13.3
Impact Factor
Theranostics 2022; 12(3):1286-1302. doi:10.7150/thno.67543 This issue Cite
Research Paper
Mitochondrion-targeted supramolecular “nano-boat” simultaneously inhibiting dual energy metabolism for tumor selective and synergistic chemo-radiotherapy
1. Key Laboratory of Radiopharmacokinetics for Innovative Drugs, Institute of Radiation Medicine, Chinese Academy of Medical Sciences & Peking Union Medical College, Tianjin 300192, P. R. China.
2. Radiation Oncology Department, Tianjin Medical University General Hospital, Tianjin 300052, P. R. China.
# These authors contributed equally to this work.
Abstract
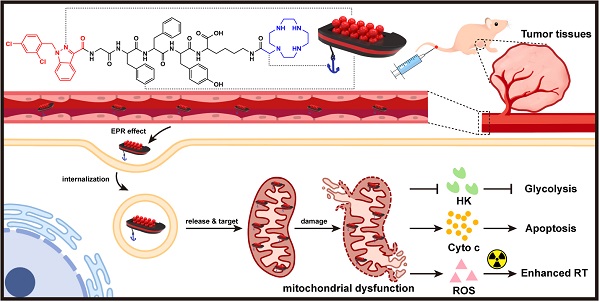
Rationale: Tumor energy metabolism has been a well-appreciated target of cancer therapy; however, the metabolism change of cancer cells between oxidative phosphorylation and glycolysis poses a challenge to the above. In this study, we constructed an innovative mitochondrion-targeted supramolecular “nano-boat” based on peptide self-assembly for tumor combined chemo-radiotherapy by simultaneously inhibiting the dual energy metabolism.
Methods: A lipophilic self-assembled peptide and a positively charged cyclen were integrated to fabricate a brand new mitochondrion-targeted nano-platform for the first time. The indices of mitochondrial dysfunction including mitochondrial membrane potential, apoptosis proteins expression and ultrastructure change were evaluated using a JC-1 probe, western blotting and biological transmission electron microscopy, respectively. Energy metabolism assays were conducted on a Seahorse XF24 system by detecting the oxygen consumption rate and the glycolytic proton efflux rate. The radio-sensitization effect was investigated by colony formation, the comet assay, and γ-H2AX staining.
Results: The supramolecular “nano-boat” could selectively kill cancer cells by much higher enrichment and reactive oxygen species generation than those in normal cells. In the cancer cells treated with the supramolecular “nano-boat”, the dysfunctional morphological changes of the mitochondrial ultrastructure including swelling and pyknosis were evidently observed, and the endogenous mitochondrial apoptosis pathway was effectively triggered by abundant of cytochrome C leaking out. Concurrently, the dual metabolic pathways of glycolysis and oxidative phosphorylation were severely inhibited. More importantly, the supramolecular “nano-boat” displayed an excellent radio-sensitization effect with a sensitization enhancement ratio value as high as 2.58, and hence, in vivo efficiently combining radiotherapy yielded an enhanced chemo-radiotherapy effect.
Conclusion: Our study demonstrated that the rationally designed peptide-based “nano-boat” could efficiently induce cancer cell apoptosis by the energy metabolism inhibition involving multiple pathways, which may provide the motivation for designing novel and universal mitochondria-targeted drug delivery systems for cancer therapy.
Keywords: cancer metabolism, peptide self-assembly, mitochondrion-targeted, selective killing, radio-sensitization
 Global reach, higher impact
Global reach, higher impact