13.3
Impact Factor
Theranostics 2022; 12(3):1030-1060. doi:10.7150/thno.64805 This issue Cite
Review
Nanocarriers for pancreatic cancer imaging, treatments, and immunotherapies
1. Department of Chemical and Biological Engineering, Iowa State University, Ames, IA.
2. Department of Biochemistry and Molecular Biology, University of Nebraska Medical Center, Omaha NE.
3. Department of Pharmaceutical Sciences and Experimental Therapeutics, College of Pharmacy, University of Iowa, Iowa City, IA.
4. Department of Veterinary Microbiology & Preventive Medicine, Iowa State University, Ames, IA.
5. Nanovaccine Institute, Iowa State University, Ames, IA.
6. Eppley Institute, University of Nebraska Medical Center, Omaha, NE.
7. Fred & Pamela Buffett Cancer Center, University of Nebraska Medical Center, Omaha NE.
#These authors contributed equally to this work.
Received 2021-7-10; Accepted 2021-12-3; Published 2022-1-1
Abstract
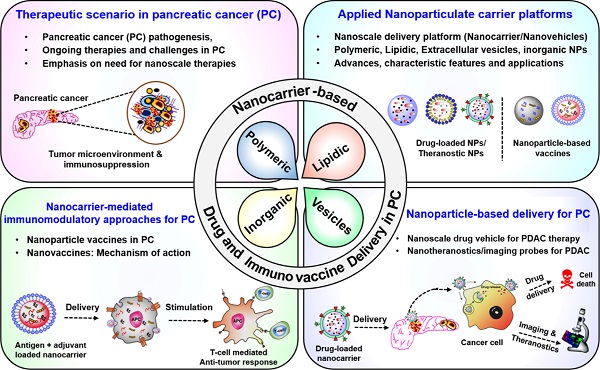
Pancreatic tumors are highly desmoplastic and immunosuppressive. Delivery and distribution of drugs within pancreatic tumors are compromised due to intrinsic physical and biochemical stresses that lead to increased interstitial fluid pressure, vascular compression, and hypoxia. Immunotherapy-based approaches, including therapeutic vaccines, immune checkpoint inhibition, CAR-T cell therapy, and adoptive T cell therapies, are challenged by an immunosuppressive tumor microenvironment. Together, extensive fibrosis and immunosuppression present major challenges to developing treatments for pancreatic cancer. In this context, nanoparticles have been extensively studied as delivery platforms and adjuvants for cancer and other disease therapies. Recent advances in nanotechnology have led to the development of multiple nanocarrier-based formulations that not only improve drug delivery but also enhance immunotherapy-based approaches for pancreatic cancer. This review discusses and critically analyzes the novel nanoscale strategies that have been used for drug delivery and immunomodulation to improve treatment efficacy, including newly emerging immunotherapy-based approaches. This review also presents important perspectives on future research directions that will guide the rational design of novel and robust nanoscale platforms to treat pancreatic tumors, particularly with respect to targeted therapies and immunotherapies. These insights will inform the next generation of clinical treatments to help patients manage this debilitating disease and enhance survival rates.
Keywords: Pancreatic ductal adenocarcinoma, solid tumors, nanoparticles, drug delivery, immunotherapy, tumor microenvironment
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal malignancies of the gastrointestinal tract, with a dismal five-year survival rate of 10%. An estimated 48,220 pancreatic cancer (PC) patients will succumb due to PDAC (8% of all cancer-related deaths), which projects PDAC as the third leading cause of cancer-related deaths in the United States [1]. Despite all the efforts, the mortality rate in male PDAC patients has continued to increase by 0.3% annually since 2000, although it has been observed to be stable in female PDAC patients. Current treatment modalities that do not include surgical intervention are largely ineffective and have minimal impact on improving patient survival rates. A majority of PDAC patients are ineligible for surgery due to late diagnosis, early metastasis, and significant local tissue invasion [2, 3]. In addition, a lack of biomarkers, high recurrence rate, and chemotherapeutic resistance are other factors that contribute to the high mortality rate of PDAC patients [4-6]. To further exacerbate the situation, most pancreatic tumors are poorly responsive to therapeutic approaches due to the highly desmoplastic and immunosuppressive tumor microenvironment (TME) [7-10]. The disrupted vascular transport within the pancreatic TME not only influences cellular composition, hypoxia, and tumor metabolic profile but also regulates the response towards systemic therapies [11-13]. In particular, pharmacological inhibitors, antibody-based therapeutics, and vaccine-induced immune responses follow a systemic route to reach the TME. Similarly, intrinsic physical and biochemical barriers associated with pancreatic tumors not only affect intratumoral delivery but also compromise the stability and activity of therapeutic agents within the pancreatic TME [12, 14].
Several attempts have been directed towards targeting tumor stroma and vasculature to improve the delivery and efficacy of therapeutic agents towards PDAC [13, 15-18]. In this regard, the past two decades have witnessed significant advances in the field of nanotechnology that have introduced not only robust approaches for efficient drug delivery in pancreatic tumors but also provided relevant approaches for the development of vaccine delivery platforms for PDAC [15, 18-21]. Considering the challenges associated with the pancreatic TME described above that limit the delivery and efficacy of both chemo- and immunotherapies for pancreatic tumors, advances in nanotechnology-based approaches can play a significant role in overcoming these challenges.
A critical analysis of these advances in nanoscale carrier development, vis-à-vis effective treatments against PDAC, is the main goal of this review. In addition, we also provide an overview of the mechanisms implicated in nanocarrier-based modulation of pancreatic tumor stroma and immune responses directed towards PDAC. Together, the knowledge and insights gained from the analyses herein can set the stage for future developments and next-generation therapies to advance patient health and significantly increase survival rates. The review describes the multiplexed barrier presented by the pancreatic TME to systemic therapies, followed by a summary of various nanoscale delivery vehicles and adjuvants. Next, advances in nanocarrier-mediated delivery of therapeutic payloads for PC are analyzed, and finally, the development of nanocarrier-driven immunomodulatory approaches for PC is discussed.
2. Pancreatic tumor microenvironment and therapy resistance
The extreme resistance of PDAC to chemotherapy, radiation therapy, and immunotherapy is attributed to its complex and obstructive tumor microenvironment. Dense desmoplastic stroma, which is the hallmark of PDAC, is comprised of various cell types, including cancer cells, cancer-associated fibroblasts (CAFs), neurons, tumor endothelial cells, tumor-associated macrophages (TAMs), and other immune cells. These cells are embedded in a collagen-rich extracellular matrix (ECM), which also contains hyaluronic acid, fibronectin, chemokines, cytokines, and extracellular proteases (Figure 1) [11, 22]. The interaction of tumor cells with various stromal cells along multiple signaling axes directs the evolution of the TME (Figure 1). The cellular and acellular components of the pancreatic TME orchestrate biochemical, biophysical, and physiological processes that contribute to therapy resistance. Specifically, the growing tumor cells and excessive collagen induce solid stress and tissue stiffness, leading to the compression of blood vessels and elevated interstitial fluid pressure (IFP). As a result, pancreatic tumors are hypovascular and exhibit decreased perfusion, convection, and diffusion, and, therefore, have impaired delivery of systemic therapies [14, 16]. Further, pancreatic tumors are highly heterogeneous in cellularity, stroma composition, and vascularity, and secondary pathophysiological effects such as acidic pH and hypoxia change the tumor metabolic profile and contribute to activation of tumor cell-intrinsic pathways of therapy resistance [11, 12, 23-27]. Recent studies have emphasized tumor cellularity as an important determinant of disease progression, epithelial-mesenchymal transition (EMT), metastasis, and therapeutic responses in PDAC patients [28, 29]. In particular, high cellularity within the TME of PDAC patients has been reported as a negative prognostic factor. On the other hand, the stromal composition and matrix density in pancreatic TME is a critical determinant of therapeutic response in PDAC [12, 13, 21]. Unlike other malignancies, pancreatic tumors are extensively fibrotic (i.e., desmoplastic) and composed of heterogeneous CAFs, which are the major architects of TME in PDAC [30, 31]. According to the conventional definition, CAFs have irreversibly activated fibroblast cells that secrete ECM components, including collagen(s), fibronectin, cytokines, and growth factors, and play an important role in tumor progression [32]. However, recent studies have further classified CAFs based on the expression of molecular markers, their activation state, and their tumor-promoting or restraining functions [30, 31, 33-35]. In terms of ECM deposition, CAFs are the major stromal cell populations that contribute 60-90% of ECM and cause elevated physical stress, a consequence of increased IFP and disrupted vascular function [36-38]. Therefore, selective targeting of pro-tumorigenic CAFs might be a potential strategy for normalization of stroma and vasculature in pancreatic tumors and represents an important step towards increasing drug delivery and efficacy in PDAC.
Therapeutic and immunological challenges in pancreatic cancer. (A) Therapeutic approaches, including chemotherapies, antibody-based therapeutics, and vaccines, have different challenges related to their delivery and in vivo stability. Chemotherapies undergo systemic clearance, metabolize in the liver, and show poor tumor-specific delivery. Similarly, pancreatic cancer is poorly immunogenic, and there is a lack of tumor-specific high-quality antigens to induce a clinically relevant anti-tumor immune response, which causes poor efficacies of vaccine-based immunotherapies in pancreatic cancer. (B) Pancreatic tumor microenvironment includes both the physical and biochemical components in the stroma, such as high ECM deposition and disrupted vasculature, which lead to poor drug delivery and interfere in immune infiltration. (C) Cellular crosstalk between cancer cells and stromal cell populations leads to various pathological hallmarks of PDAC, including PC progression, metastasis, drug resistance, and immunosuppression. Different cell types of pancreatic tumor microenvironment have been mentioned in the figure (lower panel). Major challenges and hallmarks of PDAC are mentioned in blue color. The colored dots represent various cytokines and chemokines that are present in the pancreatic TME and participate in cellular crosstalk in the local milieu. ECM: Extracellular matrix; TAM: Tumor-associated macrophage; CAFs: Cancer-associated fibroblasts; DCs: Dendritic cells.
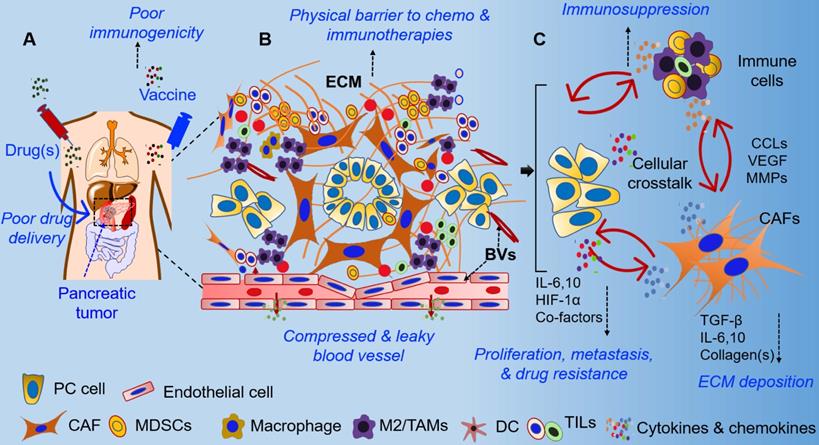
The pancreatic TME is highly immunosuppressive and considered to be unfavorable for immunotherapies in the majority of PDAC patients [7, 8]. Recently, next-generation sequencing and next-generation tissue microarray analysis suggested that ~65% of human pancreatic tumors exhibit “immune escape” phenotypes [39]. Unsurprisingly, the overall response rate to immunotherapies is poor in PDAC patients, attributed to local tissue stress and vascular disruption in the immunosuppressive TME [8, 40, 41]. Besides activated CAFs, various other immune cell populations (including regulatory T and B cells, TAMs, myeloid-derived suppressor cells, and their secreted cytokines) contribute to immunosuppression in pancreatic tumors [42-44]. In addition, the TME has been reported to alter the phenotype of infiltrating anti-tumor immune cells to that of “anergic,” “exhausted,” and/or “dysfunctional” phenotypes [45-48]. Similarly, myeloid cells, TAMs, and tumor-associated NK cells have been reported to play pro-tumorigenic roles in pancreatic tumors, resulting in poor responses to immunotherapies [49-52]. Nevertheless, selective targeting of stromal components, including hyaluronan, collagen(s), CAFs, and stroma-promoting signaling pathways, have been reported to improve the anti-tumor immune response in PDAC [49, 53-56]. Recent studies have focused on the use of nanocarriers and targeting for modulation of the stroma to enhance immune infiltration and for the re-activation of immune effector functions in pancreatic tumors [57, 58]. For example, targeting hyaluronan synthesis by incorporating an inhibitor in a nanocarrier resulted in ECM remodeling and improved γδ-T cell infiltration [59]. Similarly, silencing of retinoic acid-inducible gene 1 (RIG1) by using a selective agonist encapsulated in lipid calcium phosphate (LCP) nanoparticles (NPs) enhanced the anti-tumor effect by silencing BCL2, which enhanced apoptosis [60]. This was positively correlated with increased Th1 proinflammatory cytokine levels, infiltration of more CD8+ T cells compared to regulatory T (Treg) cells, and the presence of more M1 over M2 macrophages. Correspondingly, a decrease in regulatory B cells in the NP-RIG1-agonist treatment group also indicated the immunomodulatory effects of the nanoformulation [60]. Further, gene delivery using the same LCP nanoplatform showed selective delivery of a plasmid encoding relaxin into metastatic liver tissues. Interestingly, forced expression of relaxin not only reduced the metastatic burden but also altered stroma and immune milieu in a liver metastasis model of PDAC [61]. Several other nanocarrier platforms have been demonstrated to effectively target pancreatic tumors and deliver immunomodulatory agents. These include trapping of IL10 and CXCL12 by using lipid protamine DNA NPs loaded with the trapped gene [62], use of oxaliplatin (OX) with encapsulated siIDO-1 (indoleamine 2,3, dioxygenase-1) [63], mesoporous silica NPs (MSNs) loaded with glucose oxidase, cancer cell surface as camouflage with anti-PD1 therapy [64], and NPs loaded with standard chemotherapies [65-67]. These nanocarriers were demonstrated in various PDAC models to modulate stroma, increase the presence of effector immune cells, and decrease the immunosuppressive cytokine milieu. There is ample evidence to show that nano-driven strategies are suitable for drug delivery and effective in stromal modulation and in potentiating immunotherapy-based approaches in PDAC. The various physical, biochemical, and immunological changes in the pancreatic TME due to treatment with nano-based formulations are summarized in Figure 1.
In contrast, pharmacological inhibitors and antibody-based therapeutics differ in structure, function, and physiological stability and, therefore, need various approaches to improve their pharmacokinetics and pharmacodynamics. For example, vaccine formulations need sustained antigen release for durable immune responses, whereas chemotherapies need increased tumor availability and slower clearance. On the other hand, antibody-based therapeutics require improved stability in vivo to evoke effective responses. Nano-driven strategies could be used to enhance stability under physiological conditions and sustain the bioavailability of therapeutic agents, as detailed in Section 4. Thus, nanotechnology provides clinically relevant platforms that reduce stromal hindrance, enhance drug delivery and stability, and improve immune cell infiltration, as well as improve the efficacy of immunotherapy-based approaches by their immunomodulatory functions in PDAC (as described in Section 4).
3. Nanocarrier-based delivery of therapeutic, imaging, and theranostic payloads for PDAC
3.1. Nanoscale drug delivery vehicles and adjuvants
Current therapeutic modalities for cancer treatments are comprised of surgery, chemotherapy, radiotherapy, immunotherapy, or rational combinations. Thereof chemotherapy is the standard-of-care treatment and is the longest-serving modality for treating various cancers, including PDAC. However, direct administration of drug payloads often causes compromised delivery, systemic toxicity, and severe side effects. In addition, poor drug pharmacokinetics (i.e., solubility, stability, and metabolism) result in limited biodistribution, low therapeutic efficacy, and inadequate responses. Alternatively, immunotherapy is emerging as a promising therapeutic option for cancers with improved responses against primary and metastatic tumors [68]. Despite these advances, direct delivery of immunotherapeutic agents (e.g., cytokines, checkpoint inhibitors, etc.) suffers from suboptimal pharmacokinetics and susceptibility to degradation, resulting in adverse effects [69, 70].
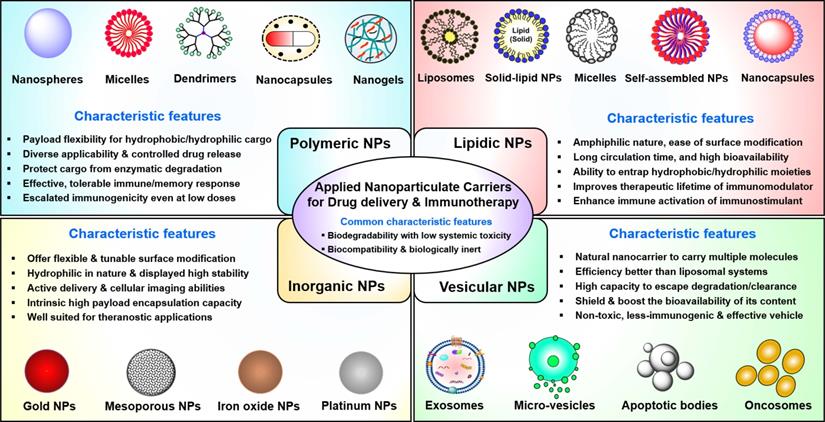
Furthermore, non-specific interactions of soluble immunotherapeutic payloads with immune cells, nucleases, and proteases not only reduce immuno-stimulatory responses but also contribute to immune-related adverse effects. Thus, there is an urgent need to develop effective delivery platforms to transport therapeutic/immunological payloads to their target cells and/or tissues, along with minimal exposure to their biological environment and reduced side effects. Previously, various nanomaterial-based carriers (i.e., nanocarriers) have been designed to overcome the issues outlined above, whereby therapeutic payloads are conjugated to or entrapped within biocompatible nanomaterials to enhance their ability to overcome sequential biological barriers associated with a TME [71-74]. The benefits of this approach include protection of payloads from degradative agents, minimization of non-specific interactions, enhanced biological stability (i.e., prolonged circulatory half-life), increased bioavailability of payloads, dose sparing, and enhancement of specific tissue targeting [75, 76]. The following sections are focused on the chemistries, characteristic features, advances, and clinical applications of nanocarriers (Figure 2) in cancer therapeutics, including PDAC. This section also discusses how different nanocarriers have been used to deliver therapeutic payloads for PDAC treatment, including chemotherapeutic and nucleic acid drugs and imaging agents. Various types of FDA-approved or clinical-stage nanomedicines used for small molecule drug delivery to PDAC are shown in Table 1.
3.1.1. Polymeric NPs
Polymeric NPs are well-studied as nanocarriers for drug delivery and immunotherapy [77]. Polymeric NPs allows for a wide range of conjugation and encapsulation options accompanied by excellent biocompatibility profiles and effective delivery at the desired site(s) of action [71]. Polymeric NPs can protect encapsulated payloads from degradation and enhance their bioavailability to tumors and other tissues by delivering maximum dose via the enhanced permeability and retention (EPR) effect [78]. Compared to liposomes, polymeric NPs show enhanced stability and resistance to drug leakage, while smaller-sized NPs have been repNanoparticle-based delivery for PCorted to lengthen the half-life of therapeutic cargos in circulation, reduce their degradation, and provide sustained release, which would enhance the accumulation of the cargo in the target tissue [79]. Additionally, the ability of polymeric NPs to adsorb or be coated with targeting ligands, combined with their inherent adjuvant properties, make them attractive candidates for induction of tumor-specific immunity (Section 4).
Engineered nanocarriers for PDAC drug delivery and immunotherapy. This figure provides schematic illustrations of major types and multiple subtypes of nanocarriers and their characteristic features that have been employed for drug/theranostic payload delivery and immunotherapy against PDAC. Clockwise from left: Polymeric, lipidic, nano/micro vesicle-based, and inorganic materials-based nanocarriers. The schematic structure of each nanocarrier subtype is depicted in the top and bottom rows. The most commonly observed features of each nanocarrier class are mentioned in the middle. NPs: Nanoparticles.
Representative examples of FDA-approved or clinical-stage nanomedicines for PDAC therapy
| Nanomedicine | Nanocarrier | Payload/coating | Cancer type | Advantages | Approval | Ref. |
|---|---|---|---|---|---|---|
| Abraxane® ABI-007 | Albumin | paclitaxel | PDAC | Increased site-specific delivery, Improved solubility | FDA | [330] |
| Lipotecan® | PEG-PGA micelle | TCL388 HCl | PDAC | Better therapeutic effect, Prolong circulation, Low toxicity | FDA | [331] |
| Genexol-PM® | mPEG-PLA micelle | Paclitaxel | Metastatic PDAC | Improved solubility/efficacy, Reduced toxicity | FDA, Korea | [332, 333] |
| Doxil® | Liposomal | doxorubicin | PDAC | Increase site-specific delivery, Decrease systematic toxicity | FDA/Phase I/II | [334] |
| Onivyde® | PEGylated Liposome | Irinotecan | PDAC | Increased delivery to a tumor site, Low systematic toxicity | FDA | [335] |
| Lipoplatin® | Liposome | Cis-platin | PDAC | Specific delivery, Reduced toxicity | Phase II/III | [336] |
| EndoTAG® -1 | Liposome | Gemcitabine | Locally advanced & metastatic PDAC | Provide great potential and better treatment options than Gemcitabine alone | Phase III | [337] |
| MSC-derived exosomes | Exosome | KRAS G12D siRNA | Metastatic PDAC | Direct specific targeting, Improved therapeutic efficacy | Phase II | [338] |
Biodegradable polymers (both natural and synthetic) have been widely used to synthesize NPs [80]. Among synthetic polymers, multiple types of commercial biodegradable polymers, including polyethylene glycol (PEG), polyesters [such as poly(lactic-co-glycolic) acid (PLGA)], and polyanhydrides [(based on monomers such as sebacic anhydride (SA), 1,3-bis(p-carboxyphenoxy propane) (CPP), 1,6-bis(p-carboxyphenoxy hexane) (CPH), and 1,8-bis(p-carboxyphenoxy hexane)-3,6-dioxaoctane (CPTEG)] have been investigated as nanocarrier platforms [81-84]. PEG has been widely used in drug delivery [85]. PEG can be used to deliver hydrophobic small molecule drugs by improving solubility compared to the drug alone. PEG is also used as a coating on other types of NPs. The process of attaching PEG to another drug or molecule is referred to as PEGylation. PEGylation reduces unwanted immune recognition, resulting in longer circulatory half-lives of small molecule drugs, which is beneficial when delivering chemotherapeutics. For example, PEGylation contributed to the success of both Doxil® and Genexol® [71]. Various types of NPs, including gold [86, 87], polymeric, and lipid NPs carrying small molecule drugs (e.g., doxorubicin) for PDAC, have been PEGylated to improve their pharmacodynamic characteristics [88, 89]. PLGA has been widely used as a nanocarrier for drug delivery because of its adaptability, suitability, and ease of manipulation with respect to its chemical and physical properties such as hydrophobicity/hydrophilicity, molecular mass, and crystallinity, which can be modified by changing the monomer ratio, terminal group chemistry, size and net surface charge [90]. The chemical properties of PLGA allow hydrolytic degradation by de-esterification. For example, polylactide and polyglycolide are composed of monomeric components that are easily metabolized by the body, and their rates of degradation as well as physicomechanical properties are tunable over a wide range by using polymers of varied molecular weights and molar ratios [91].
Biodegradable polyanhydride-based NPs have also been widely studied as drug delivery vehicles [92-101]. Copolymers based on SA, CPP, CPH, and CPTEG display tunable surface erosion kinetics (controlled by the hydrophobicity, which in turn depends upon the copolymer composition), leading to highly controlled and sustainable drug release [102]. These materials are easy to functionalize because of their carboxylic acid end groups, which has led to targeted delivery approaches that help navigate tough-to-penetrate biological barriers such as tumors, bacterial membranes, and the blood-brain barrier [103-107]. Polyester NP-encapsulated chemotherapeutic drugs have been broadly investigated [108, 109]. Among them, poly(L-glutamic acid) (PGA), PTX (Xyotax) [110], and PGA-camptothecin (CT-2106) [108] are FDA approved or are in clinical trials as anti-cancer nanomedicines. PLGA NPs have also been used in a targeted approach to deliver Taxol (PTX) to PC cells in both in vitro and in vivo settings [111]. In these studies, PEG blocks were used to increase the “stealth” of the NPs. Release studies showed that over 90% of Taxol was released within one week. This targeted delivery approach showed decreased tumor volume compared to controls in vivo. Multi-functional gene therapy platforms based on poly[oligo(ethylene glycol) methyl ether methacrylate] NPs combine shielding (provided by the short PEG block) and RNA binding capability (provided by the cationic PDEAEM moiety) with enhanced retention, high RNA loading, and increased cellular uptake, all of which translated to NP accumulation at the tumor site and growth inhibition [112]. The design of siRNA-adjuvanted GEM-based PC treatment involved the use of a cationic ε-polylysine copolymer NP core, enabling efficient loading of HIF1α siRNA and GEM. The NPs were further coated with a PEGylated lipid bilayer to prevent rapid degradation of the payload and avoid particle aggregation. The synergistic antitumor effect was demonstrated in both a subcutaneous xenograft tumor model and an intravenously administered orthotopic tumor metastasis model [89]. The same group also designed RRM2 siRNA-adsorbed 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) cationic liposomes loaded with GEM, which were shown to significantly sensitize cancer cells to GEM treatment in a subcutaneous PANC-1 murine model [113].
3.1.2. Micelles, dendrimers, and nanogels
Polymeric micelles, which are self-assembled amphiphilic core-shell particles, are efficient in delivering highly hydrophobic drugs. Bioconjugation or physical entrapping of the hydrophobic drug into micelles can provide minimal drug leakage, maximizing drug solubility and half-life in blood circulation and improving delivery [114, 115]. Block copolymers are most often used to produce micelles because of their amphiphilic properties, which allow the formation of a hydrophobic core and hydrophilic outer portion. Micelles work well to deliver hydrophobic drugs because the drugs are trapped in the hydrophobic core. Hydrophilic drugs can also be delivered using micelles when they are associated with the outer portion of the micelle.
Kumar et al. developed a block copolymer micelle based on PEG block-poly(2-methyl-2-carboxyl-propylene carbonate-graft-dodecanol-graft-tetraethylenepentamine) to deliver Vismodegib (small molecule hedgehog pathway inhibitor) and microRNA (miRNA) to treat PDAC in an orthotopic murine model [109]. The elimination half-life of the drug and biodistribution of Vismodegib was improved using these micelles. Micelles assembled from cationic polymers can be complexed with nucleic acids and be readily internalized by target cells. By bioconjugation or insertion of functional moieties into the multiblock polymer, micelles can improve targeting and delivery of multiple payloads simultaneously to pancreatic tumors. For example, Pittella et al. designed PEGylated calcium phosphate (CaP) hybrid micelles that could deliver siRNA to PC [116]. In the micelle design, a PEG layer shield, a CaP nanocore for polymer binding, and a pH-sensitive cis-aconitic amide incorporated endosome-disrupting copolymer were integrated to enable pancreatic tumor targeting and pH-responsive endosomal escape of siRNA. The micelles were tested in a transgenic murine model and shown to improve siRNA accumulation at the tumor site (demonstrated by luciferase gene silencing). In another study, micelles were prepared for the co-delivery of DTX and Atg7 siRNA to inhibit PC cell autophagy and sensitize cancer cells to chemotherapy. Micelles synthesized from a Pluronic® P123 backbone and integrin-binding iRGD were shown to target nude mouse PANC-1 xenograft tumors and released the trapped DTX and siRNA. Increasing micelle stability in blood circulation is another strategy to improve anti-cancer efficacy [117]. Uchida et al. prepared micelles attached to a cholesterol moiety to increase blood circulation stability by hydrophobic interaction. The micelles loaded with mRNA encoding anti-angiogenic protein sFlt-1 were shown to be therapeutically beneficial in a BxPC-3 pancreatic tumor model that shares histopathological features with human PC [118]. Chemoresistance and invasion of pancreatic cancer stem cells (CSCs) mediated by miRNA has been proposed as a mechanism for PC drug resistance and frequent recurrence. Micelles conjugated with GEM and a miRNA-205 mimic were tested against CSCs. Co-delivery was demonstrated to be synergistic in reducing cancer growth in both GEM-resistant CSCs and xenograft pancreatic tumors [119].
Polymeric nanogels are three-dimensional (physically or chemically) crosslinked networks with high water content. As potential carriers, polymeric nanogels can improve stability and provide longer retention and greater loading capacity of the therapeutic payload [120]. In recent work by members of our team, temperature- and pH-responsive pentablock copolymers, consisting of a temperature-responsive Pluronic® F127 middle block and pH-responsive poly(diethylaminoethylmethacrylate) (PDEAEM) end blocks were developed for dual delivery of miR-345 and GEM [121]. Recent reports have also discussed the use of nanogels as targeted nanomedicines to increase treatment effectiveness and improve outcomes of PC therapy [122, 123].
Dendritic polymers have also been used as delivery systems owing to their multivalent characteristics, defined molecular weight, monodisperse size, and water solubility. Further, the globular structure of dendrimers with an available internal cavity (central core) and modifiable surface functionality makes them attractive vehicles for payload delivery [124]. Both in vitro and in vivo studies testing therapeutic efficacy of doxorubicin (DOX)-loaded dendrimeric polymer compared to i.v. delivered DOX revealed a 10-fold improvement in cellular uptake and a 9-fold reduction in cellular toxicity [125].
3.1.3 Lipidic NPs
Lipidic NPs are well-established and easy-to-produce nanocarrier delivery systems. Liposomes, the first NP platform to be applied in medicine, are composed of nanosized synthetic vesicles consisting of one or multiple spherical shell bilayers, encompassing an aqueous core [126]. Compared to some polymeric NPs, lipidic NPs are less toxic and exhibit higher biocompatibility because their structural components display similarities with plasma membrane lipids and human cholesterol. Lipidic NPs are classified as liposomes, solid-lipid nanoparticles (SLN), phospholipid micelles, or nanocapsules [127]. Liposomes possess the unique characteristic of loading hydrophobic drug moieties within the shell layer while entrapping hydrophilic payloads within the aqueous core to protect them from degradation and metabolism. Compared to free drugs, liposomes can help in modifying pharmacokinetics and biodistribution of encapsulated drugs by augmenting drug circulation time, tumor exposure, and retention, thereby boosting the overall therapeutic effect on cancer cells [128, 129]. Several stimuli-responsive liposomes have been developed to achieve target-selective delivery of the entrapped drug. A change in temperature/pH (e.g., endosome) can trigger the intracellular release of drugs and improve the therapeutic efficacy of lipidic nanomedicines [130-132]. Similarly, SLNs also show attractive physicochemical properties, high biocompatibility, and the capability to deliver hydrophobic compounds [133].
With multiple continuing efforts for developing potential cancer nanotherapeutics, several liposomal drug products are available in the market, including Doxil®, DaunoXome®, Depocyt®, Myocet®, and others. Resembling liposomal characteristics, SLNs have shown attractive physicochemical properties, high biocompatibility, and the capability to deliver hydrophobic compounds. SLNs offer the precise release of the immune reagents, mitigate off-target CTL response, and effectively harness immune responses by activating either a humoral or cellular immune response against cancer cells [134]. Stimuvax, Tecemotide, and sHER2+AS15 are notable examples of liposome-based cancer nanovaccines that have progressed through phase-II/III clinical trials to treat PDAC, along with other carcinomas [130, 132].
3.1.4. Extracellular vesicle-based NPs
Extracellular vesicles (EVs) are cell-derived, nanosized membrane vesicles. Based on their size and biogenesis processes, EVs are subdivided into four subtypes: exosomes (30-100 nm), microvesicles (50 nm-1 μm), apoptotic bodies (20 nm-5 μm), and large oncosomes (1-5 μm) [135]. These subtypes differ in their origin, composition, and biochemical properties. As natural transporters, EVs have gained considerable scientific interest in cancer therapeutics because of their ability to shuttle biomolecular cargoes between cells [136, 137]. Exosomes have been demonstrated to establish a pre-metastatic niche in PDAC and dictate metastatic organotropism [138, 139]. Due to their natural origin (via biogenesis) and ability to target specific organs, EVs have multiple advantages over conventional drug delivery systems, including high biocompatibility, prolonged stability, ability to pass through natural barriers, intrinsic cell targeting, reduced toxicity, and low immunogenicity. To date, EVs have been shown to deliver proteins, nucleic acids, small molecules, drugs, and CRISPR/Cas9 systems [140]. Among these membrane-derived vesicles, exosomes are the most applied EVs in cancer theranostics due to their high versatility [141]. Exosomes from diverse cellular origins, including tumor cells, fibroblasts, macrophages, and mesenchymal stem cells (MSCs), have been loaded with therapeutic cargoes, including chemotherapeutic drugs and siRNA, for delivery to PC cells. Compared to liposomes, exosomes contain cell-of-origin-derived transmembrane-anchored proteins, which can regulate their clearance from phagocytosis. A recent study demonstrated that exosomes could be engineered to prevent their clearance via phagocytosis and inhibit KRAS by selectively delivering short interfering (siRNA) or short hairpin RNA (sh RNA) to PC cells [142]. It was observed that CD47 on the exosome regulates their clearance by circulating monocytes. Exosomes isolated from CD47-knockout mouse fibroblasts and loaded with siRNAs or shRNA targeting mutant KrasG12D efficiently delivered cargo to orthotopically implanted and autochthonous pancreatic tumors and resulted in decreased tumor growth and metastasis, resulting in improved survival [142]. Paclitaxel-treated immortalized MSCs were found to incorporate, package, and release the active drug in the exosomes. The drug-loaded exosomes were demonstrated to be taken up by PC cells in vitro and inhibit their growth [143]. Similarly, exosomes isolated from bone marrow MSCs were loaded with gemcitabine monophosphate by reversible electroporation and paclitaxel by sonication. The dual drug-loaded exosomes exhibited superior penetration, anti-tumor, and anti-stromal effects on orthotopic pancreatic tumors as compared to the clinically approved Gem+Nab-paclitaxel (Abraxane) or GEM-alone loaded exosomes [144]. Macrophage-derived exosomes have also been examined for packaging and delivering chemotherapeutic agents to PC cells. Exosomes isolated from a human macrophage cell line THP-1 and loaded with GEM and Deferasirox, an oral iron chelator, effectively inhibited the proliferation of GEM-resistance PC cells in 2D and 3D cultures in vitro [145]. Recently, exosomes isolated from Panc-1 PC cells were loaded with GEM either by direct incubation with the drug or sonication [146]. These GEM-loaded autologous exosomes resulted in a significant decrease in tumor volume and prolonged survival of mice with no evidence of non-target tissue toxicity as compared to the free drug [146]. The utility of exosomes as vectors for delivering therapeutic agents for PC has been described in detail in a recent review article by Oliveria et al. [147].
3.1.5. Inorganic NPs
Inorganic NPs have been widely applied to the treatment and diagnosis of cancer. Compared to polymeric NPs, inorganic NPs can be manufactured with more defined morphology, size, and surface chemistry. Based on the electrochemical and magnetic properties of the materials, techniques such as magnetic resonance imaging, surface plasmon resonance spectroscopy, and surface-enhanced Raman scattering spectroscopy provide characterization with high resolution and low tissue background [148]. A variety of inorganic NPs has been employed in nanotherapeutic applications [149]. Among them, gold nanoparticles (AuNPs), MSNs, and iron oxide NPs have emerged as leading candidates because they are biologically inert and flexible to surface modification. Additionally, their hydrophilic nature, resistance to microbial growth, high stability, and low toxicity provide added advantages. AuNPs have emerged as a potential tool for anticancer therapy due to their characteristic visibility and ease of functionalization. MSNs are also promising payload carriers with good biocompatibility and distinct porous architecture, which enables high cargo loading efficiency [150]. Magnetic NPs, based on superparamagnetic iron oxide (SPION), possess high magnetization and moderate biocompatibility, and have shown great promise in cancer therapeutics [151]. SPIONs allow the transport of therapeutic cargos and other payload moieties, i.e., imaging probe and radiotherapy payloads [152].
Various AuNP-based conjugates are being evaluated in vitro and in preclinical animal model studies to deliver routinely used chemotherapeutic drugs, such as docetaxel (DTX) and 5-fluorouracil [153]. Two AuNP-drug nanoconjugates, namely, AuraLase and NU0129, are in clinical trials for lung cancer and glioblastoma therapy, respectively [154]. Moustaoui et al. used PEGylated Au(III) NPs to deliver DOX to PDAC cells in vitro and demonstrated that DOX release was pH-dependent [86]. Studies with drug-loaded SPION showed enhanced cellular permeability and augmented tumor-targeting abilities via surface peptide interactions, supporting their utility for cancer treatment. The FDA has already approved magnetic SPION-based formulations (e.g., Feraheme®, Feridex I.V.®, and Gastromark®) as magnetic resonance imaging (MRI) contrast agents. However, investigations concerning theranostic applications of SPIONs are still at the preclinical stage because key issues related to magnetic NPs are yet to be addressed [155]. Lee et al. developed pH- and lysozyme-dependent iron oxide NPs for the release of GEM, using orthotopic tumor models as well as MiaPaCa-2 cells [156] The NPs showed a statistically significant reduction in tumor growth in the mouse models and provided superior imaging capabilities in MRI.
A nanocarrier for the dual delivery of siRNA and drug was prepared from graphene quantum dots (GQDs) by Yang and co-workers [157]. The nanocarrier was functionalized with biodegradable charged polyester vectors to encapsulate siRNA targeting KRAS mRNA. The resulting GQDs integrated photothermal therapy, siRNA release, and enhanced DOX efficacy against a MiaPaCa-2 PC cell line. AuNPs have also been used as nanocarriers for siRNA targeting nerve growth factors in PC. For example, novel fluorescent gold nanoclusters were characterized for size, siRNA release, and gene silencing performance and shown to significantly inhibit tumor growth and decrease neurite density [158]. Another study demonstrated the dual delivery of GEM and miRNA-21 inhibitor (miR-21i) using dendrimer-entrapped AuNPs [159]. The internal cavities and terminal amine groups of the dendrimer provided the capacity for GEM loading and miR-21i electrostatic compression. The co-delivery of miR-21i and GEM aided by ultrasound-targeted microbubble destruction was tested in a xenograft PC model. Most inorganic nanomaterials offer reasonable biocompatibility, moderate stability, and unique diagnostic and therapeutic opportunities that organic or traditionally used NPs cannot offer. Despite these advantages, inorganic NPs have limited success in entering clinical trials due to their low solubility and concerns related to their toxicity, biodistribution, and subsequent clearance. Recent examples showed that combining the potential of inorganic NPs with organic materials by functionalizing/coating biocompatible materials to the surface of inorganic NPs can provide avenues for the use of inorganic NPs scaffolds in the clinic [160-162].
3.1.6. Natural NPs
Natural polymers such as albumin, chitosan, heparin, and others have been formulated as NPs to deliver therapeutic drugs, proteins, and oligonucleotides. These natural polymers are particularly attractive for drug delivery owing to their non-toxic, non-immunogenic, and biodegradable properties [80]. For example, albumin-based NPs provide multiple benefits, including high binding capacities for both hydrophobic and hydrophilic drugs, relatively facile preparation, and their ability to be specifically modified to facilitate targeted delivery [163, 164].
Thiolated type B gelatin NPs were used to deliver GEM to PC in vitro and in vivo [165]. The IC50 value in PANC-1 cultures decreased when gelatin NPs were used. Tumor growth reduction was also observed during in vivo studies. Human serum albumin NPs loaded with PTX (i.e., Nab-PTX), combined with GEM, are an FDA-approved treatment for PDAC [166]. This Nab-PTX-GEM was the first combination therapeutic to include GEM that increased patient survival time [163]. In addition, the hydrophobicity of Nab-PTX was decreased compared to PTX, which led to better solubility in the bloodstream and improved pharmacokinetics [167]. Nab-PTX-GEM combination therapy has also shown therapeutic efficacy as a first-line treatment for metastatic PDAC by improving overall response rate and survival compared to GEM alone [163, 168]. Additionally, numerous phase I, II, and III clinical trials are ongoing for Nab-PTX-GEM treatments combined with radiotherapy and other drugs [163]. Nano-liposomal irinotecan (Onivyde) is being used in the treatment of PDAC patients. The liposomal formulation of irinotecan led to increased cellular uptake compared to free irinotecan [169]. In addition, lipid NPs encapsulating GEM were used in an in vitro study on BxPC-3 spheroid cultures [170]. The NPs were responsive to the hypoxic tumor microenvironment by reducing the lipid, which then released GEM. Other liposomal-drug products that are commercially available in the market include Doxil®, DaunoXome®, Depocyt®, and Myocet®.
3.1.7. Hybrid NPs
Built upon the advantages of distinct nanoparticular platforms, hybridization is another strategy to incorporate two or more nanomaterials to overcome multifaceted challenges [171]. Gao et al. produced hollow, biodegradable mesoporous organosilica NPs, which are pH-sensitive to the more acidic microenvironment of pancreatic tumors, and the NPs effectively released the drug within the tumor [172]. This nano-system showed controlled delivery of both GEM and pirfenidone in both in vitro and in vivo studies. In addition, ultrasound-triggered microbubble destruction was used to increase penetration into the tumor tissue. Li et al. produced lipid-polymer hybrid NPs to deliver FOLFIRINOX to pancreatic tumors [173] using a layer-by-layer approach with a polymer core and a PEGylated lipid shell. This NP formulation showed good stability in serum and decreased side effects in in vivo studies compared to free FOLFIRINOX. AuraLase, a silica-gold nanocomposite, is currently in clinical trials for thermal ablation therapy for solid/metastatic lung tumors [154].
3.2. Nanoparticle-based molecular imaging and theranostic probes for PDAC
Imaging is an integral component of the diagnosis and management of PDAC patients. Among various imaging modalities employed, multidetector computed tomography (CT) angiography is highly sensitive and the most preferred method for initial diagnosis, staging, and resectability assessment [174] due to its widespread availability and low cost. Magnetic resonance imaging (MRI) has comparable sensitivity in staging PDAC, and magnetic resonance cholangiopancreatography (MRCP) enables detailed evaluation of the biliary and pancreatic ductal system [175]. While MRI is not as widely used as CT for initial diagnosis, it is more efficient in detecting small tumors, metastatic lesions in liver peritoneum and lymph nodes (LN), and identifying malignant cystic lesions of the pancreas [175, 176]. Endoscopic ultrasound (EUS) is highly sensitive in detecting small tumors that are often missed by other imaging modalities, and it also provides an opportunity to collect samples (fine needle aspirates) for cytological or biomarker analysis to facilitate the most conclusive diagnosis [177]. Metabolic PET imaging, which relies on the differential uptake of 18F-labeled fluoro-deoxy glucose (FDG) by rapidly growing tumor cells, enables whole-body imaging to detect both primary tumors and metastasis and is used alone or in combination with CT and MRI for evaluating the response to therapy in PDAC patients [178, 179]. The principles, utility, and current status of various imaging modalities are elegantly reviewed in several recent articles [178, 180-182]. Imaging modalities like abdominal ultrasound utilize microbubbles as contrast agents, which have been functionalized by targeting molecules to facilitate molecular imaging. Jugniot et al. [183] have comprehensively reviewed the current clinical and preclinical status of targeted microbubbles for PC. A detailed discussion on the subject is beyond the scope of the current review.
Recently, nanoparticles have been engineered to deliver imaging agents alone or in combination with chemotherapeutic drugs and used for imaging or theranostic applications, respectively (Table 2). Several multi-functionalized NPs have been demonstrated to be capable of delivering multiple imaging probes to counter the limitations of single molecule-based imaging modalities to augment image resolution, enhance temporal resolution, and improve tissue penetration and probe sensitivity [184].
Various NPs-imaging probes based on iron oxide, carbon oxide, inorganic metal NPs, and liposomes have been evaluated to deliver imaging agents for diverse imaging modalities, including MRI, CT, PET, and SPECT for PDAC [185, 186]. These have been elegantly reviewed in several recent articles [185, 187]. Zhao et al. developed a multimodal (MRI, CT, and PAI) contrast probe using gold nanorod-silica core-shell NPs layered with gadolinium oxide (AuGR-SiO2-Gd). In vitro, AuGR-SiO2-Gd NPs exhibited significantly more enhancement in MRI contrast than Gadvovist, a commercial MRI agent, and higher X-ray attenuation, compared to the commonly used contrast agent Visipaque (Iodixanol) on agarose gel phantoms. In vivo, AuGR-SiO2-Gd NPs revealed a positive contrast in MRI and a negative contrast within the tumor area in genetically engineered mice in CT and photoacoustic imaging (PAI) [188]. The utility of conjugating radiolabeled anti-TAA with AuNPs for PET imaging of pancreatic tumors has recently been demonstrated [189]. Fully humanized, anti-CA 19.9 mAb conjugated to p-isothiocyanatobenzyl-desferrioxamine (p-SCN-DFO) to chelate a PET-emitting radionuclide (89Zr) was subsequently attached to activated Au-NPs. Radiolabeled mAb-AuNPs allowed for efficient detection of orthotopic pancreatic tumors and established the utility of depleting the mononuclear phagocyte system for reducing the non-specific hepatic uptake of nanoparticles. NP-based nanoprobes have also been developed to differentiate tumors from uninvolved healthy tissue for surgical navigation. Qi et al. synthesized hyaluronic acid (HA) NPs encapsulating near-infrared (NIR) dye-indocyanine (ICG), which allowed improved discrimination of primary orthotopic tumors from the healthy pancreas and better detection of splenic metastasis as compared to free ICG [190]. Other nano-imaging agents based on various imaging agents and NP compositions that have been evaluated in pancreatic cancer are summarized in Table 2.
Tumor-associated antigens (TAA) investigated for the delivery of therapeutic payloads in PDAC
| TAA | Nanoparticulate carrier | Surface modifier/encapsulation of | Therapeutic/imaging Cargo | Application | Phase of Investigation | Modality | Ref. |
|---|---|---|---|---|---|---|---|
| MUC1 | PLGA | MUC1 Ab (TAB004) | Paclitaxel | Ab-mediated Drug Delivery | In vivo | Therapy | [88] |
| Iron oxide | MUC-1 peptide (EPPT) | Gemcitabine/Cy 5.5 dye | MRI/Drug delivery | In vivo | Therapy | [339] | |
| MUC4 | CPG & CPTEG | MUC4β protein | MUC4β | Immunotherapy | In vitro | Therapy | [81] |
| MnMEIO-silane-NH2-mPEG | Anti-MUC4 Ab | MnMEIO | MRI | In vitro & in vivo | Imaging | [340] | |
| MUC5AC | Liposome | RA-96 Fab | Indocyanine green (ICG) | Tumor imaging | In vivo | Imaging | [186] |
| CEA | Lipid-polymer | CEA Ab | Paclitaxel | Drug Delivery | In vitro | Therapy | [264] |
| CA19-9 | mPEG-PLGA-PLL | CA19-9 Ab | Paclitaxel | Drug delivery | In vitro | Therapy | [267] |
| Liposome | CA19-9 Ab | Doxorubicin | Ab-mediated drug delivery | In vitro & in vivo | Therapy | [263] | |
| KRAS G12D | Glycol-Poly-L-lysine copolymer | Human scFv (CD44v6) Ab | siRNA | siRNA delivery (Gene therapy) | In vivo | Therapy | [341] |
| VEGF | PEG-CCP block copolymer | siRNA | siRNA | mRNA knockdown | In vitro | Therapy | [270] |
| Graphene oxide | siRNA | siRNA & Doxorubicin | Combination therapy | In vivo | Therapy | [342] | |
| Mesothelin | Iron oxide@SiO2 | Anti-mesothelin Ab | IONPs | MRI | In vitro | Imaging | [265] |
| EGFR | CPT-PLGA | Cetuximab | Camptothecin | Antibody-mediated drug delivery | In vitro | Therapy | [202] |
| BSA | Erlotinib | Parvifloron D | Targeting of EGFR | In vitro | Therapy | [271] | |
| Magnetic albumin | Cetuximab | Gemcitabine | MRI/Drug delivery | In vitro | Theranostic | [343] | |
| Silica NPs | Cetuximab | ZnPcOBP (Zinc Phthalocyanine) | PDT/PTT | In vitro | Therapy | [344] | |
| Liposomal formulation | EGFR (Cet) Ab | Benzoporphyrin derivative | In vivo photoacoustic imaging, PDT/PTT | in vitro & in vivo | Therapy/ imaging | [345] | |
| HER2 | Chitosan | HER-2Ab | Gemcitabine | Drug delivery | In vitro | Therapy | [268] |
| Iron oxide | HER-2 Ab | Gemcitabine | MRI/Drug delivery | In vivo | Theranostic | [193] | |
| Retinoic acid | Gold | Retinoic acid | siRNA | TME modulation & HSP47 targeting | In vitro & in vivo | Therapy | [21] |
| Iron oxide | Retinoic acid | Gemcitabine | TME modulation | In vitro | Therapy | [346] | |
| PEG | PEG-Retinoic acid (PGRA) | Gemcitabine | TME modulation | In vitro | Therapy | [347] | |
| CA19-9 | Liposomes | CA19-19 diabody | 124I | Emission tomography | In vivo | Imaging | [348] |
| Carbon QDs | CA19-9 Ab | QDs | Fluorescence | Ex vivo | Imaging | [349] | |
| Gold | 5B1 Ab | 89Zr | PET | In vivo | Imaging | [189] | |
| CD44 | Iron oxide | CD44 Ab | Hyaluronic acid | MRI | In vivo | Imaging | [350] |
| uPAR | Iron oxide | ATF peptide | Gemcitabine | MRI//drug delivery | In vivo | Theranostic | [156] |
| Shh | Iron oxide | Shh (5E1) Ab | Cyclopamine | MRI//drug delivery | In vivo | Theranostic | [351] |
| Plectin-1 | Iron oxide | Plectin-1 peptide | IONPs | MRI | In vivo | Imaging | [352] |
| Iron oxide | Plectin-1 Ab | Cy7 dye | MRI/Fluorescence | In vitro & in vivo | Imaging | [353] | |
| IGF-1 | Iron oxide | IGF-1 Ab | Doxorubicin | MRI/Drug delivery | In vivo | Theranostic | [195] |
| Galectin-1 | Iron oxide | t-PA-ligand | IONPs | MRI | In vivo | Imaging | [354] |
| Iron oxide | Galectin-1 Ab | IONPs | MTAI | In vivo | Imaging | [355] | |
| Glypican-1 | Gold | Hyaluronic acid | Oridonin | NIRF/MRI/Drug delivery | In vivo | Theranostic | [356] |
| Neuropilin | Hsp 16.5 nanocages | iRGD peptide | Gadolinium | MRI | In vivo | Imaging | [357] |
| CEA CA19-9 | mPEG-PLGA | CEA & CA19-9 Ab | Paclitaxel | Ab mediated drug delivery | In vitro | Therapy | [266] |
| EGFR, STAT3 | PLGA | EGFR, STAT3 Ab | Alantolactone & Erlotinib | Dual targeting of EGFR & STAT3 | In vitro | Therapy | [269] |
| MUC4, CEA, CD44 | Iron oxide-PEG | MUC4, CEA & CA19-9 | Paclitaxel | US/Drug delivery | In vivo | Theranostic | [358] |
| Cathepsin E (CTSE) | AuNPs | U11 peptides, 5-ALA (CTSE-sensitive prodrug), Cy5.5 dye | 5-ALA and fluorescent dye Cy5.5 | Optical imaging, PDT/PTT | In vivo & ex vivo | Therapy/ imaging | [359] |
Theranostic NPs have also been evaluated in several studies for targeting PDAC. Gemcitabine, which is the first-line therapy for PDAC, has been encapsulated in various NP formulations, including microbubbles (for ultrasound imaging) [191], luminescent photothermal NPs [192], and PLGA nanospheres containing fluorescent iron oxide NPs [193]. Urokinase plasminogen activator receptor (uPAR)-targeted, PEGylated iron oxide NPs labeled with NIR dye (NIR 830-maleimide) and loaded with doxorubicin (DOX) or cisplatin were also evaluated in a syngeneic orthotopic model of PDAC. These NPs, when administered via the intraperitoneal route, enabled tumor visualization by NIR optical imaging and MRI and resulted in tumor growth inhibition [194]. Similarly, human insulin-like growth factor receptor (IGF1)-targeted, NIR dye-labeled iron oxide NPs with DOX as therapeutic payload exhibited anti-tumor effects on orthotopic patient-derived xenografts (PDXs) and enabled NIR optical imaging and MRI [195]. Additional examples of the recently published molecular imaging and theranostic nanoprobes for PDAC are shown in Table 2. Overall, NP-based imaging and theranostic agents have shown promise in preclinical studies.
3.3. Nanoscale delivery system for targeted therapy in PDAC
As discussed in Section 2, the pancreatic TME is a critical determinant of resistance to chemotherapy and immunotherapy. Nanocarriers have been designed to target tumor stroma by delivering inhibitors of signaling pathways involved in stromagenesis. In this regard, three secreted hedgehog proteins (Sonic, Indian, and Desert) and their downstream signaling molecules have been extensively studied and exploited to modulate tumor stroma [196, 197]. Strategies employing nano-enabled siRNA and miRNA delivery systems targeting these pathways have been used in PC models [121], as detailed below. Efforts have also been directed to design NPs to exploit and/or modulate other pathophysiological or molecular hallmarks of PDAC, such as acidic pH, hypoxia, and stromal proteases.
3.3.1. Stimuli-responsive NPs
Stimuli-responsive NPs take advantage of several unique PC features, including hypoxia, low tissue pH, and upregulated enzymes represented by cathepsins and matrix metallopeptidases, which are related to EMT. Gurka et al. designed a pH-responsive nanocarrier to co-deliver an extracellular signal-regulated kinase inhibitor and GEM [198]. The triblock copolymer partially unfolds in response to the lower pH in the PC microenvironment, resulting in controlled release of payload and suppression of PC cell growth. Kulkarni et al. prepared hypoxia-responsive polymersome and lipid NPs for the targeted release of chemotherapeutics to PC cells [199, 200]. In both studies, an azobenzene group was incorporated into the polymer that undergoes a reduction in response to elevated levels of reducing enzymes corresponding to hypoxia in the PC microenvironment. The hypoxia-responsive release of chemotherapeutics resulted in reduced cancer cell viability. In another study, a sequential release of GEM was realized using a dual enzymatic responsive nanocarrier [201]. The PEG shield was first cleaved by the matrix metalloproteinase-9 overexpressed in the PC microenvironment and cathepsin-B upregulated in lysosomes.
3.3.2. Antibody-mediated targeting
Tumor-specific antibodies can be incorporated into nanocarriers to target tumors in an antigen-specific manner and to promote site-specific accumulation. Antibodies targeting various upregulated receptors, including vascular endothelial growth factor (VEGF), epidermal growth factor receptor (EGFR), and carbohydrate antigens (e.g., CA19-9, CA125 Sialyl Tn), have been extensively tested for targeting nanomedicines to PC. McDaid et al. used the clinically approved anti-EGFR antibody (i.e., Cetuximab) for targeting PLGA NPs in order to lower off-target cytotoxicity and enhance drug efficacy in EGFR-resistant PC [202] The conjugation-induced targeting and apoptosis were demonstrated in several different cancer cell models, indicating a generalizable approach for nano-enabled enhanced drug efficacy. In another study, (1,2-diaminocyclohexane) platinum(II) (DACHPt)-based polymeric micelles loaded with OX were conjugated with an antigen-binding fragment of a novel tissue factor antibody [203]. The antibody-conjugated micelles were rapidly internalized by PC cell line BxPC3 and localized in lysosomes and late endosomes. Further, a murine tumor model with subcutaneous BxPC3 xenografts was used to test the antitumor efficacy of DACHPt micelles. The antibody-conjugated DACHPt micelles exhibited superior tumor inhibition compared to non-targeted micelles and soluble drugs against established pancreatic tumors.
3.3.3. Ligand-promoted targeting
In addition to antibodies, other biological molecules and ligand-targeted drug systems have been explored for cancer targeting [204]. He et al. prepared a combination NP system with ECM-targeting aptamer, cell-penetrating peptide, and redox responsive release [18]. Lin et al. conjugated an anti-EGFR peptide GE11 to a liposome nanocarrier to facilitate targeting specificity [205]. The ligand targeting strategy was synergized with the co-delivery of HIF1α siRNA and GEM. The combined formulation enhanced drug uptake, increased apoptosis, and reduced tumor burden in a murine model. The aptamer GBI was released upon interaction with ECM component tenascin-C and exposed the cell-penetrating peptide for tumor cell internalization. The NP system was tested on PC spheroids and tumor-bearing nude mice, demonstrating improved drug efficacy and tumor regression. Lee et al. prepared polymer-coated magnetic iron oxide NPs conjugated with a urokinase plasminogen activator targeting peptide. This NP system realized the dual function of targeted GEM release and MRI contrast enhancement in a PC xenograft murine model [156].
4. Nanocarrier-driven immunomodulatory approaches for PDAC
Cancer emergence and progression often imply the failure of the immune system to detect tumor antigens and destroy malignant cells [206]. Current vaccine approaches, which are based on protein, peptides, nucleic acids, or adoptive transfer of immune cells such as dendritic cells (DCs) or T cells, fail to achieve or stimulate the desired magnitude and/or the correct arm (i.e., phenotype) of the immune response to confer anti-tumor immunity with therapeutic benefits. While promising, these cell-based immunotherapies rely heavily on continual in vitro stimulation or cultivation of cells, which may induce immunological exhaustion, resulting in inadequate ex vivo expansion and/or shortened survival rate upon infusion, and ultimately low rates of successful clinical responses [207]. The urgent demand to obtain precise control over the induction of desired arm(s) of the immune response has brought more attention towards the rational design of nanocarrier-based cancer vaccines (such as polymeric nanovaccines). These research efforts are based on a deep knowledge of how the immune system interacts with nanocarriers to generate strong and durable immune responses to effectively combat tumor cells [208, 209]. The successful development of such nanocarrier-based cancer vaccines relies on addressing critical challenges, including (i) efficient delivery of tumor antigen(s) to antigen-presenting cells (APCs); (ii) suitability of vaccines to activate appropriate pathways within APCs and other immune cells; (iii) appropriate packaging and delivery of diverse vaccine components (antigens and immunological adjuvants) to generate optimal antigen-specific antitumor immune responses; and (iv) minimizing adverse reactions such as systemic inflammatory responses [210, 211].
4.1. Polymer chemistry and immune activation
Various natural and synthetic biodegradable and biocompatible polymers have been widely investigated and used to fabricate nano- and microparticles encapsulating single or multiple vaccine components. Most notably, the biodegradable and biocompatible copolymer PLGA has been extensively explored for controlled delivery of biologically active molecules (including vaccine constituents) [212]. An important advantage of employing PLGA in vaccine delivery is its adaptability, suitability, and ease of manipulation of its chemical and physical properties, such as hydrophobicity/hydrophilicity, molecular mass, and crystallinity through changes in the monomer ratio, terminal group chemistry, size, and net charge [82, 90, 209]. Thus, the physicochemical properties of PLGA-based particulate vaccines can be rationally optimized to allow targeted delivery of tumor antigens for the generation of antitumor immune responses. The terminal group characteristics make PLGA amenable to surface modifications for improved targeting [213]. For example, a study performed with tumor lysate-targeted PLGA particles coated with biotinylated streptavidin stimulated stronger tumor-specific immune responses when compared to uncoated counterparts [214].
Polyanhydride particles have been reported to have an adjuvant effect in that they can stimulate DCs through binding to Toll-like receptors (TLRs) [83, 215]. Another important characteristic of polyanhydrides is their tunable degradation rate and unique surface erosion mechanism dictated by copolymer composition [216-218]. We have shown that varying the molar composition of polyanhydride copolymers can also have a significant effect on the properties of particles and, subsequently, the antitumor immune responses [218]. One major factor is hydrophobicity, which plays a key role in the opsonization and cellular uptake of particles. For example, increasing the molar ratio of CPH in polyanhydride copolymer composition resulted in a significant increase in the hydrophobicity of particles and, in turn, stimulated more potent antitumor immune responses and improved their in vivo performance [218]. Similarly, poly(phosphazenes), a class of biodegradable polymers, have been explored for their TLR stimulatory effects. Studies revealed that poly(phosphazenes) displayed strong avidity to soluble immune receptor proteins (e.g., mannose receptor) and certain TLR proteins [219, 220]. Another example of a biodegradable polymeric biomaterial that has been recently investigated for vaccine delivery is poly(diaminosulfide) (PNSN) [221, 222]. Particularly, the use of PNSN for cancer vaccines in a murine tumor model showed that mice vaccinated with tumor antigen-loaded PNSN particles had high levels of CTLs, and the formulation conferred protective immunity against the tumor challenge [223]. Poly(beta-amino esters) have also been studied for their application as cancer vaccine vectors. These polymers have a unique branched architecture that provides a large chemical space for complexation and functionalization. Due to their cationic properties, poly(beta-amino esters) enhances cellular uptake and endosomal escape via the proton sponge effect [224, 225]. Polymeric nanocarriers can provide effective solutions to these obstacles, and degradable polymers used for cancer vaccines are summarized in Table 3.
Degradable synthetic biomaterials used in vaccine platforms
| Polymer | Chemical Formula | Properties/Functions | Ref. |
|---|---|---|---|
| Poly(lactide-co-glycolide) | [C3H4O2]x[C2H2O2]y | Can be targeted to antigen-presenting cells, and their particulate nature can increase uptake and cross-presentation | [90, 214] |
| Polyanhydride | [CO-R-CO2]n | Surface erosion (tunable release rates) and inherent adjuvant properties | [83, 215, 217, 218] |
| Poly(phosphazene) | [N=PR1R2]n | Water-soluble and function as adjuvants | [219, 220] |
| Poly(diaminosulfide) | [R-N-S-N-R]n | Highly stable in neutral aqueous solutions while at lower pH conditions, the N-S-N linkage degrades faster, generating accelerated release kinetics | [221-223] |
| Poly(beta-amino ester) | [R2N-RCO2R]n | Readily phagocytosed and promotes in situ expression of chimeric antigen receptor genes | [224, 225] |
4.2. Mechanisms of immune induction by nanocarriers
The mechanisms by which nanocarriers induce antitumor immune responses are dictated by how biomaterials interact with the host immune system. Interaction of nanocarriers with blood or interstitial fluid results in the rapid formation of a protein layer on the biomaterial surface, known as the “protein corona” [226]. Nanocarrier surface chemistry, charge, and morphology have been shown to extensively impact immune activation, as reviewed elsewhere [227-229]. In addition, the identity of NPs is redefined by the protein corona due to its impact on pattern recognition receptor (PRR) engagement, activation of the complement cascade, and cellular internalization. Following protein deposition on NP surfaces, leukocytes sense the biomaterial surface by surface receptors, which leads to downstream signaling events, including activation of inflammation-related transcription factors (e.g., NF-κB and NFAT) [230]. These transcription factors further regulate a series of immune activation events such as cytokine and chemokine expression, which not only directly impact immune cell behavior, but also orchestrate global immune activation via modulation of vascular permeability and dilation. Another outcome of leukocyte interaction with biomaterials is the increase in oxidative stress because of enhanced mitochondrial activity (i.e., metabolic changes) and PRR-induced anti-microbial immunity [231]. Recent studies also used the level of reactive oxygen species (ROS) as a measure of immune activation, which could be altered by biomaterial-based immunomodulation [232, 233]. Among mononuclear cells, macrophages and DCs serve as the most effective APCs for T cell activation. In particular, DCs are the primary cell type responsible for cross-presentation and induction of antigen-specific CD8+ T cells. As one of the most heterogeneous cell populations, distinct T cell subsets can be identified by their activation status, antigen experience, and effector functions and play important roles in inducing optimal immune responses. Although T cells are rarely shown to adhere directly onto biomaterial surfaces and be activated hereby, their activation can be tuned by biomaterial-leukocyte interactions [234, 235]. The multiple advantages provided by the physicochemical and mechanistic aspects of nanocarrier-mediated immunomodulation are summarized below and shown in Figure 3.
4.2.1. Enhanced APC internalization
Nanocarriers with tumor-associated antigens (TAAs) are preferentially internalized by APCs, thus offering an increased magnitude of APC activation and dose sparing and leading to enhanced antigen processing and T cell activation [236, 237]. One of the determining factors of the endocytic uptake pathway by APCs is the size of particles that deliver the cancer vaccine components. It has been found that nano-sized particles are readily internalized by pinocytosis, whereas micron-sized particles are taken up by the phagocytotic process [238]. A study that compared the uptake of different sizes of antigen-loaded PLGA particles (0.3, 1, 7, and 17 µm) found that smaller particles were readily internalized by DCs, and this was associated with stronger stimulation of in vivo antigen-specific immune responses when tested in murine tumor model [90]. Other studies have shown that nanoparticles less than 100 nm can potentially traffic on their own to the draining lymph nodes (DLNs), where they can be captured by the LN APCs, which may result in more efficient antigen cross-presentation and CTL priming [239, 240]. In contrast, larger particles normally remain at the vaccination site and are phagocytosed by the migratory APCs, which then migrate to the closest DLN [239, 240]. PDAC is often characterized with strong local immunosuppression and distant immunoremodeling [241], which renders ineffective antigen presentation by APCs and decreased co-stimulatory signaling to T cells. Systemic or intratumoral APC activation can be exploited to enhance T cell immunotherapy. Lorkowski et al. prepared lipid-based immune-stimulatory NPs (immune-NPs) for the co-activation of STING pathway and TLR4. The immune-NP is designed to target the tumor local innate immune cells and promote APC activation and proliferation. A high percentage of NP cellular uptake was observed in multiple organs and orthotopic Panc02 tumor concomitantly with increased tumor-infiltrating APCs [242], which is instrumental for T cell priming and recognition of cancer cells.
4.2.2. Biomaterials with inherent adjuvanticity
Some biomaterial-based nanocarriers can provide immunostimulation, resembling conventional vaccine adjuvants. For example, NPs modified with hydroxyl and amino groups induced complement system-mediated immunostimulation [240, 243]. Polyanhydride NPs also showed chemistry-dependent APC activation (e.g., elevated CD80/86 expression, cytokine secretion) [244]. It has been suggested that such non-specific biomaterial-induced adjuvant effects could be attributed to a hydrophobicity-based danger-associated molecular pattern (DAMP)-like mechanism [245].
Advantages of polymeric nanoadjuvants for PDAC immunotherapy. Clockwise from the top, the figure shows how polymeric NPs: enhance exogenous antigen internalization by DCs, which can promote antigen transportation to secondary lymphoid organs and increase antigen persistence; improve antigen cross-presentation by increasing cytosolic delivery of encapsulated payloads in DCs, thus leading to more effective antigen-specific CD8+ T cell activation; enhance ICD and sensitize PDAC to immune cell recognition; induce higher levels of CD8+ T cell activation by licensed DCs or ICD based on in situ vaccination; enable more efficient removal of stroma, and enhance the reversal of immunosuppressive TME. NPs: Nanoparticles; ECM: Extracellular matrix; CAFs: Cancer-associated fibroblasts; DCs: Dendritic cells.
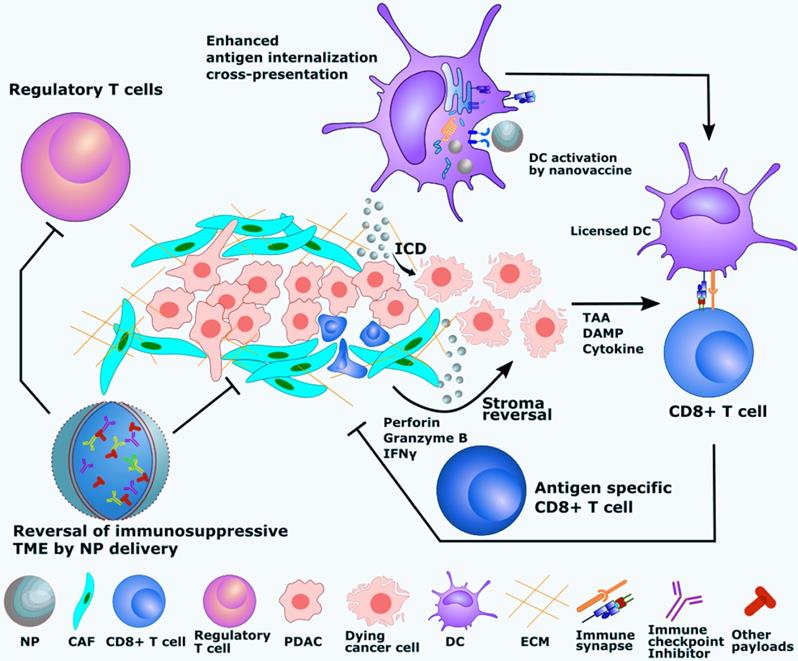
4.2.3. Enhanced cross-presentation and induction of CTLs
Extracellular antigens need to be internalized and presented to major histocompatibility complex (MHC) class I molecules for effective induction of CTLs. Nanocarriers can enhance cytosolic delivery of TAAs, leading to endosomal escape and processing via proteasome into peptides loaded onto MHC I molecules for the induction of anti-tumor, antigen-specific CD8+ T cell immunity. Several endosomal escape mechanisms have been proposed [209]. Among these mechanisms for nanocarrier-induced endosomal release is the “proton sponge hypothesis” [246]. This strategy has already been demonstrated on PDAC models using polyethyleneimine modified aluminum hydroxide NP as a vaccine carrier to a Panc02-OVA tumor [247]. The resulting nanovaccine induced antigen-specific immunity to Panc02 cells and regression of the established pancreatic tumor. Another study used liposome NPs to target mouse CD169+ DCs via ganglioside, a natural ligand of CD169. This NP was shown to increase antigen cross-presentation and target Axl+ DCs derived from PDAC patients [130]. In addition to the reversal of PDAC TME, the use of targeted APC activation could be a powerful approach to further recruit CTLs.
4.2.4. Lymph node delivery
Studies have shown that antigen accumulation at DLNs significantly enhances T cell activation [210]. Conventional routes of vaccine administration induce suboptimal activation of CD8+ T cells due to insufficient antigen-loaded cDC1 migration to LNs [248]. More recent studies with tumor models demonstrated that the co-delivery of adjuvant and antigen to LN is critical for optimal immune activation, making a strong case for NPs capable of loading multiple components [211, 212]. A Japanese study analyzed LN metastasis in 429 PDAC patients and identified high incidence in advanced PDACs [249]. Because of the limited therapeutic measures available to PDAC patients with DLN metastasis, immunomodulatory interventions to LN should get more attention. A case has been made by using PLA microspheres loaded with IL12 (IL12 MSs) to repolarize the pancreatic DLN immune profile in an orthotopic KCKO PDAC model [250]. In this study, IL12 microspheres were tested in combination with stereotactic body radiotherapy (SBRT) and/or lymphatic ablation. IL12 microspheres + SBRT inhibited tumor growth and induced immune profile alteration, including expression of CXCL10, IFNγ, and granzyme B. Interestingly, the DLN excision partially abrogated these effects.
4.2.5. Immunogenic cell death (ICD)
ICD is a specific type of cell death characterized by the release of DAMPs, inflammatory signaling molecules, and in the case of cancer cells, TAAs [251]. ICD provides a combination of antigens, cytokines, and co-stimulatory molecules required for APC activation, and therefore can be instrumental for T cell priming. NPs loaded with cytotoxic reagents have been utilized for anti-tumor therapies by inducing ICD [58, 252]. In addition to the tumoricidal effects, certain types of chemotherapeutics such as DOX and OX have also been reported to elicit ICD and thereby function as in situ vaccination against tumors [251, 253]. A study that investigated the in situ immunization against both B cell (A20) and T cell (EL4) lymphoma tumor models with PLGA particles co-encapsulating DOX and CpG-ODN showed that the combination regimen was effective at generating systemic responses and reducing tumor burden, which was further enhanced by anti-OX40/anti-CTLA4 monoclonal antibody (mAb) therapies to improve T cell activation and overcome immunosuppression [254]. Another recently reported example is the in situ immune stimulation against the B16.F10 melanoma tumor model with PEGylated PLGA NPs encapsulating DOX with or without anti-PD1 [255]. The median survival time of animals was extended to 55 days post-tumor challenge in comparison to 15 and 30 days for naïve and soluble DOX treated mice, respectively [255]. Upon combining the DOX-loaded PEGylated PLGA NPs with anti-PD1 therapy, there was a synergistic effect, and the median survival time was not reached since 60% of mice remained tumor-free at the completion of the study [255]. ICD-inducing nanoplatforms have also been tested in PDAC models. A supramolecular nanocarrier was used to co-deliver photosensitizer and prodrug in a Panc02 tumor model [256]. The NPs were made from self-assembly of cyclodextrin-grafted hyaluronic acid, pyropheophorbide a (photosensitizer), and JQ1 (prodrug). The resulting NPs downregulated Panc02 tumor-associated immunosuppression and elicited ROS-driven ICD. By 40 days post-treatment, the multiple-component NP plus laser excitation significantly prolonged the survival of Panc02-bearing mice compared with control or monotherapy groups. Inhibition of tumor recurrence and metastasis was also observed up to the endpoint of the tumor study. Another study employed OX as the inducer of ICD in the Panc02 tumor model where OX was co-encapsulated with a siRNA against galectin-9/dectin-1 axis into bone marrow mesenchymal stem cell-derived exosomes [255]. The combination therapy was shown to reverse the M2-like polarization of macrophages in the tumor and significantly inhibited orthotopic Panc02 tumor growth throughout the 28-day course study. More studies using nano-enabled mechanisms in PDAC models are summarized in Table 4.
Nanoscale immunotherapy studies related to PDAC
| Nano-enabled mechanism | Nanomaterial composition | Main results | Tumor model | Ref. |
|---|---|---|---|---|
| Reversal of immunosuppressive TME | ||||
| Enhanced cellular uptake and tumor penetration | mPEG-PEI-coated AuNP loaded with ATRA and siHSP47 for stromal modulation | Reversal of activated pancreatic stellate cell; ECM reduction Improved chemotherapy | PANC-1/pancreatic stellate cell co-inoculated subcutaneous xenografts | [21] |
| Nanocarrier enhanced co-delivery and drug efficacy | Self-assembled nanovesicles or lipid bilayer coated mesoporous silica NPs encapsulating inhibitor for immunosuppressive IDO pathway | Induced immunity against subcutaneously injected and orthotopic tumor challenge Increased CTLs, Decreased Tregs | Orthotopic pancreatic implant KPC model | [58] |
| Enhanced biodistribution and tumor accumulation | Liposome-protamine-DNA NP encapsulating plasmid encoding CXCL12 and IL10 trap | Activation of various suppressed immune cells in TME | Orthotopic, KPC PC, and 4T1 triple-negative breast cancer models | [61] |
| Reduced toxicity, enhanced transfection, and ECM targeting | Calcium phosphate core with thin-film from cholesterol, DOTAP, and PEG conjugated with ECM targeting FHK peptide | Successful transfection, Increased CTL tumor infiltration, Tumor site accumulation, Tumor site accumulation vascular normalization | Orthotopic Panc02 and KPC cell line derived pancreatic tumors | [67] |
| Exosome enhanced endocytosis via anchor protein | Exosomes derived from mesenchymal cells carrying siRNA for KRAS | Exosome enabled superior antitumor performance in various in vitro and in vivo cancer models | PANC-1 orthotopic xenograft tumor; KTC and KPC genetically engineered mouse PDAC models | [142] |
| Improved pharmacokinetics and toxicity | Liposome-protamine-DNA NP encapsulating plasmid encoding CXCL12 and PD-L1 trap | Improved antitumor response against KPC, allografts, and suppressed metastases;Enhanced T cell infiltration | Orthotopic pancreatic implant KPC allograft | [276] |
| Exosome accumulation at the tumor and enhanced payload efficacy | Exosomes derived from mesenchymal cells co-loaded with siRNA and OX | Accumulation of exosomes at the tumor site; Exosome-enhanced downregulation of immunosuppression and ICD; Improved profile of tumor-infiltrating immune cells | Orthotopic Panc02 syngeneic PDAC tumor model | [360] |
| Micelle pH-sensitive co-delivery of GEM | GEM and paclitaxel codelivery micelles based on a polyethylene glycol-polyarginine-polylysine (PEG-pArg-pLys) platform | Improved chemotherapy and immune cell infiltration; Stroma disruption; Decreased metastasis | MiaPaCa-2 tumor orthotopic PDAC xenograft model | [361] |
| PDAC nanovaccines | ||||
| Conjugated ligand enhanced internalization Enhanced cross-presentation | Ganglioside-liposome (EPC/EPG/cholesterol-based liposomes) nanovaccine loaded with WT1 or gp100 antigen targeting CD169 | CD169 dependent liposome internalization by model DC Activated antigen-specific T cell line Activated patient-derived DCs | Samples derived from PDAC or melanoma human patients | [130] |
| Viral protein-induced immune stimulation | Insect cell produced MSLN antigen containing VLPs | Activation of MSLN specific CTL Decrease of tumor-infiltrating Tregs Therapeutic vaccine-induced tumor inhibition | Orthotopic PDAC syngeneic Panc02 pancreatic cancer mouse model | [279] |
| ICD TME targeting | Nanoparticulated mushroom Schizophyllan complexed with a humanized TLR9 agonistic CpG DNA | Proved need for innate immune component IL12p40 and type I interferon Phagocyte targeting in TME Tumor site accumulation | PC peritoneal dissemination model | [362] |
| Cationic liposome enhanced CpG delivery; Enhanced cytosolic delivery | Peptide-CpG-DNA -liposome lipoplex vaccine encapsulating TM4SF5 antigen | Antibody-mediated cancer cell inhibition Prophylactic tumor prevention | Transfected Panc02 human TM4SF5 expressing cancer model | [363] |
| Micelle enhanced stability and gene delivery | PEG catiomer and DNA polyplex micelles encapsulating gene encoding SART3 antigen, adjuvant CD40L, and GM-CSF | Observed cytotoxicity and proliferation for splenic CTL and NK cells; Therapeutic vaccination against various tumors; Analysis by CD4/CD8 T cell depletion assay | Various cancer cell line and tumor model | [364] |
| Enhanced antigen delivery, cytosolic delivery, and cross-presentation | Polyethyleneimine modified aluminum hydroxide NPs | In vitro DC activation and cross-presentation assay Vaccine-induced activation and proliferation of IFN-γ expressing CTL; Inhibition of established Panc02 tumor | Panc02 subcutaneous syngeneic pancreatic tumor model | [247] |
| Other nanoscale immunotherapeutic strategies | ||||
| Enhanced biodistribution and prolonged delivery | Lipid calcium phosphate NPs encapsulating dsRNA | Induction of Th1 response Increased CTL activation over Treg Analysis by CD4/CD8 T cell depletion assay Inhibition of established pancreatic tumors | Orthotopic KPC allograft PC tumor model and subcutaneous allograft BPD6 melanoma tumor | [60] |
| Enhanced NP biodistribution and cellular uptake | Lipid, cholesterol, and PEG-based NPs encapsulating STING and TLR4 agonist | Increase of tumor-infiltrating immune cells Inhibition of established subcutaneous Panc02 tumor | Orthotopic and subcutaneous Panc02 syngeneic pancreatic tumor model | [242] |
| LN delivery and prolonged release | PLA microspheres loaded with IL12 | Intratumoral injection of IL12-MSs altered DLN cytokine profile; IL12-MS plus SBRT efficacy was reduced by DLN ablation | Orthotopic KCKO tumor model | [250] |
| Co-loading by self-assembled NP and tumor-targeting | Supramolecular NP self-assembled from cyclodextrin, photosensitizer, and prodrug Hyaluronic acid-Pyropheophobide and JQ1 | Blockade of immunosuppression molecules; ROS-driven ICD; Local and systemic tumor inhibition; Enhancement of immunogenicity; Promote intertumoral infiltration of cytotoxic T lymphocytes | Subcutaneous and orthotopic Panc02 syngeneic pancreatic tumor model | [256] |
| Exosomal targeting of Notch pathway protein | Pancreatic cell-derived exosomal NPs | Decreased Notch signaling and mitochondria-dependent apoptosis; In vitro inhibition of human PC cell line growth | Various human PC cell lines | [365] |
| Nanocarrier enhanced delivery and reduced toxicity | PEG-PLGA NPs encapsulating ICD inducer oxaliplatin | Induced IFNγ expressing tumor-infiltrating CD8 T cell | Subcutaneous Panc02 syngeneic pancreatic tumor model | [366] |
4.3. Nanocarriers for PDAC immunotherapy
The approaches and concepts described in Sections 4.1 and 4.2 have led to the design and study of nanocarrier-enabled PDAC immunotherapies. From the identification of novel TAAs to the preparation of nanoformulations to the characterization of immune activation and anti-tumor performance in various PDAC tumor models, researchers are moving forward to synergistic nano-driven immunotherapies for optimal anti-tumor efficacy. Table 4 lists nanocarrier-enabled PDAC immunotherapies applied in distinct modalities (e.g., TME reversal, nanovaccine) that have been evaluated in various types of PC models.
4.4. PDAC tumor-associated antigens (TAAs)
Various TAAs have been investigated for both targeted delivery of therapeutic and diagnostic agents and for developing immunotherapy for PDAC. The majority of TAAs initially described for various cancers, including PC, are cell surface glycoproteins and cell surface receptors that are either aberrantly glycosylated and/or overexpressed and were initially used as biomarkers. Subsequently, several of these TAAs were exploited for payload delivery of therapeutic and imaging agents or direct targets for immunotherapy, antibodies, and small molecule drugs (Table 2). PDAC is characterized by overexpression of several mucins, high molecular weight glycoproteins that are either cell-surface tethered or secreted [257]. Most cancer-associated mucins are encoded by multi-exon genes and characterized by variable number tandem repeat (VNTR) domains that are heavily O-glycosylated and secreted. By virtue of their overexpression, extensive splicing, mutations, and aberrant glycosylation in cancer, carcinoma mucins are promising neoantigens, while the presence of repetitive VNTR epitopes makes them excellent targets for payload delivery [257, 258]. Consequently, several membrane-tethered (MUC1, MUC4, and MUC16) and secretory (MUC5AC) mucins have been explored as immunogens that were delivered using nanocarriers and for the development of immunotherapies in PDAC [258-262]. Several other cell surface glycoproteins (carcinoembryonic antigen-CEA), mucin-associated carbohydrate epitopes (Sialyl T, Sialyl Lewisa), and mucin-interacting proteins (mesothelin, galectins) have also been investigated for PDAC immunotherapy [263-267]. Similarly, antibodies and ligands of several growth factor receptors (EGFR, HER-2, and VEGFR) have been used for the delivery of therapeutic and diagnostic payloads using nanocarriers in PDAC [202, 268-272]. In addition to cell surface TAAs, tumor-specific intracellular targets (K-RasG12D, telomerase) have been explored for the immunotherapy of PDAC. Various PDAC TAAs that have been explored for payload delivery are summarized in Table 2, while preclinical and clinical studies investigating their utility for immunotherapy are reviewed elsewhere [10, 273-275].
Due to the uniquely immunosuppressive TME associated with PDAC, immunomodulation strategies have focused on normalizing the desmoplastic stroma and restoring immune cell function and infiltration (Table 4). A promising nanoscale strategy was described by two studies from the Huang group [62, 276]. Plasmid genes encoding IL10, CXCL12, or PDL1 protein traps were encapsulated and delivered by liposome-protamine-based NPs for transfection. The delivery of high-affinity trap protein was designed to compete with the binding of the cytokines to their cognate receptors to interfere with factors that contribute to the immunosuppressive TME. In both studies, the NPs were shown to accumulate preferentially at the site of the tumor and induce downregulation of immunosuppressive response in an orthotopic tumor model of developed by implantation of KPC cells [62, 276]. Nanoscale formulations have also been exploited for targeting the delivery of TAAs as nanovaccines. Liposome-based nanocarriers represent the most well-studied platform in human cancer vaccine clinical trials [277, 278]. A liposome carrier was conjugated with ganglioside, a CD169 ligand, to facilitate the targeting and delivery of PDAC antigen WT1 to CD169+ APCs in patients. The resulting liposomal NP simultaneously delivered TLR4 ligand monophosphoryl lipid A (MPLA) and WT1 antigen to various types of DCs and was shown to induce the in vitro expression of IFNγ from a T cell line [130]. Another study used PDAC mesothelin (MSLN) antigen-containing virus-like particles (VLPs). VLPs are considered effective carriers for immunotherapy due to their intrinsic ability for immunostimulation. Therapeutic vaccination with VLPs containing MSLN induced CTL responses and limited the expansion of Treg cells following orthotopic implantation of Panc02 tumor cells, resulting in inhibition of tumor progression [279].
4.5. Combination nano-platforms for CTL induction and tumor control
A key advantage of nanoscale delivery systems is their ability to combine multiple components/payloads. Nanocarriers can optimize the delivery of diverse payloads, coordinate co-delivery of multiple payloads, enhance their synergistic effects, and provide immunostimulation. For example, LCP NPs were loaded with 5'triphosphate double-stranded BCL2 siRNA and conjugated to aminoethyl anisamide (AEAA) [60]. LCP NPs are ideally suited for ppp-dsDNA delivery because the phosphate-rich payload is easily loaded into the phosphate-rich particles [280]. AEAA is the ligand for the sigma-2 receptor that is upregulated in many PCs [281]. Its conjugation to the NP improved localization into tumor cells and tumor-adjacent fibroblasts in a KPC mouse model. This is the ideal location for ppp-BCL2 dsRNA delivery because it simultaneously acts as a RIG-I agonist inducing pro-CTL Th1-skewing (e.g., IFNα/β) cytokines and silences the anti-apoptotic BCL2 gene, making the tumor more vulnerable to the immune response.
Another nano-system combines the activity of the small molecule IDO1 inhibitor indoximod (IND) with OX-loaded NPs [58]. IND can help reverse the immunosuppressive TME by maintaining local tryptophan levels [282], but the drug has poor retention in the TME when delivered orally [283]. To overcome this, a phospholipid group was added to IND, creating a prodrug that self assembles into spherical nanovesicles in an aqueous solution. This lipid bilayer was stabilized with MSNs, which are ideal for the release of loaded OX, an inducer of ICD associated with KPC cells [58]. This approach not only improved the pharmacokinetic stability and tumor penetrance of both drugs, but co-delivery produced a synergistic improvement in the TME CD8+/Foxp3+ ratio, DC intercalation, and tumor control in a KPC mouse model.
4.6. Overcoming immune exclusion of CTLs
While the generation of tumor-specific CTLs is an important step in anti-cancer immunotherapy, the effectiveness of the immune response is limited by access to the tumor itself. As mentioned in Section 2, a hallmark of PDAC is the presence of extremely desmoplastic stroma, making most pancreatic cancers immune-excluding tumors or “cold tumors” and shielding them from CTL activity. However, depletion of the stroma alone has proven detrimental in some PDAC models, allowing the escape of a less differentiated, more aggressive tumor phenotype and the infiltration of undesirable regulatory B and T cells [33, 284]. One strategy to address this issue is to improve CTL infiltration without disrupting the fibrous component of the desmoplastic stroma by normalizing intratumoral vasculature. To this end, cyclopamine (CPA), an SHH pathway inhibitor, and PTX, a chemotherapeutic agent, were encapsulated into biodegradable polymeric micelles [285]. SHH activates CAFs and contributes to the development of desmoplastic stroma, but excessive ablation leads to increased metastasis [33]. However, small doses of CPA delivered by the micelles increased intratumoral vascularization without reducing collagen content and further controlled tumor growth with localized PTX delivery. This led to increased CTL infiltration, slowed tumor progression, and increased sensitivity to anti-PD-1 in murine PDAC models without the systemic toxicity associated with PTX [53, 285].
Another approach is to disrupt the fibrous tissue and tumor growth simultaneously to prevent increased metastasis. This has been accomplished in an animal model using a cholesterol-modified CXCR4 antagonist (PCX) that self-assembles into NPs. These particles have been shown to limit tumor invasiveness by blocking CXCL12/CXCR4 signaling and can be simultaneously used as a vector for transfection [286]. In one study, PCX NPs were used to transfect tumor cells with a siRNA against NCOA3, a key regulator of PDAC pathology (e.g., it regulates mucin, enhances inflammation, and promotes tumor growth) [287]. In another study, PCX was used to encapsulate two RNA therapeutics: anti-miR-210 and siKRASG12D [288]. miR-210 is a hypoxia-induced miRNA important in the induction and activity of activated pancreatic stellate cells (PSCs) [289], while KRAS mutations are central drivers of most PDACs [290, 291]. In both studies, PCX NPs increased perfusion of the tumor without detrimental effects, reducing both primary tumor size and metastatic events.
While some models of stroma-only targeting have reduced PDAC survival, a multi-faceted “nano-sapper” strategy utilizing a CaP liposome carrier has been shown to co-deliver phosphor-alpha-mangostin (PM), a prodrug that reduces liver fibrosis [292], and the pleiotropic inflammatory cytokine LIGHT [293, 294] delivered via plasmid vector [67]. The CaP nanocarrier was decorated with an FHK peptide to target the liposome against tenascin-c expressing PSC surrounding the tumor [295, 296]. While this regimen did not target tumor cells directly, it effectively attenuated the physical barrier to allow increased CD4+ and CD8+ T cell infiltration. This treatment also reduced overall tumor infiltration of regulatory immune cells and inhibited tumor progression in murine PDAC models.
Other approaches in PC immunotherapy have focused on SLNs, and hybrid MSNs. SLNs facilitate a more precise release of the immune reagents, mitigate off-target CTL responses, and effectively harness the humoral and cellular immune responses against cancer cells [134]. Stimuvax (i.e., MUC1-specific), Tecemotide (i.e., MUC1-specific), and sHER2+AS15 are notable examples of liposome-based cancer nanovaccines that have progressed through phase-II/III clinical trials to treat melanoma and NSCLC, breast cancer, and PDAC, respectively. Recently, various immunomodulator or agonist-loaded biodegradable MSNs have been studied for cancer immunotherapy [297, 298]. In one study, MSNs entrapping Ca/Mg/Zn was used as a biodegradable adjuvant to stimulate Th1-skwed immune responses to ovalbumin (OVA) and protect against E.G7-OVA lymphoma [298]. Researchers have also used PD-1-based tumor targeting in concordance with inhibition of TGF-β pathways and showed improved survival in cancer-bearing mice [299]. Additionally, using a pilot ex vivo study, T cells were loaded with SPION in order to facilitate T cell accumulation in the tumor using an external magnetic field [296].
4.7. Nanoparticulate systems to promote immune checkpoint therapy for PDAC
Despite the growing number of PDAC clinical trials involving immune checkpoint inhibitors (ICI) [300], pembrolizumab is the only FDA-approved anti-PD1 inhibitor for a fraction of PDAC patients with repair-deficient mismatch and instability-high microsatellite [301]. The application of ICI was hindered by the non-inflamed nature of the pancreatic tumor and therefore resulted in a low frequency of existing tumor-specific T cells, which was further complicated by the immunosuppressive TME. ICI can also require repeated and high doses, which can lead to immunotoxicity [302] and other adverse events [303]. Even combination with chemotherapy or other types of immunotherapies provided limited improvement. A recently completed phase II trial using GVAX and Listeria-based vaccine reported efficacy with nivolumab (anti-PD1) on patients with metastatic PDAC [304]. The nivolumab (3 mg/kg) was administrated intravenously every 3 weeks for 6 cycles. Albeit promising CD8+ T cell increases and reduced immunosuppression in patients, the use of nivolumab did not increase overall survival. A higher-grade adverse event rate (≥ 3) was also reported, emphasizing opportunities to optimize ICI delivery and manage immune activation-related toxicity. In this regard, the use of nanocarriers could be a useful approach to enhance ICI. Many efforts have focused on targeting specific cell subsets [305, 306], normalizing TME [307], and increasing tumor immunogenicity [308, 309]. More recently, several studies have described nanocarrier-enhanced ICI immunotherapy against PDAC models in mice [256, 310, 311]. In addition, Yu et al. demonstrated a comprehensive stimuli-responsive NP system to induce hyperthermia with the goal of overcoming tumor barriers and promoting ICI [311]. The anti-PD-L1 molecule was released from the dual-responsive liposome NPs to overexpressed fibroblast activation protein and irradiation. The NPs were shown to accumulate at the orthotopic tumor, increase T cell tumor infiltration, and reduce both primary and metastatic tumors. Additional nano-abled approaches are being studied for promoting ICI in PDAC.
5. Perspectives and outlook
Despite decades of progress in our understanding, PDAC remains one of the most lethal and challenging human malignancies to treat. Although more basic research and clinical studies are needed to develop potent treatments for PDAC, the advent of nanocarrier-based treatments holds immense promise to enhance patient survival rates. Some areas of future research in this area and their associated challenges are provided below.
The pancreatic TME is one of the critical drivers of therapy resistance. The obstructive stroma not only impedes the delivery of chemotherapeutic agents, but in addition various secretory components, particularly the ECM, proteases, and cytokines also orchestrate the development of an immunosuppressive milieu. While stromal targeting was envisioned to revolutionize the landscape of PC therapy, the early enthusiasm was tempered by the failure of clinical trials targeting pro-fibrogenic pathways, particularly SHH using pharmacological agents [295, 312, 313] and enzymatic degradation of ECM using PEGylated hyaluronidase [Pegvorhyaluronidase alfa (PEGPH20)] [314, 315]. However, the focus now has shifted towards stromal modulation rather than depletion (unlike SHH inhibitors and hyaluronidase, which can also lead to altered tumor immune microenvironments). In fact, several anti-stromal therapies have demonstrated an altered immune landscape in tumors, including enhanced T cell infiltration and changes in macrophage polarization, thereby sensitizing the tumor to immune checkpoint blockade agents [316]. Several clinical trials are now examining anti-stromal agents in combination with immune checkpoint blockade agents. Nanomedicine is providing some answers, such as NP-encapsulated SHH inhibitors that provided stromal modulation [285]. Similarly, Losartan, an angiotensin II receptor antagonist, has shown promise in vascular and stromal remodeling and has been demonstrated to enhance the delivery and efficacy of chemotherapy [317, 318]. Variable performance of anti-stromal therapies can be attributed to the heterogeneity and plasticity of stromal cells, particularly CAFs, whose diverse origins and phenotypes are only beginning to be understood. While NP-based therapies are believed to accumulate in the sleeves of the tumor vasculature due to the EPR effect, the dense stroma limits their delivery. Thus, evaluating NPs in conjunction with stromal modulators like Losartan and other agents that enhance perfusion can potentially improve intratumoral delivery.
Advancements in polymer design and chemistry have culminated in the synthesis of versatile polymeric delivery systems for encapsulation of TAAs and immune adjuvants. Specifically, controlled reversible-deactivation radical polymerization mechanisms such as reversible addition-fragmentation chain transfer (RAFT) polymerization and atom transfer radical polymerization have resulted in the production of well-characterized polymers and facilitated the polymerization of multifunctional monomers [319]. For example, RAFT polymerization has been exploited to synthesize versatile amphiphilic copolymers comprising a polycation-rich polymer (dimethyl aminoethyl methacrylate for CpG ODN complexation) with a pyridyl disulfide functional group (for antigen conjugation) and a hydrophobic endosome-lytic component (for endosomal escape), which form self-assembled micelles and enhance antigen cross-presentation by promoting cytosolic delivery. These micelles enable dual delivery of both tumor antigen and immunostimulatory CpG ODN [320]. Additionally, RAFT polymerization has been employed to synthesize mannose- and acetylglucosamine-containing glycopolymer delivery systems capable of targeting C-type lectin receptors, which are expressed on APCs such as DCs and macrophages [321, 322]. Such molecular targeting capabilities would confer an additional level of targeting specificity toward the rational formulation of PDAC immunotherapies.
Nano-enabled strategies can also improve PDAC sensitization to chemotherapy by combining the delivery of chemotherapeutics agents, small molecule drugs, and gene therapy. However, despite this diversity, these strategies rely upon the induction of the patient's natural anti-tumor immune response. While there has been some success with this approach, a potential avenue for improvement is the concomitant induction of a PDAC-specific CTL response. In this regard, combining targeted nano-driven immunotherapy with PDAC stromal penetration and accumulation of chemotherapeutic agents within tumors could be beneficial. The success of such approaches is predicated upon tailoring them for PC patients via genetic screening and characterizing tumors in clinical trials to better understand tumor resistance to chemotherapeutic drugs. Combining nanocarrier treatments or immunotherapies with ICI is a highly promising approach for PDAC that can abrogate the immunosuppressive TME, enhance tumor immunogenicity, and extend patient survival times. We anticipate an increase in the number of clinical studies that will pursue such strategies.
The development of multifunctional NPs with diverse and complementary functionality can be used to enhance the efficacy of PDAC treatments. Multifunctional NPs can accommodate synergies in terms of co-delivery of diverse payloads (e.g., therapeutics, immune-stimulatory molecules, and TAAs), targeting capabilities, and theranostic functionality. If successful, these multifunctional NPs can provide dose-sparing, reduce cost, and lower the toxicity of PDAC therapeutics. Such versatile approaches can also be used to treat metastasized tumors leading to a “systems approach” that anticipates adverse events and broadens therapy options.
Therapeutic strategies using EVs and exosomes have progressed rapidly in recent years [323]. Exosomes are being envisioned as an alternative natural delivery system for targeted therapeutics. Additionally, the introduction of exosome delivery, as well as molecular and nanotechnological advances in precision medicine, is enabling the scientific community to develop improved treatment options [324]. This is exemplified by recent efforts to utilize reassembled pancreatic tumor cell-derived exosomes for delivering photosensitizer to tumors for photoacoustic imaging-guided photodynamic and immunotherapy [325]. Despite these advancements, several challenges exist with respect to the large-scale production and purification of EVs and particularly exosomes. While MSC-derived exosomes have been used in preclinical and clinical studies, their interactions with the components of the immune system remain poorly understood. Other hurdles include the need for a gold-standard method to isolate and precisely identify exosomes and an ideal low-cost strategy with high reproducibility and efficient exosome purification. Furthermore, most exosome engineering applications targeting PDAC treatment are largely limited to pre-clinical studies. Advancing research to overcome these shortcomings could pave the way to novel therapies in PDAC and other cancers.
Nanocarrier-based approaches have been predominantly employed to deliver miRNA, siRNA, and plasmids for gene silencing and expression. These approaches have their own limitations. While the gene-silencing effects of siRNA are short-lived in vivo, miRNAs regulate multiple targets that can lead to off-target effects. CRISPR-Cas9 based approaches have emerged as highly selective and effective gene-expression manipulation platforms. Recently nanocarrier-based delivery of CRISPR/Cas9 system has been used for manipulation of tumor microenvironment for modulation of response to immunotherapy in conjunction with chemotherapy and photodynamic therapy in melanoma [326, 327]. It will be of interest to evaluate similar systems for the modulation of pancreatic TME and augment response to chemotherapy and immunotherapy.
PDAC is immunologically cold due to the lack of neoantigens and other immunosuppressive mechanisms that operate in the TME. Recently, it has emerged that it is not the neoantigen quantity but quality that dictates the effectiveness of immune response and patient outcome [262]. The advent of personalized medicine and advances in computational and data analytics has encouraged greater levels of genomic, transcriptomic, and epigenomic profiling, TCR sequencing, and neoantigen profiling in conjunction with mutational loads. Integration of such data emanating from preclinical and clinical studies can help identify meaningful antigens that may not be abundant but are effective, and the nature of immune responses likely to be elicited by these antigens can be predicted. Modeling these aspects in preclinical studies can pay rich dividends not only in developing effective vaccines but also in monitoring/characterizing immune responses more dynamically in clinical trials. Informatics methods can also help determine the compatibility of a given neoantigen with nanocarrier platforms, leading to rational design approaches.
The field of nanocarrier-based therapies has dramatically expanded over the past several years. However, only a few anticancer nanomedicines, including drug-antibody conjugates, have thus far made it to the clinic. In total, there are nearly 250 clinical trials in the United States evaluating the potential clinical benefit of NP-based formulations. Most of these clinical studies are performed as combination therapies, while only a few studies are focused on the use of NP-based formulations as monotherapies. Advancing NP-based delivery systems from the laboratory bench to the bedside requires addressing challenges. One of these challenges is that results generated from in vivo efficacy and safety studies typically performed in animal models do not necessarily reflect equivalent outcomes in humans since the in vivo fate of a tested nanomedicine or nanovaccine and its interaction with blood components can be highly variable [328, 329]. Another limitation is the difficulty that can be encountered in attempting to scale up the production of complex nanocarrier systems, which would be necessary for translation to the clinic. A further challenge that may impact the progress of nanomedicines to the clinic is that there are few contract facilities that have the capacity to reproducibly synthesize complex nanomedicines under cGMP conditions for use in clinical trials. The traditional approach of developing and exploring new anti-cancer nanomedicines involves varying certain parameters such as size and surface charge or chemistry. New strategies such as microfluidics or nanofluidics can help generate large libraries, which can facilitate the systematic screening of multiple parameters to maximize opportunities for the rational design of anti-cancer nano-formulations. Such approaches can enhance the success rate in terms of clinical performance and high-impact care for PC patients.
Abbreviations
APC: antigen-presenting cells; ATF: amino-terminal fragment; AuNPs: gold nanoparticles; BBB: blood-brain barrier; CAF: cancer-associated fibroblast; CAR-T cell: chimeric antigen receptor T cell; DEX: dexamethasone; DOX: doxorubicin; DSPE: 1,2-distearoyl-sn-glycerol-3 phosphoethanolamine-N-amino; DTX: docetaxel; ECM: extracellular matrix; EMA: european medicine agency; EMT: epithelial to mesenchymal transition; FDA: food and drug administration; FOLFIRINOX: folinic acid, 5-fluorouracil, irinotecan, and oxaliplatin; GEM: gemcitabine; IFP: interstitial fluid pressure; KRAS: kirsten rat sarcoma viral oncogene homolog; mCRPC: metastatic-castration resistance prostate cancer; MnMEIO: manganese-doped magnetism-engineered iron oxide; MRI: magnetic resonance imaging; MSC: mesenchymal stem cells; MSN: mesoporous silica nanoparticle; MTAI: microwave induced thermoacoustic imaging; Nab-PTX: nano-albumin bound-paclitaxel; NIRF: near-infrared fluorescence imaging; NK cell: natural killer cell; NP: nanoparticle; NSCLC: non-small cell lung cancer; OX: oxaliplatin; PC: pancreatic cancer; PD-1: programmed cell death receptor-1; PDAC: pancreatic ductal adenocarcinoma; PDEAEM: poly(diethylaminoethylmethacrylate); PDT: photodynamic therapy; PEG: polyethylene glycol; PGA: poly(L-glutamic acid); PLA: polylactic acid; PLGH: poly (DL-lactide-co-glycolide); PTT: photothermal therapy; RIG-1: retinoic acid-inducible gene-1; SHH: sonic hedgehog; SPION: superparamagnetic iron oxide nanoparticles; TAM: tumor-associated macrophage; TME: tumor microenvironment; t-PA: tissue plasminogen activator derived peptides; uPAR: urokinase plasminogen activator receptor.
Acknowledgements
The authors acknowledge financial support from multiple NIH-NCI grants (U01 CA213862, P01 CA217798, R01 CA195586, R01 CA247471, R01 CA247763, R21 CA223429, P30 CA036727, U54 GM115458, and P30 CA086862). The authors also acknowledge support from the Iowa State University Nanovaccine Institute. B.N is grateful to the Vlasta Klima Balloun Faculty Chair, and S.K.M. is grateful to the Carol Vohs Johnson Chair. S.K.B thanks the Dr. Alfred and Linda Hartmann Chair.
Author Contributions
MJ, BN, and AKS conceived the idea and designed the framework of the review article. LL, PGK, SKG, and MN contributed equally to this manuscript, LL, PGK, SKG, MN, and EIW completed the manuscript, tables, and designing of the figures with the help of JCC and BW. PK and MJ have done the final formatting with the references and figures. SKM, MJW, SK, JCS, and SKB critically reviewed the manuscript and provided feedback.
Competing Interests
Balaji Narasimhan and Michael J. Wannemuehler are co-founders of ImmunoNanoMed Inc., a start-up with business interests in the development of nano-based vaccines against infectious diseases. Narasimhan also has a financial interest in Degimflex LLC. Surya K. Mallapragada is a co-founder of Degimflex LLC., a start-up with business interests in the development of flexible degradable electronic films for biomedical applications. She also has a financial interest in ImmunoNanoMed Inc. Surinder K. Batra is a co-founder of Sanguine Diagnostics and Therapeutics Inc, a company focused on developing mucin-based diagnostic and therapeutic strategies for human cancers. The other authors disclosed no potential conflicts of interest.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7-33
2. Clancy TE. Surgery for pancreatic cancer. Hematol Oncol Clin North Am. 2015;29:701-16
3. Strobel O, Neoptolemos J, Jäger D, Büchler MW. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol. 2019;16:11-26
4. Singhi AD, Koay EJ, Chari ST, Maitra A. Early detection of pancreatic cancer: Opportunities and challenges. Gastroenterology. 2019;156:2024-40
5. Zeng S, Pöttler M, Lan B, Grützmann R, Pilarsky C, Yang H. Chemoresistance in pancreatic cancer. Int J Mol Sci. 2019;20:4505
6. Zhu H, Li T, Du Y, Li M. Pancreatic cancer: Challenges and opportunities. BMC Med. 2018;16:214
7. Balachandran VP, Beatty GL, Dougan SK. Broadening the impact of immunotherapy to pancreatic cancer: Challenges and opportunities. Gastroenterology. 2019;156:2056-72
8. Looi CK, Chung FF, Leong CO, Wong SF, Rosli R, Mai CW. Therapeutic challenges and current immunomodulatory strategies in targeting the immunosuppressive pancreatic tumor microenvironment. J Exp Clin Cancer Res. 2019;38:162
9. Cannon A, Thompson C, Hall BR, Jain M, Kumar S, Batra SK. Desmoplasia in pancreatic ductal adenocarcinoma: Insight into pathological function and therapeutic potential. Genes Cancer. 2018;9:78-86
10. Banerjee K, Kumar S, Ross KA, Gautam S, Poelaert B, Nasser MW. et al. Emerging trends in the immunotherapy of pancreatic cancer. Cancer Lett. 2018;417:35-46
11. Ligorio M, Sil S, Malagon-Lopez J, Nieman LT, Misale S, Di Pilato M. et al. Stromal microenvironment shapes the intratumoral architecture of pancreatic cancer. Cell. 2019;178:160-75
12. Neesse A, Bauer CA, Öhlund D, Lauth M, Buchholz M, Michl P. et al. Stromal biology and therapy in pancreatic cancer: Ready for clinical translation? Gut. 2019;68:159-71
13. Vennin C, Murphy KJ, Morton JP, Cox TR, Pajic M, Timpson P. Reshaping the tumor stroma for treatment of pancreatic cancer. Gastroenterology. 2018;154:820-38
14. Nia HT, Munn LL, Jain RK. Mapping physical tumor microenvironment and drug delivery. Clin Cancer Res. 2019;25:2024-26
15. Yu Q, Qiu Y, Li J, Tang X, Wang X, Cun X. et al. Targeting cancer-associated fibroblasts by dual-responsive lipid-albumin nanoparticles to enhance drug perfusion for pancreatic tumor therapy. J Control Release. 2020;321:564-75
16. Wang J, Chan DKW, Sen A, Ma WW, Straubinger RM. Tumor priming by SMO inhibition enhances antibody delivery and efficacy in a pancreatic ductal adenocarcinoma model. Mol Cancer Ther. 2019;18:2074-84
17. Nagathihalli NS, Castellanos JA, Shi C, Beesetty Y, Reyzer ML, Caprioli R. et al. Signal transducer and activator of Transcription 3, mediated remodeling of the tumor microenvironment results in enhanced tumor drug delivery in a mouse model of pancreatic cancer. Gastroenterology. 2015;149:1932-43
18. He X, Chen X, Liu L, Zhang Y, Lu Y, Zhang Y. et al. Sequentially triggered nanoparticles with tumor penetration and intelligent drug release for pancreatic cancer therapy. Adv Sci (Weinh). 2018;5:1701070
19. Liu X, Jiang J, Meng H. Transcytosis - an effective targeting strategy that is complementary to "EPR effect" for pancreatic cancer nano drug delivery. Theranostics. 2019;9:8018-25
20. Ji T, Li S, Zhang Y, Lang J, Ding Y, Zhao X. et al. An MMP-2 responsive liposome integrating antifibrosis and chemotherapeutic drugs for enhanced drug perfusion and efficacy in pancreatic cancer. ACS Appl Mater Interfaces. 2016;8:3438-45
21. Han X, Li Y, Xu Y, Zhao X, Zhang Y, Yang X. et al. Reversal of pancreatic desmoplasia by re-educating stellate cells with a tumour microenvironment-activated nanosystem. Nat Commun. 2018;9:3390
22. Dougan SK. The pancreatic cancer microenvironment. Cancer J. 2017;23:321-5
23. Cros J, Raffenne J, Couvelard A, Poté N. Tumor heterogeneity in pancreatic adenocarcinoma. Pathobiology. 2018;85:64-71
24. Cheng ZX, Wang DW, Liu T, Liu WX, Xia WB, Xu J. et al. Effects of the HIF-1α and NF-κB loop on epithelial-mesenchymal transition and chemoresistance induced by hypoxia in pancreatic cancer cells. Oncol Rep. 2014;31:1891-8
25. Dauer P, Nomura A, Saluja A, Banerjee S. Microenvironment in determining chemo-resistance in pancreatic cancer: Neighborhood matters. Pancreatology. 2017;17:7-12
26. Shukla SK, Purohit V, Mehla K, Gunda V, Chaika NV, Vernucci E. et al. MUC1 and HIF-1alpha signaling crosstalk induces anabolic glucose metabolism to impart Gemcitabine resistance to pancreatic cancer. Cancer Cell. 2017;32:71-87
27. Zhang Z, Han H, Rong Y, Zhu K, Zhu Z, Tang Z. et al. Hypoxia potentiates Gemcitabine-induced stemness in pancreatic cancer cells through AKT/NOTCH1 signaling. J Exp Clin Cancer Res. 2018;37:291
28. Lee J, Lee J-C, Hwang J-H, Kim J, Kim H, Cho I-K. Higher tumor cellularity is a negative prognostic indicator in resected pancreatic ductal adenocarcinoma. Ann Oncol. 2017;28:III71
29. Heid I, Steiger K, Trajkovic-Arsic M, Settles M, Eßwein MR, Erkan M. et al. Co-clinical assessment of tumor cellularity in pancreatic cancer. Clin Cancer Res. 2017;23:1461-70
30. Helms E, Onate MK, Sherman MH. Fibroblast heterogeneity in the pancreatic tumor microenvironment. Cancer Discov. 2020;10:648
31. Pereira BA, Vennin C, Papanicolaou M, Chambers CR, Herrmann D, Morton JP. et al. CAF subpopulations: A new reservoir of stromal targets in pancreatic cancer. Trends Cancer. 2019;5:724-41
32. Von Ahrens D, Bhagat TD, Nagrath D, Maitra A, Verma A. The role of stromal cancer-associated fibroblasts in pancreatic cancer. J Hematol Oncol. 2017;10:76
33. Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA. et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735-47
34. Chen X, Song E. Turning foes to friends: Targeting cancer-associated fibroblasts. Nat Rev Drug Discov. 2019;18:99-115
35. Elyada E, Bolisetty M, Laise P, Flynn WF, Courtois ET, Burkhart RA. et al. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 2019;9:1102
36. Gascard P, Tlsty TD. Carcinoma-associated fibroblasts: Orchestrating the composition of malignancy. Genes Dev. 2016;30:1002-19
37. Bolm L, Cigolla S, Wittel UA, Hopt UT, Keck T, Rades D. et al. The role of fibroblasts in pancreatic cancer: Extracellular matrix versus paracrine factors. Transl Oncol. 2017;10:578-88
38. D'Arcangelo E, Wu NC, Cadavid JL, McGuigan AP. The life cycle of cancer-associated fibroblasts within the tumour stroma and its importance in disease outcome. Br J Cancer. 2020;122:931-42
39. Wartenberg M, Cibin S, Zlobec I, Vassella E, Eppenberger-Castori S, Terracciano L. et al. Integrated genomic and immunophenotypic classification of pancreatic cancer reveals three distinct subtypes with prognostic/predictive significance. Clin Cancer Res. 2018;24:4444-54
40. Li K-Y, Yuan J-L, Trafton D, Wang J-X, Niu N, Yuan C-H. et al. Pancreatic ductal adenocarcinoma immune microenvironment and immunotherapy prospects. Chronic Dis Transl Med. 2020;6:6-17
41. Parayath N, Padmakumar S, Nair SV, Menon D, Amiji MM. Strategies for targeting cancer immunotherapy through modulation of the tumor microenvironment. Regen Eng Transl Med. 2020;6:29-49
42. Xu C, Sui S, Shang Y, Yu Z, Han J, Zhang G. et al. The landscape of immune cell infiltration and its clinical implications of pancreatic ductal adenocarcinoma. J Adv Res. 2020;24:139-48
43. Karamitopoulou E. Tumour microenvironment of pancreatic cancer: Immune landscape is dictated by molecular and histopathological features. Br J Cancer. 2019;121:5-14
44. Da Silva A, Bowden M, Zhang S, Masugi Y, Thorner AR, Herbert ZT. et al. Characterization of the neuroendocrine tumor immune microenvironment. Pancreas. 2018;47:1123-29
45. Li J, Byrne KT, Yan F, Yamazoe T, Chen Z, Baslan T. et al. Tumor cell-intrinsic factors underlie heterogeneity of immune cell infiltration and response to immunotherapy. Immunity. 2018;49:178-93.e7
46. Wu AA, Drake V, Huang H-S, Chiu S, Zheng L. Reprogramming the tumor microenvironment: Tumor-induced immunosuppressive factors paralyze T cells. Oncoimmunology. 2015;4:e1016700
47. Sowell RT, Kaech SM. Probing the diversity of T cell dysfunction in cancer. Cell. 2016;166:1362-4
48. Anderson KG, Stromnes IM, Greenberg PD. Obstacles posed by the tumor microenvironment toT cell activity: A case for synergistic therapies. Cancer Cell. 2017;31:311-25
49. Blair AB, Kim VM, Muth ST, Saung MT, Lokker N, Blouw B. et al. Dissecting the stromal signaling and regulation of myeloid cells and memory effector T cells in pancreatic cancer. Clin Cancer Res. 2019;25:5351-63
50. Cai L, Michelakos T, Deshpande V, Arora KS, Yamada T, Ting DT. et al. Role of tumor-associated macrophages in the clinical course of pancreatic neuroendocrine tumors (pannets). Clin Cancer Res. 2019;25:2644-55
51. Zhang Y, Velez-Delgado A, Mathew E, Li D, Mendez FM, Flannagan K. et al. Myeloid cells are required for PD-1/PD-L1 checkpoint activation and the establishment of an immunosuppressive environment in pancreatic cancer. Gut. 2017;66:124-36
52. Felix K, Gaida MM. Neutrophil-derived proteases in the microenvironment of pancreatic cancer -active players in tumor progression. Int J Biol Sci. 2016;12:302-13
53. Zhao J, Xiao Z, Li T, Chen H, Yuan Y, Wang YA. et al. Stromal modulation reverses primary resistance to immune checkpoint blockade in pancreatic cancer. ACS Nano. 2018;12:9881-93
54. Tjomsland V, Niklasson L, Sandström P, Borch K, Druid H, Bratthäll C. et al. The desmoplastic stroma plays an essential role in the accumulation and modulation of infiltrated immune cells in pancreatic adenocarcinoma. Clin Dev Immunol. 2011;2011:212810
55. Sano M, Ijichi H, Takahashi R, Miyabayashi K, Fujiwara H, Yamada T. et al. Blocking CXCLs-CXCR2 axis in tumor-stromal interactions contributes to survival in a mouse model of pancreatic ductal adenocarcinoma through reduced cell invasion/migration and a shift of immune-inflammatory microenvironment. Oncogenesis. 2019;8:8
56. Elahi-Gedwillo KY, Carlson M, Zettervall J, Provenzano PP. Antifibrotic therapy disrupts stromal barriers and modulates the immune landscape in pancreatic ductal adenocarcinoma. Cancer Res. 2019;79:372-86
57. Liu Y, Guo J, Huang L. Modulation of tumor microenvironment for immunotherapy: Focus on nanomaterial-based strategies. Theranostics. 2020;10:3099-117
58. Lu J, Liu X, Liao YP, Salazar F, Sun B, Jiang W. et al. Nano-enabled pancreas cancer immunotherapy using immunogenic cell death and reversing immunosuppression. Nat Commun. 2017;8:1-14
59. Suto A, Kudo D, Yoshida E, Nagase H, Suto S, Mimura J. et al. Increase of tumor infiltrating γδ T-cells in pancreatic ductal adenocarcinoma through remodeling of the extracellular matrix by a hyaluronan synthesis suppressor, 4-Methylumbelliferone. Pancreas. 2019;48:292-8
60. Das M, Shen L, Liu Q, Goodwin TJ, Huang L. Nanoparticle delivery of RIG-I agonist enables effective and safe adjuvant therapy in pancreatic cancer. Mol Ther. 2019;27:507-17
61. Hu M, Wang Y, Xu L, An S, Tang Y, Zhou X. et al. Relaxin gene delivery mitigates liver metastasis and synergizes with check point therapy. Nat Commun. 2019;10:2993
62. Shen L, Li J, Liu Q, Song W, Zhang X, Tiruthani K. et al. Local blockade of Interleukin 10 and C-X-C motif chemokine ligand 12 with nano-delivery promotes antitumor response in murine cancers. ACS Nano. 2018;12:9830-41
63. Huang H, Jiang CT, Shen S, Liu A, Gan YJ, Tong QS. et al. Nanoenabled reversal of IDO1-mediated immunosuppression synergizes with immunogenic chemotherapy for improved cancer therapy. Nano Lett. 2019;19:5356-65
64. Xie W, Deng WW, Zan M, Rao L, Yu GT, Zhu DM. et al. Cancer cell membrane camouflaged nanoparticles to realize starvation therapy together with checkpoint blockades for enhancing cancer therapy. ACS Nano. 2019;13:2849-57
65. Sun J, Wan Z, Chen Y, Xu J, Luo Z, Parise RA. et al. Triple drugs co-delivered by a small Gemcitabine-based carrier for pancreatic cancer immunochemotherapy. Acta Biomater. 2020;106:289-300
66. Liu XJ, Li L, Liu XJ, Li Y, Zhao CY, Wang RQ. et al. Mithramycin-loaded mPEG-PLGA nanoparticles exert potent antitumor efficacy against pancreatic carcinoma. Int J Nanomedicine. 2017;12:5255-69
67. Huang Y, Chen Y, Zhou S, Chen L, Wang J, Pei Y. et al. Dual-mechanism based CTLs infiltration enhancement initiated by nano-sapper potentiates immunotherapy against immune-excluded tumors. Nat Commun. 2020;11:622
68. Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: The beginning of the end of cancer? BMC Med. 2016;14:73
69. Ventola CL. Cancer immunotherapy, part 3: Challenges and future trends. P T. 2017;42:514-21
70. Ventola CL. Cancer immunotherapy, part 2: Efficacy, safety, and other clinical considerations. P T. 2017;42:452-63
71. Cao J, Huang D, Peppas NA. Advanced engineered nanoparticulate platforms to address key biological barriers for delivering chemotherapeutic agents to target sites. Adv Drug Deliv Rev. 2020;167:170-188
72. Saeed M, Gao J, Shi Y, Lammers T, Yu H. Engineering nanoparticles to reprogram the tumor immune microenvironment for improved cancer immunotherapy. Theranostics. 2019;9:7981-8000
73. Meng H, Nel AE. Use of nano engineered approaches to overcome the stromal barrier in pancreatic cancer. Adv Drug Deliv Rev. 2018;130:50-57
74. Tong Q-S, Miao W-M, Huang H, Luo J-Q, Liu R, Huang Y-C. et al. A tumor-penetrating nanomedicine improves the chemoimmunotherapy of pancreatic cancer. Small. 2021;17:e2101208
75. Irvine DJ, Dane EL. Enhancing cancer immunotherapy with nanomedicine. Nat Rev Immunol. 2020;20:321-34
76. Park W, Heo Y-J, Han DK. New opportunities for nanoparticles in cancer immunotherapy. Biomat Res. 2018;22:24
77. Kopeček J, Yang J. Polymer nanomedicines. Adv Drug Deliv Rev. 2020;156:40-64
78. Greish K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol Biol. 2010;624:25-37
79. Cooper DL, Conder CM, Harirforoosh S. Nanoparticles in drug delivery: Mechanism of action, formulation and clinical application towards reduction in drug-associated nephrotoxicity. Expert Opin Drug Deliv. 2014;11:1661-80
80. George A, Shah PA, Shrivastav PS. Natural biodegradable polymers based nano-formulations for drug delivery: A review. Int J Pharm. 2019;561:244-64
81. Banerjee K, Gautam SK, Kshirsagar P, Ross KA, Spagnol G, Sorgen P. et al. Amphiphilic polyanhydride-based recombinant MUC4β-nanovaccine activates dendritic cells. Genes Cancer. 2019;10:52-62
82. Joshi VB, Geary SM, Carrillo-Conde BR, Narasimhan B, Salem AK. Characterizing the antitumor response in mice treated with antigen-loaded polyanhydride microparticles. Acta Biomaterialia. 2013;9:5583-89
83. Wafa EI, Geary SM, Goodman JT, Narasimhan B, Salem AK. The effect of polyanhydride chemistry in particle-based cancer vaccines on the magnitude of the anti-tumor immune response. Acta Biomater. 2017;50:417-27
84. Liu L, Kshirsagar P, Christiansen J, Gautam SK, Aithal A, Gulati M. et al. Polyanhydride nanoparticles stabilize pancreatic cancer antigen MUC4β. J Biomed Mater Res A. 2021;109:893-902
85. Knop K, Hoogenboom R, Fischer D, Schubert US. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew Chem Int Ed Engl. 2010;49:6288-308
86. Moustaoui H, Movia D, Dupont N, Bouchemal N, Casale S, Djaker N. et al. Tunable design of gold(III)-Doxorubicin complex-PEGylated nanocarrier. The golden Doxorubicin for ontological applications. ACS Appl Mater Interfaces. 2016;8:19946-57
87. Spadavecchia J, Movia D, Moore C, Maguire CM, Moustaoui H, Casale S. et al. Targeted polyethylene glycol gold nanoparticles for the treatment of pancreatic cancer: From synthesis to proof-of-concept in vitro studies. Int J Nanomedicine. 2016;11:791-822
88. Wu ST, Fowler AJ, Garmon CB, Fessler AB, Ogle JD, Grover KR. et al. Treatment of pancreatic ductal adenocarcinoma with tumor antigen specific-targeted delivery of Paclitaxel loaded PLGA nanoparticles. BMC Cancer. 2018;18:457
89. Zhao X, Li F, Li Y, Wang H, Ren H, Chen J. et al. Co-delivery of HIF1α siRNA and Gemcitabine via biocompatible lipid-polymer hybrid nanoparticles for effective treatment of pancreatic cancer. Biomaterials. 2015;46:13-25
90. Joshi VB, Geary SM, Salem AK. Biodegradable particles as vaccine delivery systems: Size matters. AAPS J. 2013;15:85-94
91. Makadia HK, Siegel SJ. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers (Basel). 2011;3:1377-97
92. Narasimhan B, Goodman JT, Ramirez JEV. Rational design of targeted next-generation carriers for drug and vaccine delivery. Annu Rev Biomed Eng. 2016;18:25-49
93. Mullis AS, Broderick SR, Binnebose AM, Peroutka-Bigus N, Bellaire BH, Rajan K. et al. Data analytics approach for rational design of nanomedicines with programmable drug release. Mol Pharm. 2019;16:1917-28
94. Lueth P, Haughney SL, Binnebose AM, Mullis AS, Peroutka-Bigus N, Narasimhan B. et al. Nanotherapeutic provides dose sparing and improved antimicrobial activity against Brucella melitensis infections. J Control Release. 2019;294:288-97
95. Brenza TM, Schlichtmann BW, Bhargavan B, Vela Ramirez JE, Nelson RD, Panthani MG. et al. Biodegradable polyanhydride-based nanomedicines for blood to brain drug delivery. J Biomed Mater Res A. 2018;106:2881-90
96. Brenza TM, Ghaisas S, Ramirez JEV, Harischandra D, Anantharam V, Kalyanaraman B. et al. Neuronal protection against oxidative insult by polyanhydride nanoparticle-based mitochondria-targeted antioxidant therapy. Nanomedicine. 2017;13:809-20
97. Phanse Y, Lueth P, Ramer-Tait AE, Carrillo-Conde BR, Wannemuehler MJ, Narasimhan B. et al. Cellular internalization mechanisms of polyanhydride particles: Implications for rational design of drug delivery vehicles. J Biomed Nanotechnol. 2016;12:1544-52
98. Binnebose AM, Haughney SL, Martin R, Imerman PM, Narasimhan B, Bellaire BH. Polyanhydride nanoparticle delivery platform dramatically enhances killing of filarial worms. PLoS Negl Trop Dis. 2015;9:1-18
99. Ulery BD, Kan HM, Williams BA, Narasimhan B, Lo KWH, Nair LS. et al. Facile fabrication of polyanhydride/anesthetic nanoparticles with tunable release kinetics. Adv Healthc Mater. 2014;3:843-47
100. Petersen LK, Determan AS, Westgate C, Bendickson L, Nilsen-Hamilton M, Narasimhan B. Lipocalin-2-loaded amphiphilic polyanhydride microparticles accelerate cell migration. J Biomater Sci Polym Ed. 2011;22:1237-52
101. Kipper MJ, Shen E, Determan A, Narasimhan B. Design of an injectable system based on bioerodible polyanhydride microspheres for sustained drug delivery. Biomaterials. 2002;23:4405-12
102. Shen E, Kipper MJ, Dziadul B, Lim MK, Narasimhan B. Mechanistic relationships between polymer microstructure and drug release kinetics in bioerodible polyanhydrides. J Control Release. 2002;82:115-25
103. Jia F, Liu X, Li L, Mallapragada S, Narasimhan B, Wang Q. Multifunctional nanoparticles for targeted delivery of immune activating and cancer therapeutic agents. J Control Release. 2013;172:1020-34
104. Mullis AS, Peroutka-Bigus N, Phadke KS, Bellaire BH, Narasimhan B. Nanomedicines to counter microbial barriers and antimicrobial resistance. Curr Opin Chem Eng. 2021;31:100672
105. Mullis AS, Schlichtmann BW, Narasimhan B, Cademartiri R, Mallapragada SK. Ligand-cascading nano-delivery devices to enable multiscale targeting of anti-neurodegenerative therapeutics. Biomed Mater. 2018;13:034102
106. Ross KA, Brenza TM, Binnebose AM, Phanse Y, Kanthasamy AG, Gendelman HE. et al. Nano-enabled delivery of diverse payloads across complex biological barriers. J Control Release. 2015;219:548-59
107. Schlichtmann BW, Hepker M, Palanisamy BN, John M, Anantharam V, Kanthasamy AG. et al. Nanotechnology-mediated therapeutic strategies against synucleinopathies in neurodegenerative disease. Curr Opin Chem Eng. 2021;31:100673
108. Wen Y, Wang Y, Liu X, Zhang W, Xiong X, Han Z. et al. Camptothecin-based nanodrug delivery systems. Cancer Biol Med. 2017;14:363-70
109. Kumar V, Mundra V, Peng Y, Wang YZ, Tan C, Mahato RI. Pharmacokinetics and biodistribution of polymeric micelles containing miRNA and small-molecule drug in orthotopic pancreatic tumor-bearing mice. Theranostics. 2018;8:4033-49
110. Sabbatini P, Aghajanian C, Dizon D, Anderson S, Dupont J, Brown JV. et al. Phase II study of CT-2103 in patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal carcinoma. J Clin Oncol. 2004;22:4523-31
111. Bahmani B, Uehara M, Ordikhani F, Li XF, Jiang LW, Banouni N. et al. Ectopic high endothelial venules in pancreatic ductal adenocarcinoma: A unique site for targeted delivery. Ebiomedicine. 2018;38:79-88
112. Teo J, McCarroll JA, Boyer C, Youkhana J, Sagnella SM, Duong HTT. et al. A rationally optimized nanoparticle system for the delivery of RNA interference therapeutics into pancreatic tumors in vivo. Biomacromolecules. 2016;17:2337-51
113. Zhao X, Wang X, Sun W, Cheng K, Qin H, Han X. et al. Precision design of nanomedicines to restore Gemcitabine chemosensitivity for personalized pancreatic ductal adenocarcinoma treatment. Biomaterials. 2018;158:44-55
114. Chen Q, Luo L, Xue Y, Han J, Liu Y, Zhang Y. et al. Cisplatin-loaded polymeric complex micelles with a modulated drug/copolymer ratio for improved in vivo performance. Acta Biomaterialia. 2019;92:205-18
115. Liang Y, Deng X, Zhang L, Peng X, Gao W, Cao J. et al. Terminal modification of polymeric micelles with π-conjugated moieties for efficient anticancer drug delivery. Biomaterials. 2015;71:1-10
116. Pittella F, Cabral H, Maeda Y, Mi P, Watanabe S, Takemoto H. et al. Systemic siRNAdelivery to a spontaneous pancreatic tumor model in transgenic mice by PEGylated calcium phosphate hybrid micelles. J Control Release. 2014;178:18-24
117. Zhang M, Zhang W, Tang G, Wang H, Wu M, Yu W. et al. Targeted codelivery of docetaxel and Atg7 siRNA for autophagy inhibition and pancreatic cancer treatment. ACS Appl Bio Mater. 2019;2:1168-76
118. Uchida S, Kinoh H, Ishii T, Matsui A, Tockary TA, Takeda KM. et al. Systemic delivery of messenger RNA for the treatment of pancreatic cancer using polyplex nanomicelles with a cholesterol moiety. Biomaterials. 2016;82:221-28
119. Mittal A, Chitkara D, Behrman SW, Mahato RI. Efficacy of Gemcitabine conjugated and miRNA-205 complexed micelles for treatment of advanced pancreatic cancer. Biomaterials. 2014;35:7077-87
120. Kousalová J, Etrych T. Polymeric nanogels as drug delivery systems. Physiol Res. 2018;67:S305-S317
121. Uz M, Kalaga M, Pothuraju R, Ju J, Junker WM, Batra SK. et al. Dual delivery nanoscale device for miR-345 and Gemcitabine co-delivery to treat pancreatic cancer. J Control Release. 2019;294:237-46
122. Soni KS, Thomas D, Caffrey T, Mehla K, Lei F, O'Connell KA. et al. A polymeric nanogel-based treatment regimen for enhanced efficacy and sequential administration of synergistic drug combination in pancreatic cancer. J Pharmacol Exp Ther. 2019;370:894-901
123. Yin Y, Hu B, Yuan X, Cai L, Gao H, Yang QJP. Nanogel: A versatile nano-delivery system for biomedical applications. Pharmaceutics. 2020;12:290-315
124. Zhang X, Zhao J, Wen Y, Zhu C, Yang J, Yao F. Carboxymethyl chitosan-poly(amidoamine) dendrimer core-shell nanoparticles for intracellular lysozyme delivery. Carbohydr Polym. 2013;98:1326-34
125. Lee CC, Gillies ER, Fox ME, Guillaudeu SJ, Fréchet JM, Dy EE. et al. A single dose of Doxorubicin-functionalized bow-tie dendrimer cures mice bearing C-26 colon carcinomas. Proc Natl Acad Sci U S A. 2006;103:16649-54
126. Bangham A. Liposomes: The babraham connection. Chem Phys Lipids. 1993;64:275-85
127. Yingchoncharoen P, Kalinowski DS, Richardson DR. Lipid-based drug delivery systems in cancer therapy: What is available and what is yet to come. Pharmacol Rev. 2016;68:701-87
128. Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine. 2015;10:975-99
129. Fritz T, Voigt M, Worm M, Negwer I, Müller SS, Kettenbach K. et al. Orthogonal click conjugation to the liposomal surface reveals the stability of the lipid anchorage as crucial for targeting. Chemistry. 2016;22:11578-82
130. Affandi AJ, Grabowska J, Olesek K, Lopez Venegas M, Barbaria A, Rodríguez E. et al. Selective tumor antigen vaccine delivery to human CD169+ antigen-presenting cells using ganglioside-liposomes. Proc Natl Acad Sci U S A. 2020;117:27528-39
131. Aghdam MA, Bagheri R, Mosafer J, Baradaran B, Hashemzaei M, Baghbanzadeh A. et al. Recent advances on thermosensitive and ph-sensitive liposomes employed in controlled release. J Control Release. 2019;315:1-22
132. Wu Y-L, Park K, Soo RA, Sun Y, Tyroller K, Wages D. et al. Inspire: A phase III study of the blp25 liposome vaccine (L-BLP25) in Asian patients with unresectable stage III non-small cell lung cancer. BMC Cancer. 2011;11:1-7
133. Mukherjee S, Ray S, Thakur RS. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J Pharm Sci. 2009;71:349-58
134. Yuba E. Liposome-based immunity-inducing systems for cancer immunotherapy. Mol Immunol. 2018;98:8-12
135. Huyan T, Li H, Peng H, Chen J, Yang R, Zhang W. et al. Extracellular vesicles - advanced nanocarriers in cancer therapy: Progress and achievements. Int J Nanomedicine. 2020;15:6485-502
136. Kosaka N, Kogure A, Yamamoto T, Urabe F, Usuba W, Prieto-Vila M. et al. Exploiting the message from cancer: The diagnostic value of extracellular vesicles for clinical applications. Exp Mol Med. 2019;51:1-9
137. Möller A, Lobb RJ. The evolving translational potential of small extracellular vesicles in cancer. Nat Rev Cancer. 2020;20:697-709
138. Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur Basant K. et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816-26
139. Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M. et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329-35
140. Meng W, He C, Hao Y, Wang L, Li L, Zhu G. Prospects and challenges of extracellular vesicle-based drug delivery system: Considering cell source. Drug Deliv. 2020;27:585-98
141. He C, Zheng S, Luo Y, Wang B. Exosome theranostics: Biology and translational medicine. Theranostics. 2018;8:237-55
142. Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA. et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498-503
143. Pascucci L, Coccè V, Bonomi A, Ami D, Ceccarelli P, Ciusani E. et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: A new approach for drug delivery. J Control Release. 2014;192:262-70
144. Zhou Y, Zhou W, Chen X, Wang Q, Li C, Chen Q. et al. Bone marrow mesenchymal stem cells-derived exosomes for penetrating and targeted chemotherapy of pancreatic cancer. Acta Pharm Sin B. 2020;10:1563-75
145. Zhao Y, Zheng Y, Zhu Y, Zhang Y, Zhu H, Liu T. M1 macrophage-derived exosomes loaded with Gemcitabine and deferasirox against chemoresistant pancreatic cancer. Pharmaceutics. 2021;13:1493
146. Li YJ, Wu JY, Wang JM, Hu XB, Cai JX, Xiang DX. Gemcitabine loaded autologous exosomes for effective and safe chemotherapy of pancreatic cancer. Acta Biomater. 2020;101:519-30
147. Oliveira C, Calmeiro J, Carrascal MA, Falcão A, Gomes C, Miguel Neves B. et al. Exosomes as new therapeutic vectors for pancreatic cancer treatment. Eur J Pharm Biopharm. 2021;161:4-14
148. Giner-Casares JJ, Henriksen-Lacey M, Coronado-Puchau M, Liz-Marzán LM. Inorganic nanoparticles for biomedicine: Where materials scientists meet medical research. Materials Today. 2016;19:19-28
149. Li L, Liu H. Biodegradable inorganic nanoparticles: An opportunity for improved cancer therapy? Nanomedicine. 2017;12:959-61
150. Ravindran Girija A, Balasubramanian S. Theragnostic potentials of core/shell mesoporous silica nanostructures. Nanotheranostics. 2019;3:1-40
151. Zhao S, Yu X, Qian Y, Chen W, Shen J. Multifunctional magnetic iron oxide nanoparticles: An advanced platform for cancer theranostics. Theranostics. 2020;10:6278-309
152. Janko C, Ratschker T, Nguyen K, Zschiesche L, Tietze R, Lyer S. et al. Functionalized superparamagnetic iron oxide nanoparticles (SPIONS) as platform for the targeted multimodal tumor therapy. Front Oncol. 2019;9:59
153. Papasani MR, Wang G, Hill RA. Gold nanoparticles: The importance of physiological principles to devise strategies for targeted drug delivery. Nanomedicine. 2012;8:804-14
154. Anselmo AC, Mitragotri S. Nanoparticles in the clinic: An update. Bioeng Transl Med. 2019;4:e10143-e59
155. Zhu L, Zhou Z, Mao H, Yang L. Magnetic nanoparticles for precision oncology: Theranostic magnetic iron oxide nanoparticles for image-guided and targeted cancer therapy. Nanomedicine (Lond). 2017;12:73-87
156. Lee GY, Qian WP, Wang L, Wang YA, Staley CA, Satpathy M. et al. Theranostic nanoparticles with controlled release of Gemcitabine for targeted therapy and MRI of pancreatic cancer. ACS Nano. 2013;7:2078-89
157. Yang C, Chan KK, Xu G, Yin M, Lin G, Wang X. et al. Biodegradable polymer-coated multifunctional graphene quantum dots for light-triggered synergetic therapy of pancreatic cancer. ACS Appl Mater Interfaces. 2019;11:2768-81
158. Lei Y, Tang L, Xie Y, Xianyu Y, Zhang L, Wang P. et al. Gold nanoclusters-assisted delivery of NGF siRNA for effective treatment of pancreatic cancer. Nat Commun. 2017;8:15130
159. Lin L, Fan Y, Gao F, Jin L, Li D, Sun W. et al. UTMD-promoted co-delivery of Gemcitabine and miR-21 inhibitor by dendrimer-entrapped gold nanoparticles for pancreatic cancer therapy. Theranostics. 2018;8:1923-39
160. Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20:101-24
161. Anselmo AC, Mitragotri S. A review of clinical translation of inorganic nanoparticles. AAPS J. 2015;17:1041-54
162. Bayda S, Hadla M, Palazzolo S, Riello P, Corona G, Toffoli G. et al. Inorganic nanoparticles for cancer therapy: A transition from lab to clinic. Curr Med Chem. 2018;25:4269-303
163. Giordano G, Pancione M, Olivieri N, Parcesepe P, Velocci M, Di Raimo T. et al. Nano albumin bound-Paclitaxel in pancreatic cancer: Current evidences and future directions. World J Gastroenterol. 2017;23:5875-86
164. Karimi M, Bahrami S, Ravari SB, Zangabad PS, Mirshekari H, Bozorgomid M. et al. Albumin nanostructures as advanced drug delivery systems. Expert Opin Drug Deliv. 2016;13:1609-23
165. Singh A, Xu J, Mattheolabakis G, Amiji M. EGFR-targeted gelatin nanoparticles for systemic administration of Gemcitabine in an orthotopic pancreatic cancer model. Nanomedicine. 2016;12:589-600
166. Desai N. Nanoparticle albumin-bound anticancer agents. In: Crommelin DJA, DeVlieger JSB, Ed. Non-biological complex drugs: The science and the regulatory landscape, Los Angeles, USA: Springer. 2015:335-54
167. Hoffman RM, Bouvet M. Nanoparticle albumin-bound-Paclitaxel: A limited improvement under the current therapeutic paradigm of pancreatic cancer. Expert Opin Pharmacother. 2015;16:943-7
168. Rajeshkumar NV, Yabuuchi S, Pai SG, Tong Z, Hou S, Bateman S. et al. Superior therapeutic efficacy of nab-Paclitaxel over Cremophor-based Paclitaxel in locally advanced and metastatic models of human pancreatic cancer. Br J Cancer. 2016;115:442-53
169. Casadó A, Sagristá ML, Mora M. Formulation and in vitro characterization of thermosensitive liposomes for the delivery of irinotecan. J Pharm Sci. 2014;103:3127-38
170. Kulkarni P, Haldar MK, Katti P, Dawes C, You S, Choi Y. et al. Hypoxia responsive, tumor penetrating lipid nanoparticles for delivery of chemotherapeutics to pancreatic cancer cell spheroids. Bioconjug Chem. 2016;27:1830-8
171. Sailor MJ, Park JH. Hybrid nanoparticles for detection and treatment of cancer. Adv Mater. 2012;24:3779-802
172. Gao F, Wu JR, Niu SW, Sun T, Li F, Bai Y. et al. Biodegradable, pH-sensitive hollow mesoporous organosilica nanoparticle (HMON) with controlled release of pirfenidone and ultrasound-target-microbubble-destruction (UTMD) for pancreatic cancer treatment. Theranostics. 2019;9:6002-18
173. Li F, Zhao X, Wang H, Zhao RF, Ji TJ, Ren H. et al. Multiple layer-by-layer lipid-polymer hybrid nanoparticles for improved FOLFIRINOX chemotherapy in pancreatic tumor models. Adv Funct Mater. 2015;25:788-98
174. Al-Hawary MM, Francis IR, Chari ST, Fishman EK, Hough DM, Lu DS. et al. Pancreatic ductal adenocarcinoma radiology reporting template: Consensus statement of the society of abdominal radiology and the american pancreatic association. Radiology. 2014;270:248-60
175. Vachiranubhap B, Kim YH, Balci NC, Semelka RC. Magnetic resonance imaging of adenocarcinoma of the pancreas. Top Magn Reson Imaging. 2009;20:3-9
176. Jones MJ, Buchanan AS, Neal CP, Dennison AR, Metcalfe MS, Garcea G. Imaging of indeterminate pancreatic cystic lesions: A systematic review. Pancreatology. 2013;13:436-42
177. Yousaf MN, Chaudhary FS, Ehsan A, Suarez AL, Muniraj T, Jamidar P. et al. Endoscopic ultrasound (EUS) and the management of pancreatic cancer. BMJ Open Gastroenterol. 2020;7:e000408
178. Evangelista L, Zucchetta P, Moletta L, Serafini S, Cassarino G, Pegoraro N. et al. The role of EDG PET/CT or PET/MRI in assessing response to neoadjuvant therapy for patients with borderline or resectable pancreatic cancer: A systematic literature review. Ann Nucl Med. 2021;35:767-76
179. Zhang Y, Huang ZX, Song B. Role of imaging in evaluating the response after neoadjuvant treatment for pancreatic ductal adenocarcinoma. World J Gastroenterol. 2021;27:3037-49
180. Tummers WS, Willmann JK, Bonsing BA, Vahrmeijer AL, Gambhir SS, Swijnenburg RJ. Advances in diagnostic and intraoperative molecular imaging of pancreatic cancer. Pancreas. 2018;47:675-89
181. Enriquez JS, Chu Y, Pudakalakatti S, Hsieh KL, Salmon D, Dutta P. et al. Hyperpolarized magnetic resonance and artificial intelligence: Frontiers of imaging in pancreatic cancer. JMIR Med Inform. 2021;9:e26601
182. McKinney M, Griffin MO, Tolat PP. Multimodality imaging for the staging of pancreatic cancer. Surg Oncol Clin N Am. 2021;30:621-37
183. Jugniot N, Bam R, Meuillet EJ, Unger EC, Paulmurugan R. Current status of targeted microbubbles in diagnostic molecular imaging of pancreatic cancer. Bioeng Transl Med. 2021;6:e10183
184. Key J, Leary JF. Nanoparticles for multimodal in vivo imaging in nanomedicine. Int J Nanomedicine. 2014;9:711-26
185. Brachi G, Bussolino F, Ciardelli G, Mattu C. Nanomedicine for imaging and therapy of pancreatic adenocarcinoma. Front Bioeng Biotechnol. 2019;7:307
186. Medina OP, Tower RJ, Medina TP, Ashkenani F, Appold L, Bötcher M. et al. Multimodal targeted nanoparticle-based delivery system for pancreatic tumor imaging in cellular and animal models. Curr Pharm Des. 2020;26:1873-4286
187. Hu X, Xia F, Lee J, Li F, Lu X, Zhuo X. et al. Tailor-made nanomaterials for diagnosis and therapy of pancreatic ductal adenocarcinoma. Adv Sci (Weinh). 2021;8:2002545
188. Zhao Y, Ye F, Brismar TB, Li X, He R, Heuchel R. et al. Multimodal imaging of pancreatic ductal adenocarcinoma using multifunctional nanoparticles as contrast agents. ACS Appl Mater Interfaces. 2020;12:53665-81
189. Sobol NB, Korsen JA, Younes A, Edwards KJ, Lewis JS. Immunopet imaging of pancreatic tumors with (89)Zr-labeled gold nanoparticle-antibody conjugates. Mol Imaging Biol. 2021;23:84-94
190. Qi B, Crawford AJ, Wojtynek NE, Holmes MB, Souchek JJ, Almeida-Porada G. et al. Indocyanine green loaded hyaluronan-derived nanoparticles for fluorescence-enhanced surgical imaging of pancreatic cancer. Nanomedicine. 2018;14:769-780
191. Delaney LJ, Eisenbrey JR, Brown D, Brody JR, Jimbo M, Oeffinger BE. et al. Gemcitabine-loaded microbubble system for ultrasound imaging and therapy. Acta Biomater. 2021;130:385-394
192. Wang Q, Zhu X, Wu Z, Sun T, Huang W, Wang Z. et al. Theranostic nanoparticles enabling the release of phosphorylated Gemcitabine for advanced pancreatic cancer therapy. J Mater Chem B. 2020;8:2410-17
193. Jaidev LR, Chellappan DR, Bhavsar DV, Ranganathan R, Sivanantham B, Subramanian A. et al. Multi-functional nanoparticles as theranostic agents for the treatment & imaging of pancreatic cancer. Acta Biomater. 2017;49:422-33
194. Gao N, Bozeman EN, Qian W, Wang L, Chen H, Lipowska M. et al. Tumor penetrating theranostic nanoparticles for enhancement of targeted and image-guided drug delivery into peritoneal tumors following intraperitoneal delivery. Theranostics. 2017;7:1689-704
195. Zhou H, Qian W, Uckun FM, Wang L, Wang YA, Chen H. et al. IGF1 receptor targeted theranostic nanoparticles for targeted and image-guided therapy of pancreatic cancer. ACS Nano. 2015;9:7976-91
196. Gu J, Saiyin H, Fu D, Li J. Stroma - a double-edged sword in pancreatic cancer: A lesson from targeting stroma in pancreatic cancer with hedgehog signaling inhibitors. Pancreas. 2018;47:382-389
197. Skoda AM, Simovic D, Karin V, Kardum V, Vranic S, Serman L. The role of the hedgehog signaling pathway in cancer: A comprehensive review. Bosn J Basic Med Sci. 2018;18:8-20
198. Gurka MK, Pender D, Chuong P, Fouts BL, Sobelov A, McNally MW. et al. Identification of pancreatic tumors in vivo with ligand-targeted, ph responsive mesoporous silica nanoparticles by multispectral optoacoustic tomography. J Control Release. 2016;231:60-7
199. Kulkarni P, Haldar MK, Katti P, Dawes C, You S, Choi Y. et al. Hypoxia responsive, tumor penetrating lipid nanoparticles for delivery of chemotherapeutics to pancreatic cancer cell spheroids. Bioconjugate Chemistry. 2016;27:1830-8
200. Kulkarni P, Haldar MK, You S, Choi Y, Mallik S. Hypoxia-responsive polymersomes for drug delivery to hypoxic pancreatic cancer cells. Biomacromolecules. 2016;17:2507-13
201. Han H, Valdepérez D, Jin Q, Yang B, Li Z, Wu Y. et al. Dual enzymatic reaction-assisted Gemcitabine delivery systems for programmed pancreatic cancer therapy. ACS Nano. 2017;11:1281-91
202. McDaid WJ, Greene MK, Johnston MC, Pollheimer E, Smyth P, McLaughlin K. et al. Repurposing of cetuximab in antibody-directed chemotherapy-loaded nanoparticles in EGFR therapy-resistant pancreatic tumours. Nanoscale. 2019;11:20261-73
203. Ahn J, Miura Y, Yamada N, Chida T, Liu X, Kim A. et al. Antibody fragment-conjugated polymeric micelles incorporating platinum drugs for targeted therapy of pancreatic cancer. Biomaterials. 2015;39:23-30
204. Srinivasarao M, Low PS. Ligand-targeted drug delivery. Chem Rev. 2017;117:12133-64
205. Lin C, Hu Z, Yuan G, Su H, Zeng Y, Guo Z. et al. HIF1α-siRNA and Gemcitabine combination-based GE-11 peptide antibody-targeted nanomedicine for enhanced therapeutic efficacy in pancreatic cancers. J Drug Target. 2019;27:797-805
206. Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018;32:1267-84
207. Wang M, Yin B, Wang HY, Wang RF. Current advances in T-cell-based cancer immunotherapy. Immunotherapy. 2014;6:1265-78
208. Ahmed KK, Geary SM, Salem AK. Applying biodegradable particles to enhance cancer vaccine efficacy. Immunol Res. 2014;59:220-8
209. Joshi VB, Geary SM, Salem AK. Biodegradable particles as vaccine antigen delivery systems for stimulating cellular immune responses. Hum Vaccin Immunother. 2013;9:2584-90
210. Batista-Duharte A, Martinez DT, Carlos IZ. Efficacy and safety of immunological adjuvants. Where is the cut-off? Biomed Pharmacother. 2018;105:616-24
211. Petrovsky N. Comparative safety of vaccine adjuvants: A summary of current evidence and future needs. Drug Saf. 2015;38:1059-74
212. Hamdy S, Haddadi A, Hung RW, Lavasanifar A. Targeting dendritic cells with nano-particulate PLGA cancer vaccine formulations. Adv Drug Deliv Rev. 2011;63:943-55
213. Mekkawy AI, Naguib YW, Alhaj-Suliman SO, Wafa EI, Ebeid K, Acri T. et al. Paclitaxel anticancer activity is enhanced by the MEK 1/2 inhibitor PD98059 in vitro and by PD98059-loaded nanoparticles in BRAFV600E melanoma-bearing mice. Int J Pharm. 2021;606:120876
214. Gross BP, Chitphet K, Wongrakpanich A, Wafa EI, Norian LA, Salem AK. Biotinylated streptavidin surface coating improves the efficacy of a PLGA microparticle-based cancer vaccine. Bioconjug Chem. 2020;31:2147-57
215. Wafa EI, Geary SM, Ross KA, Goodman JT, Narasimhan B, Salem AK. Single dose of a polyanhydride particle-based vaccine generates potent antigen-specific antitumor immune responses. J Pharmacol Exp Ther. 2019;370:855-63
216. Torres MP, Vogel BM, Narasimhan B, Mallapragada SK. Synthesis and characterization of novel polyanhydrides with tailored erosion mechanisms. J Biomed Mater Res A. 2006;76:102-10
217. Vela Ramirez JE, Tygrett LT, Hao J, Habte HH, Cho MW, Greenspan NS. et al. Polyanhydride nanovaccines induce germinal center B cell formation and sustained serum antibody responses. J Biomed Nanotechnol. 2016;12:1303-11
218. Wafa EI, Geary SM, Ross KA, Goodman JT, Narasimhan B, Salem AK. Pentaerythritol-based lipid a bolsters the antitumor efficacy of a polyanhydride particle-based cancer vaccine. Nanomedicine. 2019;21:102055
219. Andrianov AK, Marin A, Fuerst TR. Molecular-level interactions of polyphosphazene immunoadjuvants and their potential role in antigen presentation and cell stimulation. Biomacromolecules. 2016;17:3732-42
220. Gao M, Zhu X, Wu L, Qiu L. Cationic polyphosphazene vesicles for cancer immunotherapy by efficient in vivo cytokine IL-12 plasmid delivery. Biomacromolecules. 2016;17:2199-209
221. Yoo J, D'Mello SR, Graf T, Salem AK, Bowden NB. Synthesis of the first poly(diaminosulfide)s and an investigation of their applications as drug delivery vehicles. Macromolecules. 2012;45:688-97
222. Yoo J, Kuruvilla DJ, D'Mello SR, Salem AK, Bowden NB. New class of biodegradable polymers formed from reactions of an inorganic functional group. Macromolecules. 2012;45:2292-300
223. Geary SM, Hu Q, Joshi VB, Bowden NB, Salem AK. Diaminosulfide based polymer microparticles as cancer vaccine delivery systems. J Control Release. 2015;220:682-90
224. Dold NM, Zeng Q, Zeng X, Jewell CM. A poly(beta-amino ester) activates macrophages independent of NF-κB signaling. Acta Biomater. 2018;68:168-77
225. Smith TT, Stephan SB, Moffett HF, McKnight LE, Ji W, Reiman D. et al. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat Nanotechnol. 2017;12:813-820
226. Barbero F, Russo L, Vitali M, Piella J, Salvo I, Borrajo ML. et al. Formation of the protein corona: The interface between nanoparticles and the immune system. Semin Immunol. 2017;34:52-60
227. Liu Y, Hardie J, Zhang X, Rotello VM. Effects of engineered nanoparticles on the innate immune system. Semin Immunol. 2017;34:25-32
228. Dwivedi PD, Tripathi A, Ansari KM, Shanker R, Das M. Impact of nanoparticles on the immune system. J Biomed Nanotechnol. 2011;7:193-4
229. Kumar S, Anselmo AC, Banerjee A, Zakrewsky M, Mitragotri S. Shape and size-dependent immune response to antigen-carrying nanoparticles. J Control Release. 2015;220:141-8
230. Courtney AH, Lo WL, Weiss A. TCR signaling: Mechanisms of initiation and propagation. Trends Biochem Sci. 2018;43:108-123
231. Scharping NE, Rivadeneira DB, Menk AV, Vignali PDA, Ford BR, Rittenhouse NL. et al. Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion. Nat Immunol. 2021;22:205-15
232. Thwe PM, Amiel E. The role of nitric oxide in metabolic regulation of dendritic cell immune function. Cancer Lett. 2018;412:236-42
233. Darling R, Senapati S, Christiansen J, Liu L, Ramer-Tait AE, Narasimhan B. et al. Polyanhydride nanoparticles induce low inflammatory dendritic cell activation resulting in CD8+ T cell memory and delayed tumor progression. Int J Nanomedicine. 2020;15:6579-92
234. Brodbeck WG, Macewan M, Colton E, Meyerson H, Anderson JM. Lymphocytes and the foreign body response: Lymphocyte enhancement of macrophage adhesion and fusion. J Biomed Mater Res A. 2005;74:222-29
235. Chang DT, Colton E, Matsuda T, Anderson JM. Lymphocyte adhesion and interactions with biomaterial adherent macrophages and foreign body giant cells. J Biomed Mater Res A. 2009;91:1210-20
236. Sun T, Zhang YS, Pang B, Hyun DC, Yang M, Xia Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew Chem Int Ed Engl. 2014;53:12320-64
237. Mazumdar S, Chitkara D, Mittal A. Exploration and insights into the cellular internalization and intracellular fate of amphiphilic polymeric nanocarriers. Acta Pharm Sin B. 2021;11:903-24
238. Champion JA, Walker A, Mitragotri S. Role of particle size in phagocytosis of polymeric microspheres. Pharm Res. 2008;25:1815-21
239. Rao DA, Forrest ML, Alani AW, Kwon GS, Robinson JR. Biodegradable PLGA based nanoparticles for sustained regional lymphatic drug delivery. J Pharm Sci. 2010;99:2018-31
240. Reddy ST, Van Der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O'Neil CP. et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25:1159-64
241. Mundry CS, Eberle KC, Singh PK, Hollingsworth MA, Mehla K. Local and systemic immunosuppression in pancreatic cancer: Targeting the stalwarts in tumor's arsenal. Biochim Biophys Acta Rev Cancer. 2020;1874:188387
242. Lorkowski ME, Atukorale PU, Bielecki PA, Tong KH, Covarrubias G, Zhang Y. et al. Immunostimulatory nanoparticle incorporating two immune agonists for the treatment of pancreatic tumors. J Control Release. 2021;330:1095-105
243. Liu Y, Yin Y, Wang L, Zhang W, Chen X, Yang X. et al. Engineering biomaterial-associated complement activation to improve vaccine efficacy. Biomacromolecules. 2013;14:3321-28
244. Ulery BD, Petersen LK, Phanse Y, Kong CS, Broderick SR, Kumar D. et al. Rational design of pathogen-mimicking amphiphilic materials as nanoadjuvants. Sci Rep. 2011;1:1-9
245. Seong S-y, Matzinger P. Hydrophobicity: An ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4:469-78
246. Bus T, Traeger A, Schubert US. The great escape: How cationic polyplexes overcome the endosomal barrier. J Mater Chem B. 2018;6:6904-18
247. Dong H, Wen Z-F, Chen L, Zhou N, Liu H, Dong S. et al. Polyethyleneimine modification of aluminum hydroxide nanoparticle enhances antigen transportation and cross-presentation of dendritic cells. Int J Nanomedicine. 2018;13:3353-65
248. Eisenbarth SC. Dendritic cell subsets in T cell programming: Location dictates function. Nat Rev Immunol. 2019;19:89-103
249. Kanda M, Fujii T, Nagai S, Kodera Y, Kanzaki A, Sahin TT. et al. Pattern of lymph node metastasis spread in pancreatic cancer. Pancreas. 2011;40:951-55
250. Han BJ, Murphy JD, Qin S, Ye J, Uccello TP, Garrett-Larsen J. et al. Microspheres encapsulating immunotherapy agents target the tumor-draining lymph node in pancreatic ductal adenocarcinoma. Immunol Invest. 2020;49:808-23
251. Li X. The inducers of immunogenic cell death for tumor immunotherapy. Tumori. 2018;104:1-8
252. Mishchenko T, Mitroshina E, Balalaeva I, Krysko O, Vedunova M, Krysko DV. An emerging role for nanomaterials in increasing immunogenicity of cancer cell death. Biochim Biophys Acta Rev Cancer. 2019;1871:99-108
253. Sun F, Cui L, Li T, Chen S, Song J, Li D. Oxaliplatin induces immunogenic cells death and enhances therapeutic efficacy of checkpoint inhibitor in a model of murine lung carcinoma. J Recept Signal Transduct Res. 2019;39:208-14
254. Makkouk A, Joshi VB, Wongrakpanich A, Lemke CD, Gross BP, Salem AK. et al. Biodegradable microparticles loaded with doxorubicin and CpG ODN for in situ immunization against cancer. AAPS J. 2015;17:184-93
255. Chitphet K, Geary SM, Chan CHF, Simons AL, Weiner GJ, Salem AK. Combining Doxorubicin-loaded PEGylated poly(lactide-co-glycolide) nanoparticles with checkpoint inhibition safely enhances therapeutic efficacy in a melanoma model. ACS Biomater Sci Eng. 2020;6:2659-67
256. Sun F, Zhu Q, Li T, Saeed M, Xu Z, Zhong F. et al. Regulating glucose metabolism with prodrug nanoparticles for promoting photoimmunotherapy of pancreatic cancer. Adv Sci (Weinh). 2021;8:2002746
257. Kaur S, Kumar S, Momi N, Sasson AR, Batra SK. Mucins in pancreatic cancer and its microenvironment. Nat Rev Gastroenterol Hepatol. 2013;10:607-20
258. Bhatia R, Gautam SK, Cannon A, Thompson C, Hall BR, Aithal A. et al. Cancer-associated mucins: Role in immune modulation and metastasis. Cancer Metastasis Rev. 2019;38:223-36
259. Aithal A, Rauth S, Kshirsagar P, Shah A, Lakshmanan I, Junker WM. et al. MUC16 as a novel target for cancer therapy. Expert Opin Ther Targets. 2018;22:675-86
260. Gautam SK, Kumar S, Cannon A, Hall B, Bhatia R, Nasser MW. et al. MUC4 mucin- a therapeutic target for pancreatic ductal adenocarcinoma. Expert Opin Ther Targets. 2017;21:657-69
261. Gautam SK, Kumar S, Dam V, Ghersi D, Jain M, Batra SK. Mucin-4 (MUC4) is a novel tumor antigen in pancreatic cancer immunotherapy. Semin Immunopathol. 2020;47:101391
262. Balachandran VP, Luksza M, Zhao JN, Makarov V, Moral JA, Remark R. et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature. 2017;551:512-516
263. Akaishi S, Kobari M, Takeda K, Matsuno S. Targeting chemotherapy using antibody-combined liposome against human pancreatic cancer cell-line. Tohoku J Exp Med. 1995;175:29-42
264. Hu CM, Kaushal S, Tran Cao HS, Aryal S, Sartor M, Esener S. et al. Half-antibody functionalized lipid-polymer hybrid nanoparticles for targeted drug delivery to carcinoembryonic antigen presenting pancreatic cancer cells. Mol Pharm. 2010;7:914-20
265. Liu F, Le W, Mei T, Wang T, Chen L, Lei Y. et al. In vitro and in vivo targeting imaging of pancreatic cancer using a Fe3O4@SiO2 nanoprobe modified with anti-mesothelin antibody. Int J Nanomedicine. 2016;11:2195-207
266. Ma J, Shen M, Xu CS, Sun Y, Duan YR, Du LF. Biodegradable double-targeted PTX-mPEG-PLGA nanoparticles for ultrasound contrast enhanced imaging and antitumor therapy in vitro. Oncotarget. 2016;7:80008-18
267. Xing L, Shi Q, Zheng K, Shen M, Ma J, Li F. et al. Ultrasound-mediated microbubble destruction (UMMD) facilitates the delivery of CA19-9 targeted and Paclitaxel loaded mPEG-PLGA-PLL nanoparticles in pancreatic cancer. Theranostics. 2016;6:1573-87
268. Arya G, Vandana M, Acharya S, Sahoo SK. Enhanced antiproliferative activity of herceptin (HER2)-conjugated Gemcitabine-loaded chitosan nanoparticle in pancreatic cancer therapy. Nanomedicine. 2011;7:859-70
269. Bao S, Zheng H, Ye J, Huang H, Zhou B, Yao Q. et al. Dual targeting EGFR and STAT3 with Erlotinib and alantolactone co-loaded PLGA nanoparticles for pancreatic cancer treatment. Front Pharmacol. 2021;12:625084
270. Pittella F, Zhang M, Lee Y, Kim HJ, Tockary T, Osada K. et al. Enhanced endosomal escape of siRNA-incorporating hybrid nanoparticles from calcium phosphate and PEG-block charge-conversional polymer for efficient gene knockdown with negligible cytotoxicity. Biomaterials. 2011;32:3106-14
271. Santos-Rebelo A, Kumar P, Pillay V, Choonara YE, Eleutério C, Figueira M. et al. Development and mechanistic insight into the enhanced cytotoxic potential of Parvifloron D albumin nanoparticles in EGFR-overexpressing pancreatic cancer cells. Cancers (Basel). 2019;11:1733
272. Showalter SL, Huang YH, Witkiewicz A, Costantino CL, Yeo CJ, Green JJ. et al. Nanoparticulate delivery of diphtheria toxin DNA effectively kills mesothelin expressing pancreatic cancer cells. Cancer Biol Ther. 2008;7:1584-90
273. Morrison AH, Byrne KT, Vonderheide RH. Immunotherapy and prevention of pancreatic cancer. Trends Cancer. 2018;4:418-28
274. Schizas D, Charalampakis N, Kole C, Economopoulou P, Koustas E, Gkotsis E. et al. Immunotherapy for pancreatic cancer: A 2020 update. Cancer Treat Rev. 2020;86:102016
275. Luo W, Yang G, Luo W, Cao Z, Liu Y, Qiu J. et al. Novel therapeutic strategies and perspectives for metastatic pancreatic cancer: Vaccine therapy is more than just a theory. Cancer Cell Int. 2020;20:66
276. Miao L, Li J, Liu Q, Feng R, Das M, Lin CM. et al. Transient and local expression of chemokine and immune checkpoint traps to treat pancreatic cancer. ACS Nano. 2017;11:8690-706
277. Alving CR, Beck Z, Matyas GR, Rao M. Liposomal adjuvants for human vaccines. Expert Opin Drug Deliv. 2016;13:807-16
278. Schwendener RA. Liposomes as vaccine delivery systems: A review of the recent advances. Ther Adv Vaccines. 2014;2:159-82
279. Zhang S, Yong LK, Li D, Cubas R, Chen C, Yao Q. Mesothelin virus-like particle immunization controls pancreatic cancer growth through CD8+ T cell induction and reduction in the frequency of CD4+foxp3ICOS- regulatory T cells. PLoS One. 2013;8:e68303
280. Satterlee AB, Huang L. Current and future theranostic applications of the lipid-calcium-phosphate nanoparticle platform. Theranostics. 2016;6:918-29
281. Van Waarde A, Rybczynska AA, Ramakrishnan NK, Ishiwata K, Elsinga PH, Dierckx RA. Potential applications for sigma receptor ligands in cancer diagnosis and therapy. Biochim Biophys Acta. 2015;1848:2703-14
282. Metz R, Rust S, Duhadaway JB, Mautino MR, Munn DH, Vahanian NN. et al. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: A novel IDO effector pathway targeted by D-1-methyl-tryptophan. Oncoimmunology. 2012;1:1460-8
283. Jia L, Schweikart K, Tomaszewski J, Page JG, Noker PE, Buhrow SA. et al. Toxicology and pharmacokinetics of 1-methyl-d-tryptophan: Absence of toxicity due to saturating absorption. Food Chem Toxicol. 2008;46:203-11
284. Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR. et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719-34
285. Zhao J, Wang H, Hsiao CH, Chow DS, Koay EJ, Kang Y. et al. Simultaneous inhibition of hedgehog signaling and tumor proliferation remodels stroma and enhances pancreatic cancer therapy. Biomaterials. 2018;159:215-28
286. Li J, Zhu Y, Hazeldine ST, Li C, Oupický D. Dual-function CXCR4 antagonist polyplexes to deliver gene therapy and inhibit cancer cell invasion. Angew Chem Int Ed Engl. 2012;51:8740-3
287. Wang Y, Kumar S, Rachagani S, Sajja BR, Xie Y, Hang Y. et al. Polyplex-mediated inhibition of chemokine receptor CXCR4 and chromatin-remodeling enzyme NCOA3 impedes pancreatic cancer progression and metastasis. Biomaterials. 2016;101:108-20
288. Xie Y, Hang Y, Wang Y, Sleightholm R, Prajapati DR, Bader J. et al. Stromal modulation and treatment of metastatic pancreatic cancer with local intraperitoneal triple miRNA/siRNA nanotherapy. ACS Nano. 2020;14:255-71
289. Kuninty PR, Bojmar L, Tjomsland V, Larsson M, Storm G, Östman A. et al. MicroRNA-199a and -214 as potential therapeutic targets in pancreatic stellate cells in pancreatic tumor. Oncotarget. 2016;7:16396-408
290. Collins MA, Bednar F, Zhang Y, Brisset JC, Galbán S, Galbán CJ. et al. Oncogenic KRAS is required for both the initiation and maintenance of pancreatic cancer in mice. J Clin Invest. 2012;122:639-53
291. Collins MA, Brisset JC, Zhang Y, Bednar F, Pierre J, Heist KA. et al. Metastatic pancreatic cancer is dependent on oncogenic KRAS in mice. PLoS One. 2012;7:e49707
292. Rahmaniah R, Yuyuntia Y, Soetikno V, Arozal W, Antarianto RD, Louisa M. Alpha mangostin inhibits hepatic stellate cells activation through TGF-β/smad and AKT signaling pathways: An in vitro study in LX2. Drug Res (Stuttg). 2018;68:153-58
293. Mortarini R, Scarito A, Nonaka D, Zanon M, Bersani I, Montaldi E. et al. Constitutive expression and costimulatory function of LIGHT/TNFSF14 on human melanoma cells and melanoma-derived microvesicles. Cancer Res. 2005;65:3428-36
294. Bjordahl RL, Steidl C, Gascoyne RD, Ware CF. Lymphotoxin network pathways shape the tumor microenvironment. Curr Opin Immunol. 2013;25:222-29
295. Kim EJ, Sahai V, Abel EV, Griffith KA, Greenson JK, Takebe N. et al. Pilot clinical trial of hedgehog pathway inhibitor GDC-0449 (Vismodegib) in combination with Gemcitabine in patients with metastatic pancreatic adenocarcinoma. Clin Cancer Res. 2014;20:5937-45
296. Mühlberger M, Janko C, Unterweger H, Schreiber E, Band J, Lehmann C. et al. Functionalization of T lymphocytes for magnetically controlled immune therapy: Selection of suitable superparamagnetic iron oxide nanoparticles. J Magn Magn Mater. 2019;473:61-67
297. An M, Li M, Xi J, Liu H. Silica nanoparticle as a lymph node targeting platform for vaccine delivery. ACS Appl Mater Interfaces. 2017;9:23466-75
298. Wang X, Li X, Ito A, Sogo Y, Watanabe Y, Tsuji NM. et al. Biodegradable metal ion-doped mesoporous silica nanospheres stimulate anticancer TH1 immune response in vivo. ACS Appl Mater Interfaces. 2017;9:43538-44
299. Liu C, Lai H, Chen T. Boosting natural killer cell-based cancer immunotherapy with selenocystine/transforming growth factor-beta inhibitor-encapsulated nanoemulsion. ACS Nano. 2020;14:11067-82
300. Henriksen A, Dyhl-Polk A, Chen I, Nielsen D. Checkpoint inhibitors in pancreatic cancer. Cancer Treat Rev. 2019;78:17-30
301. Bian J, Almhanna K. Pancreatic cancer and immune checkpoint inhibitors-still a long way to go. Transl Gastroenterol Hepatol. 2021;6:6
302. Marin-Acevedo JA, Chirila RM, Dronca RS. Immune checkpoint inhibitor toxicities. Mayo Clin Proc. 2019;94:1321-29
303. Khan S, Gerber DE. Autoimmunity, checkpoint inhibitor therapy and immune-related adverse events: A review. Semin Cancer Biol. 2020;64:93-101
304. Tsujikawa T, Crocenzi T, Durham JN, Sugar EA, Wu AA, Onners B. et al. Evaluation of cyclophosphamide/GVAX pancreas followed by listeria-mesothelin (CRS-207) with or without nivolumab in patients with pancreatic cancer. Clin Cancer Res. 2020;26:3578-88
305. Ordikhani F, Uehara M, Kasinath V, Dai L, Eskandari SK, Bahmani B. et al. Targeting antigen-presenting cells by anti-PD-1 nanoparticles augments antitumor immunity. JCI Insight. 2018;3:e122700
306. Li SY, Liu Y, Xu CF, Shen S, Sun R, Du XJ. et al. Restoring anti-tumor functions of T cells via nanoparticle-mediated immune checkpoint modulation. J Control Release. 2016;231:17-28
307. Ramesh A, Malik V, Ranjani HA, Smith H, Kulkarni AA. Rational combination of an immune checkpoint inhibitor with CSF1R inhibitor-loaded nanoparticle enhances anticancer efficacy. Drug Deliv Transl Res. 2021: 11: 2317-27.
308. Qi J, Jin F, Xu X, Du Y. Combination cancer immunotherapy of nanoparticle-based immunogenic cell death inducers and immune checkpoint inhibitors. Int J Nanomedicine. 2021;16:1435-56
309. Chen Q, Xu L, Liang C, Wang C, Peng R, Liu Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat Commun. 2016;7:13193
310. Wang Y, Gao Z, Du X, Chen S, Zhang W, Wang J. et al. Co-inhibition of the TGF-beta pathway and the PD-L1 checkpoint by pH-responsive clustered nanoparticles for pancreatic cancer microenvironment regulation and anti-tumor immunotherapy. Biomater Sci. 2020;8:5121-32
311. Yu Q, Tang X, Zhao W, Qiu Y, He J, Wan D. et al. Mild hyperthermia promotes immune checkpoint blockade-based immunotherapy against metastatic pancreatic cancer using size-adjustable nanoparticles. Acta Biomater. 2021;133:244-56
312. Ko AH, LoConte N, Tempero MA, Walker EJ, Kelley RK, Lewis S. et al. A phase I study of FOLFIRINOX plus IPI-926, a hedgehog pathway inhibitor, for advanced pancreatic adenocarcinoma. Pancreas. 2016;45:370-5
313. Catenacci DVT, Junttila MR, Karrison T, Bahary N, Horiba MN, Nattam SR. et al. Randomized phase Ib/II study of Gemcitabine plus Placebo or Vismodegib, a hedgehog pathway inhibitor, in patients with metastatic pancreatic cancer. J Clin Oncol. 2015;33:4284-92
314. Van Cutsem E, Tempero MA, Sigal D, Oh D-Y, Fazio N, Macarulla T. et al. Randomized phase III trial of Pegvorhyaluronidase alfa with Nab-Paclitaxel plus Gemcitabine for patients with hyaluronan-high metastatic pancreatic adenocarcinoma. J Clin Oncol. 2020;38:3185-94
315. Van Mackelenbergh MG, Stroes CI, Spijker R, Van Eijck CHJ, Wilmink JW, Bijlsma MF. et al. Clinical trials targeting the stroma in pancreatic cancer: A systematic review and meta-analysis. Cancers (Basel). 2019;11:1-23
316. Hosein AN, Brekken RA, Maitra A. Pancreatic cancer stroma: An update on therapeutic targeting strategies. Nat Rev Gastroenterol Hepatol. 2020;17:487-505
317. Kumar V, Boucher Y, Liu H, Ferreira D, Hooker J, Catana C. et al. Noninvasive assessment of losartan-induced increase in functional microvasculature and drug delivery in pancreatic ductal adenocarcinoma. Transl Oncol. 2016;9:431-37
318. Murphy JE, Wo JY, Ryan DP, Clark JW, Jiang W, Yeap BY. et al. Total neoadjuvant therapy with FOLFIRINOX in combination with losartan followed by chemoradiotherapy for locally advanced pancreatic cancer: A phase 2 clinical trial. JAMA Oncology. 2019;5:1020-27
319. Corrigan N, Jung K, Moad G, Hawker CJ, Matyjaszewski K, Boyer C. Reversible-deactivation radical polymerization (controlled/living radical polymerization): From discovery to materials design and applications. Prog Polym Sci. 2020: 101311.
320. Wilson JT, Keller S, Manganiello MJ, Cheng C, Lee CC, Opara C. et al. pH-responsive nanoparticle vaccines for dual-delivery of antigens and immunostimulatory oligonucleotides. ACS Nano. 2013;7:3912-25
321. Yilmaz G, Becer CR. Glyconanoparticles and their interactions with lectins. Polym Chem. 2015;6:5503-14
322. Song EH, Manganiello MJ, Chow YH, Ghosn B, Convertine AJ, Stayton PS. et al. In vivo targeting of alveolar macrophages via RAFT-based glycopolymers. Biomaterials. 2012;33:6889-97
323. Dai J, Su Y, Zhong S, Cong L, Liu B, Yang J. et al. Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther. 2020;5:1-10
324. Tran PHL, Xiang D, Tran TTD, Yin W, Zhang Y, Kong L. et al. Exosomes and nanoengineering: A match made for precision therapeutics. Adv Mater. 2020;32:1-8
325. Jang Y, Kim H, Yoon S, Lee H, Hwang J, Jung J. et al. Exosome-based photoacoustic imaging guided photodynamic and immunotherapy for the treatment of pancreatic cancer. J Control Release. 2021;330:293-304
326. Tu K, Deng H, Kong L, Wang Y, Yang T, Hu Q. et al. Reshaping tumor immune microenvironment through acidity-responsive nanoparticles featured with CRISPR/Cas9-mediated programmed Death-Ligand 1 attenuation and chemotherapeutics-induced immunogenic cell death. ACS Appl Mater Interfaces. 2020;12:16018-30
327. Yang C, Fu Y, Huang C, Hu D, Zhou K, Hao Y. et al. Chlorin e6 and CRISPR/Cas9dual-loading system with deep penetration for a synergistic tumoral photodynamic-immunotherapy. Biomaterials. 2020;255:120194
328. Hua S, de Matos MBC, Metselaar JM, Storm G. Current trends and challenges in the clinical translation of nanoparticulate nanomedicines: Pathways for translational development and commercialization. Front Pharmacol. 2018;9:790
329. Hare JI, Lammers T, Ashford MB, Puri S, Storm G, Barry ST. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv Drug Deliv Rev. 2017;108:25-38
330. Philip PA, Lacy J, Portales F, Sobrero A, Pazo-Cid R, Manzano Mozo JL. et al. Nab-Paclitaxel plus Gemcitabine in patients with locally advanced pancreatic cancer (LAPACT): A multicentre, open-label phase 2 study. Lancet Gastroenterol Hepatol. 2020;5:285-94
331. Ghamande SA, Lin C, Cho DC, Coleman TA, Chaudhary I, Cleary JM. et al. A phase I study of the novel DNA Topoisomerase-1 inhibitor, TLC388, administered intravenously to patients with advanced solid tumors. J Clin Oncol. 2011;29:e13618
332. Lee S-W, Kim Y-M, Cho CH, Kim YT, Kim SM, Hur SY. et al. An open-label, randomized, parallel, phase II trial to evaluate the efficacy and safety of a cremophor-free polymeric micelle formulation of Paclitaxel as first-line treatment for ovarian cancer: A korean gynecologic oncology group study (KGOG-3021). Cancer Res Treat. 2018;50:195-203
333. Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M. et al. Increased survival in pancreatic cancer with nab-paclitaxel plus Gemcitabine. N Engl J Med. 2013;369:1691-703
334. Garcia-Sampedro A, Gaggia G, Ney A, Mahamed I, Acedo P. The state-of-the-art of phase II/III clinical trials for targeted pancreatic cancer therapies. J Clin Med. 2021;10:566
335. Ludwig-Maximilians. First-line therapy in metastatic PDAC [Clinicaltrials.Gov identifier: Nct03487016]
336. Jehn CF, Boulikas T, Kourvetaris A, Kofla G, Possinger K, Lüftner D. First safety and response results of a randomized phase III study with liposomal platin in the treatment of advanced squamous cell carcinoma of the head and neck (SCCHN). Anticancer Res. 2008;28:3961-4
337. Li-Tzong Chen M-HS. EndoTAG-1 plus Gemcitabine versus Gemcitabine alone in patients with measurable locally advanced and/or metastatic adenocarcinoma of the pancreas failed on FOLFIRINOX treatment [NCT03126435]. J Clin Oncol. 2020;38:15
338. Lee BC, Kang I, Yu KR. Therapeutic features and updated clinical trials of mesenchymal stem cell (MSC)-derived exosomes. J Clin Med. 2021;10:711
339. Wang P, Yoo B, Sherman S, Mukherjee P, Ross A, Pantazopoulos P. et al. Predictive imaging of chemotherapeutic response in a transgenic mouse model of pancreatic cancer. Int J Cancer. 2016;139:712-18
340. Wu SC, Chen YJ, Lin YJ, Wu TH, Wang YM. Development of a Mucin4-targeting SPIOcontrast agent for effective detection of pancreatic tumor cells in vitro and in vivo. J Med Chem. 2013;56:9100-9
341. Zeng L, Li J, Li J, Zhang Q, Qian C, Wu W. et al. Effective suppression of the kirsten rat sarcoma viral oncogene in pancreatic tumor cells via targeted small interfering RNA delivery using nanoparticles. Pancreas. 2015;44:250-9
342. Sun Q, Wang X, Cui C, Li J, Wang Y. Doxorubicin and anti-VEGF siRNA co-delivery via nano-graphene oxide for enhanced cancer therapy in vitro and in vivo. Int J Nanomedicine. 2018;13:3713-28
343. Wang L, An Y, Yuan C, Zhang H, Liang C, Ding F. et al. GEM-loaded magnetic albumin nanospheres modified with Cetuximab for simultaneous targeting, magnetic resonance imaging, and double-targeted thermochemotherapy of pancreatic cancer cells. Int J Nanomedicine. 2015;10:2507-19
344. Er Ö, Colak SG, Ocakoglu K, Ince M, Bresolí-Obach R, Mora M. et al. Selective photokilling of human pancreatic cancer cells using Cetuximab-targeted mesoporous silica nanoparticles for delivery of Zinc phthalocyanine. Molecules. 2018;23:2749
345. Obaid G, Bano S, Mallidi S, Broekgaarden M, Kuriakose J, Silber Z. et al. Impacting pancreatic cancer therapy in heterotypic in vitro organoids and in vivo tumors with specificity-tuned, NIR-activable photoimmunonanoconjugates: Towards conquering desmoplasia? Nano Lett. 2019;19:7573-87
346. Yalçin S, Erkan M, Ünsoy G, Parsian M, Kleeff J, Gündüz U. Effect of Gemcitabine and retinoic acid loaded PAMAM dendrimer-coated magnetic nanoparticles on pancreatic cancer and stellate cell lines. Biomed Pharmacother. 2014;68:737-43
347. Kakwere H, Ingham ES, Tumbale SK, Ferrara KW. Gemcitabine-Retinoid prodrug loaded nanoparticles display in vitro antitumor efficacy towards drug-resilient human PANC-1 pancreatic cancer cells. Mater Sci Eng C Mater Biol Appl. 2020;117:111251
348. Girgis MD, Federman N, Rochefort MM, McCabe KE, Wu AM, Nagy JO. et al. An engineered anti-CA19-9 cys-diabody for positron emission tomography imaging of pancreatic cancer and targeting of polymerized liposomal nanoparticles. J Surg Res. 2013;185:45-55
349. Alarfaj NA, El-Tohamy MF, Oraby HF. CA19-9 pancreatic tumor marker fluorescence immunosensing detection via immobilized carbon quantum dots conjugated gold nanocomposite. Int J Mol Sci. 2018;19:1162
350. Luo Y, Li Y, Li J, Fu C, Yu X, Wu L. Hyaluronic acid-mediated multifunctional iron oxide-based MRI nanoprobes for dynamic monitoring of pancreatic cancer. RSC Advances. 2019;9:10486-93
351. Guimaraes AR, Rakhlin E, Weissleder R, Thayer SP. Magnetic resonance imaging monitors physiological changes with antihedgehog therapy in pancreatic adenocarcinoma xenograft model. Pancreas. 2008;37:440-4
352. Kelly KA, Bardeesy N, Anbazhagan R, Gurumurthy S, Berger J, Alencar H. et al. Targeted nanoparticles for imaging incipient pancreatic ductal adenocarcinoma. PLoS medicine. 2008;5:e85
353. Chen X, Zhou H, Li X, Duan N, Hu S, Liu Y. et al. Plectin-1 targeted dual-modality nanoparticles for pancreatic cancer imaging. EBioMedicine. 2018;30:129-37
354. Rosenberger I, Strauss A, Dobiasch S, Weis C, Szanyi S, Gil-Iceta L. et al. Targeted diagnostic magnetic nanoparticles for medical imaging of pancreatic cancer. J Control Release. 2015;214:76-84
355. Qin H, Qin B, Yuan C, Chen Q, Xing D. Pancreatic cancer detection via Galectin-1-targeted thermoacoustic imaging: Validation in an in vivo heterozygosity model. Theranostics. 2020;10:9172-85
356. Qiu W, Chen R, Chen X, Zhang H, Song L, Cui W. et al. Oridonin-loaded and GPC1-targeted gold nanoparticles for multimodal imaging and therapy in pancreatic cancer. Int J Nanomedicine. 2018;13:6809-27
357. Kawano T, Murata M, Kang JH, Piao JS, Narahara S, Hyodo F. et al. Ultrasensitive MRI detection of spontaneous pancreatic tumors with nanocage-based targeted contrast agent. Biomaterials. 2018;152:37-46
358. Zou J, Chen S, Li Y, Zeng L, Lian G, Li J. et al. Nanoparticles modified by triple single chain antibodies for MRI examination and targeted therapy in pancreatic cancer. Nanoscale. 2020;12:4473-90
359. Li H, Wang P, Deng Y, Zeng M, Tang Y, Zhu W-H. et al. Combination of active targeting, enzyme-triggered release and fluorescent dye into gold nanoclusters for endomicroscopy-guided photothermal/photodynamic therapy to pancreatic ductal adenocarcinoma. Biomaterials. 2017;139:30-38
360. Zhou W, Zhou Y, Chen X, Ning T, Chen H, Guo Q. et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials. 2021;268:120546
361. Chen X, Zhou W, Liang C, Shi S, Yu X, Chen Q. et al. Codelivery nanosystem targeting the deep microenvironment of pancreatic cancer. Nano Lett. 2019;19:3527-34
362. Kitahata Y, Kanuma T, Hayashi M, Kobayashi N, Ozasa K, Kusakabe T. et al. Circulating nano-particulate TLR9 agonist scouts out tumor microenvironment to release immunogenic dead tumor cells. Oncotarget. 2016;7:48860-9
363. Park S, Kim D, Wu G, Jung H, Park JA, Kwon HJ. et al. A peptide-CpG-DNA-liposome complex vaccine targeting TM4SF5 suppresses growth of pancreatic cancer in a mouse allograft model. Onco Targets Ther. 2018;11:8655-72
364. Furugaki K, Cui L, Kunisawa Y, Osada K, Shinkai K, Tanaka M. et al. Intraperitoneal administration of a tumor-associated antigen SART3, CD40L, and GM-CSF gene-loaded polyplex micelle elicits a vaccine effect in mouse tumor models. PLoS One. 2014;9:e101854
365. Ristorcelli E, Beraud E, Mathieu S, Lombardo D, Verine A. Essential role of Notch signaling in apoptosis of human pancreatic tumoral cells mediated by exosomal nanoparticles. Int J Cancer. 2009;125:1016-26
366. Zhao X, Yang K, Zhao R, Ji T, Wang X, Yang X. et al. Inducing enhanced immunogenic cell death with nanocarrier-based drug delivery systems for pancreatic cancer therapy. Biomaterials. 2016;102:187-97
Author contact
![]() Corresponding authors: Maneesh Jain, Ph.D., E-mail: mjainedu, Phone: 402-559-7667; Balaji Narasimhan, Ph.D., E-mail: nbalajiedu, Phone: 515-294-8019; Aliasger K. Salem, Ph. D., E-mail: aliasger-salemedu, Phone: 319-335-8796.
Corresponding authors: Maneesh Jain, Ph.D., E-mail: mjainedu, Phone: 402-559-7667; Balaji Narasimhan, Ph.D., E-mail: nbalajiedu, Phone: 515-294-8019; Aliasger K. Salem, Ph. D., E-mail: aliasger-salemedu, Phone: 319-335-8796.
 Global reach, higher impact
Global reach, higher impact