13.3
Impact Factor
Theranostics 2022; 12(3):1117-1131. doi:10.7150/thno.66026 This issue Cite
Review
Modulation of lymphatic transport in the central nervous system
1. Department of Neurosurgery, The first hospital of Jilin University, Changchun, 130021, P.R. China
2. Department of Anesthesiology, The first hospital of Jilin University, Changchun, 130021, P.R. China
3. Joint Laboratory of Opto-Functional Theranostics in Medicine and Chemistry, The First Hospital of Jilin University, Changchun, 130021, P.R. China
4. State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun 130012, P.R. China
Received 2021-8-12; Accepted 2021-12-3; Published 2022-1-1
Abstract
Over the past decade, repeated studies demonstrated that the vertebrate brain had a specialized lymphatic transport pathway, which overturned the traditional concept of central nervous system (CNS) immune privilege. Despite the lack of lymphatic vessels, the glymphatic system and the meningeal lymphatic vessels provide a unique pathway for solutes transport and metabolites clearance in the brain. Sleep, circadian rhythm, arterial pulsation, and other physiological factors modulate this specialized lymphatic drainage pathway. It has also changed significantly under pathological conditions. These modulatory mechanisms may arise critical targets for the therapeutic of CNS disorders. This review highlights the latest research progress on the modulation of lymphatic transport in the CNS under physiological and pathological conditions. Furthermore, we examined the possible upstream and downstream relation networks between these regulatory mechanisms.
Keywords: central nervous system, glymphatic system, lymphatic vessels, regulation, sleep
Introduction
The lymphatic transport system in vertebrates maintains body fluid homeostasis, immune surveillance, and lipid reabsorption in the peripheral organs; it also plays an essential role in the pathological process underlying inflammation, cardiovascular disease, tumor metastasis, and in the onset of other diseases [1]. The human brain consumes about 20% of total energy [2]. It, therefore, needs a more effective fluid transport system to ensure a stable microenvironment supporting its active metabolic status. Studies have recently identified and characterized a specialized lymphatic transport system in the central nervous system (CNS) [3-6]. It is responsible for immune monitoring, solute transport, metabolites clearance, and other functions similar to those of the peripheral lymphoid system; also involved in the pathological process of CNS disorders, such as tumors, head trauma, stroke, and degenerative diseases [7].
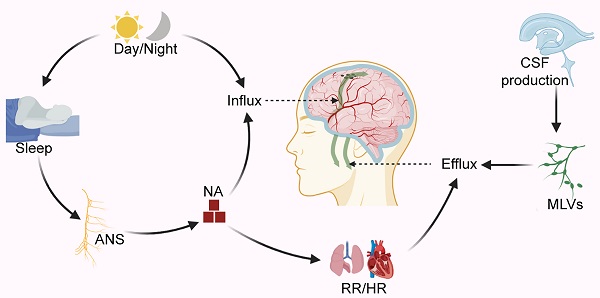
For a long time, researchers have suggested that cerebrospinal fluid (CSF) may be the "sink" through which brain metabolites is cleared [8]. Since CSF is the carrier solution for metabolites clearance in the brain, these studies are similar to those investigating CSF dynamics in the CNS. As traditionally thought to buffer the brain and spinal cord, CSF is now accepted as the lymph of the CNS. The lymphatic transport system in the CNS has been studied primarily in the context of two discoveries: the glymphatic system (GS) and meningeal lymphatic vessels (MLVs) (Figure 1). The GS, named after the 'glial' and 'lymphatic' waste clearance systems, depicts a fluid pathway through which CSF flows in and out of the brain [3]: CSF flows into the brain parenchyma (it is also referred to as glymphatic inflow in this review) through the artery paravascular space (aPVS), then exchanges solutes with interstitial fluid (ISF) in the interstitial system (ISS), and finally flows out of the brain via the venous paravascular space (vPVS) [3, 9] (Figure 1D). Although the brain lacks lymphatic vessels, studies have identified unique lymphatic structures in the basal and dorsal meninges [5, 10-12] (Figure 1B-C). These MLVs are also engaged in the outflow of CSF, metabolites, and immune cells from the brain [5, 10-12]. Multiple pathways may contribute to CSF drainage into the extracranial lymphatic vessels (in this review, we generally name them lymphatic efflux). Recent studies showed that CSF mainly flowed out of the cranial cavity via the MLVs [6, 10]. Moreover, the GS and MLVs have interrelated anatomical structures and functions; some researchers name them the glymphatic-lymphatic fluid transport system [9, 13].
Numerous studies have focused on this specialized lymphatic transport system's physiological modulation and pathological changes [14-18]. This review highlights the current research progress on the modulation of lymphatic transport in the CNS, focusing on two main routes: glymphatic inflow and lymphatic efflux (Figure 1). In particular, the main topic of these studies reviewed herein is the glymphatic inflow, a critical pathway that transports lymph into the brain to perform. Importantly, no single physiological and pathological factor can explain this complex regulatory process in isolation. We summarize the internal connection between the upstream and downstream physiological modulation and the potential relationship between physiological modulation and pathological changes in the GS.
Modulation of the glymphatic inflow
Natural sleep
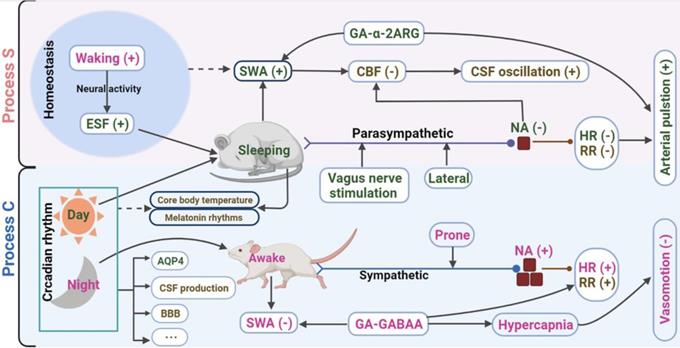
Sleep is a naturally occurring physiological state of decreasing arousal. During non-rapid eye movement (NREM) sleep, more CSF flows into ISS, and the brain's metabolites clearance is significantly more efficient than that in awake [14]. Low sleep quality is a risk factor for CNS diseases, such as Alzheimer's disease (AD), migraine, and dementia, which may be related to the brain's less clearance of solutes/metabolites [18-21]. For the complex regulatory mechanism of sleep, various regulation models have been proposed in the past few decades. The two-process model of sleep regulation, which assumes the interaction between the homeostatic recovery process (process S) and circadian pacemaker process (process C), is widely recognized in sleep research [22]. Since the glymphatic inflow is closely related to sleep, some factors regulating sleep may be potential factors modulating glymphatic inflow, and these factors may also have internal relations (Figure 2).
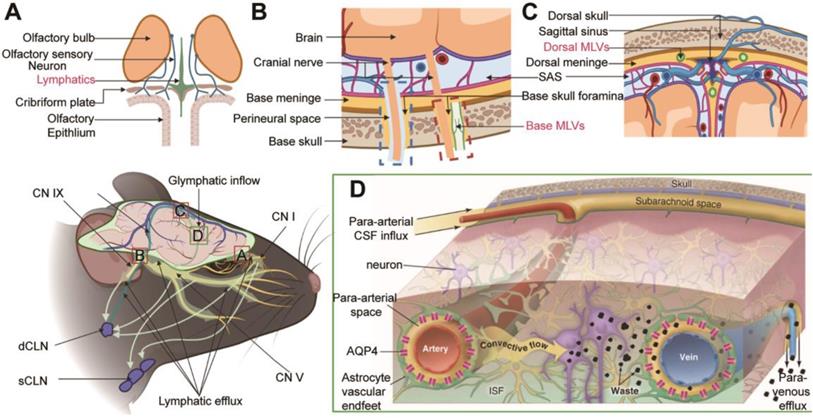
Schematic diagram of lymphatic transport in the CNS. (A) Lymphatic vessels near the cribriform plate. (B) Two uncertain pathways for CSF drainage at the base skull: the peripheral pathways (blue dashed box) and the base MLVS that overlap with the nerve anatomically (red dashed box). (C) Dorsal MLVs near sagittal sinus. (D) Overview of the lymphatic transport of CSF and ISF through the glymphatic system. Note: CN I, olfactory nerve; CN V, trigeminal nerve; CN IX, glossopharyngeal nerve; CN X, vagus nerve. Adapted with permission from Ref. [124] for A, copyright 2019 Nature Publishing Group; Ref. [125] for D, copyright 2013 American Association for the Advancement of Science. Created with BioRender.com.
The possible upstream and downstream relation networks between physiological modulatory factors. Note: The factors in dark green font represent promoted effects on the glymphatic influx, according to the current study; pink, inhibition; brown, unclear. Two dark blue boxes display the markers of processes S and C. "+", increased; "-", decreased. GA-α-2ARG, general anesthesia with α-2 adrenergic receptor agonist; GA-GABAA, general anesthesia with a positive allosteric modulator of GABAA; HR, heart rate; NA, norepinephrine; RR, respiratory rate. Created with BioRender.com.
Slow-wave activity and general anesthesia
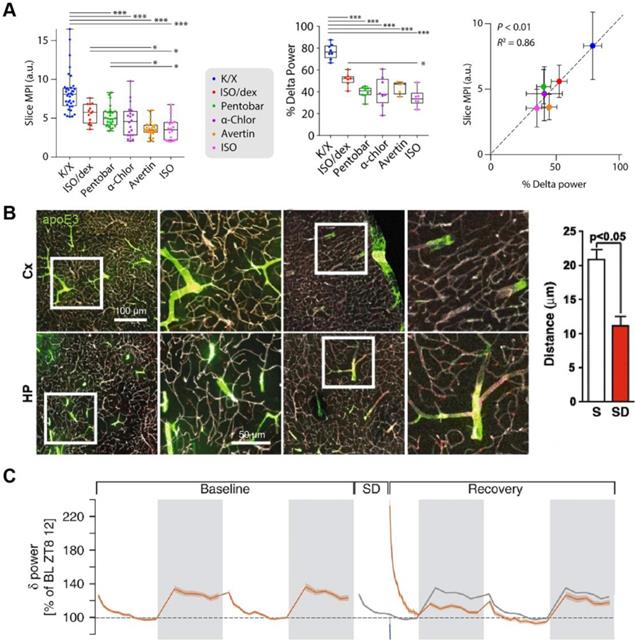
NREM sleep electroencephalography (EEG) slow-wave activity (SWA, 0.5-4Hz; a combination of slow oscillation and delta oscillations) represents the principal marker of the process S during sleep [22]. A pivotal study demonstrates that oscillations of CSF flow are interlinked with SWA in human sleep [23]. The addition of SWA accompanied by pulsatile CSF flow during sleep may be the internal mechanism leading to higher metabolite clearance [23]. During NREM sleep, the power density of SWA increases, and the glymphatic inflow increases consistently [14]. General anesthesia (GA) is a non-physiological and reversible drug-induced state, which is different from the neurophysiological mechanism of natural sleep [24]. Different general anaesthetics or sedatives have distinct inhibitory effects on the GS. Some anesthetics show positive functions similar to those observed during spontaneous sleep, while others are significant adverse, and these diverse effects are associated with SWA [15, 25, 26] (Figure 3A).
Still, there are some unclear and even confusing issues. As a marker of neurons' spontaneous and rhythmic electrical activities, EEG has been a leading tool to study brain function in health and disease. However, much less is known about its content, given the complex relationship between EEG features and microcircuit structure [27]. Existing terms typically refer to the frequency band that the rhythm occupies rather than its mechanism [28]. SWA during sleep is synchronized with the relatively short resting period of cortical neurons. At the same time, anesthesia-induced SWA is related to the significant enhancement of inhibiting postsynaptic currents (IPSCs) in the cortical loop, which is not synchronized with the long-term resting period of cortical neurons [24]. As a parameter to model process S in the two-process model, some literature has indicated that SWA regulates independently of sleep, which may be an epiphenomenon of sleep [29, 30]. Future insightful studies on the upstream neurophysiology of the origin of SWA will enable us to understand the differential effects of sleep and anesthesia on the GS.
Besides, some studies found that the glymphatic inflow decreased following sleep deprivation [31, 32] (Figure 3B). It is a confusing conclusion because the change in SWA during re-sleep after sleep deprivation is complicated. NREM sleep is affected by the prior sleep-wake history, and the SWA increases in the ensuing sleep stage after sleep deprivation, while they decrease gradually with prolonged sleep time [33-35] (Figure 3C). The increased SWA after sleep deprivation contradicts the inhibition of glymphatic inflow observed by these results. Of course, it is more likely a comprehensive process because these studies also discovered that the decreased glymphatic inflow after sleep deprivation was associated with the decline of paravascular polarization of the aquaporin-4 (AQP4) [31, 32]. Compared to humans, mice exhibit fragmented sleep patterns, characterized by unabiding sleep bouts, frequent awakenings, and a short (only 10-20 minutes) ultradian NREM / REM cycle duration [36]. Therefore, if the SWA modulates the GS, monitoring the EEG in an accurately defined sleep phase is necessary to reevaluate the relationship between sleep deprivation, SWA, and the GS.
Relationship between the glymphatic inflow and SWA. (A) SWA correlates with the various efficiencies of different anesthesia on the glymphatic influx. (B) The inflow distance of fluorescently-tagged apoE3 decreased in sleep-deprived mice. (C) The SWA of the ensuing sleep after sleep deprivation increased. Adapted with permission from Ref. [26] for A), copyright 2019 American Association for the Advancement of Science; Ref. [31] for B), copyright 2016 BioMed Central; and Ref. [35] for C), copyright 2020 Nature Publishing Group.
Furthermore, current researches on glymphatic transport rely on tracers of larger molecular solutes to simulate CSF transport, which may underestimate the flow of CSF water into brain parenchyma. The classical tracer study measured AQP4-dependent paracellular flow of the liquid exchange of ISS confirmed that tracers inflow were related to molecular solute size [3]. The use of H217O to capture both paracellular flow and diffusive transcellular exchange of water showed a faster and more active glymphatic transport [37]. The increased extracellular volume fraction is another possible reason for the increased CSF tracers inflow during sleep or ketamine/xylazine (K/X) cocktail anesthesia [14]. As the H217O tracer freely travels through the intracellular and extracellular pathways, extracellular volume fraction will also have a minor impact on the water transport of CSF [37]. Both paracellular flow and diffusive transcellular exchange of water are AQP4-dependent [38, 39].
Autonomic nervous system
During NREM sleep, the inhibition of the adrenergic system increases the area of the ISS; consequently, it reduces the fluid resistance and increases the solute transport efficiency [14]. The discharge of local neurons will spread to vast or local brain regions, which control multiple downstream targets [28]. Investigating the downstream effects after recording the electrophysiological activities may help us understand the physiological regulation of the glymphatic inflow.
The sympathetic tone is reduced during natural sleep, whereas the parasympathetic tone is increased [40]. Sleep initiation comes from the activation of γ-aminobutyric acid (GABA) and galanin receptors and the projective inhibition of synapses in the ascending reticular activated system neurons [36, 41]. Xylazine and dexmedetomidine inhibit the release of norepinephrine from the locus coeruleus by binding to the α-2 adrenergic receptors; this effect is consistent with the inhibition of norepinephrine release during natural sleep [42]. Both xylazine and dexmedetomidine show higher CSF tracers inflow efficiency than other anaesthetics [26]. Pentobarbital, α-chloralose, tribromoethanol, and isoflurane can enhance GABA-induced chloride influx and IPSCs by activating the GABA-A receptors [43-45], and these anaesthetics significantly inhibit glymphatic inflow [15, 26]. CSF tracers inflow decreases considerably in the case of acute hypertension induced by epinephrine [46]. Vagal nerve stimulation effectively cures migraines and AD, enhancing the glymphatic inflow [47]. Mammals prefer the lateral position during sleep. The glymphatic influx was more effective in the right lateral decubitus than in the prone position [48]. Moreover, the prone position increases sympathetic tone, while the vagal tone is increased in the right lateral position, which may be the reason for the increased glymphatic inflow [48, 49].
Summarizing these results, we speculate that the autonomic nervous system (ANS) has a wide range of modulatory effects on the glymphatic inflow. The ANS also regulates physiological parameters such as respiration, heart rate, and vascular pulsation. Some studies examined the impact of physiological parameters on GS, which were reviewed later in this paper.
Endogenous sleep factors
Endogenous sleep factors (ESF) such as adenosine, nitric oxide, and prostaglandin D2 have significant efficiency in regulating NREM sleep homeostasis (process S) [41]. Previous studies investigated the effects of these sleep factors on cerebral blood flow (CBF) [50, 51]. Owing to the coupling relationship between CBF and glymphatic influx [23], these ESF may also be potential molecules regulating the glymphatic inflow. In rats, low dose alcohol intake can promote glymphatic influx and metabolites clearance by inducing nitric oxide production and vasodilation [52]. Cocaine reduces adenosine transporter activity [53], and mice administered with cocaine have impaired glymphatic pathways [54]. Indeed, the potential value of these endogenous molecules has been noticed in the CNS diseases such as Alzheimer's disease, brain injury, stroke [55-57]. Future studies need to generate more evidence to reveal the mechanism and contribution of ESF toward regulating the clearance systems in the brain. Modulating NREM sleep through the intervention of these ESF may be an effective strategy to enhance brain clearance function.
Circadian rhythm
The circadian rhythm controlled by the suprachiasmatic nucleus of the hypothalamus plays the role of a pacemaker in the two-process model of sleep regulation [22, 58]. The GS is further modulated by circadian rhythm. The glymphatic inflow reaches the peak during possible daytime sleeping of mice [17]. The circadian rhythm of AQP4 perivascular polarization could be an internal mechanism of glymphatic influx affected by circadian rhythm [17]. Circadian rhythm governs CSF production, blood-brain barrier (BBB) permeability, and plasma norepinephrine concentration [59-61]. We need further ascertain whether these factors, modulated by the circadian rhythm, contribute to the circadian rhythm of the glymphatic inflow. In addition, it is also worth studying whether the two markers of process C, core body temperature and melatonin rhythms, can regulate the glymphatic inflow.
Cerebral artery pulsation and physiological parameters
Many physiological factors, including intracranial pressure (ICP), blood pressure, heart rate, and respiratory, regulate the fluid transport of the cerebrovascular system and PVS [62]. Since PVS provides a path for the glymphatic inflow, these factors may also be potential driving forces for the glymphatic inflow (Figure 2).
Arterial pulsation
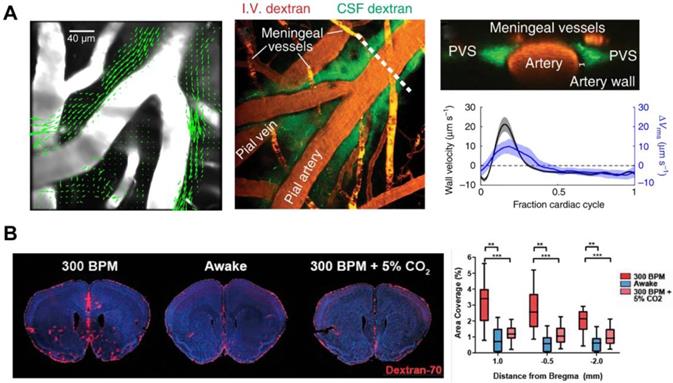
It is still controversial about the fluid transport mode in the ISS: convection or diffusion. Some modeling studies showed that arterial pulsation alone could not provide sufficient driving force to account for convective transport due to the narrow aPVS [63, 64]. Nonetheless, in vivo two-photon imaging have demonstrated that aPVS was a flat, double tubular eccentric structure, 1.4 times (whereas fixation reduced this ratio to 0.14) the cross-sectional area of the adjacent artery with low fluid resistance [46] (Figure 4A). The transport of intrathecal contrast agents through the brain is faster than expected from diffusion alone in humans [65]. Exploiting an isotopically enriched MRI tracer, H217O, a recent study revealed that the glymphatic transport was dramatically faster and more extensive than previously thought [37], further supporting the convective movement of paracellular CSF water. These studies advantageously support that arterial pulsation is the primary driving force of ISF convection. The flow of CSF in PVS is a whole pulsatile flow, which follows the same direction as that of the blood flow and has a similar frequency with the cardiac cycle [46] (Figure 4A). In humans, phase-contrast magnetic resonance imaging studies have shown that enhanced intracranial arterial pulsation promoted the influx of contrast molecules into aPVS [66]. When the internal carotid artery was ligated to inhibit the pulse of the cortical perforating artery, the glymphatic inflow was impaired; in contrast, dobutamine-enhanced pulsation of the perforating artery was positively correlated with the increased glymphatic influx [67]. Hypertension is also a risk factor for neurodegenerative diseases such as Alzheimer's and Parkinson's [68, 69]. Epinephrine-induced acute hypertension leads to arteriosclerosis, decreased vascular compliance, increased CSF reflux, and reduced CSF transport efficiency in aPVS [46].
Respiration rate and heart rate
The flow of CSF in the ventricular system and subarachnoid spaces (SAS) is mainly regulated by respiration, while the contribution of cardiac pulsations on this process is low [70]. To date, there is no direct evidence to prove that respiration modulates the glymphatic inflow, although respiration is the main factor influencing CSF drainage to the peripheral lymph nodes [62]. Given that rapid CSF efflux inhibits the glymphatic influx, further experiments are needed to identify the effect of respiration on the glymphatic inflow [71]. Mice treated with different anaesthetics showed that increased glymphatic influx was associated with decreased heart rate [26]. This study also indicated that respiration and blood pressure were not associated with glymphatic influx [26]. However, it should be noted that GA significantly reduces the respiratory dynamics, leading to hypercapnia, which can inhibit the outflow to the lymph nodes and inflow to the brain of CSF [72-74] (Figure 4B). Considering the contribution of the glymphatic influx to the metabolite clearance and immune-inflammatory response in the brain, further study on these parameters may provide newer insight into the treatment of stress conditions occurring after brain injury.
Intracranial pressure
Decompressive craniectomy reduces ICP and impairs the glymphatic inflow, which cranioplasty reversed in mice [75]. In mice's early stage of ischemic stroke, PVS dilated because of vasoconstriction, doubling the glymphatic influx speed [76]. The increase of the glymphatic inflow may cause the increased ICP induced by cerebral edema in the case of ischemic stroke; however, there is no direct conclusion characterizing the effect of increased ICP on the glymphatic influx. Another study reported that the glymphatic inflow was impaired after traumatic brain injury (TBI) [77]. Although increased ICP is a severe complication of brain injury, this study did not attribute impaired glymphatic inflow to the increased ICP but instead to the impairment of AQP4 polarization [77]. The decrease of ICP impairs the glymphatic inflow, and the increased ICP on it is still unclear. Some studies showed increased ICP after TBI generated meningeal lymphatic dysfunction, and the meningeal lymphatic drainage was impaired after stroke [16, 78, 79].
The driving force of brain waste clearance. (A) aPVS has a large cross-sectional area, and the particles flow convectively in the direction of blood. The peak in-wall velocity and the delay time are consistent with the peak and delay of the spatial root means square velocity curve. (B) Hypercapnic ventilation significantly reduces CSF tracer influx in K/X-anesthetized mice. Adapted with permission from Ref. [46] for A), copyright 2018 Nature Publishing Group; Ref. [74] for B), copyright 2020 SAGE Publications Inc.
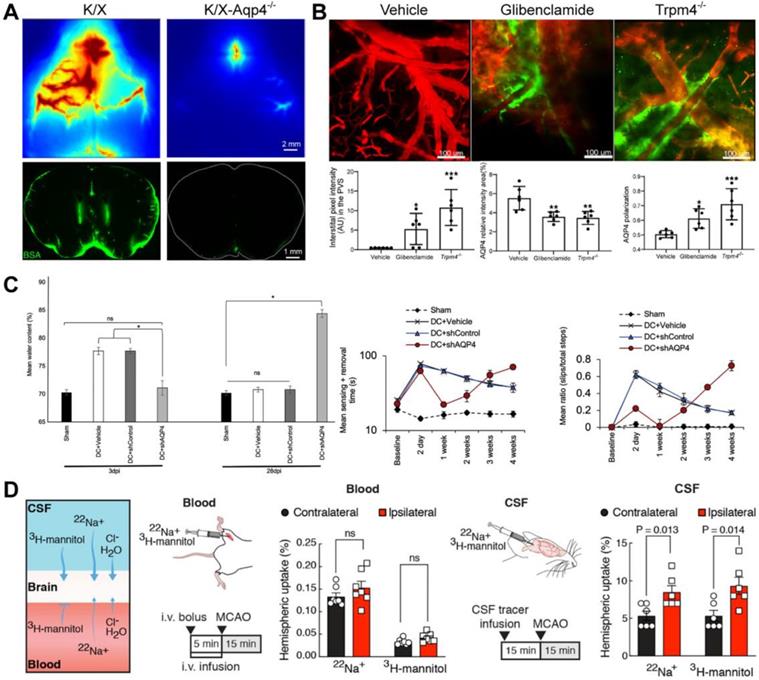
Effects of AQP4 on different CNS diseases. (A) The deletion of the AQP4 gene reduces CSF tracer influx. (B) Glibenclamide alleviates brain oedema, increases the polarization of AQP4, and reverses the impairment of GS function after SE. (C) AQP4 knockout reduces oedema and restores sensorimotor function on the 3-day post-SCI, while it increases oedema and deteriorates sensorimotor function on the 28-day post-SCI. (D) Labelling either the blood or CSF compartment shows that CSF is the source of the edema fluid. Adapted with permission from Ref. [90] for A), copyright 2018 American Society for Clinical Investigation; Ref. [93] for B), copyright 2021 American Society for Clinical Investigation; Ref. [97] for C), copyright 2020 Cell Press; and Ref. [76] for D, copyright 2020 American Association for the Advancement of Science.
Vasomotion
Some researchers believe that the efficiency provided by arterial pulsation should not exceed 15-25%, and vasomotion is a more efficient driving force [80-82]. As another factor regulating the dynamic change of vascular diameter except for arterial pulsation, vasomotion is a spontaneous low-frequency (0.1-0.4 Hz) rhythmic cerebral vasoconstriction and dilation of the vascular smooth muscle cells motions [83]. Functional hyperemia induced by visual stimulation increases the amplitude of vasomotion and promotes the clearance of fluorescent molecules in mice brains [82]. APP / PS1 mice show impaired vasomotion and decreased clearance of fluorescent molecules during functional hyperemia [82]. The dysfunction of the smooth muscle cells may be part of the possible causes of clearance dysfunction in the case of cerebral amyloid angiopathy [82]. In addition, the inhibition of vasomotion during GA may be the reason for the decline of brain clearance function [74, 82]. Vasomotion is not an isolation parameter and also intrinsically relates to other physiological parameters. According to the underlying principle of resting-state blood oxygen level-dependent signal in functional magnetic resonance imaging of rodents, the level of cerebral oxygen regulated by physiological parameters such as respiration and heart rate directly affects the movement of vascular smooth muscle [84].
Aquaporin-4
Mammalian aquaporins, transmembrane proteins that promote the bidirectional transport of water across the cell membrane, are the primary water homeostasis regulators and participate in molecular transport and membrane protein expression, cell adhesion, and cell volume regulation [85]. A subgroup of aquaporins water channels (e.g., aquaglyceroporin) also facilitate transmembrane diffusion of small, polar solutes except for water molecules [86, 87]. AQP4, the most expressed aquaporins in CNS, is enriched on astrocytes end-feet and plays a vital role in the GS [38]. According to the astrocyte-neuron lactate shuttle hypothesis, neural activity is related to brain lactate production to meet the higher energy requirements [2, 88]. Lactate flow out of the brain through AQP4-dependent glymphatic clearance in the CSF mixture during rest [89]. AQP4-null mice show markedly reduced CSF tracer influx into the PVS and clearance of metabolites in the ISS [3, 90] (Figure 5A). Notably, the perivascular polarization of AQP4 may play a more critical role. Snta1 knockout mice with regular expression and loss of polarization of AQP4 have the same inhibitory effect on glymphatic flow as AQP4 knockout mice [91]. As described in 2.1.4, the circadian polarization change of AQP4 rather than the expression is related to the circadian change of GS function [17]. The polar expression of AQP4 increases after voluntary exercise, which promotes the clearance of amyloid-β in mice [92]. In some CNS diseases, glymphatic flow inhibition is also related to AQP4 depolarization. Inhibition of brain edema increases the polarization of AQP4 and reverses the impairment of GS function after status epilepticus (SE) [93] (Figure 5B). After TBI, the expression of AQP4 is delocalized, which impairs the glymphatic flow and increases the deposition of tau protein [77]. The loss of AQP4 polarization and the impairment of the GS function still co-occur after 28 days of brain injury [77]. AQP4 deficiency also aggravates the pathology of neurodegenerative diseases [94]. Idiopathic normal pressure hydrocephalus, a subtype of dementia, is characterized by the loss of AQP4 polarization and the impairment of the glymphatic inflow, similar to those observed in AD mice [95, 96].
AQP4 also plays a vital role during brain edema. CNS edema is associated with increases in total AQP4 expression and AQP4 subcellular translocation after TBI, spinal cord injury (SCI), and stroke [97, 98]. Trifluoperazine alleviates post-injured edema by inhibiting the expression and polarization of AQP4 after TBI, SCI, and ischemic stroke [97, 98]. Importantly, this effect is achieved by targeting the calmodulin-mediated subcellular polarization of AQP4 [97]. The perivascular polarized expression of AQP4 plays a negative role by driving the cytotoxic edema caused by water influx in the early post-injury stage. Still, it also plays a positive role in promoting the clearance of vascular edema later [97]. In a rat SCI model, AQP4 knockout reduced edema and restored sensorimotor function in the early stage but increased oedema and deteriorated sensorimotor function in the late stage [97] (Figure 5C).
Is there any intrinsic relationship between brain edema and GS transport? The source of water for brain edema is also a controversial issue. A recent study showed that CSF flowed into the ischemic area through PVS (the same inflow pathway as the GS), driving acute tissue swelling (Figure 5D) [76]. Does the bulk influx of CSF into PVS synchronously lead to an enhancement of GS function and increase of solute clearance of ISS during acute ischemic (within 30 minutes as the study focused on [76])? Brain edema is long-term and complex pathophysiology, including cytotoxic edema, ionic edema, and subsequent vasogenic edema with the breakdown of the BBB [97]. The GS function is impaired rather than enhanced, accompanied by pathological brain edema in the early stage (1-7 days) after TBI, ischemic stroke, and epilepsy in wild mice [77, 93, 99]. The deletion or depolarization of AQP4 is adverse for vascular edema clearance and GS function recovery in brain injury. Obviously, brain edema and glymphatic transports are two different pathophysiological processes. Who led to the later differentiated outcome when they shared the same initial pathway (the glymphatic inflow)? In addition to the increase of ICP and the destruction of BBB caused by brain edema, the regional differential expression, inconsistent expression and polarization, and the repolarization of subcellular of AQP4 after brain injury also needs to be further characterized. AQP4 expression is strongly downregulated in the infarct and overexpressed in the penumbra after acute ischemic stroke in mice [100]. Both patients with temporal lobe epilepsy associated with unilateral hippocampal sclerosis and mice in the early days after SE showed up-regulated AQP4 expression and decreased AQP4 polarization [93, 101]. Other studies have shown that the increased AQP4 membrane localization in primary human astrocytes was not accompanied by a change in AQP4 protein expression levels [102, 103].
AQP4 inhibitors may benefit from the reversible blockade of AQP4 within one week after brain injury [97]. Since AQP4 gene-deficient mice showed a coincident decrease between brain edema and GS clearance function accompanied by decreased AQP4 expression and polarization [102, 104], modulators of AQP4 might also be potentially effective drugs that reversibly inhibit cytotoxic edema in the early stage of brain injury but, after that, recover vascular edema clearance and GS function by AQP4 relocalization. However, despite intense efforts over many years, many highly hoped pore-blockers of AQP4 previously developed were later challenged because the inhibitory effects of most of these molecules were not repeatable in other analyses [38, 39]. TGN-020 has originally designated an AQP4 inhibitor based on data from the Xenopus laevis oocyte swelling assay. However, when tested in cell-based assays, it lacks AQP4 inhibitory function [105, 106]. Targeting AQP4 subcellular relocalization (a dynamic process independent of changes in AQP4 expression [39]) may be an alternative strategy. Trifluoperazine eliminates brain edema in the CNS by inhibiting calmodulin, which drives AQP4 cell-surface localization by binding to the carboxyl terminus of AQP4 [97]. Glibenclamide alleviates pathological brain edema after SE by inhibiting the SUR1-TRMP4 channel complex on the astrocyte membrane and recovers GS function [93]. Technical innovations will solve the attrition challenges in drug screening and confounding factors in water permeation analysis, such as calcein fluorescence queuing, human microvessel-on-a-chip platforms, high-throughput screening, and computer-aided drug design [107-110]. The latter two have recently been applied to discover novel drugs for neurodegenerative diseases. They are likely to provide a novel insight that can help new treatments' findings targeting AQP4 in the future.
Modulation of lymphatic efflux
CSF production
CSF is mainly produced by the choroid plexus of the ventricular system and other tissues (such as the BBB) [111]. Various physiological factors and molecules affect the production rate of CSF, and the production of CSF increases in some pathological conditions such as stroke and meningitis [112, 113]. As the leading participant in the glymphatic inflow, it is vital to understand whether the CSF production modulates the GS.
The direct measurement of CSF production in mice by blocking the aqueduct of Sylvius indicated that isoflurane caused a higher rate of CSF production than that of the K/X cocktail [113]. However, the increased CSF production does not indicate the increased glymphatic inflow since previous studies have shown that the K/X cocktail resulted in more glymphatic influx than isoflurane [26, 113]. CSF production in female mice is higher than in male mice, but there is no sex difference in the glymphatic influx among the healthy young, middle-aged, or old mice [113, 114]. These results expounded no association between the glymphatic inflow and CSF production. Still, this conclusion needs to be further studied since isoflurane can also cause changes of many factors (physiological parameters, fluid dynamics and vascular compliance), which may offset the benefits of increased CSF production.
In contrast, although there is no direct research evidence, other studies have demonstrated that the increased CSF production was consistent with the increased glymphatic influx. The production of CSF increases during the mid-rest phase and the treatment with non-selective β-adrenergic receptor antagonists in mice [113, 115, 116]. Coincidently, the glymphatic influx increases during these conditions [14, 17]. Both CSF production and glymphatic influx are reduced in elderly and AD mice [18, 113, 117, 118]. The loss of CSF induced by cisternostomy and the reduction of CSF production treated with acetazolamide decrease the clearance of fluorescent protein and TBI injury markers in mice [89, 119].
We speculate that the regulation of CSF production under physiological conditions may be intrinsically related to the modulation of sleep, ANS, physiological parameters, and cerebrovascular, all of which ensure a stable and adequate brain clearance. Changes in CSF production during pathological conditions may be related to brain clearance disorder's compensatory or decompensated mechanism.
Pathway of lymphatic efflux
CSF in the ventricles converges into the fourth ventricle through the interventricular foramen, the third ventricle, the midbrain aqueduct, and eventually flows into the cisterna magna circulate in SAS. CSF in the fourth ventricle can also flow into the central canal of the spinal cord. The drainage of CSF into the spinal cord is far less than that into the cranial cavity under physiological conditions [120, 121]. Initial studies that ignored the effect of injection volume and rate on ICP suggested that the primary drainage route of CSF involved its return to the sagittal sinus via the arachnoid granules [122]. Actually, no CSF flow into the superficial cerebral venous system through the arachnoid granules in physiological ICP conditions [6]. There are three widely accepted pathways for CSF efflux from the cranial cavity (Figure 1): (1) along the olfactory nerve through the cribriform plate to the nasal mucosa, efflux into the superficial and deep cervical lymph nodes (sCLNs, dCLNs) (Figure 1A); (2) through the peripheral pathways of the trigeminal nerve, glossopharyngeal nerve, vagus nerve, and other crucial cranial nerves, drain into the sCLNs and dCLNs eventually (Figure 1B, blue dashed box); and (3) drainage through the MLVs into the dCLNs [4, 6, 123] (Figure 1C). Notably, recent studies have characterized specialized lymphatic structures on the dura mater passing through the vicinity of the cribriform plate and cranial foramen of the cranial base nerve [11, 124] (Figure 1A-B). The cranial nerves overlap anatomically with the base MLVs. These lymphatic vessels may be independent of cerebral nerves, and the excision of nerves will not affect MLVs' integrity and drainage efficiency [11] (Figure 1B, red dashed box).
The novel pathway, GS, elaborates that some CSF flow into the brain parenchyma before efflux out of the skull cavity [125] (Figure 1D). BBB, composed of tightly connected endothelial cells, astrocytes, and pericytes, provides a robust physical barrier for CNS and prevents foreign molecules' interference on neuronal activity and signal transduction [126]. On the side, this barrier makes it impossible for the brain' metabolites to be discharged in the same way as the peripheral microcirculation. The GS provides a practical clearance pathway to the brain in a state of continuous high metabolic activity, especially those far away from BBB. It is certain that not all CSF have the opportunity to flow into the brain, and most of it directly outflows into the extracranial lymphatic vessels during awake [9, 71]. Similar to the peripheral lymphatic system, the CSF transport rate is higher in the awake state than during sleep, and the rapid CSF outflow leads to a decreased glymphatic influx [71]. According to the principle of mass conservation, the increased CSF efflux will inevitably lead to a reduced glymphatic influx in homeostatic conditions.
In addition, these traditional and novel drainage pathways also have some outstanding open scientific issues that need to be further characterized. For example, is there an essential connection between the conventional drainage path of the perineural space and the MLVs located around these nerves? The exact contribution of these outflow pathways to CSF drainage remains clarified. What anatomical pathway does the ISF pass into the dorsal and base MLVs?
Meningeal lymphatic vessels
Although the mechanism of how CSF is transported to MLVs is unclear, many studies showed that CSF primary outflowed cranial cavity through MLVs [6, 10]. Some studies demonstrated that MLVs also regulated the GS. In elderly mice, the density of the dorsal MLVs is reduced, and abnormal branching hyperplasia of MLVs at the base skull is noted, resulting in decreased CSF outflow to the dCLNs and reduced glymphatic inflow into the brain [6, 11, 127, 128]. After blocking the MLVs outflow pathway of CSF with ligation of dCLNs, photochemical ablation of the MLVs or MLVs developmental defects, both glymphatic influx and brain solute clearance are decreased in mice [4, 10, 127-130]. Overexpression of vascular endothelial growth factor C promotes the proliferation and remodeling of MLVs, which effectively reverses the reduced glymphatic influx caused by the aging-related degeneration of MLVs [127, 131].
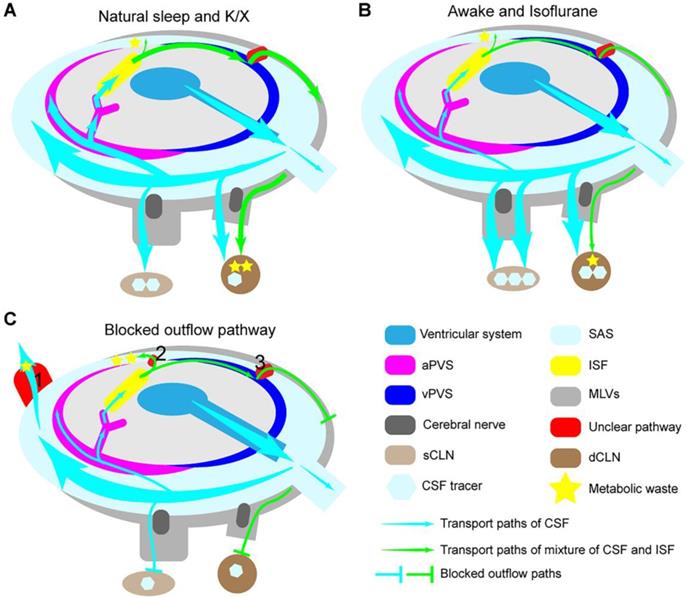
The degeneration of MLVs in aging mice and the blockade of the CSF outflow pathway does not increase brain water content and ISF pressure [4]. This conclusion indicates that the MLVs' status as the primary CSF exclusion pathway will be challenged when dysfunctional. Other routes must diverge some CSF (Figure 6C, red box 1). When blocking the MLVs drainage pathway, the elimination of metabolic waste in the brain is not entirely blocked, which means the presence of other routes for the elimination of metabolic waste [132] (Figure 6C, red box 2). Small molecules injected into the ventricle or cisterna magna symmetrically drain into the bilateral dCLNs and sCLNs through the cribriform plate [4] (Figure 6A). Moreover, the tracers injected into the brain parenchyma primarily flow into the ipsilateral dCLNs, twice the contralateral, and less than 30% are circulated into the cisterna magna [4, 133] (Figure 6C). T cells injected through cistern magna will drain into the dCLNs and sCLNs, while those injected into the brain parenchyma could not be emptied into the sCLNs through the cribriform plate [10]. Blocking the main outflow path of CSF reduces glymphatic influx synchronously, while the rapid outflow of CSF is inversely proportional to the glymphatic inflow; these are two contradictory conclusions (Figure 6B-C). Therefore, we propose the following hypothesis that may explain these results: the outflow pathway of CSF in the SAS may differ from that in the brain. Other complementary courses might become active when pathologically blocked outflow pathways (Figure 6C). CSF in the SAS may flow out of the cranial cavity symmetrically from the widespread outflow pathway, and the mixed fluid in the brain tends to drain into the ipsilateral side through a specific path. Further study on the anatomical track of CSF passing into the MLVs may help us understand these unclear ways.
Moreover, the proliferation and remodeling of MLVs have both sides in CNS disorders. Structural and functional disorders of MLVs will impair the brain clearance system and reduce the immune-inflammatory response, which may be a potential therapeutic target for CNS autoimmune response such as multiple sclerosis [124]. In contrast, promoting the proliferation and remodeling of MLVs may be an effective treatment direction for intracranial tumors and neurodegeneration [127, 131, 134, 135].
The modulation of lymphatic fluid efflux in the cranial cavity. (A) During natural sleep or K/X anesthesia, more CSF flows into the brain and efficiently removes accumulated metabolic waste through the GS pathway. (B) During awake or isoflurane anesthesia, the rapid outflow of CSF reduces the glymphatic inflow and brain waste clearance. (C) Blocking the outflow pathways impairs the glymphatic influx and reduces lymph drainage but does not increase ICP and interstitial hydraulic pressure. Some supplementary pathways may be involved in the shunt of CSF (red box 1) and ISF (red box 2) under some pathological conditions.
Conclusion
In this review, we systematically review the physiological regulatory mechanisms of the lymphatic transport in the CNS based on the two-process model of sleep regulation. It is now clear that no single pathological feature can explain this complex modulatory process in isolation. These mechanisms are related and interact upstream and downstream (Figure 2). Sleep is regulated by homeostasis (process S) and circadian rhythm (process C). The two-process and related factors (SWA, ESF, sleep deprivation, etc.) are all involved in the modulation of the glymphatic inflow. The mechanism of the differential regulatory effects of different anesthesia and sleep in the upstream is unclear, and there may be an interpretable mechanism in the downstream. During natural sleep, the reduced sympathetic tone and increased parasympathetic tone inhibit noradrenergic receptors. Then, following a decrease in respiratory rate and heart rate. The reduction of respiratory rate leads to increased CO2 partial pressure; after that, through internal chemical regulation mechanisms, the cerebrovascular dilation and vascular compliance increases, along with the lowering of heart rate, which eventually increases the arterial pulsation amplitude [136].
Unlike physiological regulation, pathological changes are often compensatory or decompensated physiological reactions occurring after disease onset. Like inflammation, which has both protective and adverse effects, we need to investigate the comprehensive impact of these physiological regulations on different diseases and stages after pathological CNS disorders and further identify the regulatory strategies that can be the most beneficial. These physiological parameters will also change during pathological stress after brain injury in different periods throughout the disease course. Many clinicians have been developing individualized and accurate therapeutic schemes by adjusting these physiological parameters to reduce secondary brain injury, and these therapeutics are also controversial. For example, which is a better therapy after severe TBI, the Lund concept, or the Brain Trauma Foundation guidelines [137]? Is preventive hypothermia beneficial after TBI [138]? As sleep has a restorative physiological function, the independent or comprehensive study of these modulatory mechanisms may identify critical targets for the recovery from CNS disorders.
Under physiological conditions, the rapid outflow of CSF reduces the glymphatic influx. Pathologically blocking MLVs does not increase cerebral water content and glymphatic influx. We speculate that the outflow pathway of CSF in the SAS may differ from that in the brain; other complementary routes may be hidden. Further studies that characterize the exact anatomical structure of these outflow pathways will help us find new therapeutic strategies that effectively regulate these pathways to counteract the brain damage caused by the changes of outflow pathways in pathological conditions.
The lymphatic transport may be involved in most pathophysiological processes in the CNS, so these directions are far from enough. We can restudy any CNS disease by targeting the specialized lymphatic transport system with new information and insight.
Abbreviations
AD: Alzheimer's disease; ANS: autonomic nervous system; aPVS: artery paravascular space; AQP4: astrocyte aquaporin-4; BBB: blood-brain barrier; CBF: cerebral blood flow; CNS: central nervous system; CSF: cerebrospinal fluid; dCLNs: deep cervical lymph nodes; EEG: electroencephalogram; ESF: Endogenous sleep factors; GA: General anaesthesia; GABA: γ-aminobutyric acid; GS: glymphatic system; ICP: intracranial pressure; IPSCs: inhibiting postsynaptic currents; ISF: interstitial fluid; ISS: interstitial system; K/X: ketamine/xylazine; MLVs: meningeal lymphatic vessels; NREM: non-rapid eye movement; SAS: subarachnoid spaces; sCLNs: superficial cervical lymph nodes; SCI: spinal cord injury; SE: status epilepticus; SWA: slow-wave activity; TBI: traumatic brain injury; vPVS: venous paravascular space.
Acknowledgements
This work was supported by the National Nature and Science Foundation of China (81871555) and the Jilin Provincial Department of Finance (JLSCZD2019-006, JLSCZD2019-067, 2018SCZWSZX-006, and 2017F006).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Oliver G, Kipnis J, Randolph GJ, Harvey NL. The Lymphatic Vasculature in the 21st Century: Novel Functional Roles in Homeostasis and Disease. Cell. 2020;182:270-96
2. Bordone MP, Salman MM, Titus HE, Amini E, Andersen JV, Chakraborti B. et al. The energetic brain - A review from students to students. J Neurochem. 2019;151:139-65
3. Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4:147ra111
4. Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M. et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212:991-9
5. Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD. et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337-41
6. Ma Q, Ineichen BV, Detmar M, Proulx ST. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat Commun. 2017;8:1434
7. Plog BA, Nedergaard M. The Glymphatic System in Central Nervous System Health and Disease: Past, Present, and Future. Annu Rev Pathol. 2018;13:379-94
8. DAVSON H, KLEEMAN CR, LEVIN E. Quantitative studies of the passage of different substances out of the cerebrospinal fluid. J Physiol. 1962;161:126-42
9. Mestre H, Mori Y, Nedergaard M. The Brain's Glymphatic System: Current Controversies. Trends Neurosci. 2020;43:458-66
10. Louveau A, Herz J, Alme MN, Salvador AF, Dong MQ, Viar KE. et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci. 2018;21:1380-91
11. Ahn JH, Cho H, Kim JH, Kim SH, Ham JS, Park I. et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature. 2019;572:62-6
12. Jacob L, Boisserand L, Geraldo L, de Brito Neto J, Mathivet T, Antila S. et al. Anatomy and function of the vertebral column lymphatic network in mice. Nat Commun. 2019;10:4594
13. Louveau A, Plog BA, Antila S, Alitalo K, Nedergaard M, Kipnis J. Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J Clin Invest. 2017;127:3210-9
14. Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M. et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373-7
15. Gakuba C, Gaberel T, Goursaud S, Bourges J, Di Palma C, Quenault A. et al. General Anesthesia Inhibits the Activity of the "Glymphatic System". Theranostics. 2018;8:710-22
16. Bolte AC, Dutta AB, Hurt ME, Smirnov I, Kovacs MA, McKee CA. et al. Meningeal lymphatic dysfunction exacerbates traumatic brain injury pathogenesis. Nat Commun. 2020;11:4524
17. Hablitz LM, Plá V, Giannetto M, Vinitsky HS, Stæger FF, Metcalfe T. et al. Circadian control of brain glymphatic and lymphatic fluid flow. Nat Commun. 2020;11:4411
18. Harrison IF, Ismail O, Machhada A, Colgan N, Ohene Y, Nahavandi P. et al. Impaired glymphatic function and clearance of tau in an Alzheimer's disease model. Brain. 2020;143:2576-93
19. Schain AJ, Melo-Carrillo A, Strassman AM, Burstein R. Cortical Spreading Depression Closes Paravascular Space and Impairs Glymphatic Flow: Implications for Migraine Headache. J Neurosci. 2017;37:2904-15
20. Nedergaard M, Goldman SA. Glymphatic failure as a final common pathway to dementia. Science. 2020;370:50-6
21. Christensen J, Yamakawa GR, Shultz SR, Mychasiuk R. Is the glymphatic system the missing link between sleep impairments and neurological disorders? Examining the implications and uncertainties. Prog Neurobiol. 2021;198:101917
22. Borbély AA, Daan S, Wirz-Justice A, Deboer T. The two-process model of sleep regulation: a reappraisal. J Sleep Res. 2016;25:131-43
23. Fultz NE, Bonmassar G, Setsompop K, Stickgold RA, Rosen BR, Polimeni JR. et al. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science. 2019;366:628-31
24. Akeju O, Brown EN. Neural oscillations demonstrate that general anesthesia and sedative states are neurophysiologically distinct from sleep. Curr Opin Neurobiol. 2017;44:178-85
25. Benveniste H, Lee H, Ding F, Sun Q, Al-Bizri E, Makaryus R. et al. Anesthesia with Dexmedetomidine and Low-dose Isoflurane Increases Solute Transport via the Glymphatic Pathway in Rat Brain When Compared with High-dose Isoflurane. Anesthesiology. 2017;127:976-88
26. Hablitz LM, Vinitsky HS, Sun Q, Stæger FF, Sigurdsson B, Mortensen KN. et al. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci Adv. 2019;5:eaav5447
27. Cohen MX. Where Does EEG Come From and What Does It Mean. Trends Neurosci. 2017;40:208-18
28. Buzsáki G, Logothetis N, Singer W. Scaling brain size, keeping timing: evolutionary preservation of brain rhythms. Neuron. 2013;80:751-64
29. Kim Y, Laposky AD, Bergmann BM, Turek FW. Repeated sleep restriction in rats leads to homeostatic and allostatic responses during recovery sleep. Proc Natl Acad Sci U S A. 2007;104:10697-702
30. Davis CJ, Clinton JM, Jewett KA, Zielinski MR, Krueger JM. Delta wave power: an independent sleep phenotype or epiphenomenon. J Clin Sleep Med. 2011;7:S16-8
31. Achariyar TM, Li B, Peng W, Verghese PB, Shi Y, McConnell E. et al. Glymphatic distribution of CSF-derived apoE into brain is isoform specific and suppressed during sleep deprivation. Mol Neurodegener. 2016;11:74
32. Zhang R, Liu Y, Chen Y, Li Q, Marshall C, Wu T. et al. Aquaporin 4 deletion exacerbates brain impairments in a mouse model of chronic sleep disruption. CNS Neurosci Ther. 2020;26:228-39
33. Franken P, Chollet D, Tafti M. The homeostatic regulation of sleep need is under genetic control. J Neurosci. 2001;21:2610-21
34. Dijk DJ. Regulation and functional correlates of slow wave sleep. J Clin Sleep Med. 2009;5:S6-15
35. Hubbard J, Gent TC, Hoekstra M, Emmenegger Y, Mongrain V, Landolt HP. et al. Rapid fast-delta decay following prolonged wakefulness marks a phase of wake-inertia in NREM sleep. Nat Commun. 2020;11:3130
36. Weber F, Dan Y. Circuit-based interrogation of sleep control. Nature. 2016;538:51-9
37. Alshuhri MS, Gallagher L, Work LM, Holmes WM. Direct imaging of glymphatic transport using H217O MRI. JCI Insight. 2021;6:e141159
38. Salman MM, Kitchen P, Halsey A, Wang MX, Tornroth-Horsefield S, Conner AC. et al. Emerging roles for dynamic aquaporin-4 subcellular relocalization in CNS water homeostasis. Brain. 2021 awab311
39. Salman MM, Kitchen P, Iliff JJ, Bill RM. Aquaporin 4 and glymphatic flow have central roles in brain fluid homeostasis. Nat Rev Neurosci. 2021;22:650-1
40. Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993;328:303-7
41. Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92:1087-187
42. Park JW, Chung HW, Lee EJ, Jung KH, Paik JY, Lee KH. α2-Adrenergic agonists including xylazine and dexmedetomidine inhibit norepinephrine transporter function in SK-N-SH cells. Neurosci Lett. 2013;541:184-9
43. Garrett KM, Gan J. Enhancement of gamma-aminobutyric acidA receptor activity by alpha-chloralose. J Pharmacol Exp Ther. 1998;285:680-6
44. Krasowski MD, Harrison NL. The actions of ether, alcohol and alkane general anaesthetics on GABAA and glycine receptors and the effects of TM2 and TM3 mutations. Br J Pharmacol. 2000;129:731-43
45. Brown EN, Purdon PL, Van Dort CJ. General anesthesia and altered states of arousal: a systems neuroscience analysis. Annu Rev Neurosci. 2011;34:601-28
46. Mestre H, Tithof J, Du T, Song W, Peng W, Sweeney AM. et al. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat Commun. 2018;9:4878
47. Cheng KP, Brodnick SK, Blanz SL, Zeng W, Kegel J, Pisaniello JA. et al. Clinically-derived vagus nerve stimulation enhances cerebrospinal fluid penetrance. Brain Stimul. 2020;13:1024-30
48. Lee H, Xie L, Yu M, Kang H, Feng T, Deane R. et al. The Effect of Body Posture on Brain Glymphatic Transport. J Neurosci. 2015;35:11034-44
49. Kuo CD, Chen GY, Lo HM. Effect of different recumbent positions on spectral indices of autonomic modulation of the heart during the acute phase of myocardial infarction. Crit Care Med. 2000;28:1283-9
50. O'Regan M. Adenosine and the regulation of cerebral blood flow. Neurol Res. 2005;27:175-81
51. Toda N, Okamura T. Cerebral blood flow regulation by nitric oxide in Alzheimer's disease. J Alzheimers Dis. 2012;32:569-78
52. Cheng Y, Liu X, Ma X, Garcia R, Belfield K, Haorah J. Alcohol promotes waste clearance in the CNS via brain vascular reactivity. Free Radic Biol Med. 2019;143:115-26
53. Kubrusly RC, Bhide PG. Cocaine exposure modulates dopamine and adenosine signaling in the fetal brain. Neuropharmacology. 2010;58:436-43
54. Chen W, Huang P, Zeng H, Lin J, Shi Z, Yao X. Cocaine-induced structural and functional impairments of the glymphatic pathway in mice. Brain Behav Immun. 2020;88:97-104
55. Ahmad AS, Ottallah H, Maciel CB, Strickland M, Doré S. Role of the L-PGDS-PGD2-DP1 receptor axis in sleep regulation and neurologic outcomes. Sleep. 2019;42:zsz073
56. Curvello V, Pastor P, Hekierski H, Armstead WM. Inhaled Nitric Oxide Protects Cerebral Autoregulation and Reduces Hippocampal Necrosis After Traumatic Brain Injury Through Inhibition of ET-1, ERK MAPK and IL-6 Upregulation in Pigs. Neurocrit Care. 2019;30:467-77
57. Franco R, Rivas-Santisteban R, Casanovas M, Lillo A, Saura CA, Navarro G. Adenosine A2A Receptor Antagonists Affects NMDA Glutamate Receptor Function. Potential to Address Neurodegeneration in Alzheimer's Disease. Cells. 2020;9:1075
58. Brancaccio M, Patton AP, Chesham JE, Maywood ES, Hastings MH. Astrocytes Control Circadian Timekeeping in the Suprachiasmatic Nucleus via Glutamatergic Signaling. Neuron. 2017;93:1420-35.e5
59. Linsell CR, Lightman SL, Mullen PE, Brown MJ, Causon RC. Circadian rhythms of epinephrine and norepinephrine in man. J Clin Endocrinol Metab. 1985;60:1210-5
60. Takahashi H, Tanaka H, Fujita N, Murase K, Tomiyama N. Variation in supratentorial cerebrospinal fluid production rate in one day: measurement by nontriggered phase-contrast magnetic resonance imaging. Jpn J Radiol. 2011;29:110-5
61. Zhang SL, Yue Z, Arnold DM, Artiushin G, Sehgal A. A Circadian Clock in the Blood-Brain Barrier Regulates Xenobiotic Efflux. Cell. 2018;173:130-9.e10
62. Dreha-Kulaczewski S, Joseph AA, Merboldt KD, Ludwig HC, Gärtner J, Frahm J. Inspiration is the major regulator of human CSF flow. J Neurosci. 2015;35:2485-91
63. Asgari M, de Zélicourt D, Kurtcuoglu V. How astrocyte networks may contribute to cerebral metabolite clearance. Sci Rep. 2015;5:15024
64. Asgari M, de Zélicourt D, Kurtcuoglu V. Glymphatic solute transport does not require bulk flow. Sci Rep. 2016;6:38635
65. Ringstad G, Valnes LM, Dale AM, Pripp AH, Vatnehol SS, Emblem KE. et al. Brain-wide glymphatic enhancement and clearance in humans assessed with MRI. JCI Insight. 2018;3:e121537
66. Shi Y, Thrippleton MJ, Blair GW, Dickie DA, Marshall I, Hamilton I. et al. Small vessel disease is associated with altered cerebrovascular pulsatility but not resting cerebral blood flow. J Cereb Blood Flow Metab. 2020;40:85-99
67. Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y. et al. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci. 2013;33:18190-9
68. Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7:137-52
69. Espay AJ, LeWitt PA, Hauser RA, Merola A, Masellis M, Lang AE. Neurogenic orthostatic hypotension and supine hypertension in Parkinson's disease and related synucleinopathies: prioritization of treatment targets. Lancet Neurol. 2016;15:954-66
70. Yamada S, Miyazaki M, Yamashita Y, Ouyang C, Yui M, Nakahashi M. et al. Influence of respiration on cerebrospinal fluid movement using magnetic resonance spin labeling. Fluids Barriers CNS. 2013;10:36
71. Ma Q, Ries M, Decker Y, Müller A, Riner C, Bücker A. et al. Rapid lymphatic efflux limits cerebrospinal fluid flow to the brain. Acta Neuropathol. 2019;137:151-65
72. Massey CA, Richerson GB. Isoflurane, ketamine-xylazine, and urethane markedly alter breathing even at subtherapeutic doses. J Neurophysiol. 2017;118:2389-401
73. Proulx ST, Ma Q, Andina D, Leroux JC, Detmar M. Quantitative measurement of lymphatic function in mice by noninvasive near-infrared imaging of a peripheral vein. JCI Insight. 2017;2:e90861
74. Goodman JR, Iliff JJ. Vasomotor influences on glymphatic-lymphatic coupling and solute trafficking in the central nervous system. J Cereb Blood Flow Metab. 2020;40:1724-34
75. Borha A, Chagnot A, Goulay R, Emery E, Vivien D, Gaberel T. Cranioplasty Reverses Dysfunction of the Solutes Distribution in the Brain Parenchyma After Decompressive Craniectomy. Neurosurgery. 2020;87:1064-9
76. Mestre H, Du T, Sweeney AM, Liu G, Samson AJ, Peng W. et al. Cerebrospinal fluid influx drives acute ischemic tissue swelling. Science. 2020;367:eaax7171
77. Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L. et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci. 2014;34:16180-93
78. Liu X, Gao C, Yuan J, Xiang T, Gong Z, Luo H. et al. Subdural haematomas drain into the extracranial lymphatic system through the meningeal lymphatic vessels. Acta Neuropathol Commun. 2020;8:16
79. Yanev P, Poinsatte K, Hominick D, Khurana N, Zuurbier KR, Berndt M. et al. Impaired meningeal lymphatic vessel development worsens stroke outcome. J Cereb Blood Flow Metab. 2020;40:263-75
80. Kiviniemi V, Wang X, Korhonen V, Keinänen T, Tuovinen T, Autio J. et al. Ultra-fast magnetic resonance encephalography of physiological brain activity - Glymphatic pulsation mechanisms. J Cereb Blood Flow Metab. 2016;36:1033-45
81. Aldea R, Weller RO, Wilcock DM, Carare RO, Richardson G. Cerebrovascular Smooth Muscle Cells as the Drivers of Intramural Periarterial Drainage of the Brain. Front Aging Neurosci. 2019;11:1
82. van Veluw SJ, Hou SS, Calvo-Rodriguez M, Arbel-Ornath M, Snyder AC, Frosch MP. et al. Vasomotion as a Driving Force for Paravascular Clearance in the Awake Mouse Brain. Neuron. 2020;105:549-61.e5
83. Mayhew JE, Askew S, Zheng Y, Porrill J, Westby GW, Redgrave P. et al. Cerebral vasomotion: a 0.1-Hz oscillation in reflected light imaging of neural activity. Neuroimage. 1996;4:183-93
84. He Y, Wang M, Chen X, Pohmann R, Polimeni JR, Scheffler K. et al. Ultra-Slow Single-Vessel BOLD and CBV-Based fMRI Spatiotemporal Dynamics and Their Correlation with Neuronal Intracellular Calcium Signals. Neuron. 2018;97:925-39.e5
85. Kitchen P, Day RE, Salman MM, Conner MT, Bill RM, Conner AC. Beyond water homeostasis: Diverse functional roles of mammalian aquaporins. Biochim Biophys Acta. 2015;1850:2410-21
86. Hara-Chikuma M, Verkman AS. Physiological roles of glycerol-transporting aquaporins: the aquaglyceroporins. Cell Mol Life Sci. 2006;63:1386-92
87. Kitchen P, Salman MM, Pickel SU, Jennings J, Törnroth-Horsefield S, Conner MT. et al. Water channel pore size determines exclusion properties but not solute selectivity. Sci Rep. 2019;9:20369
88. Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91:10625-9
89. Lundgaard I, Lu ML, Yang E, Peng W, Mestre H, Hitomi E. et al. Glymphatic clearance controls state-dependent changes in brain lactate concentration. J Cereb Blood Flow Metab. 2017;37:2112-24
90. Plog BA, Mestre H, Olveda GE, Sweeney AM, Kenney HM, Cove A. et al. Transcranial optical imaging reveals a pathway for optimizing the delivery of immunotherapeutics to the brain. JCI Insight. 2018;3:e120922
91. Mestre H, Hablitz LM, Xavier AL, Feng W, Zou W, Pu T. et al. Aquaporin-4-dependent glymphatic solute transport in the rodent brain. Elife. 2018;7:e40070
92. He XF, Liu DX, Zhang Q, Liang FY, Dai GY, Zeng JS. et al. Voluntary Exercise Promotes Glymphatic Clearance of Amyloid Beta and Reduces the Activation of Astrocytes and Microglia in Aged Mice. Front Mol Neurosci. 2017;10:144
93. Liu K, Zhu J, Chang Y, Lin Z, Shi Z, Li X. et al. Attenuation of cerebral edema facilitates recovery of glymphatic system function after status epilepticus. JCI Insight. 2021;6:e151835
94. Xu Z, Xiao N, Chen Y, Huang H, Marshall C, Gao J. et al. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Aβ accumulation and memory deficits. Mol Neurodegener. 2015;10:58
95. Hasan-Olive MM, Enger R, Hansson HA, Nagelhus EA, Eide PK. Loss of perivascular aquaporin-4 in idiopathic normal pressure hydrocephalus. Glia. 2019;67:91-100
96. Reeves BC, Karimy JK, Kundishora AJ, Mestre H, Cerci HM, Matouk C. et al. Glymphatic System Impairment in Alzheimer's Disease and Idiopathic Normal Pressure Hydrocephalus. Trends Mol Med. 2020;26:285-95
97. Kitchen P, Salman MM, Halsey AM, Clarke-Bland C, MacDonald JA, Ishida H. et al. Targeting Aquaporin-4 Subcellular Localization to Treat Central Nervous System Edema. Cell. 2020;181:784-99.e19
98. Sylvain NJ, Salman MM, Pushie MJ, Hou H, Meher V, Herlo R. et al. The effects of trifluoperazine on brain edema, aquaporin-4 expression and metabolic markers during the acute phase of stroke using photothrombotic mouse model. Biochim Biophys Acta Biomembr. 2021;1863:183573
99. Lin L, Hao X, Li C, Sun C, Wang X, Yin L. et al. Impaired glymphatic system in secondary degeneration areas after ischemic stroke in rats. J Stroke Cerebrovasc Dis. 2020;29:104828
100. Frydenlund DS, Bhardwaj A, Otsuka T, Mylonakou MN, Yasumura T, Davidson KG. et al. Temporary loss of perivascular aquaporin-4 in neocortex after transient middle cerebral artery occlusion in mice. Proc Natl Acad Sci U S A. 2006;103:13532-6
101. Salman MM, Sheilabi MA, Bhattacharyya D, Kitchen P, Conner AC, Bill RM. et al. Transcriptome analysis suggests a role for the differential expression of cerebral aquaporins and the MAPK signalling pathway in human temporal lobe epilepsy. Eur J Neurosci. 2017;46:2121-32
102. Abir-Awan M, Kitchen P, Salman MM, Conner MT, Conner AC, Bill RM. Inhibitors of Mammalian Aquaporin Water Channels. Int J Mol Sci. 2019;20:1589
103. Ciappelloni S, Bouchet D, Dubourdieu N, Boué-Grabot E, Kellermayer B, Manso C. et al. Aquaporin-4 Surface Trafficking Regulates Astrocytic Process Motility and Synaptic Activity in Health and Autoimmune Disease. Cell Rep. 2019;27:3860-72.e4
104. Verkman AS, Anderson MO, Papadopoulos MC. Aquaporins: important but elusive drug targets. Nat Rev Drug Discov. 2014;13:259-77
105. Verkman AS, Smith AJ, Phuan PW, Tradtrantip L, Anderson MO. The aquaporin-4 water channel as a potential drug target in neurological disorders. Expert Opin Ther Targets. 2017;21:1161-70
106. MacAulay N. Molecular mechanisms of brain water transport. Nat Rev Neurosci. 2021;22:326-44
107. Aldewachi H, Al-Zidan RN, Conner MT, Salman MM. High-Throughput Screening Platforms in the Discovery of Novel Drugs for Neurodegenerative Diseases. Bioengineering. 2021;8:30
108. Salman MM, Al-Obaidi Z, Kitchen P, Loreto A, Bill RM, Wade-Martins R. Advances in Applying Computer-Aided Drug Design for Neurodegenerative Diseases. Int J Mol Sci. 2021;22:4688
109. Kitchen P, Salman MM, Abir-Awan M, Al-Jubair T, Törnroth-Horsefield S, Conner AC. et al. Calcein Fluorescence Quenching to Measure Plasma Membrane Water Flux in Live Mammalian Cells. STAR Protoc. 2020;1:100157
110. Salman MM, Marsh G, Kusters I, Delincé M, Di Caprio G, Upadhyayula S. et al. Design and Validation of a Human Brain Endothelial Microvessel-on-a-Chip Open Microfluidic Model Enabling Advanced Optical Imaging. Front Bioeng Biotechnol. 2020;8:573775
111. Brinker T, Stopa E, Morrison J, Klinge P. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS. 2014;11:10
112. Karimy JK, Zhang J, Kurland DB, Theriault BC, Duran D, Stokum JA. et al. Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nat Med. 2017;23:997-1003
113. Liu G, Mestre H, Sweeney AM, Sun Q, Weikop P, Du T. et al. Direct Measurement of Cerebrospinal Fluid Production in Mice. Cell Rep. 2020;33:108524
114. Giannetto M, Xia M, Stæger FF, Metcalfe T, Vinitsky HS, Dang J. et al. Biological sex does not predict glymphatic influx in healthy young, middle aged or old mice. Sci Rep. 2020;10:16073
115. Kervezee L, Hartman R, van den Berg DJ, Shimizu S, Emoto-Yamamoto Y, Meijer JH. et al. Diurnal variation in P-glycoprotein-mediated transport and cerebrospinal fluid turnover in the brain. AAPS J. 2014;16:1029-37
116. Lilius TO, Blomqvist K, Hauglund NL, Liu G, Stæger FF, Bærentzen S. et al. Dexmedetomidine enhances glymphatic brain delivery of intrathecally administered drugs. J Control Release. 2019;304:29-38
117. Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D. et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014;76:845-61
118. Silverberg GD, Heit G, Huhn S, Jaffe RA, Chang SD, Bronte-Stewart H. et al. The cerebrospinal fluid production rate is reduced in dementia of the Alzheimer's type. Neurology. 2001;57:1763-6
119. Plog BA, Dashnaw ML, Hitomi E, Peng W, Liao Y, Lou N. et al. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J Neurosci. 2015;35:518-526
120. Bozanovic-Sosic R, Mollanji R, Johnston MG. Spinal and cranial contributions to total cerebrospinal fluid transport. Am J Physiol Regul Integr Comp Physiol. 2001;281:R909-16
121. Ma Q, Decker Y, Müller A, Ineichen BV, Proulx ST. Clearance of cerebrospinal fluid from the sacral spine through lymphatic vessels. J Exp Med. 2019;216:2492-502
122. WELCH K, POLLAY M. Perfusion of particles through arachnoid villi of the monkey. Am J Physiol. 1961;201:651-4
123. Rasmussen MK, Mestre H, Nedergaard M. The glymphatic pathway in neurological disorders. Lancet Neurol. 2018;17:1016-24
124. Hsu M, Rayasam A, Kijak JA, Choi YH, Harding JS, Marcus SA. et al. Neuroinflammation-induced lymphangiogenesis near the cribriform plate contributes to drainage of CNS-derived antigens and immune cells. Nat Commun. 2019;10:229
125. Nedergaard M. Neuroscience. Garbage truck of the brain. Science. 2013;340:1529-30
126. Wevers NR, Kasi DG, Gray T, Wilschut KJ, Smith B, van Vught R. et al. A perfused human blood-brain barrier on-a-chip for high-throughput assessment of barrier function and antibody transport. Fluids Barriers CNS. 2018;15:23
127. Da Mesquita S, Louveau A, Vaccari A, Smirnov I, Cornelison RC, Kingsmore KM. et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer's disease. Nature. 2018;560:185-91
128. Zhou Y, Cai J, Zhang W, Gong X, Yan S, Zhang K. et al. Impairment of the Glymphatic Pathway and Putative Meningeal Lymphatic Vessels in the Aging Human. Ann Neurol. 2020;87:357-69
129. Wang L, Zhang Y, Zhao Y, Marshall C, Wu T, Xiao M. Deep cervical lymph node ligation aggravates AD-like pathology of APP/PS1 mice. Brain Pathol. 2019;29:176-92
130. Zou W, Pu T, Feng W, Lu M, Zheng Y, Du R. et al. Blocking meningeal lymphatic drainage aggravates Parkinson's disease-like pathology in mice overexpressing mutated α-synuclein. Transl Neurodegener. 2019;8:7
131. Song E, Mao T, Dong H, Boisserand L, Antila S, Bosenberg M. et al. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature. 2020;577:689-94
132. Patel TK, Habimana-Griffin L, Gao X, Xu B, Achilefu S, Alitalo K. et al. Dural lymphatics regulate clearance of extracellular tau from the CNS. Mol Neurodegener. 2019;14:11
133. Cserr HF, Cooper DN, Suri PK, Patlak CS. Efflux of radiolabeled polyethylene glycols and albumin from rat brain. Am J Physiol. 1981;240:F319-28
134. Goodman JR, Adham ZO, Woltjer RL, Lund AW, Iliff JJ. Characterization of dural sinus-associated lymphatic vasculature in human Alzheimer's dementia subjects. Brain Behav Immun. 2018;73:34-40
135. Hu X, Deng Q, Ma L, Li Q, Chen Y, Liao Y. et al. Meningeal lymphatic vessels regulate brain tumor drainage and immunity. Cell Res. 2020;30:229-43
136. Coote JH. Respiratory and circulatory control during sleep. J Exp Biol. 1982;100:223-44
137. Grände PO. Critical Evaluation of the Lund Concept for Treatment of Severe Traumatic Head Injury, 25 Years after Its Introduction. Front Neurol. 2017;8:315
138. Engrand N, Pharaboz A, Dinkelacker V. Prophylactic Hypothermia for Severe Traumatic Brain Injury. JAMA. 2019;321:1725
Author contact
![]() Corresponding authors: Shoujun Zhu, sjzhuedu.cn; Haifeng Wang, hfwangedu.cn.
Corresponding authors: Shoujun Zhu, sjzhuedu.cn; Haifeng Wang, hfwangedu.cn.
 Global reach, higher impact
Global reach, higher impact