13.3
Impact Factor
Theranostics 2022; 12(3):1220-1246. doi:10.7150/thno.65427 This issue Cite
Research Paper
Garlic exosome-like nanoparticles reverse high-fat diet induced obesity via the gut/brain axis
1. James Graham Brown Cancer Center, Department of Microbiology & Immunology, University of Louisville, KY 40202, USA.
2. Department of Physiology, University of Louisville School of Medicine, Louisville, KY 40202, USA.
3. Department of Medicine, University of Louisville, Louisville, KY 40202, USA.
4. Department of Pharmacology and Toxicology, University of Louisville, Louisville, KY 40202, USA.
5. Department of Computer Engineering and Computer Science, University of Louisville, KY 40202, USA.
6. KBRIN Bioinformatics Core, University of Louisville, Louisville, KY 40202, USA.
7. Kidney Disease Program and Clinical Proteomics Center, University of Louisville, Louisville, KY 40202, USA.
8. Robley Rex Veterans Affairs Medical Center, Louisville, KY 40206, USA.
Abstract
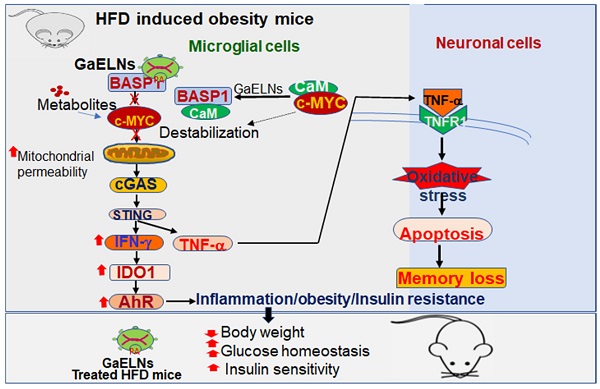
Background: Obesity is becoming a global epidemic and reversing the pathological processes underlying obesity and metabolic co-morbidities is challenging. Obesity induced chronic inflammation including brain inflammation is a hallmark of obesity via the gut-brain axis. The objective of this study was to develop garlic exosome-like nanoparticles (GaELNs) that inhibit systemic as well as brain inflammatory activity and reverse a HFD induced obesity in mice.
Methods: GELNs were isolated and administrated orally into HFD fed mice. GaELNs were fluorescent labeled for monitoring their in vivo trafficking route after oral administration and quantified the number particles in several tissues. The brain inflammation was determined by measuring inflammatory cytokines by ELISA and real-time PCR. Mitochondrial membrane permeability of microglial cells was determined using JC-10 fluorescence dye. The in vivo apoptotic cell death was quantified by TUNEL assay. The brain metabolites were identified and quantified by LC-MS analysis. Memory function of the mice was determined by several memory functional analysis. The effect of GaELNs on glucose and insulin response of the mice was determined by glucose and insulin tolerance tests. c-Myc localization and interaction with BASP1 and calmodulin was determined by confocal microscopy.
Results: Our results show that GaELNs is preferentially taken up microglial cells and inhibits the brain inflammation in HFD mice. GaELN phosphatidic acid (PA) (36:4) is required for the uptake of GaELNs via interaction with microglial BASP1. Formation of the GaELNs/BASP1 complex is required for inhibition of c-Myc mediated expression of STING. GaELN PA binds to BASP1, leading to inhibition of c-Myc expression and activity through competitively binding to CaM with c-Myc transcription factor. Inhibition of STING activity leads to reducing the expression of an array of inflammatory cytokines including IFN-γ and TNF-α. IFN-γ induces the expression of IDO1, which in turn the metabolites generated as IDO1 dependent manner activate the AHR pathway that contributes to developing obesity. The metabolites derived from the GaELNs treated microglial cells promote neuronal differentiation and inhibit mitochondrial mediated neuronal cell death. GaELNs treated HFD mice showed improved memory function and increased glucose tolerance and insulin sensitivity in these mice.
Conclusion: Collectively, these results demonstrate how nanoparticles from a healthy diet can inhibit unhealthy high-fat diet induced brain inflammation and reveal a link between brain microglia/diet to brain inflammatory disease outcomes via diet-derived exosome-like nanoparticles.
Keywords: Garlic derived exosome-like nanoparticles, phosphatidic acid, microglial cells, BASP1, c-Myc, IDO1, AHR, STING, mitochondria, gut/brain axis, brain inflammation, obesity.
 Global reach, higher impact
Global reach, higher impact