13.3
Impact Factor
Theranostics 2021; 11(19):9587-9604. doi:10.7150/thno.65277 This issue Cite
Research Paper
Lysyl hydroxylase 1 (LH1) deficiency promotes angiotensin II (Ang II)-induced dissecting abdominal aortic aneurysm
1. State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100037, China.
2. Department of Pathology, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100037, China.
3. State Key Laboratory of Cardiovascular Disease, Center of Vascular Surgery, Fuwai Hospital, National Center for Cardiovascular Disease, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100037, China.
4. Department of Vascular Surgery, Fuwai Yunnan Cardiovascular Hospital, Affiliated Cardiovascular Hospital of Kunming Medical University, Kunming, 650102, China.
5. National Health Commission Key Laboratory of Cardiovascular Regenerative Medicine, Fuwai Central-China Hospital, Central-China Branch of National Center for Cardiovascular Diseases, Zhengzhou 450046, China.
*These authors contributed equally to this work.
Abstract
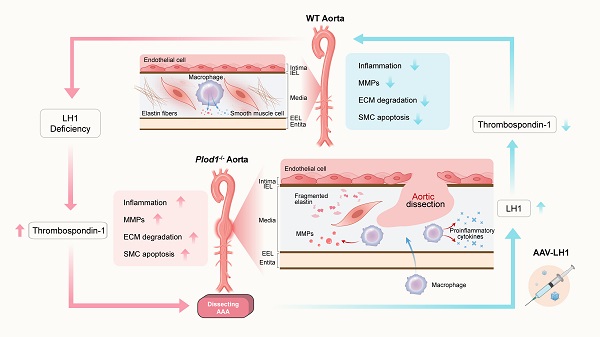
Rationale: The progressive disruption of extracellular matrix (ECM) proteins, particularly early elastin fragmentation followed by abnormalities in collagen fibril organization, are key pathological processes that contribute to dissecting abdominal aortic aneurysm (AAA) pathogenesis. Lysyl hydroxylase 1 (LH1) is essential for type I/III collagen intermolecular crosslinking and stabilization. However, its function in dissecting AAA has not been explored. Here, we investigated whether LH1 is significantly implicated in dissecting AAA progression and therapeutic intervention.
Methods and Results: Sixteen-week-old male LH1-deficient and wild-type (WT) mice on the C57Bl/6NCrl background were infused with angiotensin II (Ang II, 1000 ng/kg per minute) via subcutaneously implanted osmotic pumps for 4 weeks. Ang II increased LH1 levels in the abdominal aortas of WT mice, whereas mice lacking LH1 developed dissecting AAA. To evaluate the related mechanism, we performed whole-transcriptomic analysis, which demonstrated that LH1 deficiency aggravated gene transcription alterations; in particular, the expression of thrombospondin-1 was markedly upregulated in the aortas of LH1-deficient mice. Furthermore, targeting thrombospondin-1 with TAX2 strongly inhibited the proinflammatory process, matrix metalloproteinase (MMP) activity and vascular smooth muscle cells (VSMCs) apoptosis, ultimately decreasing the incidence of dissecting AAA. Restoration of LH1 protein expression in LH1-deficient mice by intraperitoneal injection of an adeno-associated virus normalized thrombospondin-1 levels, subsequently alleviating dissecting AAA formation and preserving aortic structure and function. Consistently, in human AAA specimens, decreased LH1 expression was associated with increased thrombospondin-1 levels.
Conclusions: LH1 deficiency contributes to dissecting AAA pathogenesis, at least in part, by upregulating thrombospondin-1 expression, which subsequently enables proinflammatory processes, MMP activation and VSMCs apoptosis. Our study provides evidence that LH1 is a potential critical therapeutic target for AAA.
Keywords: angiotensin II, dissecting abdominal aortic aneurysm, lysyl hydroxylase, mice, thrombospondin-1
 Global reach, higher impact
Global reach, higher impact