In addition to protein cargo, EVs carry a rich diverse RNA cargo, involved in many EV functions. The enrichment of distinct RNAs in EVs responsive to different cellular statuses relies on the sophisticated RNA sorting system. Recent studies suggest that the selective sorting of RNA in EVs is attributed mainly to RNA binding proteins (RBPs), accounting for about 25% of the protein content in EVs [106,107]. RBPs usually recognize RNAs with specific “tags”, such as certain motifs, modifications, structures, or sequences, and sort them into EVs [107,108]. For instance, hnRNPA2B1, a well-known regulator of RNA metabolism, can package specific miRNAs (miR-198, miR-30b-3p) and long noncoding RNAs (lncRNAs) (AFAP1-AS1, LNMAT2) into EVs by the direct interaction between its RNA-binding domains and the GGAG motif of the RNAs [109-113]. Besides hnRNPA2B1, other members of the hnRNP family, including hnRNPA1, hnRNPC1, hnRNPH1, hnRNPK, hnRNPQ, and hnRNPU, have been implicated in RNA sorting and enrichment in EVs [114-120]. Additionally, YBX1 (miR-223 in HEK293T cells), human antigen R (HuR) (miR-122 in human hepatic cells), and other RBPs have been reported to play a role in the selective miRNA enrichment in EVs [121-123].
The exploration of EV cargo sorting machinery is not restricted to EVs per se. An exciting correlation of autophagy with EV biogenesis and content loading has been recently reported, in which the LC3-conjugation machinery is proposed to govern the RBP capture and thus specify RNAs in secreted EVs [124-126], adding another layer of complexity to EV cargo sorting. In summary, EVs are emerging as an important mediator of intercellular communication. Elucidation of the mechanism underlying EV generation and content packaging has been an active area of research.
EV biogenesis machinery in DM and diabetic complications
EVs are crucial information transmitters between original and recipient cells, and their abnormalities contribute to the development of DM and diabetic complications. Exploring the mechanisms underlying EV biogenesis and cargo sorting is critical in developing novel therapies for various diseases. So far, the role of EV biogenesis and sorting machinery in diabetic pathology has not been systemically reviewed. Here we summarize current knowledge about the EV machinery involved in the pathogenic process of DM and its complications (Table 1 and Figure 3).
EV generation
ESCRTs
ESCRT complexes participate in the generation of the majority of EVs. Multiple components of ESCRT complexes have been shown to play a role in various metabolic processes, especially glucose and lipid metabolism, indicating their potential involvement in DM and diabetic complications. Therefore, it is a reasonable assumption that EVs may partially mediate ESCRT functions in metabolism and metabolic diseases, albeit direct evidence is currently limited.
ESCRT complexes are involved in the transportation of lipid droplets and translocation of GLUT4 and glycogen synthase kinase 3β (GSK3β) in adipocytes, thereby mediating the regulation of neutral lipids micro-autophagy consumption, adipogenesis, and insulin-stimulated glucose uptake [213-215]. Disruption of these cellular biological processes is involved in the pathogenesis of DM. Lipotoxicity, a common risk factor for IR and T2D, can induce TSG101 expression in adipocytes and thus promote the biogenesis of exosomes [216]. Subsequently, TSG101 upregulation triggers the sorting of CD36 into EVs, which then are delivered into hepatocytes and evoke hepatic lipid accumulation [216]. Furthermore, several factors interacting with ESCRT components may regulate EV formation, such as MLKL and HSP20 [217-219]. MLKL, a critical factor involved in plasma membrane disruption and necroptosis, is upregulated in multiple tissues, including the adipose, liver, muscle, kidney, and cardiomyocytes under diabetic conditions [220-224]. MLKL can engage in the biogenesis of both exosomes and MVs by binding ESCRT proteins (TSG101, MVB128, VPS28, VPS37A, VPS25, CHMP3, CHMP4B, and CHMP2A) [218,219]. Interestingly, MLKL can also regulate insulin sensitivity in diabetic mice independent of its proinflammatory and necroptotic roles [220]. These observations indicate that non-necroptotic functions of MLKL might be mediated by its effect on EV formation.
In contrast to MLKL, HSP20 is downregulated in T1D and T2D and its reduction is considered a primary driver for DM-induced organ damage. HSP20 function, at least partially, is attributed to its regulatory activity on exosomes. Specific overexpression of HSP20 in cardiomyocytes can increase the generation/secretion of exosomes enriched in HSP20, p-AKT, survivin, and SOD1 through interacting with TSG101, thereby attenuating cardiac dysfunction, hypertrophy, and microvascular rarefaction under diabetic conditions [217,225]. Besides the role of ESCRT components in metabolism and metabolic disease, it is anticipated that the crosstalk between ESCRTs and EVs may be involved in the pathogenesis of DM and its complications.
Expressions and implications of EV biogenesis and sorting machinery under diabetic conditions.
| Genes | Level/activity [Reference] | Sample: resource | Function |
|---|---|---|---|
| aSMase | ↑ [127-129] | AT: T2D patients with FLD, ob/ob mice | Promoting thrombosis and inflammation |
| ↑ [130,131] | Serum: T2D patients, db/db mice | Promoting endothelial dysfunction | |
| ↑ [132,133] | Plasma, RECs, CD34+ CACs: T2D patients | Promoting inflammation and CACs migration | |
| ↑ [134] | RPECs: STZ rats | Impairing mitochondrial function | |
| ↑ [135] | Liver and brain: HFD mice | Promoting hepatic IR and neurodegeneration | |
| ↑ [136] | Kidney: GK rats | Promoting ER stress | |
| nSMase | ↑ [127-129] | AT: T2D patients with FLD, ob/ob mice | Promoting thrombosis and inflammation |
| ↑ [135] | Liver, brain: HFD mice | Promoting hepatic IR and neurodegeneration | |
| ↑ [137] | Skeletal muscle: Wistar fatty rats | Promoting IR in the muscle | |
| ↑ [138] | Vastus lateralis muscle: obese IGT patients | UD | |
| ↑ [139,140] | Islet β cells: Akita mice | Promoting β cell apoptosis | |
| ↑ [141] | Atrial appendage: obese T2D patients | UD | |
| Sdc1 | ↑ [142] | Liver: obese Zucker fa/fa rats | Promoting hepatic IR |
| ↑ [143-145] | Neutrophils, serum: T2D patients | UD | |
| ↓ [146] | Serum, small intestine: STZ mice | Promoting epithelial barrier damage | |
| ↑ [147,148] | Plasma, serum: T1D DN patients | Promoting inflammation and microalbuminuria | |
| ↑ [149,150] | Vitreous fluid: PDR Patients | Promoting angiogenesis | |
| ↓ [151] | Skeletal muscle, heart: HFD ob/ob mice | UD | |
| Sdc4 | ↑ [152,153] | Heart, skeletal muscle: STZ rats | Promoting cardiac dysfunction |
| ↑ [154] | Kidney: KK/Ta mice | UD | |
| ↓ [151] | Skeletal muscle, heart: HFD ob/ob mice | Promoting growth factor resistance | |
| HPSE | ↑ [155-158] | Islet: NOD/Lt mice, T1D patients, STZ mice | Promoting β cell death |
| ↑ [159] | Serum: obese patients with prediabetes | Promoting endothelial injury and inflammation | |
| ↑ [160,161] | Urine, plasma: T2D patients | UD | |
| ↑ [162-167] | Kidney: STZ mice and rats, DN patients | Promoting renal damage, protein excretion | |
| ↑ [150,168,169] | Vitreous fluid, serum, retina: PDR patients, STZ rats | Promoting inflammation, angiogenesis, and subendothelial barrier damage | |
| ↑ [146] | Serum, small intestine: STZ mice | Promoting epithelial barrier damage | |
| ↑ [170] | Carotid artery: DM patients, STZ rats | Promoting atherosclerosis | |
| Calpain | ↑ [171] | Heart: STZ rats | Promoting apoptosis |
| ↑ [172-175] | Heart: HFD, STZ, OVE26 mice | Promoting myocardial hypertrophy, and fibrosis | |
| ↑ [176] | Aortas: STZ and OVE26 mice | Promoting ROS and peroxynitrite production | |
| ↑ [177,178] | Platelet: T2D patients, STZ mice | Promoting platelets hyperaggregability | |
| ↑ [179,180] | Plasma: T2D patients | Promoting platelet activation and inflammation | |
| ↑ [181] | Platelet: T2D patients | Promoting MVs release and inflammation | |
| ↑ [182] | Dorsal root ganglion: STZ rats | Promoting oxidative stress and inflammation | |
| ↑ [183] | Penis: STZ mice | Promoting erectile dysfunction | |
| ↑ [184] | Lens epithelial cells: DR patients | UD | |
| Calpain-1 | ↑ [185] | Heart: STZ rats | Promoting oxidative stress and apoptosis |
| ↑ [186,187] | Vascular mesentery: STZ and ZDF rats | Promoting endothelial inflammation | |
| ↑ [188] | Retina: STZ rats, HFD rats | Promoting retinal ganglion cell death | |
| Calpain-10 | ↑ [189] | Islet: T2D patients | Biomarker for islet dysfunction |
| ↑ [190] | Kidney: STZ rats, HFD rats | Promoting apoptosis and renal dysfunction | |
| ↓ [191] | Kidney: STZ rats, ob/ob mice | Promoting apoptosis and renal dysfunction | |
| SNARE | a ↓ [192-195] | Islet: T2D patients, GK rats, ZDF rats | Impairing insulin secretion |
| b ↓ [196] | AT: STZ-NA rats | Promoting IR | |
| c ↑ [197] | Skeletal muscle: Zucker rats, STZ rats | Promoting IR | |
| d ↓ [198] | Hippocampus: STZ rats | UD | |
| e ↑ [199] | Serum: T1D patients | Promoting insulitis as autoantigen | |
| CD63 | ↑ [200,201] | Platelets: T2D patients | UD |
| ↑ [202] | Kidney: DN patients | Promoting renal cell apoptosis | |
| CD82 | ↑ [203] | Skin: DM patients | Promoting chronic inflammation |
| HuR | ↑ [204-207] | Kidney: DN patients, db/db mice, STZ rats | Promoting pyroptosis, inflammation, and EMT |
| ↑ [208] | Retina: STZ rats | Promoting angiogenesis | |
| ↑ [209] | BMMØ, heart: db/db mice | Promoting cardiac fibrosis and dysfunction | |
| ↑ [210] | Heart: diabetic cardiomyopathy patients | Promoting pyroptosis and inflammation | |
| hnRNPK | PTMf ↑ [211] | Islet: db/db mice | Promoting oxidative stress and apoptosis |
| ↓ [212] | Kidney: Akita mice | Promoting RAS activation and hypertension |
AT: adipose tissue; BMMØ: bone marrow‐derived macrophage; CACs: circulating angiogenic cells; DN: diabetic nephropathy; FLD: fatty liver disease; GK rats: Goto-Kakizaki rats; HFD: high-fat diet; IGT: impaired glucose tolerance; HPSE: heparanase; NOD/Lt mice: nonobese diabetic mice harboring a hybrid rat insulin-promoter/SV40 large T-antigen gene spontaneously develop β-cell adenomas; OVE26: FVB(Cg)-Tg(Ins2-CALM)26OveTg(Cryaa-Tag)1Ove/PneJ transgenic mice; PDR: proliferative diabetic retinopathy; PTM: posttranslational modification; RAS: renin-angiotensin system; Sdc1: syndecan 1; Sdc4: syndecan 4; SNARE: soluble N-ethylmaleimide-sensitive fusion attachment protein receptor; STZ+NA: streptozotocin+ nicotinamide; UD: undetermined; ZDF: Zucker fat diabetic. a: synaptotagmin, VAMP-2, syntaxin-1A and -2 and SNAP-25; b: SNAP23, syntaxin-4 and VAMP-2; c: VAMP-2, syntaxin-4; d: syntaxin-1; e: VAMP2; f: phosphorylation.
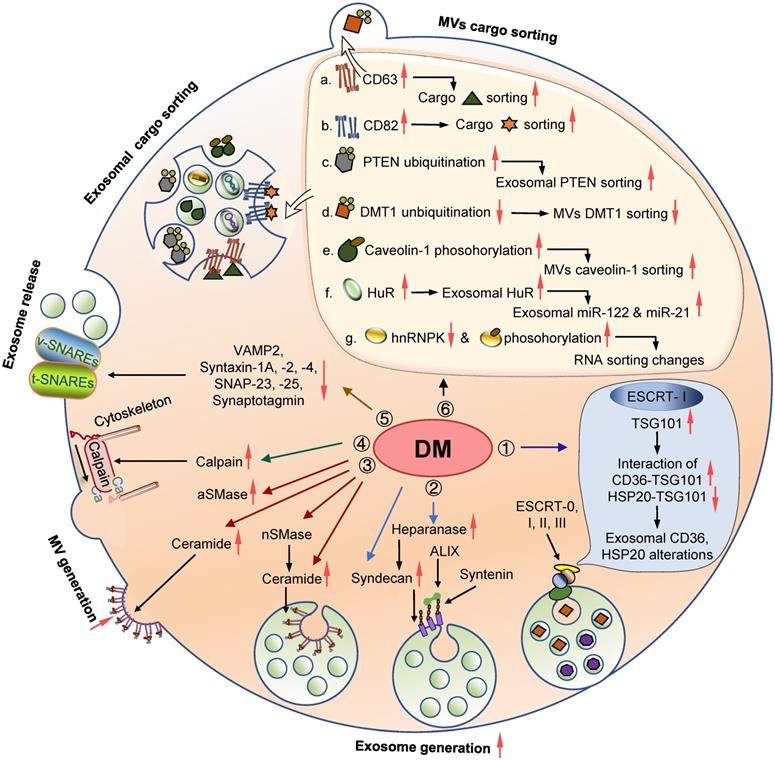
Involvement of the EV biogenesis and cargo sorting machineries in DM and diabetic complications. Diabetic conditions trigger the alteration in the expression and activity of the molecules involved in the process of EV biogenesis and cargo sorting. 1. Lipotoxicity induces TSG101 expression and influences its interaction with CD36 and HSP20, leading to their exosomal sorting dysregulation; 2. Elevated syndecans and heparinase in DM animals and patients can potentially activate of the syntenin-syndecan-ALIX pathway and promote exosomes biogenesis; 3. Elevated ceramide levels and nSMase/aSMase expression and activity may induce EV generation; 4. High glucose may impact the expression and activity of calpain 1 and 2, leading to elevated microvesicle generation; 5. Reduced SNARE components in diabetic conditions may influence exosomes release; 6. Altered expression of some regulators associated with EVs cargo sorting, as well as certain PTMs of specific proteins, may also affect EVs proteome and RNA profile under DM conditions. Abbreviations: aSMase: acid sphingomyelinase; DM: diabetes mellitus; ESCRT: endosomal sorting complex required for transport; EVs: extracellular vesicles; HuR: human antigen R; MV: microvesicle; nSMase: neutral sphingomyelinase; SNARE: soluble N-ethylmaleimide-sensitive fusion attachment protein receptor; t-SNAREs: target-membrane SNAREs; v-SNAREs: vesicle- membrane SNAREs.
Ceramide and SMases
EVs are enriched in cholesterol and sphingolipids, such as sphingomyelin and hexosylceramide, and have a remarkable ceramide enrichment. Neutral sphingomylinase (nSMase) and acid SMase (aSMase) potentially mediate the budding of vesicles into MVEs and plasma membrane, respectively, and thus promote the generation of exosomes and MVs [55,226]. Accumulating evidence suggests a role of the SMase-ceramide pathway in the pathogenesis of DM and its complications, although direct experimental data supporting EV contribution are lacking [127-141].
An elevated level of circulating ceramide is associated with the severity of IR in obesity [227]. Specifically, membranous ceramide can influence the structural organization of plasma membrane and insulin receptor translocation, impairing insulin signaling [228, 229]. In parallel, ceramide metabolism is over-represented in the plasma and markedly associated with the progression of T1D, consistent with its crucial role in immune regulation [230]. Ceramide also serves as a critical lipotoxic mediator and drives the development of vascular dysfunction and damage [231-233]. Similarly, abnormality and dysfunction of both aSMase and nSMase have been reported in DM and its complications (Table 1) [127-141]. The pathogenic roles of these enzymes have generally been attributed to mediating sphingomyelin hydrolysis and ceramide in the AT, retina, liver, kidney, and other tissues. For example, aSMase and nSMase are increased in obese epididymal fat, along with altered levels of sphingomyelin, ceramide, and downstream ceramide metabolites in AT and plasma, promoting the expression of prothrombotic and proinflammatory genes and subsequently contributing to obesity-associated metabolic and cardiovascular diseases, such as atherosclerosis [127-129]. Thus, inhibition of SMase-ceramide is considered an effective therapy for IR and DM by inhibiting inflammatory responses [131,132,234]. However, the contribution of EVs in SMase-ceramide-mediated functions remains unknown and awaits future investigation.
Syndecan-syntenin pathway
The syndecan-syntenin-ALIX axis has been shown to regulate the formation of ILVs and exosomes [235]. Syndecan, syntenin, and ALIX co-exist in a subset of exosomes. The PDZ domains of syntenin have a high affinity to syndecan, which recruits syntenin to membranes, while the N-terminal domain of syntenin directly interacts with ALIX. Heparanase, the only catalytic enzyme of syndecan, trims its heparan sulfate and significantly promotes exosome budding and generation [236]. Syntenin can also recognize ligands with PDZ-binding motifs, which are specifically sorted into the exosomes. For example, syntenin directly binds the exposed PDZ-binding motif of KRS and targets it into exosomes, thereby contributing to caspase-8-triggered inflammation [80]. These recent findings collectively suggest an important role of the syndecan-syntenin pathway in the biogenesis and function of exosomes.
Syndecan is a ubiquitous transmembrane protein and plays important physiological and pathological roles in development, differentiation, and human diseases, including DM and its complications (Table 1) [142-154]. Generally, syndecan, particularly syndecan-1 and syndecan-4, are upregulated in diabetic humans and animals compared with euglycemic controls. Syndecan-1 is induced in the liver of obese Zucker fa/fa rats and potentially promotes lipid uptake, resulting in hepatic IR and dyslipidemia [142]. Moreover, elevated syndecan-1 expression is associated with body mass index (BMI) and serum apoA1 in T2D, suggesting its involvement in vascular inflammation and injury [143-145]. In T1D, syndecan-1 expression is positively correlated with microalbuminuria and inflammatory indicators, implying a role in DN pathogenesis [147, 148]. Syndecan-4 is also increased in the heart and kidney of diabetic mice and rats and has a role in diabetic cardiomyopathy and DN [152-154].
The expression and activity of heparanase, a unique endoglycosidase known to degrade heparan sulfate chains, including those of syndecan-1, are increased under diabetic conditions [155-170]. Notably, heparanase derived from insulitis leukocytes can degrade heparan sulfate of β cells and thus promote islet cell death in T1D. In mice, inhibition of heparanase can effectively delay the onset of T1D induced by STZ and NOD [155-158]. Also, the level of heparanase in the circulation and urine is positively correlated with glucose and HbA1c [159-161] and is also closely associated with albuminuria in DM, indicating its crucial role in diabetic renal injury [162-167]. Specifically, heparanase can potentially lead to the loss of heparan sulfate in the glomerular basement membrane, induce glomerular inflammation, and promote renal fibrosis in DN [150,162-167]. Similarly, elevated heparanase has also been implicated in diabetic microangiopathies, such as diabetic retinopathy (DR) [150,168,169], and carotid artery atherosclerosis [170].
Together, these findings highlight the key roles of syndecan and heparanase in the pathogenesis of DM and its complications. Given the importance of the syndecans-syndecan-ALIX pathway in exosome biogenesis and cargo sorting, it is conceivable that exosomes could, at least partially, mediate syndecans and heparanase functions under diabetic conditions despite a lack of direct evidence.
Calpain
Calpains are a superfamily of Ca2+-dependent intracellular cysteine proteases and have a role in generating MVs via remodeling the cytoskeleton and facilitating the budding of the plasma membrane. Emerging data suggest that calpains, particularly calpain 1, 2, and 10, contribute to the genetic causes and biochemical defects of T2D, albeit a clear involvement of EVs in calpain-modulated T2D phenotypes remains elusive [171-191].
CAPN10 encoding calpain 10 is the first positionally cloned gene for T2D [237-244]. Its polymorphisms are closely associated with chronic diabetic vascular complications, such as DN, DR, diabetic neuropathy, and cardiovascular diseases [245]. By utilizing multiple calpain inhibitors, recent studies have uncovered the function of calpains in IAPP-mediated cell dysfunction, insulin secretion in islet cells, insulin-stimulated glucose uptake, and glycogen synthesis in adipocytes and skeletal muscle cells [246,247]. Notably, O-GlcNAcylation modification may facilitate the exosomal release of calpain 2 in hepatocytes under the high glucose (HG) condition [248]. Exosomal calpain 2 can cleave the ectodomain of the insulin receptor and thus impair insulin action, providing a credible link between calpain 2, exosomes, and T2D etiology [248]. Moreover, activation of calpain 1 and 2 contributes to accelerated atherothrombosis development in T2D by regulating different substrates in platelets and ECs [177-181]. Since MVs loaded with elevated calpain 1 can be delivered to ECs and induce vascular inflammation [180,181], MVs might contribute to the phenotypes mentioned above.
SNARE proteins
The assembly of the SNARE complex mediates MVE fusion with the plasma membrane and allows exosome secretion into extracellular space. The role of SNAREs in glucose metabolism and T2D pathology has been extensively reported, although the involvement of EVs in the SNAREs-mediated effects remains unclear and awaits further investigation [192-198].
SNAREs fundamentally maintain glucose homeostasis via participating in insulin and glucagon-like peptide 1 (GLP-1) secretion and GLUT4-mediated glucose uptake [249-251]. Many SNARE components, including VAMP2, syntaxin-1A, -2 and -4, SNAP-23 and -25, and synaptotagmin, are decreased in human and rodent T2D islets [192-195], and are associated with β cell hypertrophy and defective insulin secretion. Abnormal expression of SNARE proteins is implicated in IR in insulin-responsive tissues like the AT and muscle, probably due to impaired GLUT4 intracellular translocation [196, 197]. In addition to dysregulated expression, abnormal location of SNAREs may have a role in systemic metabolism and T2D development. For example, abnormal sorting of VAMP2 into lipid droplets leads to inadequate trafficking of GLUT4 on the plasma membrane and IR in adipocytes [252]. Additionally, VAMP2 is elevated in serum and possibly induces autoimmune response and consequent insulitis, suggesting it as a potential autoantigen of T1D [199].
EV cargo sorting
Protein cargo sorting
Proteomic analysis has uncovered that diabetic condition alters the protein composition of EVs of different origins [253-257]. Therefore, the cellular expression and function of EV protein sorting machinery in response to diabetic stimulations could be attributed to proteomic alterations of EVs in DM. In addition to ESCRTs mentioned above, tetraspanins CD63 and CD82 that participate in EV protein cargo sorting [83-85], have also been implicated in developing DM and its complications [200-203, 258].
Under glucolipotoxic conditions, CD63 mediates stress-induced nascent granule degradation of insulin in β cells, thereby mitigating insulin secretion and accelerating T2D [258]. Moreover, AGEs can induce the expression of CD63, the marker of platelet activation, and the CD63+ platelet level is elevated in T2D patients with progression of carotid wall thickness [200, 201]. CD63 is also upregulated in diabetic patients with DN and contributes to renal cell death by inhibiting the Wnt/β-catenin signaling pathway [202]. CD82 is highly expressed in diabetic skin tissue and possibly associated with diabetic chronic inflammatory and hypoxic state [203], albeit its precise role and mechanism in diabetic dermopathy remain elusive. Taken together, altered expression and function of CD63 and CD82 under diabetic conditions may contribute to selective enrichment of cargoes in EVs and consequently induce changes in the EV proteome profile.
Additionally, several PTMs of proteins are required for their sorting in EVs, possibly involved in ubiquitination of PTEN and DMT1, phosphorylation of caveolin 1, and other diabetic pathological changes [259-265]. Specifically, the concentration of polyubiquitinated PTEN, which plays an important role in regulating renal fibrosis, is increased in the serum and urine of DN patients [260]. It has been reported that ubiquitination at lysine 13 of PTEN is required for the selective enrichment of PTEN in exosomes [90], which may partially mediate the pathological role of PTEN in DN. Similarly, the release of DMT1 from MVs is mediated by Nedd4-2 ubiquitin ligase, suggesting a role of ubiquitination in the cargo sorting of EV proteins in the gut explant [91]. Moreover, in vivo and in vitro studies have found that HG leads to elevated DMT1 levels in intestinal epithelial cells partially by inhibiting DMT1 ubiquitination and promoting DMT1 membrane translocation, resulting in increased iron uptake and iron loading [259]. Together, it is reasonable to hypothesize that the ubiquitinated DMT1 located at the plasma membrane is sorted into the budding vesicles and secreted into the extracellular environment. In contrast, the deubiquitinated DMT1 is trapped within cells, leading to elevated expression of DMT1 in the diabetic intestine. Also, phosphorylation of caveolin-1, a scaffolding protein involved in protein sorting of MVs [100], has been shown to be important in DN development [261-265]. Hypoxia induces the phosphorylation of caveolin-1 that can directly interact with hnRNPA2B1, facilitating the sorting of hnRNPA2B1 and its-associated miRNAs into MVs [100]. Under diabetic conditions, HG promotes caveolin-1 phosphorylation in podocytes and glomerular mesangial cells (GMCs), resulting in renal cell apoptosis, inflammation, EMT, and glomerular matrix accumulation [261-265]. Moreover, circulating MVs derived from diabetic rats can be delivered into vascular ECs and lead to elevated caveolin-1 levels in recipient cells [266].
RNA cargo sorting
RNA cargo affects many EV functions in various diseases, including DM and diabetic complications. Diabetic conditions induce alterations of mRNAs, miRNAs, lncRNAs, and circular RNAs (circRNAs) in EVs [267-270], primarily due to the dysregulation of numerous RBPs. HuR, an extensively studied RBP, is involved in EV RNA sorting by directly recognizing and binding RNAs bearing AU-rich elements, such as miR-122 and miR-21 [121]. Both HuR and its associated miRNAs have been implicated in the diabetic heart, DN, and DR developing [204-210].
In the context of DM, target proteins post-transcriptionally modified by HuR have been shown to play a role in the pathogenesis of diabetic complications like DN, DR, and diabetic cardiomyopathy [204-210]. For instance, HuR can post-transcriptionally modulate the expression of several regulators involved in renal injury, such as claudin-1, IL-17, NOD2, NLRP3, CTGF, TGF-β1, and Snail [204-207]. Similarly, pyroptosis, inflammation, oxidative stress, and EMT have been mechanically involved in HuR-mediated DN development and progression. Moreover, it has recently been shown that HuR can be delivered into cardiomyocytes and thus elicit inflammatory and profibrogenic responses, highlighting its importance in the diabetic heart [209].
It has been reported that miR-122 and miR-21, two miRNAs sorted by HuR into EVs [121], have a role in the diabetic heart [271-273]. MiR-21 is significantly decreased in cardiomyocytes of diabetic mice and contributes to diastolic cardiac dysfunction by directly targeting gelsolin and consequent oxidative stress. In contrast, circulating levels of miR-21 and miR-122 are increased in T2D patients with heart failure [272,273], probably resulting from increased EV secretion triggered by HuR upregulation. Thus, miR-21 and miR-122 may be selectively encapsulated in the EVs via HuR and secreted extracellularly, leading to an increase in their extracellular levels while causing a decrease in their intracellular levels, possibly mediating the pathogenic effect of HuR in diabetic cardiomyopathy. Similarly, these two miRNAs could play a role in DN and DR [274-277]. For instance, it has been shown that miR-21 encapsulated in EVs exerts a pro-angiogenic effect on ECs and promotes DR development [278, 279]. Further research is required to clarify whether the HuR function in the pathogenesis of diabetic complications depends on EV-miRNA sorting.
HnRNPK is another RBP involved in the RNA sorting of EV and its phosphorylation can be induced by glucolipotoxicity, a classic metabolic abnormality associated with T2D [211,212]. Phosphorylated hnRNPK can significantly modulate the expression of oxidative and inflammatory genes in β cells [211]. HnRNPK expression is decreased in the kidney of T1D mice and can potentially mediate RAS activation and hypertension in T1D [212]. The altered expression and post-translational modification of hnRNPK might lead to different RNA selection in EVs, possibly contributing to hnRNPK function in DM.
Collectively, it is reasonable to conclude that EVs may mediate specific pathogenic roles of EV generation machinery in the initiation and progression of DM and diabetic complications. Thus, elucidating the association between EV biogenesis and diabetic pathogenesis represents an attractive direction for future investigation, which would pave the way for developing novel targeted therapeutics for DM and diabetic complications.
Roles of EVs in DM
EVs are emerging as novel effectors of intercellular and interorgan communication and play active roles in multiple pathophysiological situations of metabolic modulation like metabolic homeostasis, maintenance, and disturbance. Of note, EV-induced phenotypic and molecular alterations in target cells are often associated with the composition and origin of these microstructures. In this section, we summarize the diverse EV functions in DM pathology and diabetic complications in the context of the cellular origin of EVs.
Adipocytes
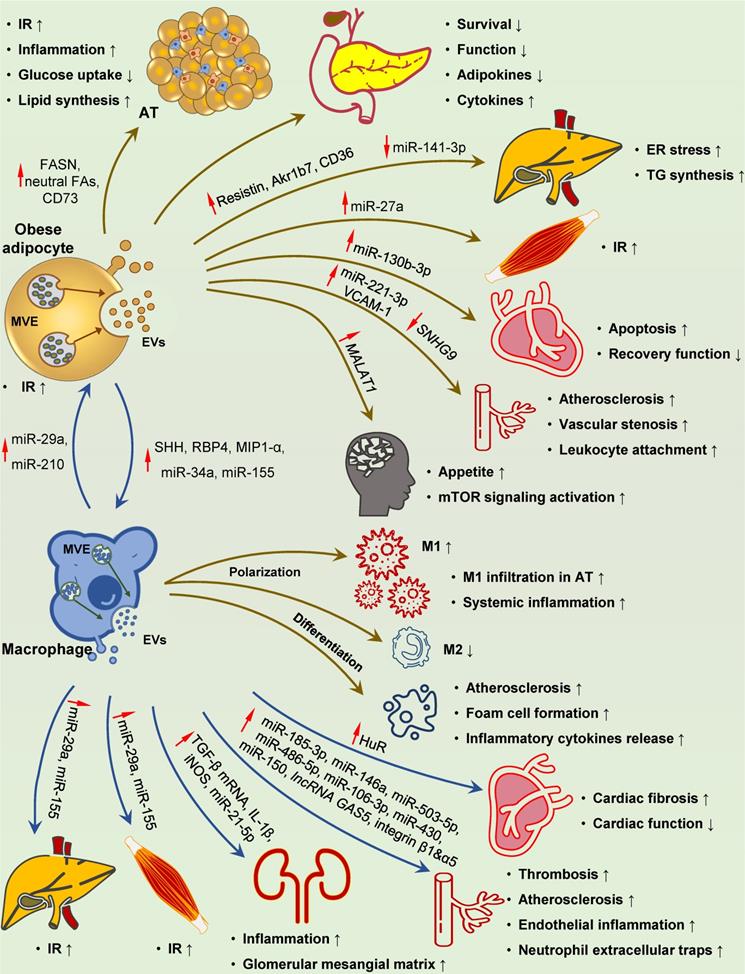
AT is central in regulating systemic insulin sensitivity, hypertrophic adipocyte-induced elevated FFA release, inflammation, and adipokine alterations that are the drivers of the whole-body IR in T2D. Lipogenic stimulus and excess fat expansion promote EV generation in obese adipocytes, which, in turn, contribute to IR and islet cell dysfunction via paracrine effect and/or distant action (Figure 4) [280-285]. These EVs induce lipid droplets deposit by directly delivering neutral fatty acids [286], and promote lipid synthesis by transmitting the key lipid synthesis enzyme FASN, lipogenic-related miRNAs and mRNAs, and CD73 [281-284, 287]. In vitro experiments showed that EVs can impair insulin response and glucose uptake in recipient adipocytes [280]. Besides the paracrine effect on local adipocytes, EVs secreted by adipocytes can result in peripheral IR and metabolic disorder by functioning as adipokine carriers [288]. Hypertrophic adipocyte-derived exosomes loaded with resistin, a canonical obesity-related adipokine, triggered hepatic ER stress and liver steatosis [289]. Several studies have described functional lncRNA (MALAT1), miRNAs (miR-27a, miR-141-3p) and proteins (CD36, and Akr1b7) encapsulated in adipocyte-derived EVs as novel adipokines that exert metabolic modulatory effects on distant organs [216, 290-293]. These newly discovered adipokines are sufficient to induce hepatic lipid accumulation and IR in the liver and skeletal muscle [216, 292, 293]. In addition to peripheral tissues, EV-encapsulated MALAT1 can be transported to pro-opiomelanocortin neurons, increasing appetite and body weight [291]. Additionally, adipocyte-derived EVs have a modulatory effect on the survival and function of distant islets by delivering specific miRNAs [285].
Macrophage infiltration in AT is a hallmark of obesity and contributes to chronic inflammation and subsequent IR [294]. Obese adipocyte-derived EVs have been demonstrated to play a role in recruiting and activating circulating monocytes and polarizing resident macrophages toward the proinflammatory phenotype [287, 295-300]. Interestingly, based on the specific interactions between surface proteins of EVs and recipient cells, EVs are preferentially taken up by circulating monocytes in vivo and promote macrophage activation and IR [52, 295]. In addition, obese adipocyte-derived EVs can shuttle bioactive molecules, such as miR-34a and miR-155, into recipient macrophages, and thus promote pro-inflammatory M1 polarization and inhibit anti-inflammatory M2 polarization [296-298].
EVs can also function as a mode of communication between adipocytes and vascular ECs, which may be dynamically influenced by metabolic status [301], and have a role in cardiovascular complications. EVs derived from obese AT often exert detrimental effects on vascular cells, including ECs, vascular smooth muscle cells (VSMCs), and cardiomyocytes [302-308]. It has been shown that miR-221-3p, miR-130b-3p, lncRNA SNHG9, and VACM-1 within the EVs can result in endothelium inflammation, vascular stenosis, unstable atherosclerotic plaque formation, and impaired cardiac recovery [304-307].
Macrophages
Inflammatory macrophages infiltrated in AT lead to low-grade tissue inflammation, which is the key cause of IR in T2D [309]. EVs derived from obese AT macrophages (ATMs) can serve as systemic inflammation factors and impair insulin signaling in distal organs (Figure 4). Metabolic regulatory miRNAs, such as miR-29a, miR-155, and miR-210, can be carried by EVs and delivered into insulin-responsive cells and organs via paracrine or endocrine routes [310-313]. These miRNAs robustly regulate insulin action on AT, liver, and skeletal muscle and cooperatively modulate systemic glucose homeostasis [310-313].
Macrophage-derived EVs also play a role in DM complications. It has been reported that HG and RAGEs induce EVs production in macrophages [314-318]. Biomolecular cargoes within these EVs, such as IL-1β, iNOS, HuR, miR-21-5p, miR-486-5p, and TGF-β mRNA can be transferred to target cells and subsequently induce renal and cardiac injury and dysfunction [314-319]. In particular, two miRNAs closely related to cardiac fibrosis and diastolic dysfunction, miR-122 and miR-1246 [320, 321], have been shown to be specifically sorted into EVs by the RBP HuR [121, 322], raising the possibility that some pathogenic effects of HuR may be mediated by miRNAs enclosed in macrophage-derived exosomes. The oxidized low-density lipoprotein (oxLDL) is known to induce M1 polarization of macrophages and foam cell formation in the arterial wall, two crucial atherogenic events in DM. Interestingly, recent studies suggest an important role of miRNAs carried by activated macrophage-derived EVs in atherosclerosis [323-332]. Thus, EVs can effectively transmit pathogenic miRNAs to target cells, including VSMCs, ECs, neutrophils, and macrophages, leading to vascular stenosis, dysfunction, and inflammation that promote atherosclerosis and thrombosis (Figure 4) [323-329]. Besides miRNAs, functional factors, including lncRNA GAS5, integrin β1A, and α5, loaded in EVs, also participate in the progression of vascular injury and cardiovascular diseases [331,332].
Macrophage-derived EVs have been implicated in multiple immune response processes [333-335] and can present dead cell-associated auto-antigens to dendritic cells, and activate an autoimmune response [333,336]. Exosomes derived from M1 macrophages can also act on T cells, amplifying Th1 response and aggravating neuritis in Guillain-Barré syndrome [337]. Notably, macrophages infiltrated in islets are the main source of free radicals and pro-inflammatory cytokines, inducing β cell death in T1D [338,339]; however, the potential contribution of EVs in this process awaits further exploration.
Involvement of adipocyte- and macrophage-derived EVs in DM-related pathological changes. Adipocyte-derived EVs play a distinct role at multiple processes in the development of DM-related pathology. These EVs with specific cargoes (FASN, neutral fatty acids, CD73, resistin, Akr1b7, CD36 and miR-27a) can circulate throughout the body and reach their destination for IR development and metabolic disturbance in the adipose, liver and skeletal muscle. Islet inflammation, damage and dysfunction can also be induced by adipocyte-derived EVs. Upon uptake by recipient cells, these EVs can deliver several pathogenic mediators to ECs, hypothalamus and heart (increased miR-221-3p and VCAM-1, reduced SNHG9 to ECs, and increased MALAT1 to hypothalamus), resulting in vascular injury, elevated appetite, and myocardial damage, respectively. SHH-, RBP4-, MIP1-α-, miR-34a- and miR-155-containing EVs taken up by macrophages can promote M1 polarization and foam cell differentiation, while inhibit M2 polarization, leading to localized adipose and systemic inflammation, and accelerated atherosclerosis. Reciprocally, inflamed macrophage-derived EVs carrying elevated miR-210 and miR-29a can be transferred to adipocytes, causing IR in the adipose tissues. EVs containing miR-29a originated from macrophages can also be delivered to the liver and skeletal muscle, leading to IR in target organs. Elevated HuR, integrin β1 and α5, IL-1β, iNOS, TGF-β mRNA, miR-21-5p, miR-185-3p, miR-146a, miR-503-5p, miR-486-5p, miR-106-3p, miR-430, miR-150, and lncRNA GAS5 in these EVs ultimately result in cardiac fibrosis and dysfunction, atherosclerosis, renal inflammation, and glomerular mesangial matrix accumulation. Abbreviations: AT: adipose tissue; ECs: endothelial cells; ER: endoplasmic reticulum; EVs: extracellular vesicles; FAs: fatty acids; HuR: human antigen R; IR: insulin resistance; SHH: sonic hedgehog; TG: triglyceride.
Hepatocytes
Lipid stress under obese conditions leads to abnormal fat accumulation and inflammation in the liver in T2D, contributing to localized and systemic IR and inflammation. EVs derived from hepatocytes with overnutrition participate in this process via paracrine and endocrine actions. For instance, increased geranylgeranylation of Rab27a in hepatocytes promotes vesicle docking toward the plasma membrane and the subsequent EV release into circulation [340]. Specifically, let-7e-5p, with the greatest increase in EVs under a high-fat diet (HFD), can be transferred to adipocytes and increase lipogenesis and adipose expansion through targeting Pgc1α [340]. When taken up by the pancreas, EVs can promote islet cell proliferation and participate in the compensatory response in the early onset of T2D [341]. In addition, these EVs are enriched in proinflammatory molecules, including S1P, TRAIL, integrin β1, ceramide, miR-122, and miR-192-5p, which can induce inflammatory cell infiltration and inflammation by attracting circulating monocytes and polarizing macrophages toward pro-inflammatory differentiation in the liver [342-348].
Moreover, EVs derived from lipid-stressed hepatocytes can mediate the crosstalk between the liver and cardiovascular system and contribute to related complications. EVs shed by steatotic hepatic cells contain elevated miR-1 and miR-122, which can induce expression of adhesion molecules and diminish mitochondrial activity in target ECs and cardiomyocytes, resulting in atherosclerosis aggravation and cardiac function impairment [349, 350].
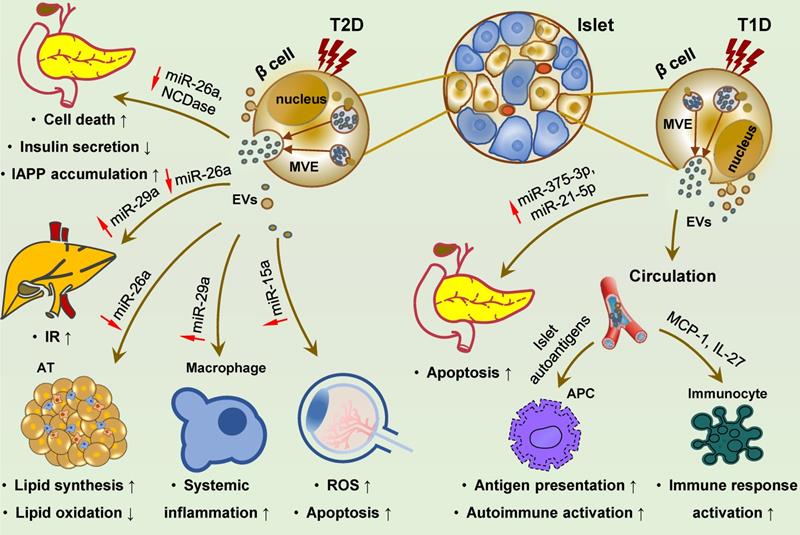
Islet cells
Insulin-releasing cells are considered the main effectors of autoimmune response, and their destruction is the main cause of T1D. In the past few years, EVs derived from islet cells under inflammatory stress have underscored their pathogenic function in autoimmune insulitis of T1D. Inflammatory cytokines induce islet autoantigen enclosure [351-354] and RNA profile alteration [270,355] in these EVs. Several known canonical diabetic antigens, for e.g., GAD65, IA-2, ZnT8, GLUT2, and proinsulin, as well as the newly identified Gag antigen, can be effectively delivered to antigen-presenting cells (APCs), leading to T cell activation and autoimmune response [351-354]. In addition, these EVs can transfer bioactive RNAs and proinflammatory molecules, such as MCP1 and IL-27, to immune cells [355-359], and thus might account for the activation of recipient immune cells, such as dendritic cells, macrophages, B lymphocytes, and T lymphocytes. EVs derived from inflamed islet cells may also impose a pro-apoptotic effect on neighboring β cells by paracrine action and horizontal transmission of pathogenic miRNAs (e.g., miR-375-3p and miR-21-5p) associated with pancreas injuries [360].
Bioactive miRNAs loaded in pancreatic β cell-derived exosomes can function as endocrine factors, whose level changes influence glucose homeostasis and T2D development. HFD, the common risk factor for obesity and T2D, can affect specific miRNA levels in β cell-derived EVs, such as an increase in miR-29 and a decrease in miR-26a [361-363]. These exosomal miRNAs can be transferred to peripheral tissues and impair insulin signaling in recipient cells, and also be transmitted to circulating monocytes and macrophages and induce chronic low-grade inflammation [361-363]. MiR-26a is widely expressed in human tissues and involved in the pathogenesis of various human diseases, including DM and its associated disorders [364-369]. Under T2D conditions, miR-26a expression is decreased in β cells, subsequently reducing circulating exosomal miR-26a, impairing insulin sensitivity and metabolic homeostasis in the liver and AT, thereby promoting the development of T2D [363]. In contrast, exosomal miR-29s and miR-29a derived from islet cells are induced by FFAs stimulation and inflammation [361,362]. These two exosomal miRNAs are delivered to the liver and inflammatory cells, resulting in hepatic IR and systemic metabolic dysregulation and inflammation [361,362]. Moreover, islet cell-derived EVs seemingly contribute to pancreatic failure in T2D and thus promote disease progression. Mechanistically, EVs may potentially facilitate IAPP aggregation and amyloid formation in pancreatic cells, resulting in cell death [370]. Additionally, pancreatic cell-derived EVs have a role in DM complications. HG stimulation significantly increases miR-15a levels in exosomes isolated from pancreatic β cells that can be readily absorbed by retinal cells and induce ROS production and apoptosis in recipient cells, leading to DR [371].
To sum up, the pancreas is the target organ of diabetic injury and also serves as the pathogenic tissue releasing damaging EVs that can effectively mediate the crosstalk among the pancreas, distant organs, and immune system (Figure 5). Given that the pancreas is an active and potent endocrine and exocrine tissue and plays a central role in systemic metabolic homeostasis and multiple diseases, its EVs are expected to be involved in diverse physiological and pathological processes.
Islet cell-derived EVs promote the development of T1D, T2D and diabetic retinopathy. Islet cell-derived EVs carry various molecular effectors that can trigger multiple signaling cascades, and may regulate the development of T1D, T2D and diabetic complications. In T2D, reduced miR-26a and NCDase in these EVs can exert a paracrine effect on ambient islet cells, resulting in cell death, dysfunction and IAPP accumulation. Distant delivery of EVs derived from islets cells with reduced miR-26a and elevated miR-29s to the liver, adipose and macrophages can promote IR and lipid accumulation in the liver, and cell expansion and systemic inflammation in the adipose, ultimately leading to T2D development. EVs with increased miR-15a are also be transmitted to retina and cause oxidative stress and cell apoptosis, promoting the occurrence of diabetic retinopathy. In T1D, islets cell-derived EVs are encapsuled with islet autoantigens and facilitate autoantigen presentation and autoimmune activation, along with activating phagocytes and promoting cytokines and chemokines release. Inflammatory islet cell-derived EVs are loaded with increased miR-375-3p and miR-21-5p, exerting a pro-apoptotic effect on surrounding β cells via paracrine action. Abbreviations: APC: antigen presenting cell; AT: adipose tissue; EVs: extracellular vesicles; IAPP: islet amyloid polypeptide; IR: insulin resistance; ROS: reactive oxygen species; T1D: type 1 diabetes; T2D: type 2 diabetes.
ECs
ECs are centrally involved in the microvascular pathology and complications in DM [372]. Specifically, diabetic vascular complications are characterized by EC dysfunction and death, and endothelium inflammation. Accumulating evidence indicates that EC-derived EVs are involved in these processes via paracrine action (Figure 6). HG and AGEs have been shown to induce MV generation and alter EV cargo sorting in ECs [253, 373, 374]. These MVs can promote apoptosis and dysfunction of recipient ECs [375-378]. For example, reduced EV miR-126 and miR-222 are sufficient to decrease endothelium repair capacity, partially accounting for the loss of protective function of EC-derived EVs [376-378]. Moreover, MVs are rich in membranous tight-junction proteins, occludin and claudin-5, resulting in a reduction of these molecules on the surface of parental ECs and impaired vessel walls [373]. Additionally, these MVs can induce the expression of adhesion molecules in target ECs and facilitate inflammatory cells to attach and infiltrate into the endothelium [379, 380].
Capillary basement membrane thickening of the glomerular, retinal, cardiac, and cutaneous arterioles is the most common microvascular structural modification in DM, resulting in organ malperfusion and classic diabetic microangiopathy [372]. In the diabetic setting, EVs derived from HG-treated ECs encapsulate elevated Notch3, versican, PDGF-BB, and circRNA-0077930, which can be taken up by surrounding VSMCs [381-386]. Consequently, recipient VSMCs acquire an anti-apoptotic, osteoblast-like and senescent phenotype, leading to intimal hyperplasia and vascular calcification [381-386]. Furthermore, ECs from different tissues can exert paracrine actions on ambient cells and promote the development of diabetic cardiomyopathy, DN, and diabetic foot. Exosomes derived from HG-treated ECs can suppress autophagy, increase apoptosis, and interfere with energy metabolism in target cardiomyocytes [387]. Exosomes derived from diabetic glomerular ECs (GECs) transmit TGF-β1 mRNA to GMCs and podocytes then induce elevated proliferation and matrix production of GMCs and fibrosis of podocytes [388, 389]. More recently, it has been shown that specific circRNAs in these exosomes, such as circRNF169 and circSTRN3, may also contribute to the dysregulation of GMCs and mesentery proliferation in DN [269]. Similarly, AGEs can boost miR-106b-5p in EVs derived from ECs that can be efficiently transported to recipient fibroblasts, leading to fibroblast autophagy and subsequent delayed wound healing [390].
Role of EC-derived EVs in the pathogenesis of diabetic complications. EVs derived from ECs are critically involved in the occurrence and progression of diabetic complications, including endothelial damage and inflammation, vascular sclerosis, diabetic cardiomyopathy, diabetic nephropathy and diabetic foot, by transferring functional biomolecules. On the one hand, by secreting occludin and claudin-5 via EVs, original ECs lose tight junctions. On the other hand, EC-derived EVs can promote apoptosis, induce the expression of adhesion molecules, and impair repairment capacity of recipient ECs, resulting in endothelial injury and inflammatory cell attachment and infiltration in endothelium. The protective function of EC-derived EVs on endothelium (ECs and VSMCs) is potentially mediated by miR-126 and miR-222, which is decreased under diabetic conditions. Notch 3, versican, PDGF-BB, LINC01005, circRNA-0077930 are delivered to VSMCs by EVs from ECs in a paracrine manner, resulting in apoptosis resistance and osteoblast-like differentiation in recipient VSMCs. EVs derived from ECs under oxLDL stress can transmit HSP70, ICAM-1, MALAT1, miR-155, miR-4306, miR-505 and miR-92a-3p into circulating system and local inflammatory cells including monocytes, macrophages and neutrophils, leading to endothelial inflammation and atherosclerosis. Glomerular EC-derived EVs are involved in the development of diabetic nephropathy via transferring TGF-β1 mRNA, circRNF16 and circSTRN3, thereby promoting renal cell proliferation, fibrosis and ECM production. EVs derived from ECs can disturb energy metabolism and induce cardiomyocyte apoptosis, facilitating the development of diabetic cardiomyopathy. MiR-106-5p is increased in the EVs from ECs, and is subsequently transmitted into dermal fibroblasts and contributes to a refractory wound in diabetic foot. Abbreviations: AGEs: advanced glycation end products; ECs: endothelial cells; ECM: extracellular matrix; EVs: extracellular vesicles; NET formation: neutrophil extracellular trap formation; oxLDL: oxidated low-density lipoprotein; VEC: vascular endothelial cells; VSMC: vascular smooth muscular cell.
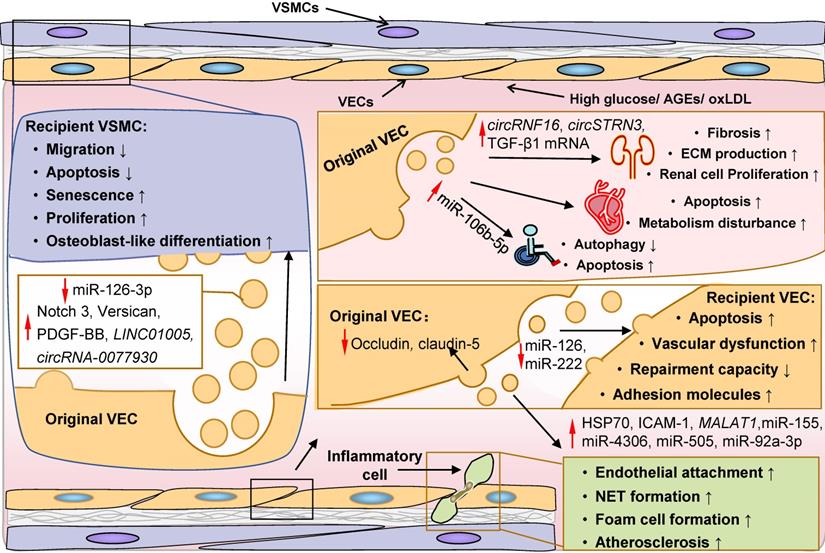
Furthermore, generation and abnormal miRNAs sorting of EVs induced by oxLDL are also considered important atherogenic events in DM. Elevated EV miRNAs, including miR-155, miR-4306, miR-505, and miR-92a-3p, are delivered into macrophages, neutrophils and surrounding ECs, leading to endothelial inflammation, dysfunction, and damage, and promoting atherosclerosis [328, 391-393]. Consequently, recipient inflammatory cells are aberrantly activated and exhibit a pro-inflammatory phenotype, while target ECs display decreased migration, proliferation, and angiogenic capacity [328,391-393]. Besides, other bioactive molecules with an atherogenic role, such as LINC01005, MALAT1, HSP70, and ICAM-1, have also been detected in EVs and may play a role in DM pathogenesis [394-397].
Other cells
Skeletal muscle is the major organ for glucose uptake, whose IR is one of the primary defects of T2D [398]. During lipid-induced IR, exosomes derived from skeletal muscle cells are enriched in saturated fatty acid palmitate, which can be taken up by insulin-sensitive tissues, particularly the pancreas and liver, representing a new paradigm of inter-organ communication and metabolic homeostasis [399]. MiR-16 encapsulated in these lipid toxic exosomes can promote the proliferation of target islet cells, acting as a compensatory IR mechanism during the onset of T2D [400]. Nevertheless, after exercise training, skeletal muscle-derived EVs of healthy individuals carry specific protein and miRNA signatures and display liver tropism [401, 402]. Bioactive miRNAs, including miR-133b, are transmitted to hepatic cells, inhibiting FoxO1 expression and leading to improved systemic metabolism [402]. The target specificity is thought to be mediated by interactions between the proteins distributed on the surface of exosomes and recipient cells [401, 402].
Gut microbiota dysbiosis has a driving role in T2D by inducing abnormal intestinal metabolites and intestinal permeability dysfunction [403]. Recent studies indicate that EVs derived from Akkermansia muciniphila, a beneficial bacterium preventing IR, contribute to the HFD-induced gut permeability elevation due to decreased intestinal tight junction function [404]. In general, intestinal barrier disruption causes an increase in EVs derived from gut microbes in the circulation and whole body [405-408]. The gut dysbiosis-related EVs appear to promote IR by transferring deleterious cargoes to recipient cells, such as HMGB1 and phosphatidylcholine [404-407].
In T1D, β cell death is primarily mediated by T cells, triggering diabetogenic insulitis [409]. In addition to inflammatory cytokines that are traditionally viewed as inducers of islet mass loss, EVs loaded with pro-inflammatory miRNAs, such as miR-142-3p, miR-142-5p, and miR-155, have been shown to specifically target pancreatic β cells and function as a novel pathogenic factor mediating autoimmune attack of β cells in T1D [410].
In T2D, platelets are considered a mediator of cellular crosstalk and a driver of inflammation [411]. EVs shed by platelets carrying soluble inflammatory cytokines have been recently implicated in these processes [412-414]. In a diabetic setting, platelets can release more EVs containing increased CXCL7 and CXCL10 that could be targeted to ECs in the aorta, kidney, and retina, resulting in increased expression of adhesion molecules, ROS production, oxidative stress, and inflammation-induced endothelial injury, thereby promoting the development of DR, DN, and atherosclerosis [412-414].
EVs derived from the kidney also have a role in mediating intercellular crosstalk in diabetic conditions. On the one hand, HG and AGEs induce shedding of MVs from podocytes potentially via activation of NOX4/ROS and the Smad3 pathway [415, 416]. These EVs mediate proximal tubular epithelial cell (PTECs) injury and apoptosis and proximal tubule fibrosis, partially due to transportation of miR-221 to target cells and subsequent regulation of Wnt/β-catenin signaling [415-419]. On the other hand, HG-treated GMC-derived exosomes can be delivered to podocytes, which induce apoptosis and inhibit cell adhesion, leading to impairment of the last line of defense of the glomerular filtration barrier [420]. These exosomes also potentially trigger an autocrine response in GMCs by delivering circ-DLGAP4 and miR-15b-5p that induce fibrosis and apoptosis [421,422]. Interestingly, HG seems to have a distinct effect on the generation of MVs and exosomes in PTECs. The MV release is increased under HG stimulation, which has a paracrine function on surrounding PTECs, promoting their fibrosis and impairing their adaptive responses combating hypoxia [423, 424]. In contrast, exosome biogenesis is decreased by HG treatment, which then exhibits a pro-proliferative effect on target fibroblasts and promotes extracellular matrix production [425].
Exosomes derived from HG-treated retinal pigment epithelial cells can promote angiogenesis by directly delivering the pro-angiogenic factor VEGF into retinal ECs [426]. Exosomes released by limbal stromal cells from non-diabetic individuals, but not from diabetic patients, can improve proliferation and migration of recipient limbal epithelial cells and maintain the integrity of cornea limbal epithelium [427]. Additionally, diabetic condition disrupts the metabolism of Schwann cells (SCs), the most abundant cells in the peripheral nervous system, and results in their neurotrophic molecules production compromise, contributing to diabetic peripheral neuropathy [428]. SC-derived exosomes act as an important neuronal support factor, nurturing peripheral axons and maintaining neuronal structure and function [429]. Conversely, diabetic SC-derived exosomes likely function as carriers of pathogenic content, reducing the nerve conduction velocity and aggravating mechanical and thermal hypoesthesia in diabetic mice [430].
Clinical applications of EVs in DM and diabetic complications
EVs as a biomarker for DM
As described previously, EVs function as paracrine and endocrine factors and facilitate the crosstalk between metabolic organs and tissues. In addition, EVs have promising potential as biomarkers due to their good stability in body fluids and the ease of isolation and detection by fast-evolving technologies. Indeed, accumulating data have demonstrated the promise of EVs for clinical applications as biomarkers in DM. Several recent reviews, extensively summarizing EVs as potential biomarkers for the early detection of DM and diabetic complications, stratification of patients, and response monitoring of treatment from different perspectives, are highly recommended [431-433]. Given the emerging role of EV RNAs in DM, here we briefly summarize the application of EV RNAs, including mRNAs and ncRNAs, as clinical biomarkers for the identification of diabetic patients and disease management (Table 2) [434-451].
For example, urinary exosomal miR-424 is robustly associated with islet autoimmunity and could efficiently discriminate patients with T1D with an area under the receiver operating characteristic (ROC) curve (AUC) of 0.803. However, serum miR-424 showed a relatively low diagnostic accuracy and sensitivity of 43% [452], suggesting urinary exosomal miR-424 as a more efficient biomarker for early detection of T1D. Another cohort study found that the combination of miR-10b and miR223-3p in serum MVs can effectively predict the occurrence of T2D in individuals with pre-diabetes with an AUC of 0.884 [451]. Importantly, this correlation has been further confirmed in the validation set with an AUC of 0.807 [451]. It has recently been pointed out that during the serum sampling process, apoptotic MVs with surface membrane phosphatidylserine could be consumed and new populations of MVs generated [453]. The authors indicated that these possible major changes in serum MVs might raise controversy over the results [453]. Compared to serum, the sampling process for plasma is simple with relatively stable contents. In this regard, it has been proposed that plasma might be a better source of MVs for biomarker investigation.
Native EVs for DM therapy
EVs have been used as carriers of therapeutic substances and the administration of exogenous EVs has great promise in diabetic treatment. The therapeutic potential of EVs in treating DM and its complications in animal trials have been summarized and discussed in recent reviews [432,454]. Here, we briefly discuss recent advances and the prospect of native EV-based therapeutics in DM and its complications.
Diagnostic index of EV RNAs in DM and diabetic complications.
| RNAs | Types [Reference] | Source | Number (ND/DM) | AUC | SEN (%) | SPE (%) | 95% CI |
|---|---|---|---|---|---|---|---|
| let-7c-5p | T2DN [444] | Urine | 15/28 | 0.818 | 96 | 53.4 | 0.718-0.919 |
| miR-21-5p | T2D [442] | Plasma | 60/57 | 0.859 | - | - | - |
| T2D-C [442] | Plasma | 57/101 | 0.744 | - | - | - | |
| T2DN [438] | Urine | 15/14 | 0.830 | - | - | 0.673-0.986 | |
| miR-23a | T2D [434] | Plasma | 36/42 | 0.828 | - | - | 0.735-0.920 |
| miR-29c-5p | T2DN [444] | Urine | 15/28 | 0.774 | - | - | - |
| miR-30a-5p | T2D-ESRD [436] | Urine | 80/40 | 0.912 | - | - | - |
| miR-30a | T2DN [443] | Urine | 56/110 | 0.897 | 76.4 | 90.9 | 0.858-0.936 |
| miR-34a | Dyslipidemia [439] | Serum | 78/42 | 0.730 | - | - | 0.630-0.830 |
| T2DN [441] | Urine | 44/136 | 0.917 | 93.3 | 86.7 | 0.874-0.96 | |
| miR-133b | T2DN [443] | Urine | 56/110 | 0.867 | 86.4 | 72.7 | 0.820-0.914 |
| miR-146a-5p | T2D [442] | Plasma | 60/57 | 0.911 | - | - | - |
| T2D-C [442] | Plasma | 57/101 | 0.673 | - | - | - | |
| miR-156 | T2DN [441] | Urine | 44/136 | 0.883 | 97.8 | 82.2 | 0.824-0.942 |
| miR-156-5p | T2DN [444] | Urine | 15/28 | 0.818 | - | - | - |
| miR-192 | T2D [434] | Plasma | 36/42 | 0.717 | - | - | 0.607-0.828 |
| MIC [440] | Urine | 30/30 | 0.802 | - | - | 0.696-0.907 | |
| miR-194 | MIC [440] | Urine | 30/30 | 0.703 | - | - | 0.581-0.826 |
| miR-215 | MIC [440] | Urine | 30/30 | 0.757 | - | - | 0.545-0.869 |
| miR-218 | T1D [437] | Urine | 30/30 | 0.817 | - | - | - |
| miR-342 | T2DN [443] | Urine | 56/110 | 0.910 | 81.8 | 80.9 | 0.873-0.948 |
| miR-424 | T1D [437] | Urine | 30/30 | 0.803 | - | - | - |
| miR-636 | T2DN [441] | Urine | 44/136 | 0.984 | 97.8 | 93.3 | 0.971-0.997 |
| miR-4534 | DN [435] | Urine | 14/14 | 0.786 | 85.7 | 78.6 | 0.607-0.965 |
| miR-10b and miR223-3p | T2D [451] | Serum | 8/9 | 0.884 | - | - | - |
| circ_0000907 | DFU [445] | Serum | 20/20 | 0.878 | 80 | 80.85 | - |
| circ_0057362 | DFU [445] | Serum | 20/20 | 0.848 | 86.005 | 70.22 | - |
| Ace | Overt DN [448] | Plasma | 100/37 | 0.75 | 73 | 72 | 0.66-0.83 |
| Incipient DN [448] | Plasma | 37/66 | 0.62 | 65.2 | 61 | 0.54-0.71 | |
| Aebp1 | T2DN [446] | Plasma | 15/15 | 0.742 | 53.3 | 86.7 | - |
| Ccl21 | T2DN [447] | Urine | 15/28 | 0.888 | - | - | 0.737-0.997 |
| Umod | T2DN [450] | Urine | 15/88 | 0.90 | 93 | 73 | - |
| Wt1 | Incipient DN [448] | Plasma | 37/66 | 0.63 | 50 | 74 | 0.55-0.72 |
| Overt DN [448] | Plasma | 100/37 | 0.83 | 67.6 | 93 | 0.74-0.92 | |
| DN [449] | Urine | 10/10 | 0.705 | - | - | - |
AUC: area under the ROC curve; CI: confidence interval; DFU: diabetic foot ulcer; DM: diabetes mellitus; DN: diabetic nephropathy; ESRD: end-stage renal disease; MIC: microalbuminuria; ND: non diabetes; SEN: sensitivity; SPE: specificity; T2DN: T2D with DN; T2D-C: T2D with complications.
Potential clinical applications of native cell-derived EVs in treating DM and its complications. EVs of native cells (such as pancreatic pathfinder cells, adipocytes, stem cells, retinal pigment epithelial cells, keratinocytes, endothelial progenitor cells, amniotic epithelial cells, endothelial cells, fibrocytes, and macrophages) show potent promise as novel therapies for T1D (via inhibiting autoimmune response) and T2D (via promoting islet cell function and survival, and/or improving peripheral insulin sensitivity). These EVs also have the potential to treat diabetic complications including atherosclerosis, diabetic retinopathy, diabetic heart, diabetic nephropathy, diabetic erectile dysfunction, diabetic neuropathy, and diabetic foot ulcer. Abbreviations: APC: antigen presenting cells; DM: diabetes mellitus; EMT: epithelial-mesenchymal transition; ECM: extracellular matrix; EVs: extracellular vesicles; GLUT4: glucose transporter 4.
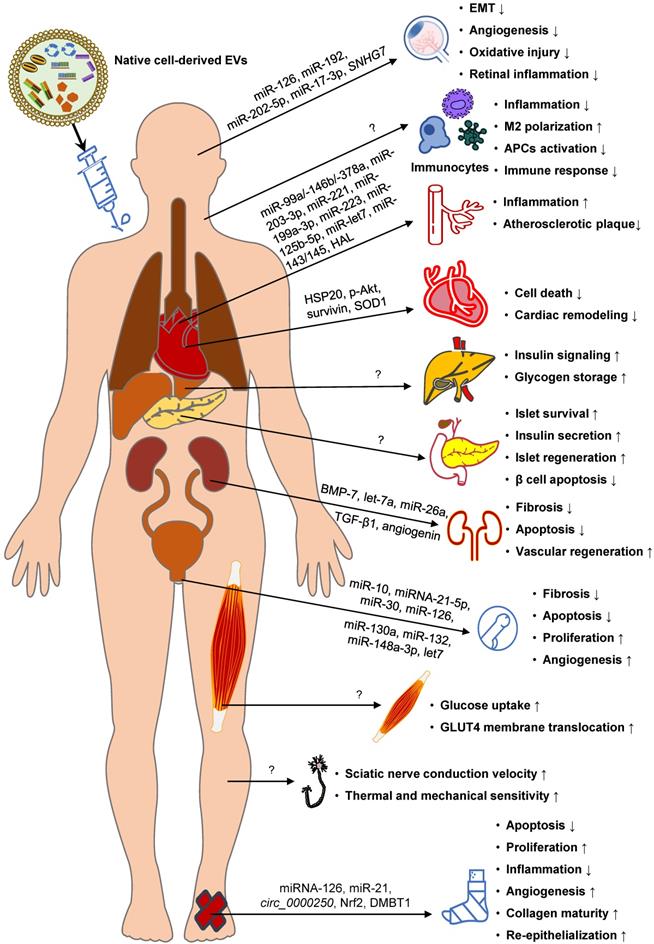
Anti-diabetic EVs have been isolated from various native cells, such as pancreatic pathfinder cells [455,456], adipocytes [457], and stem cells [458-462] (Figure 7). The preclinical data collected so far indicate that these EVs can improve peripheral insulin sensitivity and pancreatic islet function, alleviate inflammation, and/or attenuate obesity, regardless of their origin. The therapeutic roles of EVs in recipient cells have been ascribed to the delivery of bioactive proteins. For example, the anti-inflammatory and anti-apoptotic roles of exosomes have been attributed to active STAT3 and VEGF [459, 460]. Depending on the source and content of EVs, they can trigger various therapeutic effects, such as inhibiting β cell apoptosis, restoring the phosphorylation of the insulin receptor substrate 1 and protein kinase B, increasing hepatic glycogen storage, polarizing M2 macrophages, and inhibiting the auto-immune response [458-462]. Moreover, numerous examples of EV-mediated functional transfer of ncRNAs have been demonstrated for various diseases and the therapeutic applications of EV ncRNAs in treating DM offer a fertile field for study.
Another important clinical application of EVs from different origins is in treating diabetic complications, such as DN [463-466], DR [467-471], diabetic erectile dysfunction [472-475], diabetic foot [476-490], diabetic cardiomyopathy [217], atherosclerosis [491-500], and diabetic peripheral neuropathy [429] (Figure 7). Currently, the therapeutic roles of EVs in treating diabetic complications are mostly attributed to the delivery of ncRNAs, especially miRNAs. For example, the angiogenic role of EVs has been ascribed to miR-21, let-7, miR-10, miR-30, miR-148a-3p, miR-126, miR-130a, and miR-132 [474-478], whereas their anti-fibrotic function has been attributed to let-7b and let-7c [474]. Some of these miRNAs have been identified in previous studies as anti-diabetes therapies, such as miR-21 [271,501,502], let-7 [503], miR-126 [504], and miR-132 [505, 506], further highlighting their great potential in DM treatment. In addition to ncRNAs, some proteins like TGF-β1, angiogenin, BMP-7, Nrf2, and DMBT1within EVs can also elicit biological therapeutic effects [463, 482, 484].
However, important limitations in eliciting functional responses must be overcome for EVs to be used as an effective clinical therapeutic tool. Efforts have been made to address the challenges of harnessing the full potential of native EVs in the treatment of DM and diabetic complications. Because the EV composition is dependent on features of their donor cells, transfecting the original cells with exogenous compounds might modulate EVs and realize the goal of improving their bioactivity and augmenting their therapeutic efficacy. For example, overexpression of siFas and anti-miR-375 in human bone marrow mesenchymal stem cells can increase their levels in exosomes, effectively inhibiting Fas and miR-375 in recipient pancreatic islet cells and thus improve islet viability and function against inflammation [462]. Similarly, overexpression of functional proangiogenic components, such as Nrf2 [482], miR-221-3p [507], mmu_circ_0000250 [480], and miR-126 [476], in parental stem cells is accompanied by upregulation of these genes in the secreted exosomes, thereby improving the therapeutic effect against diabetic foot ulcer.
Furthermore, biomaterials, such as the thermosensitive and/or antibacterial hydrogel, have been developed to prolong the half-life of EVs and can serve as the controlled drug delivery system of EVs for treating chronic wounds [483, 508-510]. Additionally, taking advantage of the high-yield EV-mimetic nanovesicles (EMNVs) as a novel drug delivery system, the nanocarriers loaded with lncRNA-H19 have been applied to treat diabetic wounds [511]. These EMNVs function effectively by restoring lncRNA-H19 expression in dermal microvascular ECs and remarkably increase vascular formation [511] to treat DM and diabetic complications in the future.
Conclusion and Perspective
EVs are major regulators of DM and diabetic complications and play an important role in IR, inflammation, and islet dysfunction. Significantly, EVs have shown promising efficacy in animal models to deliver bioactive proteins and RNAs and can be harnessed as effective therapies for DM and diabetic complications. Despite these tremendous advances, the basic and clinical research of EVs in DM and diabetic complications is still at an infant stage.
Although it is generally recognized that EVs communicate between cells and organs by delivering messages and exchanging information, many questions remain to be resolved. First, it is still challenging to categorize and characterize EV subclasses with high heterogeneity [512], mainly due to technological limitations in separating and analysing vesicles. Second, due to the complexity of EV contents, their functions, individually or collectively, are far from being fully elucidated. Many attempts have been made to address this issue, for e.g., by developing a single-vesicle array and imaging method to track EV uptake [513,514]. Third, limited information is available about the molecular mechanisms underlying the target specificity of EVs with different origins so far. This process is believed to be largely mediated by membranous interactions between EVs originating from different cell types and target cells. Finally, it is important to monitor the fate of EVs after docking at recipient cells and determine the mechanisms underlying the usage of their cargoes.
From the clinical perspective, therapeutic applications of EVs have multiple challenges that need to be addressed. Biological detection of EVs requires adequate enrichment together with high sensitivity. Nanomaterials, such as magnetic nanoparticles, have been used to improve the sensitivity of EV detection [515, 516] by effectively increasing the interface between biological molecules and nanomaterials to facilitate the capture of target EVs, significantly raising the efficiency of EV isolation. Also, improving the specificity of EV separation required for the high specificity of biomarkers represents another challenge. Appropriate modifications of magnetic nanoparticles by attaching biological probes, such as antibodies targeting EV surface markers, can efficiently improve specificity [517,518].
However, there are several outstanding issues regarding the use of EVs as effective therapies for DM and diabetic complications, including scale-up of the production, shelf stability, prolonging the half-life of therapeutic EVs, toxicity, off-target effects, and the delivery specificity. Despite these problems, EV-based biomarker discovery and clinic application are feasible and promising with constantly developing technologies. Due to their unique biological characteristics, EVs still have a great potential for accurate early diagnosis of DM and overcoming diabetic complications.
Abbreviation
aSMase: acid sphingomyelinase; AGEs: advanced glycation end products; APCs: antigen-presenting cells; AT: adipose tissue; AUC: area under the receiver operating characteristic curve; BMI: body mass index; circRNAs: circular RNAs; CNS: central nervous system; DM: diabetes mellitus; DN: diabetic nephropathy; DR: diabetic retinopathy; ECs: endothelial cells; EMNVs: EV-mimetic nanovesicles; ER: endoplasmic reticulum; ESCRT: endosomal sorting complex required for transport; EVs: extracellular vesicles; FFAs: free fatty acids; GECs: glomerular ECs; GLP-1: glucagon-like peptide 1; GLUT: glucose transporter; GMCs: glomerular mesangial cells; GSK3β: glycogen synthase kinase 3β; HG: high glucose; HFD: high-fat diet; HuR: human antigen R; IAPP: islet amyloid polypeptide; ILVs: intraluminal vesicles; IR: insulin resistance; lncRNAs: long noncoding RNAs; MVs: microvesicles; MVEs: multivesicular endosomes; ncRNAs: non-coding RNAs; NETosis: neutrophil extracellular traps formation; nSMase: neutral sphingomyelinase; oxLDL: oxidated low-density lipoprotein; PTECs: proximal tubular epithelial cells; PTMs: posttranslational modifications; RAGEs: receptors for AGE; RBPs: RNA binding proteins; ROC: receiver operating characteristic; ROS: reactive oxygen species; SCs: Schwann cells; SNARE: soluble N-ethylmaleimide-sensitive fusion attachment protein receptor; t-SNAREs: target-membrane SNAREs; T1D: type 1 diabetes; T2D: type 2 diabetes; UBL3: ubiquitin-like 3; v-SNAREs: vesicle-membrane SNAREs; VSMCs: vascular smooth muscle cells.
Acknowledgements
We thank members of the laboratory of X.F. for helpful discussion.
Funding
This work was supported by the National Natural Science Foundation of China (92157205, 81970561, 82172986), the Ministry of Science and Technology of China (2018ZX09201018-005), National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (Z20191005 and Z20201003), and the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (ZYJC18049 and ZYGD18017).
Author Contributions
X.F. and Y.T. conceived the idea; J.L. and Y.Z. performed the literature search and draft the manuscript; X.F., Y.T., N.T. and W.H. supervised and revised the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Williams R, Colagiuri S, Chan J, Gregg EW, Yang X. IDF Atlas 9th Edition 2019: IDF Atlas 9th Edition. 2019. 2019
2. Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E. et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215-22
3. Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015;350:g7607
4. Saran R, Robinson B, Abbott KC, Agodoa L, Bragg-Gresham J, Balkrishnan R. et al. US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2019;73:A7-8
5. Flaxman SR, Bourne R, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV. et al. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5:e1221-34
6. Moxey PW, Gogalniceanu P, Hinchliffe RJ, Loftus IM, Jones KJ, Thompson MM. et al. Lower extremity amputations-a review of global variability in incidence. Diabet Med. 2011;28:1144-53
7. Katsarou A, Gudbjornsdottir S, Rawshani A, Dabelea D, Bonifacio E, Anderson BJ. et al. Type 1 diabetes mellitus. Nat Rev Dis Primers. 2017;3:17016
8. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88-98
9. de Goffau MC, Luopajarvi K, Knip M, Ilonen J, Ruohtula T, Harkonen T. et al. Fecal microbiota composition differs between children with beta-cell autoimmunity and those without. Diabetes. 2013;62:1238-44
10. Kolb H, Eizirik DL. Resistance to type 2 diabetes mellitus: a matter of hormesis? Nat Rev Endocrinol. 2011;8:183-92
11. Asterholm IW, Scherer PE. Enhanced metabolic flexibility associated with elevated adiponectin levels. Am J Pathol. 2010;176:1364-76
12. Frohman LA. CNS peptides and glucoregulation. Annu Rev Physiol. 1983;45:95-107
13. Sandoval DA, Obici S, Seeley RJ. Targeting the CNS to treat type 2 diabetes. Nat Rev Drug Discov. 2009;8:386-98
14. Tups A, Benzler J, Sergi D, Ladyman SR, Williams LM. Central Regulation of Glucose Homeostasis. Compr Physiol. 2017;7:741-64
15. Morton GJ, Schwartz MW. Leptin and the central nervous system control of glucose metabolism. Physiol Rev. 2011;91:389-411
16. Roder PV, Wu B, Liu Y, Han W. Pancreatic regulation of glucose homeostasis. Exp Mol Med. 2016;48:e219
17. Barrera JG, Sandoval DA, D'Alessio DA, Seeley RJ. GLP-1 and energy balance: an integrated model of short-term and long-term control. Nat Rev Endocrinol. 2011;7:507-16
18. Stefan N, Haring HU. The role of hepatokines in metabolism. Nat Rev Endocrinol. 2013;9:144-52
19. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977
20. Harcourt BE, Penfold SA, Forbes JM. Coming full circle in diabetes mellitus: from complications to initiation. Nat Rev Endocrinol. 2013;9:113-23
21. Roden M, Shulman GI. The integrative biology of type 2 diabetes. Nature. 2019;576:51-60
22. Donath MY. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat Rev Drug Discov. 2014;13:465-76
23. Ghorpade DS, Ozcan L, Zheng Z, Nicoloro SM, Shen Y, Chen E. et al. Hepatocyte-secreted DPP4 in obesity promotes adipose inflammation and insulin resistance. Nature. 2018;555:673-7
24. Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87:507-20
25. Stenvers DJ, Scheer F, Schrauwen P, la Fleur SE, Kalsbeek A. Circadian clocks and insulin resistance. Nat Rev Endocrinol. 2019;15:75-89
26. Cai D, Khor S. "Hypothalamic Microinflammation" Paradigm in Aging and Metabolic Diseases. Cell Metab. 2019;30:19-35
27. Li B, Leung J, Chan L, Yiu WH, Tang S. A global perspective on the crosstalk between saturated fatty acids and Toll-like receptor 4 in the etiology of inflammation and insulin resistance. Prog Lipid Res. 2020;77:101020
28. Pi X, Xie L, Patterson C. Emerging Roles of Vascular Endothelium in Metabolic Homeostasis. Circ Res. 2018;123:477-94
29. Winer DA, Luck H, Tsai S, Winer S. The Intestinal Immune System in Obesity and Insulin Resistance. Cell Metab. 2016;23:413-26
30. Tsuchiya K, Accili D. Liver sinusoidal endothelial cells link hyperinsulinemia to hepatic insulin resistance. Diabetes. 2013;62:1478-89
31. Johansson M, Mattsson G, Andersson A, Jansson L, Carlsson PO. Islet endothelial cells and pancreatic beta-cell proliferation: studies in vitro and during pregnancy in adult rats. Endocrinology. 2006;147:2315-24
32. Huang CJ, Lin CY, Haataja L, Gurlo T, Butler AE, Rizza RA. et al. High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated beta-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes. 2007;56:2016-27
33. Krogvold L, Skog O, Sundström G, Edwin B, Buanes T, Hanssen KF. et al. Function of Isolated Pancreatic Islets From Patients at Onset of Type 1 Diabetes: Insulin Secretion Can Be Restored After Some Days in a Nondiabetogenic Environment In Vitro: Results From the DiViD Study. Diabetes. 2015;64:2506-12
34. Marchetti P, Bugliani M, Lupi R, Marselli L, Masini M, Boggi U. et al. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia. 2007;50:2486-94
35. Gonzalez-Duque S, Azoury ME, Colli ML, Afonso G, Turatsinze JV, Nigi L. et al. Conventional and Neo-antigenic Peptides Presented by beta Cells Are Targeted by Circulating Naive CD8+ T Cells in Type 1 Diabetic and Healthy Donors. Cell Metab. 2018;28:946-60
36. Vaarala O, Atkinson MA, Neu J. The "perfect storm" for type 1 diabetes: the complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes. 2008;57:2555-62
37. Vatanen T, Franzosa EA, Schwager R, Tripathi S, Arthur TD, Vehik K. et al. The human gut microbiome in early-onset type 1 diabetes from the TEDDY study. Nature. 2018;562:589-94
38. Rouxel O, Da SJ, Beaudoin L, Nel I, Tard C, Cagninacci L. et al. Cytotoxic and regulatory roles of mucosal-associated invariant T cells in type 1 diabetes. Nat Immunol. 2017;18:1321-31
39. Costa FR, Francozo MC, de Oliveira GG, Ignacio A, Castoldi A, Zamboni DS. et al. Gut microbiota translocation to the pancreatic lymph nodes triggers NOD2 activation and contributes to T1D onset. J Exp Med. 2016;213:1223-39
40. van de Weijer T, Schrauwen-Hinderling VB, Schrauwen P. Lipotoxicity in type 2 diabetic cardiomyopathy. Cardiovasc Res. 2011;92:10-8
41. Murea M, Freedman BI, Parks JS, Antinozzi PA, Elbein SC, Ma L. Lipotoxicity in diabetic nephropathy: the potential role of fatty acid oxidation. Clin J Am Soc Nephrol. 2010;5:2373-9
42. Sun B, Luo Z, Zhou J. Comprehensive elaboration of glycemic variability in diabetic macrovascular and microvascular complications. Cardiovasc Diabetol. 2021;20:9
43. Singh VP, Bali A, Singh N, Jaggi AS. Advanced Glycation End Products and Diabetic Complications. Korean J Physiol Pharmacol. 2014;18:1
44. Ruegsegger GN, Creo AL, Cortes TM, Dasari S, Nair KS. Altered mitochondrial function in insulin-deficient and insulin-resistant states. J Clin Invest. 2018;128:3671-81
45. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058-70
46. Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93:137-88
47. Ly LD, Xu S, Choi SK, Ha CM, Thoudam T, Cha SK. et al. Oxidative stress and calcium dysregulation by palmitate in type 2 diabetes. Exp Mol Med. 2017;49:e291
48. Ujihara N, Sakka Y, Takeda M, Hirayama M, Ishii A, Tomonaga O. et al. Association between plasma oxidized low-density lipoprotein and diabetic nephropathy. Diabetes Res Clin Pract. 2002;58:109-14
49. Gao L, Yang TT, Zhang JS, Liu HX, Cai DC, Wang LT. et al. THBS1/CD47 Modulates the Interaction of gamma-Catenin With E-Cadherin and Participates in Epithelial-Mesenchymal Transformation in Lipid Nephrotoxicity. Front Cell Dev Biol. 2020;8:601521
50. Gutwein P, Abdel-Bakky MS, Doberstein K, Schramme A, Beckmann J, Schaefer L. et al. CXCL16 and oxLDL are induced in the onset of diabetic nephropathy. J Cell Mol Med. 2009;13:3809-25
51. Kim YS, Jung DH, Sohn E, Kim J, Kim JS. Glycoxidised LDL induced the upregulation of Axl receptor tyrosine kinase and its ligand in mouse mesangial cells. Plos One. 2012;7:e50297
52. van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213-28
53. Meldolesi J. Exosomes and Ectosomes in Intercellular Communication. Curr Biol. 2018;28:R435-44
54. Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750
55. Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F. et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244-7
56. Dogrammatzis C, Deschamps T, Kalamvoki M. Biogenesis of Extracellular Vesicles during Herpes Simplex Virus 1 Infection: Role of the CD63 Tetraspanin. J Virol. 2019;93:e1818-50
57. Gauthier SA, Pérez-González R, Sharma A, Huang F, Alldred MJ, Pawlik M. et al. Enhanced exosome secretion in Down syndrome brain - a protective mechanism to alleviate neuronal endosomal abnormalities. Acta Neuropathol Com. 2017;5:65
58. Wei D, Zhan W, Gao Y, Huang L, Gong R, Wang W. et al. RAB31 marks and controls an ESCRT-independent exosome pathway. Cell Res. 2021;31:157-77
59. Puri N, Roche PA. Ternary SNARE complexes are enriched in lipid rafts during mast cell exocytosis. Traffic. 2006;7:1482-94
60. Sun C, Wang P, Dong W, Liu H, Sun J, Zhao L. LncRNA PVT1 promotes exosome secretion through YKT6, RAB7, and VAMP3 in pancreatic cancer. Aging. 2020;12:10427-40
61. Wei Y, Wang D, Jin F, Bian Z, Li L, Liang H. et al. Pyruvate kinase type M2 promotes tumour cell exosome release via phosphorylating synaptosome-associated protein 23. Nat Commun. 2017;8:14041
62. Yu Z, Shi M, Stewart T, Fernagut PO, Huang Y, Tian C. et al. Reduced oligodendrocyte exosome secretion in multiple system atrophy involves SNARE dysfunction. Brain. 2020;143:1780-97
63. Yang L, Peng X, Li Y, Zhang X, Ma Y, Wu C. et al. Long non-coding RNA HOTAIR promotes exosome secretion by regulating RAB35 and SNAP23 in hepatocellular carcinoma. Mol Cancer. 2019;18:78
64. Savina A, Furlan M, Vidal M, Colombo MI. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem. 2003;278:20083-90
65. Morrell AE, Brown GN, Robinson ST, Sattler RL, Baik AD, Zhen G. et al. Mechanically induced Ca(2+) oscillations in osteocytes release extracellular vesicles and enhance bone formation. Bone Res. 2018;6:6
66. Liu ML, Reilly MP, Casasanto P, McKenzie SE, Williams KJ. Cholesterol enrichment of human monocyte/macrophages induces surface exposure of phosphatidylserine and the release of biologically-active tissue factor-positive microvesicles. Arterioscler Thromb Vasc Biol. 2007;27:430-5
67. Larson MC, Woodliff JE, Hillery CA, Kearl TJ, Zhao M. Phosphatidylethanolamine is externalized at the surface of microparticles. Biochim Biophys Acta. 2012;1821:1501-7
68. Inal JM, Jorfi S. Coxsackievirus B transmission and possible new roles for extracellular vesicles. Biochem Soc Trans. 2013;41:299-302
69. Rothmeier AS, Marchese P, Petrich BG, Furlan-Freguia C, Ginsberg MH, Ruggeri ZM. et al. Caspase-1-mediated pathway promotes generation of thromboinflammatory microparticles. J Clin Invest. 2015;125:1471-84
70. Li CJ, Fang QH, Liu ML, Lin JN. Current understanding of the role of Adipose-derived Extracellular Vesicles in Metabolic Homeostasis and Diseases: Communication from the distance between cells/tissues. Theranostics. 2020;10:7422-35
71. Pavlyukov MS, Yu H, Bastola S, Minata M, Shender VO, Lee Y. et al. Apoptotic Cell-Derived Extracellular Vesicles Promote Malignancy of Glioblastoma Via Intercellular Transfer of Splicing Factors. Cancer Cell. 2018;34:119-35
72. Migneault F, Dieude M, Turgeon J, Beillevaire D, Hardy MP, Brodeur A. et al. Apoptotic exosome-like vesicles regulate endothelial gene expression, inflammatory signaling, and function through the NF-kappaB signaling pathway. Sci Rep. 2020;10:12562
73. Zargarian S, Shlomovitz I, Erlich Z, Hourizadeh A, Ofir-Birin Y, Croker BA. et al. Phosphatidylserine externalization, "necroptotic bodies" release, and phagocytosis during necroptosis. Plos Biol. 2017;15:e2002711
74. Spencer DM, Dye JR, Piantadosi CA, Pisetsky DS. The release of microparticles and mitochondria from RAW 264.7 murine macrophage cells undergoing necroptotic cell death in vitro. Exp Cell Res. 2018;363:151-9
75. Lorey MB, Rossi K, Eklund KK, Nyman TA, Matikainen S. Global Characterization of Protein Secretion from Human Macrophages Following Non-canonical Caspase-4/5 Inflammasome Activation. Mol Cell Proteomics. 2017;16:S187-99
76. Liu ML, Lyu X, Werth VP. Recent progress in the mechanistic understanding of NET formation in neutrophils. Febs J. 2021 [Epub ahead of print]
77. Peng L, Wang Y, Yang B, Qin Q, Song E, Song Y. Polychlorinated biphenyl quinone regulates MLKL phosphorylation that stimulates exosome biogenesis and secretion via a short negative feedback loop. Environ Pollut. 2021;274:115606
78. Pathan M, Fonseka P, Chitti SV, Kang T, Sanwlani R, Van Deun J. et al. Vesiclepedia 2019: a compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res. 2019;47:D516-9
79. Schuldner M, Dorsam B, Shatnyeva O, Reiners KS, Kubarenko A, Hansen HP. et al. Exosome-dependent immune surveillance at the metastatic niche requires BAG6 and CBP/p300-dependent acetylation of p53. Theranostics. 2019;9:6047-62
80. Kim SB, Kim HR, Park MC, Cho S, Goughnour PC, Han D. et al. Caspase-8 controls the secretion of inflammatory lysyl-tRNA synthetase in exosomes from cancer cells. J Cell Biol. 2017;216:2201-16
81. Nkosi D, Sun L, Duke LC, Patel N, Surapaneni SK, Singh M. et al. Epstein-Barr Virus LMP1 Promotes Syntenin-1- and Hrs-Induced Extracellular Vesicle Formation for Its Own Secretion To Increase Cell Proliferation and Migration. Mbio. 2020;11:e520-89
82. Perez-Hernandez D, Gutierrez-Vazquez C, Jorge I, Lopez-Martin S, Ursa A, Sanchez-Madrid F. et al. The intracellular interactome of tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes. J Biol Chem. 2013;288:11649-61
83. Huang C, Hays FA, Tomasek JJ, Benyajati S, Zhang XA. Tetraspanin CD82 interaction with cholesterol promotes extracellular vesicle-mediated release of ezrin to inhibit tumour cell movement. J Extracell Vesicles. 2020;9:1692417
84. Cheerathodi M, Nkosi D, Cone AS, York SB, Meckes DJ. Epstein-Barr Virus LMP1 Modulates the CD63 Interactome. Viruses. 2021;13:675
85. Hurwitz SN, Nkosi D, Conlon MM, York SB, Liu X, Tremblay DC. et al. CD63 Regulates Epstein-Barr Virus LMP1 Exosomal Packaging, Enhancement of Vesicle Production, and Noncanonical NF-kappaB Signaling. J Virol. 2017;91:e2216-51
86. Ageta H, Ageta-Ishihara N, Hitachi K, Karayel O, Onouchi T, Yamaguchi H. et al. UBL3 modification influences protein sorting to small extracellular vesicles. Nat Commun. 2018;9:3936
87. Ageta H, Tsuchida K. Post-translational modification and protein sorting to small extracellular vesicles including exosomes by ubiquitin and UBLs. Cell Mol Life Sci. 2019;76:4829-48
88. Anand S, Foot N, Ang CS, Gembus KM, Keerthikumar S, Adda CG. et al. Arrestin-Domain Containing Protein 1 (Arrdc1) Regulates the Protein Cargo and Release of Extracellular Vesicles. Proteomics. 2018;18:e1800266
89. Burke MC, Oei MS, Edwards NJ, Ostrand-Rosenberg S, Fenselau C. Ubiquitinated proteins in exosomes secreted by myeloid-derived suppressor cells. J Proteome Res. 2014;13:5965-72
90. Putz U, Howitt J, Doan A, Goh CP, Low LH, Silke J. et al. The tumor suppressor PTEN is exported in exosomes and has phosphatase activity in recipient cells. Sci Signal. 2012;5:a70
91. Mackenzie K, Foot NJ, Anand S, Dalton HE, Chaudhary N, Collins BM. et al. Regulation of the divalent metal ion transporter via membrane budding. Cell Discov. 2016;2:16011
92. Kunadt M, Eckermann K, Stuendl A, Gong J, Russo B, Strauss K. et al. Extracellular vesicle sorting of alpha-Synuclein is regulated by sumoylation. Acta Neuropathol. 2015;129:695-713
93. Mariscal J, Vagner T, Kim M, Zhou B, Chin A, Zandian M. et al. Comprehensive palmitoyl-proteomic analysis identifies distinct protein signatures for large and small cancer-derived extracellular vesicles. J Extracell Vesicles. 2020;9:1764192
94. Romancino DP, Buffa V, Caruso S, Ferrara I, Raccosta S, Notaro A. et al. Palmitoylation is a post-translational modification of Alix regulating the membrane organization of exosome-like small extracellular vesicles. Biochim Biophys Acta Gen Subj. 2018;1862:2879-87
95. Itoh S, Mizuno K, Aikawa M, Aikawa E. Dimerization of sortilin regulates its trafficking to extracellular vesicles. J Biol Chem. 2018;293:4532-44
96. Verweij FJ, de Heus C, Kroeze S, Cai H, Kieff E, Piersma SR. et al. Exosomal sorting of the viral oncoprotein LMP1 is restrained by TRAF2 association at signalling endosomes. J Extracell Vesicles. 2015;4:26334
97. Kobayashi E, Aga M, Kondo S, Whitehurst C, Yoshizaki T, Pagano JS. et al. C-Terminal Farnesylation of UCH-L1 Plays a Role in Transport of Epstein-Barr Virus Primary Oncoprotein LMP1 to Exosomes. Msphere. 2018;3:e18-30
98. Roche JV, Survery S, Kreida S, Nesverova V, Ampah-Korsah H, Gourdon M. et al. Phosphorylation of human aquaporin 2 (AQP2) allosterically controls its interaction with the lysosomal trafficking protein LIP5. J Biol Chem. 2017;292:14636-48
99. Valapala M, Vishwanatha JK. Lipid raft endocytosis and exosomal transport facilitate extracellular trafficking of annexin A2. J Biol Chem. 2011;286:30911-25
100. Lee H, Li C, Zhang Y, Zhang D, Otterbein LE, Jin Y. Caveolin-1 selectively regulates microRNA sorting into microvesicles after noxious stimuli. J Exp Med. 2019;216:2202-20
101. Surman M, Hoja-Lukowicz D, Szwed S, Drozdz A, Stepien E, Przybylo M. Human melanoma-derived ectosomes are enriched with specific glycan epitopes. Life Sci. 2018;207:395-411
102. Liang Y, Eng WS, Colquhoun DR, Dinglasan RR, Graham DR, Mahal LK. Complex N-linked glycans serve as a determinant for exosome/microvesicle cargo recruitment. J Biol Chem. 2014;289:32526-37
103. Harada T, Yamamoto H, Kishida S, Kishida M, Awada C, Takao T. et al. Wnt5b-associated exosomes promote cancer cell migration and proliferation. Cancer Sci. 2017;108:42-52
104. Palicharla VR, Maddika S. HACE1 mediated K27 ubiquitin linkage leads to YB-1 protein secretion. Cell Signal. 2015;27:2355-62
105. Lee HS, Jeong J, Lee KJ. Characterization of vesicles secreted from insulinoma NIT-1 cells. J Proteome Res. 2009;8:2851-62
106. Sork H, Corso G, Krjutskov K, Johansson HJ, Nordin JZ, Wiklander OPB. et al. Heterogeneity and interplay of the extracellular vesicle small RNA transcriptome and proteome. Sci Rep. 2018;8:10813
107. Fabbiano F, Corsi J, Gurrieri E, Trevisan C, Notarangelo M, D'Agostino VG. RNA packaging into extracellular vesicles: An orchestra of RNA-binding proteins? J Extracell Vesicles. 2020;10:e12043
108. Mateescu B, Kowal EJ, van Balkom BW, Bartel S, Bhattacharyya SN, Buzas EI. et al. Obstacles and opportunities in the functional analysis of extracellular vesicle RNA - an ISEV position paper. J Extracell Vesicles. 2017;6:1286095
109. Wu B, Su S, Patil DP, Liu H, Gan J, Jaffrey SR. et al. Molecular basis for the specific and multivariant recognitions of RNA substrates by human hnRNP A2/B1. Nat Commun. 2018;9:420
110. Villarroya-Beltri C, Gutierrez-Vazquez C, Sanchez-Cabo F, Perez-Hernandez D, Vazquez J, Martin-Cofreces N. et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980
111. Yin J, Ge X, Shi Z, Yu C, Lu C, Wei Y. et al. Extracellular vesicles derived from hypoxic glioma stem-like cells confer temozolomide resistance on glioblastoma by delivering miR-30b-3p. Theranostics. 2021;11:1763-79
112. Han M, Gu Y, Lu P, Li J, Cao H, Li X. et al. Exosome-mediated lncRNA AFAP1-AS1 promotes trastuzumab resistance through binding with AUF1 and activating ERBB2 translation. Mol Cancer. 2020;19:26
113. Chen C, Luo Y, He W, Zhao Y, Kong Y, Liu H. et al. Exosomal long noncoding RNA LNMAT2 promotes lymphatic metastasis in bladder cancer. J Clin Invest. 2020;130:404-21
114. Balaguer N, Moreno I, Herrero M, Gonzalez M, Simon C, Vilella F. Heterogeneous nuclear ribonucleoprotein C1 may control miR-30d levels in endometrial exosomes affecting early embryo implantation. Mol Hum Reprod. 2018;24:411-25
115. Statello L, Maugeri M, Garre E, Nawaz M, Wahlgren J, Papadimitriou A. et al. Identification of RNA-binding proteins in exosomes capable of interacting with different types of RNA: RBP-facilitated transport of RNAs into exosomes. Plos One. 2018;13:e195969
116. Lu P, Li H, Li N, Singh RN, Bishop CE, Chen X. et al. MEX3C interacts with adaptor-related protein complex 2 and involves in miR-451a exosomal sorting. Plos One. 2017;12:e185992
117. Hobor F, Dallmann A, Ball NJ, Cicchini C, Battistelli C, Ogrodowicz RW. et al. A cryptic RNA-binding domain mediates Syncrip recognition and exosomal partitioning of miRNA targets. Nat Commun. 2018;9:831
118. Santangelo L, Giurato G, Cicchini C, Montaldo C, Mancone C, Tarallo R. et al. The RNA-Binding Protein SYNCRIP Is a Component of the Hepatocyte Exosomal Machinery Controlling MicroRNA Sorting. Cell Rep. 2016;17:799-808
119. Zietzer A, Hosen MR, Wang H, Goody PR, Sylvester M, Latz E. et al. The RNA-binding protein hnRNPU regulates the sorting of microRNA-30c-5p into large extracellular vesicles. J Extracell Vesicles. 2020;9:1786967
120. Chen C, Zheng H, Luo Y, Kong Y, An M, Li Y. et al. SUMOylation promotes extracellular vesicle-mediated transmission of lncRNA ELNAT1 and lymph node metastasis in bladder cancer. J Clin Invest. 2021;131:e146431
121. Mukherjee K, Ghoshal B, Ghosh S, Chakrabarty Y, Shwetha S, Das S. et al. Reversible HuR-microRNA binding controls extracellular export of miR-122 and augments stress response. EMBO Rep. 2016;17:1184-203
122. Yanshina DD, Kossinova OA, Gopanenko AV, Krasheninina OA, Malygin AA, Venyaminova AG. et al. Structural features of the interaction of the 3'-untranslated region of mRNA containing exosomal RNA-specific motifs with YB-1, a potential mediator of mRNA sorting. Biochimie. 2018;144:134-43
123. Shurtleff MJ, Temoche-Diaz MM, Karfilis KV, Ri S, Schekman R. Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. Elife. 2016;5:e19276
124. Guo H, Chitiprolu M, Roncevic L, Javalet C, Hemming FJ, Trung MT. et al. Atg5 Disassociates the V1V0-ATPase to Promote Exosome Production and Tumor Metastasis Independent of Canonical Macroautophagy. Dev Cell. 2017;43:716-30
125. Murrow L, Malhotra R, Debnath J. ATG12-ATG3 interacts with Alix to promote basal autophagic flux and late endosome function. Nat Cell Biol. 2015;17:300-10
126. Leidal AM, Huang HH, Marsh T, Solvik T, Zhang D, Ye J. et al. The LC3-conjugation machinery specifies the loading of RNA-binding proteins into extracellular vesicles. Nat Cell Biol. 2020;22:187-99
127. Kolak M, Westerbacka J, Velagapudi VR, Wagsater D, Yetukuri L, Makkonen J. et al. Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes. 2007;56:1960-8
128. Błachnio-Zabielska AU, Pułka M, Baranowski M, Nikołajuk A, Zabielski P, Górska M. et al. Ceramide metabolism is affected by obesity and diabetes in human adipose tissue. J Cell Physiol. 2012;227:550-7
129. Samad F, Hester KD, Yang G, Hannun YA, Bielawski J. Altered adipose and plasma sphingolipid metabolism in obesity: a potential mechanism for cardiovascular and metabolic risk. Diabetes. 2006;55:2579-87
130. Gorska M, Baranczuk E, Dobrzyn A. Secretory Zn2+-dependent sphingomyelinase activity in the serum of patients with type 2 diabetes is elevated. Horm Metab Res. 2003;35:506-7
131. Jiang M, Huang S, Duan W, Liu Q, Lei M. Inhibition of acid sphingomyelinase activity ameliorates endothelial dysfunction in db/db mice. Biosci Rep. 2019;39:1-10
132. Chakravarthy H, Navitskaya S, O'Reilly S, Gallimore J, Mize H, Beli E. et al. Role of Acid Sphingomyelinase in Shifting the Balance Between Proinflammatory and Reparative Bone Marrow Cells in Diabetic Retinopathy. Stem Cells. 2016;34:972-83
133. Kady N, Yan Y, Salazar T, Wang Q, Chakravarthy H, Huang C. et al. Increase in acid sphingomyelinase level in human retinal endothelial cells and CD34(+) circulating angiogenic cells isolated from diabetic individuals is associated with dysfunctional retinal vasculature and vascular repair process in diabetes. J Clin Lipidol. 2017;11:694-703
134. Levitsky Y, Hammer SS, Fisher KP, Huang C, Gentles TL, Pegouske DJ. et al. Mitochondrial Ceramide Effects on the Retinal Pigment Epithelium in Diabetes. Int J Mol Sci. 2020;21:3830
135. Lyn-Cook LJ, Lawton M, Tong M, Silbermann E, Longato L, Jiao P. et al. Hepatic ceramide may mediate brain insulin resistance and neurodegeneration in type 2 diabetes and non-alcoholic steatohepatitis. J Alzheimers Dis. 2009;16:715-29
136. Liu T, Duan W, Nizigiyimana P, Gao L, Liao Z, Xu B. et al. Alpha-mangostin attenuates diabetic nephropathy in association with suppression of acid sphingomyelianse and endoplasmic reticulum stress. Biochem Bioph Res Co. 2018;496:394-400
137. Murase K, Odaka H, Suzuki M, Tayuki N, Ikeda H. Pioglitazone time-dependently reduces tumour necrosis factor-alpha level in muscle and improves metabolic abnormalities in Wistar fatty rats. Diabetologia. 1998;41:257-64
138. Straczkowski M, Kowalska I, Baranowski M, Nikolajuk A, Otziomek E, Zabielski P. et al. Increased skeletal muscle ceramide level in men at risk of developing type 2 diabetes. Diabetologia. 2007;50:2366-73
139. Lei X, Zhang S, Barbour SE, Bohrer A, Ford EL, Koizumi A. et al. Spontaneous development of endoplasmic reticulum stress that can lead to diabetes mellitus is associated with higher calcium-independent phospholipase A2 expression: a role for regulation by SREBP-1. J Biol Chem. 2010;285:6693-705
140. Lei X, Zhang S, Bohrer A, Barbour SE, Ramanadham S. Role of calcium-independent phospholipase A(2)beta in human pancreatic islet beta-cell apoptosis. Am J Physiol Endocrinol Metab. 2012;303:E1386-95
141. Baranowski M, Blachnio-Zabielska A, Hirnle T, Harasiuk D, Matlak K, Knapp M. et al. Myocardium of type 2 diabetic and obese patients is characterized by alterations in sphingolipid metabolic enzymes but not by accumulation of ceramide. J Lipid Res. 2010;51:74-80
142. Olsson U, Egnell AC, Lee MR, Lunden GO, Lorentzon M, Salmivirta M. et al. Changes in matrix proteoglycans induced by insulin and fatty acids in hepatic cells may contribute to dyslipidemia of insulin resistance. Diabetes. 2001;50:2126-32
143. Wang J, Zhang Y, Guan J, Zhou L, Sheng Y, Zhang Y. et al. Enhanced syndecan-1 expression on neutrophils in patients with type 2 diabetes mellitus. Acta Diabetol. 2012;49:41-6
144. Wang JB, Guan J, Shen J, Zhou L, Zhang YJ, Si YF. et al. Insulin increases shedding of syndecan-1 in the serum of patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2009;86:83-8
145. Wang J, Zhang Y, Zhang Y, Guan J, Chen L, Fu C. et al. Negative correlation between serum syndecan-1 and apolipoprotein A1 in patients with type 2 diabetes mellitus. Acta Diabetol. 2013;50:111-5
146. Qing Q, Zhang S, Chen Y, Li R, Mao H, Chen Q. High glucose-induced intestinal epithelial barrier damage is aggravated by syndecan-1 destruction and heparanase overexpression. J Cell Mol Med. 2015;19:1366-74
147. Kolseth IB, Reine TM, Parker K, Sudworth A, Witczak BJ, Jenssen TG. et al. Increased levels of inflammatory mediators and proinflammatory monocytes in patients with type I diabetes mellitus and nephropathy. J Diabetes Complications. 2017;31:245-52
148. Svennevig K, Kolset SO, Bangstad HJ. Increased syndecan-1 in serum is related to early nephropathy in type 1 diabetes mellitus patients. Diabetologia. 2006;49:2214-6
149. Abu EA, Nawaz MI, De Hertogh G, Alam K, Siddiquei MM, Van den Eynde K. et al. S100A4 is upregulated in proliferative diabetic retinopathy and correlates with markers of angiogenesis and fibrogenesis. Mol Vis. 2014;20:1209-24
150. Abu EA, Alam K, Nawaz MI, Mohammad G, Van den Eynde K, Siddiquei MM. et al. Upregulated Expression of Heparanase in the Vitreous of Patients With Proliferative Diabetic Retinopathy Originates From Activated Endothelial Cells and Leukocytes. Invest Ophthalmol Vis Sci. 2015;56:8239-47
151. Das S, Singh G, Baker AB. Overcoming disease-induced growth factor resistance in therapeutic angiogenesis using recombinant co-receptors delivered by a liposomal system. Biomaterials. 2014;35:196-205
152. Strunz C, Roggerio A, Cruz PL, Pacanaro AP, Salemi V, Benvenuti LA. et al. Down-regulation of fibroblast growth factor 2 and its co-receptors heparan sulfate proteoglycans by resveratrol underlies the improvement of cardiac dysfunction in experimental diabetes. J Nutr Biochem. 2017;40:219-27
153. Strunz CM, Matsuda M, Salemi VM, Nogueira A, Mansur AP, Cestari IN. et al. Changes in cardiac heparan sulfate proteoglycan expression and streptozotocin-induced diastolic dysfunction in rats. Cardiovasc Diabetol. 2011;10:35
154. Fan Q, Shike T, Shigihara T, Tanimoto M, Gohda T, Makita Y. et al. Gene expression profile in diabetic KK/Ta mice. Kidney Int. 2003;64:1978-85
155. Ziolkowski AF, Popp SK, Freeman C, Parish CR, Simeonovic CJ. Heparan sulfate and heparanase play key roles in mouse beta cell survival and autoimmune diabetes. J Clin Invest. 2012;122:132-41
156. Parish CR, Freeman C, Ziolkowski AF, He YQ, Sutcliffe EL, Zafar A. et al. Unexpected new roles for heparanase in Type 1 diabetes and immune gene regulation. Matrix Biol. 2013;32:228-33
157. Simeonovic CJ, Popp SK, Starrs LM, Brown DJ, Ziolkowski AF, Ludwig B. et al. Loss of intra-islet heparan sulfate is a highly sensitive marker of type 1 diabetes progression in humans. Plos One. 2018;13:e191360
158. Song WY, Jiang XH, Ding Y, Wang Y, Zhou MX, Xia Y. et al. Inhibition of heparanase protects against pancreatic beta cell death in streptozotocin-induced diabetic mice via reducing intra-islet inflammatory cell infiltration. Br J Pharmacol. 2020;177:4433-47
159. Arfian N, Setyaningsih W, Romi MM, Sari D. Heparanase upregulation from adipocyte associates with inflammation and endothelial injury in diabetic condition. BMC Proc. 2019;13:17
160. Shafat I, Ilan N, Zoabi S, Vlodavsky I, Nakhoul F. Heparanase levels are elevated in the urine and plasma of type 2 diabetes patients and associate with blood glucose levels. Plos One. 2011;6:e17312
161. Zhao Y, Liu J, Ten S, Zhang J, Yuan Y, Yu J. et al. Plasma heparanase is associated with blood glucose levels but not urinary microalbumin excretion in type 2 diabetic nephropathy at the early stage. Renal Failure. 2017;39:698-701
162. van den Hoven MJ, Rops AL, Bakker MA, Aten J, Rutjes N, Roestenberg P. et al. Increased expression of heparanase in overt diabetic nephropathy. Kidney Int. 2006;70:2100-8
163. An X, Zhang L, Yuan Y, Wang B, Yao Q, Li L. et al. Hyperoside pre-treatment prevents glomerular basement membrane damage in diabetic nephropathy by inhibiting podocyte heparanase expression. Sci Rep. 2017;7:6413
164. Rops ALWM, van den Hoven MJ, Veldman BA, Salemink S, Vervoort G, Elving LD. et al. Urinary heparanase activity in patients with Type 1 and Type 2 diabetes. Nephrol Dial Transpl. 2012;27:2853-61
165. Maxhimer JB, Somenek M, Rao G, Pesce CE, Baldwin DJ, Gattuso P. et al. Heparanase-1 gene expression and regulation by high glucose in renal epithelial cells: a potential role in the pathogenesis of proteinuria in diabetic patients. Diabetes. 2005;54:2172-8
166. Wijnhoven TJ, van den Hoven MJ, Ding H, van Kuppevelt TH, van der Vlag J, Berden JH. et al. Heparanase induces a differential loss of heparan sulphate domains in overt diabetic nephropathy. Diabetologia. 2008;51:372-82
167. Gil N, Goldberg R, Neuman T, Garsen M, Zcharia E, Rubinstein AM. et al. Heparanase is essential for the development of diabetic nephropathy in mice. Diabetes. 2012;61:208-16
168. Abu EA, Siddiquei MM, Nawaz MI, De Hertogh G, Mohammad G, Alam K. et al. Coexpression of heparanase activity, cathepsin L, tissue factor, tissue factor pathway inhibitor, and MMP-9 in proliferative diabetic retinopathy. Mol Vis. 2016;22:424-35
169. Ma P, Luo Y, Zhu X, Li T, Hu J, Tang S. Retinal heparanase expression in streptozotocin-induced diabetic rats. Can J Ophthalmol. 2010;45:46-51
170. Rao G, Ding HG, Huang W, Le D, Maxhimer JB, Oosterhof A. et al. Reactive oxygen species mediate high glucose-induced heparanase-1 production and heparan sulphate proteoglycan degradation in human and rat endothelial cells: a potential role in the pathogenesis of atherosclerosis. Diabetologia. 2011;54:1527-38
171. Dong LY, Yao LP, Zhao J, Jin KK, Qiu XX. Captopril inhibits calpainmediated apoptosis of myocardial cells in diabetic rats and improves cardiac function. Mol Med Rep. 2018;18:2300-6
172. Li Y, Ma J, Zhu H, Singh M, Hill D, Greer PA. et al. Targeted Inhibition of Calpain Reduces Myocardial Hypertrophy and Fibrosis in Mouse Models of Type 1 Diabetes. Diabetes. 2011;60:2985-94
173. Ni R, Zheng D, Xiong S, Hill DJ, Sun T, Gardiner RB. et al. Mitochondrial Calpain-1 Disrupts ATP Synthase and Induces Superoxide Generation in Type 1 Diabetic Hearts: A Novel Mechanism Contributing to Diabetic Cardiomyopathy. Diabetes. 2016;65:255-68
174. Li Y, Li Y, Feng Q, Arnold M, Peng T. Calpain activation contributes to hyperglycaemia-induced apoptosis in cardiomyocytes. Cardiovasc Res. 2009;84:100-10
175. Li S, Zhang L, Ni R, Cao T, Zheng D, Xiong S. et al. Disruption of calpain reduces lipotoxicity-induced cardiac injury by preventing endoplasmic reticulum stress. Biochim Biophys Acta. 2016;1862:2023-33
176. Chen B, Zhao Q, Ni R, Tang F, Shan L, Cepinskas I. et al. Inhibition of calpain reduces oxidative stress and attenuates endothelial dysfunction in diabetes. Cardiovasc Diabetol. 2014;13:88
177. Randriamboavonjy V, Pistrosch F, Bolck B, Schwinger RH, Dixit M, Badenhoop K. et al. Platelet sarcoplasmic endoplasmic reticulum Ca2+-ATPase and mu-calpain activity are altered in type 2 diabetes mellitus and restored by rosiglitazone. Circulation. 2008;117:52-60
178. Elgheznawy A, Shi L, Hu J, Wittig I, Laban H, Pircher J. et al. Dicer cleavage by calpain determines platelet microRNA levels and function in diabetes. Circ Res. 2015;117:157-65
179. Randriamboavonjy V, Isaak J, Elgheznawy A, Pistrosch F, Fromel T, Yin X. et al. Calpain inhibition stabilizes the platelet proteome and reactivity in diabetes. Blood. 2012;120:415-23
180. Kyselova A, Elgheznawy A, Wittig I, Heidler J, Mann AW, Ruf W. et al. Platelet-derived calpain cleaves the endothelial protease-activated receptor 1 to induce vascular inflammation in diabetes. Basic Res Cardiol. 2020;115:75
181. Giannella A, Ceolotto G, Radu CM, Cattelan A, Iori E, Benetti A. et al. PAR-4/Ca(2+)-calpain pathway activation stimulates platelet-derived microparticles in hyperglycemic type 2 diabetes. Cardiovasc Diabetol. 2021;20:77
182. Kharatmal SB, Singh JN, Sharma SS. Calpain inhibitor, MDL 28170 confer electrophysiological, nociceptive and biochemical improvement in diabetic neuropathy. Neuropharmacology. 2015;97:113-21
183. Li H, Chen LP, Wang T, Wang SG, Liu JH. Calpain inhibition improves erectile function in diabetic mice via upregulating endothelial nitric oxide synthase expression and reducing apoptosis. Asian J Androl. 2018;20:342-8
184. Ahn YJ, Kim MS, Chung SK. Calpain and Caspase-12 Expression in Lens Epithelial Cells of Diabetic Cataracts. Am J Ophthalmol. 2016;167:31-7
185. Ni R, Zheng D, Xiong S, Hill DJ, Sun T, Gardiner RB. et al. Mitochondrial Calpain-1 Disrupts ATP Synthase and Induces Superoxide Generation in Type 1 Diabetic Hearts: A Novel Mechanism Contributing to Diabetic Cardiomyopathy. Diabetes. 2016;65:255-68
186. Smolock AR, Mishra G, Eguchi K, Eguchi S, Scalia R. Protein kinase C upregulates intercellular adhesion molecule-1 and leukocyte-endothelium interactions in hyperglycemia via activation of endothelial expressed calpain. Arterioscler Thromb Vasc Biol. 2011;31:289-96
187. Stalker TJ, Gong Y, Scalia R. The calcium-dependent protease calpain causes endothelial dysfunction in type 2 diabetes. Diabetes. 2005;54:1132-40
188. Shanab AY, Nakazawa T, Ryu M, Tanaka Y, Himori N, Taguchi K. et al. Metabolic stress response implicated in diabetic retinopathy: the role of calpain, and the therapeutic impact of calpain inhibitor. Neurobiol Dis. 2012;48:556-67
189. Ling C, Groop L, Guerra SD, Lupi R. Calpain-10 expression is elevated in pancreatic islets from patients with type 2 diabetes. Plos One. 2009;4:e6558
190. Wang T, Gao Y, Wang X, Shi Y, Xu J, Wu B. et al. Calpain-10 drives podocyte apoptosis and renal injury in diabetic nephropathy. Diabetes Metab Syndr Obes. 2019;12:1811-20
191. Covington MD, Schnellmann RG. Chronic high glucose downregulates mitochondrial calpain 10 and contributes to renal cell death and diabetes-induced renal injury. Kidney Int. 2012;81:391-400
192. Zhang W, Khan A, Ostenson CG, Berggren PO, Efendic S, Meister B. Down-regulated expression of exocytotic proteins in pancreatic islets of diabetic GK rats. Biochem Biophys Res Commun. 2002;291:1038-44
193. Nagamatsu S, Nakamichi Y, Yamamura C, Matsushima S, Watanabe T, Ozawa S. et al. Decreased expression of t-SNARE, syntaxin 1, and SNAP-25 in pancreatic beta-cells is involved in impaired insulin secretion from diabetic GK rat islets: restoration of decreased t-SNARE proteins improves impaired insulin secretion. Diabetes. 1999;48:2367-73
194. Ostenson CG, Gaisano H, Sheu L, Tibell A, Bartfai T. Impaired gene and protein expression of exocytotic soluble N-ethylmaleimide attachment protein receptor complex proteins in pancreatic islets of type 2 diabetic patients. Diabetes. 2006;55:435-40
195. Chan CB, MacPhail RM, Sheu L, Wheeler MB, Gaisano HY. Beta-cell hypertrophy in fa/fa rats is associated with basal glucose hypersensitivity and reduced SNARE protein expression. Diabetes. 1999;48:997-1005
196. Rezaei FA, Saidijam M, Goodarzi MT, Yadegar AR, Asadi S, Zarei S. et al. Effect of Resveratrol Supplementation on the SNARE Proteins Expression in Adipose Tissue of Stroptozotocin-Nicotinamide Induced Type 2 Diabetic Rats. Iran J Med Sci. 2015;40:248-55
197. Maier VH, Melvin DR, Lister CA, Chapman H, Gould GW, Murphy GJ. v- and t-SNARE protein expression in models of insulin resistance: normalization of glycemia by rosiglitazone treatment corrects overexpression of cellubrevin, vesicle-associated membrane protein-2, and syntaxin 4 in skeletal muscle of Zucker diabetic fatty rats. Diabetes. 2000;49:618-25
198. Gaspar JM, Baptista FI, Galvao J, Castilho AF, Cunha RA, Ambrosio AF. Diabetes differentially affects the content of exocytotic proteins in hippocampal and retinal nerve terminals. Neuroscience. 2010;169:1589-600
199. Hirai H, Miura J, Hu Y, Larsson H, Larsson K, Lernmark A. et al. Selective screening of secretory vesicle-associated proteins for autoantigens in type 1 diabetes: VAMP2 and NPY are new minor autoantigens. Clin Immunol. 2008;127:366-74
200. Gawlowski T, Stratmann B, Ruetter R, Buenting CE, Menart B, Weiss J. et al. Advanced glycation end products strongly activate platelets. Eur J Nutr. 2009;48:475-81
201. Fateh-Moghadam S, Li Z, Ersel S, Reuter T, Htun P, Plockinger U. et al. Platelet degranulation is associated with progression of intima-media thickness of the common carotid artery in patients with diabetes mellitus type 2. Arterioscler Thromb Vasc Biol. 2005;25:1299-303
202. Zhang RD, Shi M. Occurrence and development of diabetic nephropathy caused by CD63 by inhibiting Wnt-beta-catenin signaling pathway. Eur Rev Med Pharmacol Sci. 2020;24:284-94
203. Cho MK, Kwon SB, Kim CH, Lee YJ, Nam HS, Lee SH. Overexpression of KAI1 Protein in Diabetic Skin Tissues. Arch Plast Surg. 2014;41:248-52
204. Li X, Zeng L, Cao C, Lu C, Lian W, Han J. et al. Long noncoding RNA MALAT1 regulates renal tubular epithelial pyroptosis by modulated miR-23c targeting of ELAVL1 in diabetic nephropathy. Exp Cell Res. 2017;350:327-35
205. Guo J, Lei M, Cheng F, Liu Y, Zhou M, Zheng W. et al. RNA-binding proteins tristetraprolin and human antigen R are novel modulators of podocyte injury in diabetic kidney disease. Cell Death Dis. 2020;11:413
206. Shang J, Wan Q, Wang X, Duan Y, Wang Z, Wei X. et al. Identification of NOD2 as a novel target of RNA-binding protein HuR: evidence from NADPH oxidase-mediated HuR signaling in diabetic nephropathy. Free Radic Biol Med. 2015;79:217-27
207. Yu C, Xin W, Zhen J, Liu Y, Javed A, Wang R. et al. Human antigen R mediated post-transcriptional regulation of epithelial-mesenchymal transition related genes in diabetic nephropathy. J Diabetes. 2015;7:562-72
208. Amadio M, Bucolo C, Leggio GM, Drago F, Govoni S, Pascale A. The PKCbeta/HuR/VEGF pathway in diabetic retinopathy. Biochem Pharmacol. 2010;80:1230-7
209. Govindappa PK, Patil M, Garikipati V, Verma SK, Saheera S, Narasimhan G. et al. Targeting exosome-associated human antigen R attenuates fibrosis and inflammation in diabetic heart. Faseb J. 2020;34:2238-51
210. Jeyabal P, Thandavarayan RA, Joladarashi D, Suresh BS, Krishnamurthy S, Bhimaraj A. et al. MicroRNA-9 inhibits hyperglycemia-induced pyroptosis in human ventricular cardiomyocytes by targeting ELAVL1. Biochem Biophys Res Commun. 2016;471:423-9
211. Good AL, Haemmerle MW, Oguh AU, Doliba NM, Stoffers DA. Metabolic stress activates an ERK/hnRNPK/DDX3X pathway in pancreatic beta cells. Mol Metab. 2019;26:45-56
212. Abdo S, Lo CS, Chenier I, Shamsuyarova A, Filep JG, Ingelfinger JR. et al. Heterogeneous nuclear ribonucleoproteins F and K mediate insulin inhibition of renal angiotensinogen gene expression and prevention of hypertension and kidney injury in diabetic mice. Diabetologia. 2013;56:1649-60
213. Ouahoud S, Fiet MD, Martinez-Montanes F, Ejsing CS, Kuss O, Roden M. et al. Lipid droplet consumption is functionally coupled to vacuole homeostasis independent of lipophagy. J Cell Sci. 2018 131
214. Romero M, Sabate-Perez A, Francis VA, Castrillon-Rodriguez I, Diaz-Ramos A, Sanchez-Feutrie M. et al. TP53INP2 regulates adiposity by activating beta-catenin through autophagy-dependent sequestration of GSK3beta. Nat Cell Biol. 2018;20:443-54
215. Koumanov F, Pereira VJ, Whitley PR, Holman GD. GLUT4 traffic through an ESCRT-III-dependent sorting compartment in adipocytes. Plos One. 2012;7:e44141
216. Yan C, Tian X, Li J, Liu D, Ye D, Xie Z. et al. A High-Fat Diet Attenuates AMPK alpha1 in Adipocytes to Induce Exosome Shedding and Nonalcoholic Fatty Liver Development In Vivo. Diabetes. 2021;70:577-88
217. Wang X, Gu H, Huang W, Peng J, Li Y, Yang L. et al. Hsp20-Mediated Activation of Exosome Biogenesis in Cardiomyocytes Improves Cardiac Function and Angiogenesis in Diabetic Mice. Diabetes. 2016;65:3111-28
218. Yoon S, Kovalenko A, Bogdanov K, Wallach D. MLKL, the Protein that Mediates Necroptosis, Also Regulates Endosomal Trafficking and Extracellular Vesicle Generation. Immunity. 2017;47:51-65
219. Gong YN, Guy C, Olauson H, Becker JU, Yang M, Fitzgerald P. et al. ESCRT-III Acts Downstream of MLKL to Regulate Necroptotic Cell Death and Its Consequences. Cell. 2017;169:286-300
220. Xu H, Du X, Liu G, Huang S, Du W, Zou S. et al. The pseudokinase MLKL regulates hepatic insulin sensitivity independently of inflammation. Mol Metab. 2019;23:14-23
221. Kang P, Wang J, Fang D, Fang T, Yu Y, Zhang W. et al. Activation of ALDH2 attenuates high glucose induced rat cardiomyocyte fibrosis and necroptosis. Free Radic Biol Med. 2020;146:198-210
222. Xu Y, Gao H, Hu Y, Fang Y, Qi C, Huang J. et al. High glucose-induced apoptosis and necroptosis in podocytes is regulated by UCHL1 via RIPK1/RIPK3 pathway. Exp Cell Res. 2019;382:111463
223. LaRocca TJ, Sosunov SA, Shakerley NL, Ten VS, Ratner AJ. Hyperglycemic Conditions Prime Cells for RIP1-dependent Necroptosis. J Biol Chem. 2016;291:13753-61
224. Malekpour-Dehkordi Z, Teimourian S, Nourbakhsh M, Naghiaee Y, Sharifi R, Mohiti-Ardakani J. Metformin reduces fibrosis factors in insulin resistant and hypertrophied adipocyte via integrin/ERK, collagen VI, apoptosis, and necrosis reduction. Life Sci. 2019;233:116682
225. Reddy VS, Kumar C, Raghu G, Reddy GB. Expression and induction of small heat shock proteins in rat heart under chronic hyperglycemic conditions. Arch Biochem Biophys. 2014;558:1-9
226. Bianco F, Perrotta C, Novellino L, Francolini M, Riganti L, Menna E. et al. Acid sphingomyelinase activity triggers microparticle release from glial cells. Embo J. 2009;28:1043-54
227. Haus JM, Kashyap SR, Kasumov T, Zhang R, Kelly KR, Defronzo RA. et al. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes. 2009;58:337-43
228. Park JW, Park WJ, Kuperman Y, Boura-Halfon S, Pewzner-Jung Y, Futerman AH. Ablation of very long acyl chain sphingolipids causes hepatic insulin resistance in mice due to altered detergent-resistant membranes. Hepatology. 2013;57:525-32
229. Blouin CM, Prado C, Takane KK, Lasnier F, Garcia-Ocana A, Ferre P. et al. Plasma membrane subdomain compartmentalization contributes to distinct mechanisms of ceramide action on insulin signaling. Diabetes. 2010;59:600-10
230. Sen P, Dickens AM, Lopez-Bascon MA, Lindeman T, Kemppainen E, Lamichhane S. et al. Metabolic alterations in immune cells associate with progression to type 1 diabetes. Diabetologia. 2020;63:1017-31
231. Turpin-Nolan SM, Bruning JC. The role of ceramides in metabolic disorders: when size and localization matters. Nat Rev Endocrinol. 2020;16:224-33
232. Choi RH, Tatum SM, Symons JD, Summers SA, Holland WL. Ceramides and other sphingolipids as drivers of cardiovascular disease. Nat Rev Cardiol. 2021
233. Chaurasia B, Summers SA. Ceramides in Metabolism: Key Lipotoxic Players. Annu Rev Physiol. 2021;83:303-30
234. Chaurasia B, Tippetts TS, Mayoral MR, Liu J, Li Y, Wang L. et al. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science. 2019;365:386-92
235. Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A. et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14:677-85
236. Roucourt B, Meeussen S, Bao J, Zimmermann P, David G. Heparanase activates the syndecan-syntenin-ALIX exosome pathway. Cell Res. 2015;25:412-28
237. Bodhini D, Radha V, Ghosh S, Sanapala KR, Majumder PP, Rao MR. et al. Association of calpain 10 gene polymorphisms with type 2 diabetes mellitus in Southern Indians. Metabolism. 2011;60:681-8
238. Kifagi C, Makni K, Mnif F, Boudawara M, Hamza N, Rekik N. et al. Association of calpain-10 polymorphisms with type 2 diabetes in the Tunisian population. Diabetes Metab. 2008;34:273-8
239. Horikawa Y, Oda N, Cox NJ, Li X, Orho-Melander M, Hara M. et al. Genetic variation in the gene encoding calpain-10 is associated with type 2 diabetes mellitus. Nat Genet. 2000;26:163-75
240. Cassell PG, Jackson AE, North BV, Evans JC, Syndercombe-Court D, Phillips C. et al. Haplotype combinations of calpain 10 gene polymorphisms associate with increased risk of impaired glucose tolerance and type 2 diabetes in South Indians. Diabetes. 2002;51:1622-8
241. Weedon MN, Schwarz PE, Horikawa Y, Iwasaki N, Illig T, Holle R. et al. Meta-analysis and a large association study confirm a role for calpain-10 variation in type 2 diabetes susceptibility. Am J Hum Genet. 2003;73:1208-12
242. Garant MJ, Kao WH, Brancati F, Coresh J, Rami TM, Hanis CL. et al. SNP43 of CAPN10 and the risk of type 2 Diabetes in African-Americans: the Atherosclerosis Risk in Communities Study. Diabetes. 2002;51:231-7
243. Evans JC, Frayling TM, Cassell PG, Saker PJ, Hitman GA, Walker M. et al. Studies of association between the gene for calpain-10 and type 2 diabetes mellitus in the United Kingdom. Am J Hum Genet. 2001;69:544-52
244. Rasmussen SK, Urhammer SA, Berglund L, Jensen JN, Hansen L, Echwald SM. et al. Variants within the calpain-10 gene on chromosome 2q37 (NIDDM1) and relationships to type 2 diabetes, insulin resistance, and impaired acute insulin secretion among Scandinavian Caucasians. Diabetes. 2002;51:3561-7
245. Buraczynska M, Wacinski P, Stec A, Kuczmaszewska A. Calpain-10 gene polymorphisms in type 2 diabetes and its micro- and macrovascular complications. J Diabetes Complications. 2013;27:54-8
246. Sreenan SK, Zhou YP, Otani K, Hansen PA, Currie KP, Pan CY. et al. Calpains play a role in insulin secretion and action. Diabetes. 2001;50:2013-20
247. Huang CJ, Gurlo T, Haataja L, Costes S, Daval M, Ryazantsev S. et al. Calcium-activated calpain-2 is a mediator of beta cell dysfunction and apoptosis in type 2 diabetes. J Biol Chem. 2010;285:339-48
248. Yuasa T, Amo-Shiinoki K, Ishikura S, Takahara M, Matsuoka T, Kaneto H. et al. Sequential cleavage of insulin receptor by calpain 2 and gamma-secretase impairs insulin signalling. Diabetologia. 2016;59:2711-21
249. Oh E, Ahn M, Afelik S, Becker TC, Roep BO, Thurmond DC. Syntaxin 4 Expression in Pancreatic beta-Cells Promotes Islet Function and Protects Functional beta-Cell Mass. Diabetes. 2018;67:2626-39
250. Wheeler SE, Stacey HM, Nahaei Y, Hale SJ, Hardy AB, Reimann F. et al. The SNARE Protein Syntaxin-1a Plays an Essential Role in Biphasic Exocytosis of the Incretin Hormone Glucagon-Like Peptide 1. Diabetes. 2017;66:2327-38
251. Bogan JS. Regulation of glucose transporter translocation in health and diabetes. Annu Rev Biochem. 2012;81:507-32
252. Bostrom P, Andersson L, Li L, Perkins R, Hojlund K, Boren J. et al. The assembly of lipid droplets and its relation to cellular insulin sensitivity. Biochem Soc Trans. 2009;37:981-5
253. Burger D, Turner M, Xiao F, Munkonda MN, Akbari S, Burns KD. High glucose increases the formation and pro-oxidative activity of endothelial microparticles. Diabetologia. 2017;60:1791-800
254. Wu SF, Noren HN, Freeman DW, Mode NA, Zonderman AB, Evans MK. Extracellular vesicles in diabetes mellitus induce alterations in endothelial cell morphology and migration. J Transl Med. 2020;18:230
255. Raimondo F, Corbetta S, Morosi L, Chinello C, Gianazza E, Castoldi G. et al. Urinary exosomes and diabetic nephropathy: a proteomic approach. Mol Biosyst. 2013;9:1139-46
256. Lee JE, Moon PG, Lee IK, Baek MC. Proteomic Analysis of Extracellular Vesicles Released by Adipocytes of Otsuka Long-Evans Tokushima Fatty (OLETF) Rats. Protein J. 2015;34:220-35
257. Zubiri I, Posada-Ayala M, Sanz-Maroto A, Calvo E, Martin-Lorenzo M, Gonzalez-Calero L. et al. Diabetic nephropathy induces changes in the proteome of human urinary exosomes as revealed by label-free comparative analysis. J Proteomics. 2014;96:92-102
258. Pasquier A, Vivot K, Erbs E, Spiegelhalter C, Zhang Z, Aubert V. et al. Lysosomal degradation of newly formed insulin granules contributes to beta cell failure in diabetes. Nat Commun. 2019;10:3312
259. Zhao L, Bartnikas T, Chu X, Klein J, Yun C, Srinivasan S. et al. Hyperglycemia promotes microvillus membrane expression of DMT1 in intestinal epithelial cells in a PKCalpha-dependent manner. Faseb J. 2019;33:3549-61
260. Li Y, Hu Q, Li C, Liang K, Xiang Y, Hsiao H. et al. PTEN-induced partial epithelial-mesenchymal transition drives diabetic kidney disease. J Clin Invest. 2019;129:1129-51
261. Sun LN, Liu XC, Chen XJ, Guan GJ, Liu G. Curcumin attenuates high glucose-induced podocyte apoptosis by regulating functional connections between caveolin-1 phosphorylation and ROS. Acta Pharmacol Sin. 2016;37:645-55
262. Sun LN, Chen ZX, Liu XC, Liu HY, Guan GJ, Liu G. Curcumin ameliorates epithelial-to-mesenchymal transition of podocytes in vivo and in vitro via regulating caveolin-1. Biomed Pharmacother. 2014;68:1079-88
263. Wu SZ, Peng FF, Li JL, Ye F, Lei SQ, Zhang BF. Akt and RhoA activation in response to high glucose require caveolin-1 phosphorylation in mesangial cells. Am J Physiol Renal Physiol. 2014;306:F1308-17
264. Jin J, Peng C, Wu SZ, Chen HM, Zhang BF. Blocking VEGF/Caveolin-1 signaling contributes to renal protection of fasudil in streptozotocin-induced diabetic rats. Acta Pharmacol Sin. 2015;36:831-40
265. Sun LN, Yang ZY, Lv SS, Liu XC, Guan GJ, Liu G. Curcumin prevents diabetic nephropathy against inflammatory response via reversing caveolin-1 Tyr14 phosphorylation influenced TLR4 activation. Int Immunopharmacol. 2014;23:236-46
266. Ishida K, Taguchi K, Hida M, Watanabe S, Kawano K, Matsumoto T. et al. Circulating microparticles from diabetic rats impair endothelial function and regulate endothelial protein expression. Acta Physiol (Oxf). 2016;216:211-20
267. Kim H, Bae YU, Jeon JS, Noh H, Park HK, Byun DW. et al. The circulating exosomal microRNAs related to albuminuria in patients with diabetic nephropathy. J Transl Med. 2019;17:236
268. Castano C, Kalko S, Novials A, Parrizas M. Obesity-associated exosomal miRNAs modulate glucose and lipid metabolism in mice. Proc Natl Acad Sci U S A. 2018;115:12158-63
269. Ling L, Tan Z, Zhang C, Gui S, Cui Y, Hu Y. et al. CircRNAs in exosomes from high glucose-treated glomerular endothelial cells activate mesangial cells. Am J Transl Res. 2019;11:4667-82
270. Krishnan P, Syed F, Jiyun KN, Mirmira RG, Evans-Molina C. Profiling of RNAs from Human Islet-Derived Exosomes in a Model of Type 1 Diabetes. Int J Mol Sci. 2019;20:5903
271. Dai B, Li H, Fan J, Zhao Y, Yin Z, Nie X. et al. MiR-21 protected against diabetic cardiomyopathy induced diastolic dysfunction by targeting gelsolin. Cardiovasc Diabetol. 2018;17:123
272. Pofi R, Giannetta E, Galea N, Francone M, Campolo F, Barbagallo F. et al. Diabetic Cardiomiopathy Progression is Triggered by miR122-5p and Involves Extracellular Matrix: A 5-Year Prospective Study. JACC Cardiovasc Imaging. 2021;14:1130-42
273. Al-Hayali MA, Sozer V, Durmus S, Erdenen F, Altunoglu E, Gelisgen R. et al. Clinical Value of Circulating Microribonucleic Acids miR-1 and miR-21 in Evaluating the Diagnosis of Acute Heart Failure in Asymptomatic Type 2 Diabetic Patients. Biomolecules. 2019;9:193
274. Pastukh N, Meerson A, Kalish D, Jabaly H, Blum A. Serum miR-122 levels correlate with diabetic retinopathy. Clin Exp Med. 2019;19:255-60
275. Lu JM, Zhang ZZ, Ma X, Fang SF, Qin XH. Repression of microRNA-21 inhibits retinal vascular endothelial cell growth and angiogenesis via PTEN dependent-PI3K/Akt/VEGF signaling pathway in diabetic retinopathy. Exp Eye Res. 2020;190:107886
276. Liu L, Wang Y, Yan R, Liang L, Zhou X, Liu H. et al. BMP-7 inhibits renal fibrosis in diabetic nephropathy via miR-21 downregulation. Life Sci. 2019;238:116957
277. Lou HD, Wang SY, Guo T, Yang Y. Role of miR-21 in rats with proliferative diabetic retinopathy via TGF-beta signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23:9-16
278. Mazzeo A, Lopatina T, Gai C, Trento M, Porta M, Beltramo E. Functional analysis of miR-21-3p, miR-30b-5p and miR-150-5p shuttled by extracellular vesicles from diabetic subjects reveals their association with diabetic retinopathy. Exp Eye Res. 2019;184:56-63
279. Mazzeo A, Beltramo E, Lopatina T, Gai C, Trento M, Porta M. Molecular and functional characterization of circulating extracellular vesicles from diabetic patients with and without retinopathy and healthy subjects. Exp Eye Res. 2018;176:69-77
280. Mleczko J, Ortega FJ, Falcon-Perez JM, Wabitsch M, Fernandez-Real JM, Mora S. Extracellular Vesicles from Hypoxic Adipocytes and Obese Subjects Reduce Insulin-Stimulated Glucose Uptake. Mol Nutr Food Res. 2018;62:1700917
281. Sano S, Izumi Y, Yamaguchi T, Yamazaki T, Tanaka M, Shiota M. et al. Lipid synthesis is promoted by hypoxic adipocyte-derived exosomes in 3T3-L1 cells. Biochem Biophys Res Commun. 2014;445:327-33
282. Muller G, Jung C, Straub J, Wied S, Kramer W. Induced release of membrane vesicles from rat adipocytes containing glycosylphosphatidylinositol-anchored microdomain and lipid droplet signalling proteins. Cell Signal. 2009;21:324-38
283. Muller G, Schneider M, Biemer-Daub G, Wied S. Microvesicles released from rat adipocytes and harboring glycosylphosphatidylinositol-anchored proteins transfer RNA stimulating lipid synthesis. Cell Signal. 2011;23:1207-23
284. Muller G, Schneider M, Biemer-Daub G, Wied S. Upregulation of lipid synthesis in small rat adipocytes by microvesicle-associated CD73 from large adipocytes. Obesity (Silver Spring). 2011;19:1531-44
285. Gesmundo I, Pardini B, Gargantini E, Gamba G, Birolo G, Fanciulli A. et al. Adipocyte-derived extracellular vesicles regulate survival and function of pancreatic beta cells. JCI Insight. 2021;6:e141962
286. Flaherty SR, Grijalva A, Xu X, Ables E, Nomani A, Ferrante AJ. A lipase-independent pathway of lipid release and immune modulation by adipocytes. Science. 2019;363:989-93
287. Camino T, Lago-Baameiro N, Bravo SB, Sueiro A, Couto I, Santos F. et al. Vesicles Shed by Pathological Murine Adipocytes Spread Pathology: Characterization and Functional Role of Insulin Resistant/Hypertrophied Adiposomes. Int J Mol Sci. 2020;21:2252
288. Zhang B, Yang Y, Xiang L, Zhao Z, Ye R. Adipose-derived exosomes: A novel adipokine in obesity-associated diabetes. J Cell Physiol. 2019;234:16692-702
289. Rong B, Feng R, Liu C, Wu Q, Sun C. Reduced delivery of epididymal adipocyte-derived exosomal resistin is essential for melatonin ameliorating hepatic steatosis in mice. J Pineal Res. 2019;66:e12561
290. Yu Y, Du H, Wei S, Feng L, Li J, Yao F. et al. Adipocyte-Derived Exosomal MiR-27a Induces Insulin Resistance in Skeletal Muscle Through Repression of PPARgamma. Theranostics. 2018;8:2171-88
291. Gao J, Li X, Wang Y, Cao Y, Yao D, Sun L. et al. Adipocyte-derived extracellular vesicles modulate appetite and weight through mTOR signalling in the hypothalamus. Acta Physiol (Oxf). 2020;228:e13339
292. Gu H, Yang K, Shen Z, Jia K, Liu P, Pan M. et al. ER stress-induced adipocytes secrete-aldo-keto reductase 1B7-containing exosomes that cause nonalcoholic steatohepatitis in mice. Free Radic Biol Med. 2021;163:220-33
293. Dang SY, Leng Y, Wang ZX, Xiao X, Zhang X, Wen T. et al. Exosomal transfer of obesity adipose tissue for decreased miR-141-3p mediate insulin resistance of hepatocytes. Int J Biol Sci. 2019;15:351-68
294. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175-84
295. Deng ZB, Poliakov A, Hardy RW, Clements R, Liu C, Liu Y. et al. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes. 2009;58:2498-505
296. Zhang Y, Mei H, Chang X, Chen F, Zhu Y, Han X. Adipocyte-derived microvesicles from obese mice induce M1 macrophage phenotype through secreted miR-155. J Mol Cell Biol. 2016;8:505-17
297. Pan Y, Hui X, Hoo R, Ye D, Chan C, Feng T. et al. Adipocyte-secreted exosomal microRNA-34a inhibits M2 macrophage polarization to promote obesity-induced adipose inflammation. J Clin Invest. 2019;129:834-49
298. Song M, Han L, Chen FF, Wang D, Wang F, Zhang L. et al. Adipocyte-Derived Exosomes Carrying Sonic Hedgehog Mediate M1 Macrophage Polarization-Induced Insulin Resistance via Ptch and PI3K Pathways. Cell Physiol Biochem. 2018;48:1416-32
299. Renovato-Martins M, Matheus ME, de Andrade IR, Moraes JA, Da SS, Citelli DRM. et al. Microparticles derived from obese adipose tissue elicit a pro-inflammatory phenotype of CD16(+), CCR5(+) and TLR8(+) monocytes. Biochim Biophys Acta Mol Basis Dis. 2017;1863:139-51
300. Eguchi A, Mulya A, Lazic M, Radhakrishnan D, Berk MP, Povero D. et al. Microparticles release by adipocytes act as "find-me" signals to promote macrophage migration. Plos One. 2015;10:e123110
301. Crewe C, Joffin N, Rutkowski JM, Kim M, Zhang F, Towler DA. et al. An Endothelial-to-Adipocyte Extracellular Vesicle Axis Governed by Metabolic State. Cell. 2018;175:695-708
302. Wadey RM, Connolly KD, Mathew D, Walters G, Rees DA, James PE. Inflammatory adipocyte-derived extracellular vesicles promote leukocyte attachment to vascular endothelial cells. Atherosclerosis. 2019;283:19-27
303. Wang F, Chen FF, Shang YY, Li Y, Wang ZH, Han L. et al. Insulin resistance adipocyte-derived exosomes aggravate atherosclerosis by increasing vasa vasorum angiogenesis in diabetic ApoE(-/-) mice. Int J Cardiol. 2018;265:181-7
304. Li X, Ballantyne LL, Yu Y, Funk CD. Perivascular adipose tissue-derived extracellular vesicle miR-221-3p mediates vascular remodeling. Faseb J. 2019;33:12704-22
305. Gan L, Xie D, Liu J, Bond LW, Christopher TA, Lopez B. et al. Small Extracellular Microvesicles Mediated Pathological Communications Between Dysfunctional Adipocytes and Cardiomyocytes as a Novel Mechanism Exacerbating Ischemia/Reperfusion Injury in Diabetic Mice. Circulation. 2020;141:968-83
306. Song Y, Li H, Ren X, Li H, Feng C. SNHG9, delivered by adipocyte-derived exosomes, alleviates inflammation and apoptosis of endothelial cells through suppressing TRADD expression. Eur J Pharmacol. 2020;872:172977
307. Wadey RM, Connolly KD, Mathew D, Walters G, Rees DA, James PE. Inflammatory adipocyte-derived extracellular vesicles promote leukocyte attachment to vascular endothelial cells. Atherosclerosis. 2019;283:19-27
308. Xie Z, Wang X, Liu X, Du H, Sun C, Shao X. et al. Adipose-Derived Exosomes Exert Proatherogenic Effects by Regulating Macrophage Foam Cell Formation and Polarization. J Am Heart Assoc. 2018;7:e7442
309. Lackey DE, Olefsky JM. Regulation of metabolism by the innate immune system. Nat Rev Endocrinol. 2016;12:15-28
310. Liu T, Sun YC, Cheng P, Shao HG. Adipose tissue macrophage-derived exosomal miR-29a regulates obesity-associated insulin resistance. Biochem Biophys Res Commun. 2019;515:352-8
311. Ying W, Riopel M, Bandyopadhyay G, Dong Y, Birmingham A, Seo JB. et al. Adipose Tissue Macrophage-Derived Exosomal miRNAs Can Modulate In Vivo and In Vitro Insulin Sensitivity. Cell. 2017;171:372-84
312. Tian F, Tang P, Sun Z, Zhang R, Zhu D, He J. et al. miR-210 in Exosomes Derived from Macrophages under High Glucose Promotes Mouse Diabetic Obesity Pathogenesis by Suppressing NDUFA4 Expression. J Diabetes Res. 2020;2020:6894684
313. Zhang Y, Shi L, Mei H, Zhang J, Zhu Y, Han X. et al. Inflamed macrophage microvesicles induce insulin resistance in human adipocytes. Nutr Metab (Lond). 2015;12:21
314. Zhu QJ, Zhu M, Xu XX, Meng XM, Wu YG. Exosomes from high glucose-treated macrophages activate glomerular mesangial cells via TGF-beta1/Smad3 pathway in vivo and in vitro. Faseb J. 2019;33:9279-90
315. Zhu M, Sun X, Qi X, Xia L, Wu Y. Exosomes from high glucose-treated macrophages activate macrophages andinduce inflammatory responses via NF-kappaB signaling pathway in vitro and in vivo. Int Immunopharmacol. 2020;84:106551
316. Kawakami R, Katsuki S, Travers R, Romero DC, Becker-Greene D, Passos LSA. et al. S100A9-RAGE Axis Accelerates Formation of Macrophage-Mediated Extracellular Vesicle Microcalcification in Diabetes Mellitus. Arteriosclerosis, Thrombosis, and Vascular Biology. 2020;40:1838-53
317. Ding X, Jing N, Shen A, Guo F, Song Y, Pan M. et al. MiR-21-5p in macrophage-derived extracellular vesicles affects podocyte pyroptosis in diabetic nephropathy by regulating A20. J Endocrinol Invest. 2021;44:1175-84
318. Bouchareychas L, Duong P, Phu TA, Alsop E, Meechoovet B, Reiman R. et al. High glucose macrophage exosomes enhance atherosclerosis by driving cellular proliferation & hematopoiesis. iScience. 2021;24:102847
319. Govindappa PK, Patil M, Garikipati V, Verma SK, Saheera S, Narasimhan G. et al. Targeting exosome-associated human antigen R attenuates fibrosis and inflammation in diabetic heart. Faseb J. 2020;34:2238-51
320. Nair N, Kumar S, Gongora E, Gupta S. Circulating miRNA as novel markers for diastolic dysfunction. Mol Cell Biochem. 2013;376:33-40
321. Liu Y, Song JW, Lin JY, Miao R, Zhong JC. Roles of MicroRNA-122 in Cardiovascular Fibrosis and Related Diseases. Cardiovasc Toxicol. 2020;20:463-73
322. Shi Y, Wang Z, Zhu X, Chen L, Ma Y, Wang J. et al. Exosomal miR-1246 in serum as a potential biomarker for early diagnosis of gastric cancer. Int J Clin Oncol. 2020;25:89-99
323. Li K, Cui M, Zhang K, Wang G, Zhai S. M1 macrophages-derived extracellular vesicles elevate microRNA-185-3p to aggravate the development of atherosclerosis in ApoE(-/-) mice by inhibiting small mothers against decapentaplegic 7. Int Immunopharmacol. 2021;90:107138
324. Nguyen MA, Karunakaran D, Geoffrion M, Cheng HS, Tandoc K, Perisic ML. et al. Extracellular Vesicles Secreted by Atherogenic Macrophages Transfer MicroRNA to Inhibit Cell Migration. Arterioscler Thromb Vasc Biol. 2018;38:49-63
325. Wang Y, Xu Z, Wang X, Zheng J, Peng L, Zhou Y. et al. Extracellular-vesicle containing miRNA-503-5p released by macrophages contributes to atherosclerosis. Aging (Albany NY). 2021;13:12239-57
326. Liu Y, Zhang WL, Gu JJ, Sun YQ, Cui HZ, Bu JQ. et al. Exosome-mediated miR-106a-3p derived from ox-LDL exposed macrophages accelerated cell proliferation and repressed cell apoptosis of human vascular smooth muscle cells. Eur Rev Med Pharmacol Sci. 2020;24:7039-50
327. Zhang YG, Song Y, Guo XL, Miao RY, Fu YQ, Miao CF. et al. Exosomes derived from oxLDL-stimulated macrophages induce neutrophil extracellular traps to drive atherosclerosis. Cell Cycle. 2019;18:2674-84
328. Yang Y, Luo H, Zhou C, Zhang R, Liu S, Zhu X. et al. Regulation of capillary tubules and lipid formation in vascular endothelial cells and macrophages via extracellular vesicle-mediated microRNA-4306 transfer. J Int Med Res. 2019;47:453-69
329. Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z. et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 2010;39:133-44
330. Liu ML, Scalia R, Mehta JL, Williams KJ. Cholesterol-induced membrane microvesicles as novel carriers of damage-associated molecular patterns: mechanisms of formation, action, and detoxification. Arterioscler Thromb Vasc Biol. 2012;32:2113-21
331. Chen L, Yang W, Guo Y, Chen W, Zheng P, Zeng J. et al. Exosomal lncRNA GAS5 regulates the apoptosis of macrophages and vascular endothelial cells in atherosclerosis. Plos One. 2017;12:e185406
332. Niu C, Wang X, Zhao M, Cai T, Liu P, Li J. et al. Macrophage Foam Cell-Derived Extracellular Vesicles Promote Vascular Smooth Muscle Cell Migration and Adhesion. J Am Heart Assoc. 2016;5(10):e004099
333. Xu Y, Liu Y, Yang C, Kang L, Wang M, Hu J. et al. Macrophages transfer antigens to dendritic cells by releasing exosomes containing dead-cell-associated antigens partially through a ceramide-dependent pathway to enhance CD4(+) T-cell responses. Immunology. 2016;149:157-71
334. Casella G, Colombo F, Finardi A, Descamps H, Ill-Raga G, Spinelli A. et al. Extracellular Vesicles Containing IL-4 Modulate Neuroinflammation in a Mouse Model of Multiple Sclerosis. Mol Ther. 2018;26:2107-18
335. Zhang Y, Liu F, Yuan Y, Jin C, Chang C, Zhu Y. et al. Inflammasome-Derived Exosomes Activate NF-kappaB Signaling in Macrophages. J Proteome Res. 2017;16:170-8
336. Lindenbergh M, Stoorvogel W. Antigen Presentation by Extracellular Vesicles from Professional Antigen-Presenting Cells. Annu Rev Immunol. 2018;36:435-59
337. Du T, Yang CL, Ge MR, Liu Y, Zhang P, Li H. et al. M1 Macrophage Derived Exosomes Aggravate Experimental Autoimmune Neuritis via Modulating Th1 Response. Front Immunol. 2020;11:1603
338. Burg AR, Tse HM. Redox-Sensitive Innate Immune Pathways During Macrophage Activation in Type 1 Diabetes. Antioxid Redox Signal. 2018;29:1373-98
339. Feduska JM, Tse HM. The proinflammatory effects of macrophage-derived NADPH oxidase function in autoimmune diabetes. Free Radic Biol Med. 2018;125:81-9
340. Zhao Y, Zhao MF, Jiang S, Wu J, Liu J, Yuan XW. et al. Liver governs adipose remodelling via extracellular vesicles in response to lipid overload. Nat Commun. 2020;11:719
341. Fu Q, Li Y, Jiang H, Shen Z, Gao R, He Y. et al. Hepatocytes derived extracellular vesicles from high-fat diet induced obese mice modulate genes expression and proliferation of islet beta cells. Biochem Biophys Res Commun. 2019;516:1159-66
342. Liu XL, Pan Q, Cao HX, Xin FZ, Zhao ZH, Yang RX. et al. Lipotoxic Hepatocyte-Derived Exosomal MicroRNA 192-5p Activates Macrophages Through Rictor/Akt/Forkhead Box Transcription Factor O1 Signaling in Nonalcoholic Fatty Liver Disease. Hepatology. 2020;72:454-69
343. Hirsova P, Ibrahim SH, Krishnan A, Verma VK, Bronk SF, Werneburg NW. et al. Lipid-Induced Signaling Causes Release of Inflammatory Extracellular Vesicles From Hepatocytes. Gastroenterology. 2016;150:956-67
344. Zhao Z, Zhong L, Li P, He K, Qiu C, Zhao L. et al. Cholesterol impairs hepatocyte lysosomal function causing M1 polarization of macrophages via exosomal miR-122-5p. Exp Cell Res. 2020;387:111738
345. Kakazu E, Mauer AS, Yin M, Malhi H. Hepatocytes release ceramide-enriched pro-inflammatory extracellular vesicles in an IRE1alpha-dependent manner. J Lipid Res. 2016;57:233-45
346. Guo Q, Furuta K, Lucien F, Gutierrez SL, Hirsova P, Krishnan A. et al. Integrin beta1-enriched extracellular vesicles mediate monocyte adhesion and promote liver inflammation in murine NASH. J Hepatol. 2019;71:1193-205
347. Liao CY, Song MJ, Gao Y, Mauer AS, Revzin A, Malhi H. Hepatocyte-Derived Lipotoxic Extracellular Vesicle Sphingosine 1-Phosphate Induces Macrophage Chemotaxis. Front Immunol. 2018;9:2980
348. Zhao Z, Zhong L, Li P, He K, Qiu C, Zhao L. et al. Cholesterol impairs hepatocyte lysosomal function causing M1 polarization of macrophages via exosomal miR-122-5p. Exp Cell Res. 2020;387:111738
349. Jiang F, Chen Q, Wang W, Ling Y, Yan Y, Xia P. Hepatocyte-derived extracellular vesicles promote endothelial inflammation and atherogenesis via microRNA-1. J Hepatol. 2020;72:156-66
350. Wang Y, Jin P, Liu J, Xie X. Exosomal microRNA-122 mediates obesity-related cardiomyopathy through suppressing mitochondrial ADP-ribosylation factor-like 2. Clin Sci (Lond). 2019;133:1871-81
351. Hasilo CP, Negi S, Allaeys I, Cloutier N, Rutman AK, Gasparrini M. et al. Presence of diabetes autoantigens in extracellular vesicles derived from human islets. Sci Rep. 2017;7:5000
352. Cianciaruso C, Phelps EA, Pasquier M, Hamelin R, Demurtas D, Alibashe AM. et al. Primary Human and Rat beta-Cells Release the Intracellular Autoantigens GAD65, IA-2, and Proinsulin in Exosomes Together With Cytokine-Induced Enhancers of Immunity. Diabetes. 2017;66:460-73
353. Rutman AK, Negi S, Gasparrini M, Hasilo CP, Tchervenkov J, Paraskevas S. Immune Response to Extracellular Vesicles From Human Islets of Langerhans in Patients With Type 1 Diabetes. Endocrinology. 2018;159:3834-47
354. Dai YD, Dias P, Margosiak A, Marquardt K, Bashratyan R, Hu WY. et al. Endogenous retrovirus Gag antigen and its gene variants are unique autoantigens expressed in the pancreatic islets of non-obese diabetic mice. Immunol Lett. 2020;223:62-70
355. Saravanan PB, Vasu S, Yoshimatsu G, Darden CM, Wang X, Gu J. et al. Differential expression and release of exosomal miRNAs by human islets under inflammatory and hypoxic stress. Diabetologia. 2019;62:1901-14
356. Bashratyan R, Sheng H, Regn D, Rahman MJ, Dai YD. Insulinoma-released exosomes activate autoreactive marginal zone-like B cells that expand endogenously in prediabetic NOD mice. Eur J Immunol. 2013;43:2588-97
357. Sheng H, Hassanali S, Nugent C, Wen L, Hamilton-Williams E, Dias P. et al. Insulinoma-released exosomes or microparticles are immunostimulatory and can activate autoreactive T cells spontaneously developed in nonobese diabetic mice. J Immunol. 2011;187:1591-600
358. Tesovnik T, Kovac J, Pohar K, Hudoklin S, Dovc K, Bratina N. et al. Extracellular Vesicles Derived Human-miRNAs Modulate the Immune System in Type 1 Diabetes. Front Cell Dev Biol. 2020;8:202
359. Giri KR, de Beaurepaire L, Jegou D, Lavy M, Mosser M, Dupont A. et al. Molecular and Functional Diversity of Distinct Subpopulations of the Stressed Insulin-Secreting Cell's Vesiculome. Front Immunol. 2020;11:1814
360. Guay C, Menoud V, Rome S, Regazzi R. Horizontal transfer of exosomal microRNAs transduce apoptotic signals between pancreatic beta-cells. Cell Commun Signal. 2015;13:17
361. Sun Y, Zhou Y, Shi Y, Zhang Y, Liu K, Liang R. et al. Expression of miRNA-29 in Pancreatic beta Cells Promotes Inflammation and Diabetes via TRAF3. Cell Rep. 2021;34:108576
362. Li J, Zhang Y, Ye Y, Li D, Liu Y, Lee E. et al. Pancreatic beta cells control glucose homeostasis via the secretion of exosomal miR-29 family. J Extracell Vesicles. 2021;10:e12055
363. Xu H, Du X, Xu J, Zhang Y, Tian Y, Liu G. et al. Pancreatic beta cell microRNA-26a alleviates type 2 diabetes by improving peripheral insulin sensitivity and preserving beta cell function. Plos Biol. 2020;18:e3000603
364. Fu X, Jin L, Wang X, Luo A, Hu J, Zheng X. et al. MicroRNA-26a targets ten eleven translocation enzymes and is regulated during pancreatic cell differentiation. Proc Natl Acad Sci U S A. 2013;110:17892-7
365. Fu X, Dong B, Tian Y, Lefebvre P, Meng Z, Wang X. et al. MicroRNA-26a regulates insulin sensitivity and metabolism of glucose and lipids. J Clin Invest. 2015;125:2497-509
366. Zeng H, Sun W, Ren X, Xia N, Zheng S, Xu H. et al. AP2-microRNA-26a overexpression reduces visceral fat mass and blood lipids. Mol Cell Endocrinol. 2021;528:111217
367. Zhang W, Fu X, Xie J, Pan H, Han W, Huang W. miR-26a attenuates colitis and colitis-associated cancer by targeting the multiple intestinal inflammatory pathways. Mol Ther Nucleic Acids. 2021;24:264-73
368. Xu H, Tian Y, Tang D, Zou S, Liu G, Song J. et al. An Endoplasmic Reticulum Stress-MicroRNA-26a Feedback Circuit in NAFLD. Hepatology. 2021;73:1327-45
369. Liu G, Du W, Xu H, Sun Q, Tang D, Zou S. et al. RNA G-quadruplex regulates microRNA-26a biogenesis and function. J Hepatol. 2020;73:371-82
370. Ribeiro D, Horvath I, Heath N, Hicks R, Forslow A, Wittung-Stafshede P. Extracellular vesicles from human pancreatic islets suppress human islet amyloid polypeptide amyloid formation. Proc Natl Acad Sci U S A. 2017;114:11127-32
371. Kamalden TA, Macgregor-Das AM, Kannan SM, Dunkerly-Eyring B, Khaliddin N, Xu Z. et al. Exosomal MicroRNA-15a Transfer from the Pancreas Augments Diabetic Complications by Inducing Oxidative Stress. Antioxid Redox Signal. 2017;27:913-30
372. Orasanu G, Plutzky J. The pathologic continuum of diabetic vascular disease. J Am Coll Cardiol. 2009;53:S35-42
373. Rom S, Heldt NA, Gajghate S, Seliga A, Reichenbach NL, Persidsky Y. Hyperglycemia and advanced glycation end products disrupt BBB and promote occludin and claudin-5 protein secretion on extracellular microvesicles. Sci Rep. 2020;10:7274
374. Chen YH, Chen ZW, Li HM, Yan XF, Feng B. AGE/RAGE-Induced EMP Release via the NOX-Derived ROS Pathway. J Diabetes Res. 2018;2018:6823058
375. Bammert TD, Hijmans JG, Reiakvam WR, Levy MV, Brewster LM, Goldthwaite ZA. et al. High glucose derived endothelial microparticles increase active caspase-3 and reduce microRNA-Let-7a expression in endothelial cells. Biochem Biophys Res Commun. 2017;493:1026-9
376. Schober A, Nazari-Jahantigh M, Wei Y, Bidzhekov K, Gremse F, Grommes J. et al. MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat Med. 2014;20:368-76
377. Jansen F, Yang X, Hoelscher M, Cattelan A, Schmitz T, Proebsting S. et al. Endothelial microparticle-mediated transfer of MicroRNA-126 promotes vascular endothelial cell repair via SPRED1 and is abrogated in glucose-damaged endothelial microparticles. Circulation. 2013;128:2026-38
378. Jansen F, Yang X, Baumann K, Przybilla D, Schmitz T, Flender A. et al. Endothelial microparticles reduce ICAM-1 expression in a microRNA-222-dependent mechanism. J Cell Mol Med. 2015;19:2202-14
379. Hijmans JG, Bammert TD, Stockelman KA, Reiakvam WR, Greiner JJ, DeSouza CA. High glucose-induced endothelial microparticles increase adhesion molecule expression on endothelial cells. Diabetol Int. 2019;10:143-7
380. Jansen F, Yang X, Franklin BS, Hoelscher M, Schmitz T, Bedorf J. et al. High glucose condition increases NADPH oxidase activity in endothelial microparticles that promote vascular inflammation. Cardiovasc Res. 2013;98:94-106
381. Jansen F, Stumpf T, Proebsting S, Franklin BS, Wenzel D, Pfeifer P. et al. Intercellular transfer of miR-126-3p by endothelial microparticles reduces vascular smooth muscle cell proliferation and limits neointima formation by inhibiting LRP6. J Mol Cell Cardiol. 2017;104:43-52
382. Jansen F, Zietzer A, Stumpf T, Flender A, Schmitz T, Nickenig G. et al. Endothelial microparticle-promoted inhibition of vascular remodeling is abrogated under hyperglycaemic conditions. J Mol Cell Cardiol. 2017;112:91-4
383. Lin X, Li S, Wang YJ, Wang Y, Zhong JY, He JY. et al. Exosomal Notch3 from high glucose-stimulated endothelial cells regulates vascular smooth muscle cells calcification/aging. Life Sci. 2019;232:116582
384. Li S, Zhan JK, Wang YJ, Lin X, Zhong JY, Wang Y. et al. Exosomes from hyperglycemia-stimulated vascular endothelial cells contain versican that regulate calcification/senescence in vascular smooth muscle cells. Cell Biosci. 2019;9:1
385. Togliatto G, Dentelli P, Rosso A, Lombardo G, Gili M, Gallo S. et al. PDGF-BB Carried by Endothelial Cell-Derived Extracellular Vesicles Reduces Vascular Smooth Muscle Cell Apoptosis in Diabetes. Diabetes. 2018;67:704-16
386. Wang S, Zhan J, Lin X, Wang Y, Wang Y, Liu Y. CircRNA-0077930 from hyperglycaemia-stimulated vascular endothelial cell exosomes regulates senescence in vascular smooth muscle cells. Cell Biochem Funct. 2020;38:1056-68
387. Hu J, Wang S, Xiong Z, Cheng Z, Yang Z, Lin J. et al. Exosomal Mst1 transfer from cardiac microvascular endothelial cells to cardiomyocytes deteriorates diabetic cardiomyopathy. Biochim Biophys Acta Mol Basis Dis. 2018;1864:3639-49
388. Wu XM, Gao YB, Cui FQ, Zhang N. Exosomes from high glucose-treated glomerular endothelial cells activate mesangial cells to promote renal fibrosis. Biol Open. 2016;5:484-91
389. Wu X, Gao Y, Xu L, Dang W, Yan H, Zou D. et al. Exosomes from high glucose-treated glomerular endothelial cells trigger the epithelial-mesenchymal transition and dysfunction of podocytes. Sci Rep. 2017;7:9371
390. Zeng T, Wang X, Wang W, Feng Q, Lao G, Liang Y. et al. Endothelial cell-derived small extracellular vesicles suppress cutaneous wound healing through regulating fibroblasts autophagy. Clin Sci (Lond). 2019;133:S20190008
391. He S, Wu C, Xiao J, Li D, Sun Z, Li M. Endothelial extracellular vesicles modulate the macrophage phenotype: Potential implications in atherosclerosis. Scand J Immunol. 2018;87:e12648
392. Chen L, Hu L, Li Q, Ma J, Li H. Exosome-encapsulated miR-505 from ox-LDL-treated vascular endothelial cells aggravates atherosclerosis by inducing NET formation. Acta Biochim Biophys Sin (Shanghai). 2019;51:1233-41
393. Liu Y, Li Q, Hosen MR, Zietzer A, Flender A, Levermann P. et al. Atherosclerotic Conditions Promote the Packaging of Functional MicroRNA-92a-3p Into Endothelial Microvesicles. Circ Res. 2019;124:575-87
394. Zhan R, Leng X, Liu X, Wang X, Gong J, Yan L. et al. Heat shock protein 70 is secreted from endothelial cells by a non-classical pathway involving exosomes. Biochem Biophys Res Commun. 2009;387:229-33
395. Zhang Z, Yi D, Zhou J, Zheng Y, Gao Z, Hu X. et al. Exosomal LINC01005 derived from oxidized low-density lipoprotein-treated endothelial cells regulates vascular smooth muscle cell phenotypic switch. Biofactors. 2020;46:743-53
396. Gao H, Wang X, Lin C, An Z, Yu J, Cao H. et al. Exosomal MALAT1 derived from ox-LDL-treated endothelial cells induce neutrophil extracellular traps to aggravate atherosclerosis. Biol Chem. 2020;401:367-76
397. Fu Z, Zhou E, Wang X, Tian M, Kong J, Li J. et al. Oxidized low-density lipoprotein-induced microparticles promote endothelial monocyte adhesion via intercellular adhesion molecule 1. Am J Physiol Cell Physiol. 2017;313:C567-74
398. DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S157-63
399. Aswad H, Forterre A, Wiklander OP, Vial G, Danty-Berger E, Jalabert A. et al. Exosomes participate in the alteration of muscle homeostasis during lipid-induced insulin resistance in mice. Diabetologia. 2014;57:2155-64
400. Jalabert A, Vial G, Guay C, Wiklander OP, Nordin JZ, Aswad H. et al. Exosome-like vesicles released from lipid-induced insulin-resistant muscles modulate gene expression and proliferation of beta recipient cells in mice. Diabetologia. 2016;59:1049-58
401. Whitham M, Parker BL, Friedrichsen M, Hingst JR, Hjorth M, Hughes WE. et al. Extracellular Vesicles Provide a Means for Tissue Crosstalk during Exercise. Cell Metab. 2018;27:237-51
402. Castano C, Mirasierra M, Vallejo M, Novials A, Parrizas M. Delivery of muscle-derived exosomal miRNAs induced by HIIT improves insulin sensitivity through down-regulation of hepatic FoxO1 in mice. Proc Natl Acad Sci U S A. 2020;117:30335-43
403. Yang G, Wei J, Liu P, Zhang Q, Tian Y, Hou G. et al. Role of the gut microbiota in type 2 diabetes and related diseases. Metabolism. 2021;117:154712
404. Chelakkot C, Choi Y, Kim DK, Park HT, Ghim J, Kwon Y. et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med. 2018;50:e450
405. Chen Y, Sun H, Bai Y, Zhi F. Gut dysbiosis-derived exosomes trigger hepatic steatosis by transiting HMGB1 from intestinal to liver in mice. Biochem Biophys Res Commun. 2019;509:767-72
406. Choi Y, Kwon Y, Kim DK, Jeon J, Jang SC, Wang T. et al. Gut microbe-derived extracellular vesicles induce insulin resistance, thereby impairing glucose metabolism in skeletal muscle. Sci Rep. 2015;5:15878
407. Kumar A, Sundaram K, Mu J, Dryden GW, Sriwastva MK, Lei C. et al. High-fat diet-induced upregulation of exosomal phosphatidylcholine contributes to insulin resistance. Nat Commun. 2021;12:213
408. Nah G, Park SC, Kim K, Kim S, Park J, Lee S. et al. Type-2 Diabetics Reduces Spatial Variation of Microbiome Based on Extracellur Vesicles from Gut Microbes across Human Body. Sci Rep. 2019;9:20136
409. Pugliese A. Autoreactive T cells in type 1 diabetes. J Clin Invest. 2017;127:2881-91
410. Guay C, Kruit JK, Rome S, Menoud V, Mulder NL, Jurdzinski A. et al. Lymphocyte-Derived Exosomal MicroRNAs Promote Pancreatic beta Cell Death and May Contribute to Type 1 Diabetes Development. Cell Metab. 2019;29:348-61
411. Pretorius E. Platelets as Potent Signaling Entities in Type 2 Diabetes Mellitus. Trends Endocrinol Metab. 2019;30:532-45
412. Zhang W, Dong X, Wang T, Kong Y. Exosomes derived from platelet-rich plasma mediate hyperglycemia-induced retinal endothelial injury via targeting the TLR4 signaling pathway. Exp Eye Res. 2019;189:107813
413. Wang GH, Ma KL, Zhang Y, Hu ZB, Liu L, Lu J. et al. Platelet microparticles contribute to aortic vascular endothelial injury in diabetes via the mTORC1 pathway. Acta Pharmacol Sin. 2019;40:468-76
414. Zhang Y, Ma KL, Gong YX, Wang GH, Hu ZB, Liu L. et al. Platelet Microparticles Mediate Glomerular Endothelial Injury in Early Diabetic Nephropathy. J Am Soc Nephrol. 2018;29:2671-95
415. Li M, Zhang T, Wu X, Chen Y, Sun L. High glucose provokes microvesicles generation from glomerular podocytes via NOX4/ROS pathway. Biosci Rep. 2019;39(11):BSR20192554
416. Sakurai A, Ono H, Ochi A, Matsuura M, Yoshimoto S, Kishi S. et al. Involvement of Elf3 on Smad3 activation-dependent injuries in podocytes and excretion of urinary exosome in diabetic nephropathy. Plos One. 2019;14:e216788
417. Munkonda MN, Akbari S, Landry C, Sun S, Xiao F, Turner M. et al. Podocyte-derived microparticles promote proximal tubule fibrotic signaling via p38 MAPK and CD36. J Extracell Vesicles. 2018;7:1432206
418. Su H, Qiao J, Hu J, Li Y, Lin J, Yu Q. et al. Podocyte-derived extracellular vesicles mediate renal proximal tubule cells dedifferentiation via microRNA-221 in diabetic nephropathy. Mol Cell Endocrinol. 2020;518:111034
419. Huang Y, Li R, Zhang L, Chen Y, Dong W, Zhao X. et al. Extracellular Vesicles From High Glucose-Treated Podocytes Induce Apoptosis of Proximal Tubular Epithelial Cells. Front Physiol. 2020;11:579296
420. Wang YY, Tang LQ, Wei W. Berberine attenuates podocytes injury caused by exosomes derived from high glucose-induced mesangial cells through TGFbeta1-PI3K/AKT pathway. Eur J Pharmacol. 2018;824:185-92
421. Bai S, Xiong X, Tang B, Ji T, Li X, Qu X. et al. Exosomal circ_DLGAP4 promotes diabetic kidney disease progression by sponging miR-143 and targeting ERBB3/NF-kappaB/MMP-2 axis. Cell Death Dis. 2020;11:1008
422. Tsai YC, Kuo MC, Hung WW, Wu LY, Wu PH, Chang WA. et al. High Glucose Induces Mesangial Cell Apoptosis through miR-15b-5p and Promotes Diabetic Nephropathy by Extracellular Vesicle Delivery. Mol Ther. 2020;28:963-74
423. Garcia-Pastor C, Benito-Martinez S, Moreno-Manzano V, Fernandez-Martinez AB, Lucio-Cazana FJ. Mechanism and Consequences of The Impaired Hif-1alpha Response to Hypoxia in Human Proximal Tubular HK-2 Cells Exposed to High Glucose. Sci Rep. 2019;9:15868
424. Ravindran S, Pasha M, Agouni A, Munusamy S. Microparticles as Potential Mediators of High Glucose-Induced Renal Cell Injury. Biomolecules. 2019;9:348
425. Wen J, Ma Z, Livingston MJ, Zhang W, Yuan Y, Guo C. et al. Decreased secretion and profibrotic activity of tubular exosomes in diabetic kidney disease. Am J Physiol Renal Physiol. 2020;319:F664-73
426. Maisto R, Oltra M, Vidal-Gil L, Martinez-Gil N, Sancho-Pelluz J, Filippo CD. et al. ARPE-19-derived VEGF-containing exosomes promote neovascularization in HUVEC: the role of the melanocortin receptor 5. Cell Cycle. 2019;18:413-24
427. Leszczynska A, Kulkarni M, Ljubimov AV, Saghizadeh M. Exosomes from normal and diabetic human corneolimbal keratocytes differentially regulate migration, proliferation and marker expression of limbal epithelial cells. Sci Rep. 2018;8:15173
428. Goncalves NP, Vaegter CB, Andersen H, Ostergaard L, Calcutt NA, Jensen TS. Schwann cell interactions with axons and microvessels in diabetic neuropathy. Nat Rev Neurol. 2017;13:135-47
429. Wang L, Chopp M, Szalad A, Lu X, Zhang Y, Wang X. et al. Exosomes Derived From Schwann Cells Ameliorate Peripheral Neuropathy in Type II Diabetic Mice. Diabetes. 2020;69:749-59
430. Jia L, Chopp M, Wang L, Lu X, Szalad A, Zhang ZG. Exosomes derived from high-glucose-stimulated Schwann cells promote development of diabetic peripheral neuropathy. Faseb J. 2018;32:j201800597R
431. Liu J, Sun X, Zhang FL, Jin H, Yan XL, Huang S. et al. Clinical Potential of Extracellular Vesicles in Type 2 Diabetes. Front Endocrinol (Lausanne). 2020;11:596811
432. Prattichizzo F, Matacchione G, Giuliani A, Sabbatinelli J, Olivieri F, de Candia P. et al. Extracellular vesicle-shuttled miRNAs: a critical appraisal of their potential as nano-diagnostics and nano-therapeutics in type 2 diabetes mellitus and its cardiovascular complications. Theranostics. 2021;11:1031-45
433. He X, Kuang G, Wu Y, Ou C. Emerging roles of exosomal miRNAs in diabetes mellitus. Clin Transl Med. 2021;11:e468
434. Liu C, Gao Y, Wu J, Zou J. Exosomal miR-23a and miR-192, Potential Diagnostic Biomarkers for Type 2 Diabetes. Clin Lab. 2021 67
435. Zhao Y, Shen A, Guo F, Song Y, Jing N, Ding X. et al. Urinary Exosomal MiRNA-4534 as a Novel Diagnostic Biomarker for Diabetic Kidney Disease. Front Endocrinol (Lausanne). 2020;11:590
436. Prabu P, Rome S, Sathishkumar C, Gastebois C, Meugnier E, Mohan V. et al. MicroRNAs from urinary extracellular vesicles are non-invasive early biomarkers of diabetic nephropathy in type 2 diabetes patients with the 'Asian Indian phenotype'. Diabetes Metab. 2019;45:276-85
437. Kong Q, Guo X, Guo Z, Su T. Urinary Exosome miR-424 and miR-218 as Biomarkers for Type 1 Diabetes in Children. Clin Lab. 2019; 65. doi:10.7754/Clin.Lab. 2020 200612
438. Zang J, Maxwell AP, Simpson DA, McKay GJ. Differential Expression of Urinary Exosomal MicroRNAs miR-21-5p and miR-30b-5p in Individuals with Diabetic Kidney Disease. Sci Rep. 2019;9:10900
439. Ibrahim AA, Wahby AA, Ashmawy I, Saleh RM, Soliman H. Association of Exosomal miR-34a with Markers of Dyslipidemia and Endothelial Dysfunction in Children and Adolescents with T1DM. J Clin Res Pediatr Endocrinol. 2020;12:401-9
440. Jia Y, Guan M, Zheng Z, Zhang Q, Tang C, Xu W. et al. miRNAs in Urine Extracellular Vesicles as Predictors of Early-Stage Diabetic Nephropathy. J Diabetes Res. 2016;2016:7932765
441. Eissa S, Matboli M, Aboushahba R, Bekhet MM, Soliman Y. Urinary exosomal microRNA panel unravels novel biomarkers for diagnosis of type 2 diabetic kidney disease. J Diabetes Complications. 2016;30:1585-92
442. Prattichizzo F, De Nigris V, Sabbatinelli J, Giuliani A, Castano C, Parrizas M. et al. CD31(+) Extracellular Vesicles From Patients With Type 2 Diabetes Shuttle a miRNA Signature Associated With Cardiovascular Complications. Diabetes. 2021;70:240-54
443. Eissa S, Matboli M, Bekhet MM. Clinical verification of a novel urinary microRNA panal: 133b, -342 and -30 as biomarkers for diabetic nephropathy identified by bioinformatics analysis. Biomed Pharmacother. 2016;83:92-9
444. Li W, Yang S, Qiao R, Zhang J. Potential Value of Urinary Exosome-Derived let-7c-5p in the Diagnosis and Progression of Type II Diabetic Nephropathy. Clin Lab. 2018;64:709-18
445. Chen ZJ, Shi XJ, Fu LJ, Liu J, Shi K, Zhang WB. et al. Serum and exosomal hsa_circ_0000907 and hsa_circ_0057362 as novel biomarkers in the early diagnosis of diabetic foot ulcer. Eur Rev Med Pharmacol Sci. 2020;24:8117-26
446. Tao Y, Wei X, Yue Y, Wang J, Li J, Shen L. et al. Extracellular vesicle-derived AEBP1 mRNA as a novel candidate biomarker for diabetic kidney disease. J Transl Med. 2021;19:326
447. Feng Y, Zhong X, Ni HF, Wang C, Tang TT, Wang LT. et al. Urinary small extracellular vesicles derived CCL21 mRNA as biomarker linked with pathogenesis for diabetic nephropathy. J Transl Med. 2021;19:355
448. Hashemi E, Dehghanbanadaki H, Baharanchi AA, Forouzanfar K, Kakaei A, Mohammadi SM. et al. WT1 and ACE mRNAs of blood extracellular vesicle as biomarkers of diabetic nephropathy. J Transl Med. 2021;19:299
449. Abe H, Sakurai A, Ono H, Hayashi S, Yoshimoto S, Ochi A. et al. Urinary Exosomal mRNA of WT1 as Diagnostic and Prognostic Biomarker for Diabetic Nephropathy. J Med Invest. 2018;65:208-15
450. Yamamoto CM, Murakami T, Oakes ML, Mitsuhashi M, Kelly C, Henry RR. et al. Uromodulin mRNA from Urinary Extracellular Vesicles Correlate to Kidney Function Decline in Type 2 Diabetes Mellitus. Am J Nephrol. 2018;47:283-91
451. Parrizas M, Mundet X, Castano C, Canivell S, Cos X, Brugnara L. et al. miR-10b and miR-223-3p in serum microvesicles signal progression from prediabetes to type 2 diabetes. J Endocrinol Invest. 2020;43:451-9
452. Snowhite IV, Allende G, Sosenko J, Pastori RL, Messinger CS, Pugliese A. Association of serum microRNAs with islet autoimmunity, disease progression and metabolic impairment in relatives at risk of type 1 diabetes. Diabetologia. 2017;60:1409-22
453. Liu ML, Werth VP, Williams KJ. Blood plasma versus serum: which is right for sampling circulating membrane microvesicles in human subjects? Ann Rheum Dis. 2020;79:e73
454. Hu W, Song X, Yu H, Sun J, Zhao Y. Therapeutic Potentials of Extracellular Vesicles for the Treatment of Diabetes and Diabetic Complications. Int J Mol Sci. 2020;21(14):5163
455. Stevenson K, Chen D, MacIntyre A, McGlynn LM, Montague P, Charif R. et al. Pancreatic-derived pathfinder cells enable regeneration of critically damaged adult pancreatic tissue and completely reverse streptozotocin-induced diabetes. Rejuvenation Res. 2011;14:163-71
456. Xu R, Greening DW, Zhu HJ, Takahashi N, Simpson RJ. Extracellular vesicle isolation and characterization: toward clinical application. J Clin Invest. 2016;126:1152-62
457. Gesmundo I, Pardini B, Gargantini E, Gamba G, Birolo G, Fanciulli A. et al. Adipocyte-derived extracellular vesicles regulate survival and function of pancreatic β cells. JCI Insight. 2021;6:e141962
458. Sun Y, Shi H, Yin S, Ji C, Zhang X, Zhang B. et al. Human Mesenchymal Stem Cell Derived Exosomes Alleviate Type 2 Diabetes Mellitus by Reversing Peripheral Insulin Resistance and Relieving beta-Cell Destruction. Acs Nano. 2018;12:7613-28
459. Zhao H, Shang Q, Pan Z, Bai Y, Li Z, Zhang H. et al. Exosomes From Adipose-Derived Stem Cells Attenuate Adipose Inflammation and Obesity Through Polarizing M2 Macrophages and Beiging in White Adipose Tissue. Diabetes. 2018;67:235-47
460. Keshtkar S, Kaviani M, Sarvestani FS, Ghahremani MH, Aghdaei MH, Al-Abdullah IH. et al. Exosomes derived from human mesenchymal stem cells preserve mouse islet survival and insulin secretion function. Excli J. 2020;19:1064-80
461. Shigemoto-Kuroda T, Oh JY, Kim DK, Jeong HJ, Park SY, Lee HJ. et al. MSC-derived Extracellular Vesicles Attenuate Immune Responses in Two Autoimmune Murine Models: Type 1 Diabetes and Uveoretinitis. Stem Cell Rep. 2017;8:1214-25
462. Wen D, Peng Y, Liu D, Weizmann Y, Mahato RI. Mesenchymal stem cell and derived exosome as small RNA carrier and Immunomodulator to improve islet transplantation. J Control Release. 2016;238:166-75
463. Jiang ZZ, Liu YM, Niu X, Yin JY, Hu B, Guo SC. et al. Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res Ther. 2016;7:24
464. Grange C, Tritta S, Tapparo M, Cedrino M, Tetta C, Camussi G. et al. Stem cell-derived extracellular vesicles inhibit and revert fibrosis progression in a mouse model of diabetic nephropathy. Sci Rep. 2019;9:4468
465. Mao R, Shen J, Hu X. BMSCs-derived exosomal microRNA-let-7a plays a protective role in diabetic nephropathy via inhibition of USP22 expression. Life Sci. 2021;268:118937
466. Duan Y, Luo Q, Wang Y, Ma Y, Chen F, Zhu X. et al. Adipose mesenchymal stem cell-derived extracellular vesicles containing microRNA-26a-5p target TLR4 and protect against diabetic nephropathy. J Biol Chem. 2020;295:12868-84
467. Zhang W, Wang Y, Kong Y. Exosomes Derived From Mesenchymal Stem Cells Modulate miR-126 to Ameliorate Hyperglycemia-Induced Retinal Inflammation Via Targeting HMGB1. Invest Ophthalmol Vis Sci. 2019;60:294-303
468. Cao X, Xue LD, Di Y, Li T, Tian YJ, Song Y. MSC-derived exosomal lncRNA SNHG7 suppresses endothelial-mesenchymal transition and tube formation in diabetic retinopathy via miR-34a-5p/XBP1 axis. Life Sci. 2021;272:119232
469. Gu C, Zhang H, Gao Y. Adipose mesenchymal stem cells-secreted extracellular vesicles containing microRNA-192 delays diabetic retinopathy by targeting ITGA1. J Cell Physiol. 2021;236:5036-51
470. Gu S, Liu Y, Zou J, Wang W, Wei T, Wang X. et al. Retinal pigment epithelial cells secrete miR-202-5p-containing exosomes to protect against proliferative diabetic retinopathy. Exp Eye Res. 2020;201:108271
471. Li W, Jin LY, Cui YB, Xie N. Human umbilical cord mesenchymal stem cells-derived exosomal microRNA-17-3p ameliorates inflammatory reaction and antioxidant injury of mice with diabetic retinopathy via targeting STAT1. Int Immunopharmacol. 2021;90:107010
472. Chen F, Zhang H, Wang Z, Ding W, Zeng Q, Liu W. et al. Adipose-Derived Stem Cell-Derived Exosomes Ameliorate Erectile Dysfunction in a Rat Model of Type 2 Diabetes. J Sex Med. 2017;14:1084-94
473. Huo W, Li Y, Zhang Y, Li H. Mesenchymal stem cells-derived exosomal microRNA-21-5p downregulates PDCD4 and ameliorates erectile dysfunction in a rat model of diabetes mellitus. Faseb J. 2020;34:13345-60
474. Zhu LL, Huang X, Yu W, Chen H, Chen Y, Dai YT. Transplantation of adipose tissue-derived stem cell-derived exosomes ameliorates erectile function in diabetic rats. Andrologia. 2018;50:e12871
475. Ouyang B, Xie Y, Zhang C, Deng C, Lv L, Yao J. et al. Extracellular Vesicles From Human Urine-Derived Stem Cells Ameliorate Erectile Dysfunction in a Diabetic Rat Model by Delivering Proangiogenic MicroRNA. Sex Med. 2019;7:241-50
476. Tao SC, Guo SC, Li M, Ke QF, Guo YP, Zhang CQ. Chitosan Wound Dressings Incorporating Exosomes Derived from MicroRNA-126-Overexpressing Synovium Mesenchymal Stem Cells Provide Sustained Release of Exosomes and Heal Full-Thickness Skin Defects in a Diabetic Rat Model. Stem Cells Transl Med. 2017;6:736-47
477. Li Q, Zhao H, Chen W, Huang P, Bi J. Human keratinocyte-derived microvesicle miRNA-21 promotes skin wound healing in diabetic rats through facilitating fibroblast function and angiogenesis. Int J Biochem Cell Biol. 2019;114:105570
478. Huang C, Luo W, Wang Q, Ye Y, Fan J, Lin L. et al. Human mesenchymal stem cells promote ischemic repairment and angiogenesis of diabetic foot through exosome miRNA-21-5p. Stem Cell Res. 2021;52:102235
479. Li X, Jiang C, Zhao J. Human endothelial progenitor cells-derived exosomes accelerate cutaneous wound healing in diabetic rats by promoting endothelial function. J Diabetes Complications. 2016;30:986-92
480. Shi R, Jin Y, Hu W, Lian W, Cao C, Han S. et al. Exosomes derived from mmu_circ_0000250-modified adipose-derived mesenchymal stem cells promote wound healing in diabetic mice by inducing miR-128-3p/SIRT1-mediated autophagy. Am J Physiol Cell Physiol. 2020;318:C848-56
481. Guo SC, Tao SC, Yin WJ, Qi X, Yuan T, Zhang CQ. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics. 2017;7:81-96
482. Li X, Xie X, Lian W, Shi R, Han S, Zhang H. et al. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp Mol Med. 2018;50:1-14
483. Yang J, Chen Z, Pan D, Li H, Shen J. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomes Combined Pluronic F127 Hydrogel Promote Chronic Diabetic Wound Healing and Complete Skin Regeneration. Int J Nanomedicine. 2020;15:5911-26
484. Chen CY, Rao SS, Ren L, Hu XK, Tan YJ, Hu Y. et al. Exosomal DMBT1 from human urine-derived stem cells facilitates diabetic wound repair by promoting angiogenesis. Theranostics. 2018;8:1607-23
485. Wei P, Zhong C, Yang X, Shu F, Xiao S, Gong T. et al. Exosomes derived from human amniotic epithelial cells accelerate diabetic wound healing via PI3K-AKT-mTOR-mediated promotion in angiogenesis and fibroblast function. Burns Trauma. 2020;8:a20
486. Wei F, Wang A, Wang Q, Han W, Rong R, Wang L. et al. Plasma endothelial cells-derived extracellular vesicles promote wound healing in diabetes through YAP and the PI3K/Akt/mTOR pathway. Aging (Albany NY). 2020;12:12002-18
487. Dalirfardouei R, Jamialahmadi K, Jafarian AH, Mahdipour E. Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J Tissue Eng Regen Med. 2019;13:555-68
488. Pomatto M, Gai C, Negro F, Cedrino M, Grange C, Ceccotti E. et al. Differential Therapeutic Effect of Extracellular Vesicles Derived by Bone Marrow and Adipose Mesenchymal Stem Cells on Wound Healing of Diabetic Ulcers and Correlation to Their Cargoes. Int J Mol Sci. 2021;22:3851
489. Geiger A, Walker A, Nissen E. Human fibrocyte-derived exosomes accelerate wound healing in genetically diabetic mice. Biochem Biophys Res Commun. 2015;467:303-9
490. Li M, Wang T, Tian H, Wei G, Zhao L, Shi Y. Macrophage-derived exosomes accelerate wound healing through their anti-inflammation effects in a diabetic rat model. Artif Cells Nanomed Biotechnol. 2019;47:3793-803
491. Bouchareychas L, Duong P, Covarrubias S, Alsop E, Phu TA, Chung A. et al. Macrophage Exosomes Resolve Atherosclerosis by Regulating Hematopoiesis and Inflammation via MicroRNA Cargo. Cell Rep. 2020;32:107881
492. Lin B, Xie W, Zeng C, Wu X, Chen A, Li H. et al. Transfer of exosomal microRNA-203-3p from dendritic cells to bone marrow-derived macrophages reduces development of atherosclerosis by downregulating Ctss in mice. Aging (Albany NY). 2021;13:15638-58
493. Guo Z, Zhao Z, Yang C, Song C. Transfer of microRNA-221 from mesenchymal stem cell-derived extracellular vesicles inhibits atherosclerotic plaque formation. Transl Res. 2020;226:83-95
494. Li L, Wang H, Zhang J, Chen X, Zhang Z, Li Q. Effect of endothelial progenitor cell-derived extracellular vesicles on endothelial cell ferroptosis and atherosclerotic vascular endothelial injury. Cell Death Discov. 2021;7:235
495. Lin Y, Liu M, Chen E, Jiang W, Shi W, Wang Z. Bone marrow-derived mesenchymal stem cells microvesicles stabilize atherosclerotic plaques by inhibiting NLRP3-mediated macrophage pyroptosis. Cell Biol Int. 2021;45:820-30
496. Lin F, Zhang S, Liu X, Wu M. Mouse bone marrow derived mesenchymal stem cells-secreted exosomal microRNA-125b-5p suppresses atherosclerotic plaque formation via inhibiting Map4k4. Life Sci. 2021;274:119249
497. Li J, Xue H, Li T, Chu X, Xin D, Xiong Y. et al. Exosomes derived from mesenchymal stem cells attenuate the progression of atherosclerosis in ApoE(-/-) mice via miR-let7 mediated infiltration and polarization of M2 macrophage. Biochem Biophys Res Commun. 2019;510:565-72
498. Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM. et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14:249-56
499. Wu G, Zhang J, Zhao Q, Zhuang W, Ding J, Zhang C. et al. Molecularly Engineered Macrophage-Derived Exosomes with Inflammation Tropism and Intrinsic Heme Biosynthesis for Atherosclerosis Treatment. Angew Chem Int Ed Engl. 2020;59:4068-74
500. Ma Q, Fan Q, Han X, Dong Z, Xu J, Bai J. et al. Platelet-derived extracellular vesicles to target plaque inflammation for effective anti-atherosclerotic therapy. J Control Release. 2021;329:445-53
501. Zhong X, Chung AC, Chen HY, Dong Y, Meng XM, Li R. et al. miR-21 is a key therapeutic target for renal injury in a mouse model of type 2 diabetes. Diabetologia. 2013;56:663-74
502. Kolling M, Kaucsar T, Schauerte C, Hubner A, Dettling A, Park JK. et al. Therapeutic miR-21 Silencing Ameliorates Diabetic Kidney Disease in Mice. Mol Ther. 2017;25:165-80
503. Brennan E, Wang B, McClelland A, Mohan M, Marai M, Beuscart O. et al. Protective Effect of let-7 miRNA Family in Regulating Inflammation in Diabetes-Associated Atherosclerosis. Diabetes. 2017;66:2266-77
504. Pishavar E, Behravan J. miR-126 as a Therapeutic Agent for Diabetes Mellitus. Curr Pharm Des. 2017;23:3309-14
505. Bijkerk R, Esguerra J, Ellenbroek JH, Au YW, Hanegraaf M, de Koning EJ. et al. In Vivo Silencing of MicroRNA-132 Reduces Blood Glucose and Improves Insulin Secretion. Nucleic Acid Ther. 2019;29:67-72
506. Li X, Li D, Wang A, Chu T, Lohcharoenkal W, Zheng X. et al. MicroRNA-132 with Therapeutic Potential in Chronic Wounds. J Invest Dermatol. 2017;137:2630-8
507. Yu M, Liu W, Li J, Lu J, Lu H, Jia W. et al. Exosomes derived from atorvastatin-pretreated MSC accelerate diabetic wound repair by enhancing angiogenesis via AKT/eNOS pathway. Stem Cell Res Ther. 2020;11:350
508. Wang C, Wang M, Xu T, Zhang X, Lin C, Gao W. et al. Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration. Theranostics. 2019;9:65-76
509. Zhang Y, Zhang P, Gao X, Chang L, Chen Z, Mei X. Preparation of exosomes encapsulated nanohydrogel for accelerating wound healing of diabetic rats by promoting angiogenesis. Mater Sci Eng C Mater Biol Appl. 2021;120:111671
510. Wang M, Wang C, Chen M, Xi Y, Cheng W, Mao C. et al. Efficient Angiogenesis-Based Diabetic Wound Healing/Skin Reconstruction through Bioactive Antibacterial Adhesive Ultraviolet Shielding Nanodressing with Exosome Release. Acs Nano. 2019;13:10279-93
511. Tao SC, Rui BY, Wang QY, Zhou D, Zhang Y, Guo SC. Extracellular vesicle-mimetic nanovesicles transport LncRNA-H19 as competing endogenous RNA for the treatment of diabetic wounds. Drug Deliv. 2018;25:241-55
512. Zabeo D, Cvjetkovic A, Lasser C, Schorb M, Lotvall J, Hoog JL. Exosomes purified from a single cell type have diverse morphology. J Extracell Vesicles. 2017;6:1329476
513. Pick H, Alves AC, Vogel H. Single-Vesicle Assays Using Liposomes and Cell-Derived Vesicles: From Modeling Complex Membrane Processes to Synthetic Biology and Biomedical Applications. Chem Rev. 2018;118:8598-654
514. Han Z, Liu S, Pei Y, Ding Z, Li Y, Wang X. et al. Highly efficient magnetic labelling allows MRI tracking of the homing of stem cell-derived extracellular vesicles following systemic delivery. J Extracell Vesicles. 2021;10:e12054
515. Zhang W, Yu ZL, Wu M, Ren JG, Xia HF, Sa GL. et al. Magnetic and Folate Functionalization Enables Rapid Isolation and Enhanced Tumor-Targeting of Cell-Derived Microvesicles. Acs Nano. 2017;11:277-90
516. Masud MK, Na J, Younus M, Hossain M, Bando Y, Shiddiky M. et al. Superparamagnetic nanoarchitectures for disease-specific biomarker detection. Chem Soc Rev. 2019;48:5717-51
517. Sancho-Albero M, Sebastian V, Sese J, Pazo-Cid R, Mendoza G, Arruebo M. et al. Isolation of exosomes from whole blood by a new microfluidic device: proof of concept application in the diagnosis and monitoring of pancreatic cancer. J Nanobiotechnology. 2020;18:150
518. Chen H, Luo D, Shang B, Cao J, Wei J, Chen Q. et al. Immunoassay-type biosensor based on magnetic nanoparticle capture and the fluorescence signal formed by horseradish peroxidase catalysis for tumor-related exosome determination. Mikrochim Acta. 2020;187:282
Author contact
![]() Corresponding author: Xianghui Fu (xfuedu.cn). Address: State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, No.17, The 3rd Section Renmin South Road, Chengdu 610041, Sichuan, China.
Corresponding author: Xianghui Fu (xfuedu.cn). Address: State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, No.17, The 3rd Section Renmin South Road, Chengdu 610041, Sichuan, China.
 Global reach, higher impact
Global reach, higher impact