13.3
Impact Factor
Theranostics 2021; 11(19):9687-9704. doi:10.7150/thno.60851 This issue Cite
Research Paper
Inhibition of tumor progression and M2 microglial polarization by extracellular vesicle-mediated microRNA-124 in a 3D microfluidic glioblastoma microenvironment
1. Research Center for Bioconvergence Analysis, Korea Basic Science Institute, Chungbuk 28119, Republic of Korea.
2. Center for Scientific Instrumentation, Korea Basic Science Institute, Chungbuk 28119, Republic of Korea.
3. Program in Biomicro System Technology, Korea University, Seoul 02841, Republic of Korea.
4. Department of Bioengineering and Nano-Bioengineering, Incheon National University, Incheon 22012, Republic of Korea.
5. Brain Science Institute, Korea Institute of Science and Technology, Seoul 02792, Republic of Korea.
6. KU-KIST Graduate School of Converging Science and Technology, Korea University, Seoul 02841, Republic of Korea.
7. Division of Bio-Medical Science & Technology, University of Science and Technology, Daejeon 34113, Republic of Korea.
8. School of Mechanical Engineering, Korea University, Seoul 02841, Republic of Korea.
9. Division of Bioengineering, Incheon National University, Incheon 22012, Republic of Korea.
10. Department of Bio-Analytical Science, University of Science and Technology, Daejeon 34113, Republic of Korea.
Abstract
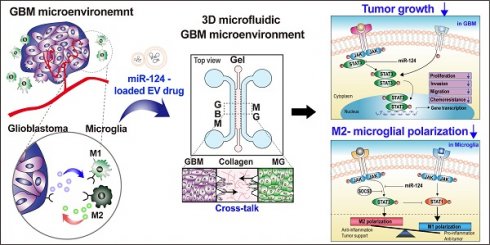
Background: Glioblastoma (GBM) is one of the most aggressive types of brain cancer. GBM progression is closely associated with microglia activation; therefore, understanding the regulation of the crosstalk between human GBM and microglia may help develop effective therapeutic strategies. Elucidation of efficient delivery of microRNA (miRNA) via extracellular vesicles (EVs) and their intracellular communications is required for therapeutic applications in GBM treatment.
Methods: We used human GBM cells (U373MG) and human microglia. MiRNA-124 was loaded into HEK293T-derived EVs (miR-124 EVs). Various anti-tumor effects (proliferation, metastasis, chemosensitivity, M1/M2 microglial polarization, and cytokine profile) were investigated in U373MG and microglia. Anti-tumor effect of miR-124 EVs was also investigated in five different patient-derived GBM cell lines (SNU-201, SNU-466, SNU-489, SNU-626, and SNU-1105). A three-dimensional (3D) microfluidic device was used to investigate the interactive microenvironment of the tumor and microglia.
Results: MiR-124 EVs showed highly efficient anti-tumor effects both in GBM cells and microglia. The mRNA expression levels of tumor progression and M2 microglial polarization markers were decreased in response to miR-124 EVs. The events were closely related to signal transducer and activator of transcription (STAT) 3 signaling in both GBM and microglia. In 3D microfluidic experiments, both U373MG and microglia migrated to a lesser extent and showed less-elongated morphology in the presence of miR-124 EVs compared to the control. Analyses of changes in cytokine levels in the microfluidic GBM-microglia environment showed that the treatment with miR-124 EVs led to tumor suppression and anti-cancer immunity, thereby recruiting natural killer (NK) cells into the tumor.
Conclusions: In this study, we demonstrated that EV-mediated miR-124 delivery exerted synergistic anti-tumor effects by suppressing the growth of human GBM cells and inhibiting M2 microglial polarization. These findings provide new insights toward a better understanding of the GBM microenvironment and provide substantial evidence for the development of potential therapeutic strategies using miRNA-loaded EVs.
Keywords: glioblastoma, microglia, extracellular vesicle, microRNA-124, three-dimensional (3D) cell culture
 Global reach, higher impact
Global reach, higher impact