13.3
Impact Factor
Theranostics 2021; 11(19):9492-9502. doi:10.7150/thno.64320 This issue Cite
Research Paper
Neuroprotective effects of minocycline and KML29, a potent inhibitor of monoacylglycerol lipase, in an experimental stroke model: a small-animal positron emission tomography study
1. Department of Advanced Nuclear Medicine Sciences, Institute of Quantum Medical Sciences, National Institutes for Quantum and Radiological Science and Technology, 4-9-1 Anagawa, Inage-ku, Chiba 263-8555, Japan.
2. SHI Accelerator Service Co., 1-17-6 Osaki, Shinagawa-ku, Tokyo 141-0032, Japan.
3. Division of Nuclear Medicine and Molecular Imaging, Massachusetts General Hospital, and Department of Radiology, Harvard Medical School, Boston, Massachusetts 02114, United States.
4. Center of Cyclotron and PET Radiopharmaceuticals, Department of Nuclear Medicine and PET/CT-MRI Center, The First Affiliated Hospital of Jinan University, 613 West Huangpu Road, Tianhe District, Guangzhou 510630, China.
Abstract
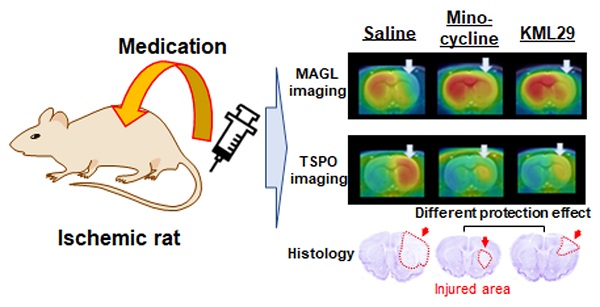
Hypoxia caused by ischemia induces acidosis and neuroexcitotoxicity, resulting in neuronal death in the central nervous system (CNS). Monoacylglycerol lipase (MAGL) is a modulator of 2-arachidonoylglycerol (2-AG), which is involved in retrograde inhibition of glutamate release in the endocannabinoid system. In the present study, we used positron emission tomography (PET) to monitor MAGL-positive neurons and neuroinflammation in the brains of ischemic rats. Additionally, we performed PET imaging to evaluate the neuroprotective effects of an MAGL inhibitor in an ischemic injury model.
Methods: Ischemic-injury rat models were induced by intraluminal right middle cerebral artery occlusion (MCAO). PET studies of the brains of the ischemic rats were performed at several experimental time points (pre-occlusion, days 2, 4, and 7 after the MCAO surgery) using [11C]SAR127303 for MAGL and [18F]FEBMP for 18 kDa translocator protein (TSPO, a hall-mark of neuroinflammation). Medication using minocycline (a well-known neuroprotective agent) or KML29 (a potent MAGL inhibitor) was given immediately after the MCAO surgery and then daily over the subsequent three days.
Results: PET imaging of the ischemic rats using [11C]SAR127303 showed an acute decline of radioactive accumulation in the ipsilateral side at two days after MCAO surgery (ratio of the area under the curve between the ipsilateral and contralateral sides: 0.49 ± 0.04 in the cortex and 0.73 ± 0.02 in the striatum). PET imaging with [18F]FEBMP, however, showed a moderate increase in accumulation of radioactivity in the ipsilateral hemisphere on day 2 (1.36 ± 0.11), and further increases on day 4 (1.72 ± 0.15) and day 7 (1.99 ± 0.06). Treatment with minocycline or KML29 eased the decline in radioactive accumulation of [11C]SAR127303 for MAGL (minocycline-treated group: 0.82 ± 0.06 in the cortex and 0.81 ± 0.05 in the striatum; KML29-treated group: 0.72 ± 0.07 in the cortex and 0.88 ± 0.04 in the striatum) and increased uptake of [18F]FEBMP for TSPO (minocycline-treated group: 1.52 ± 0.21 in the cortex and 1.56 ± 0.11 in the striatum; KML29-treated group: 1.63 ± 0.09 in the cortex and 1.50 ± 0.17 in the striatum). In MCAO rats, minocycline treatment showed a neuroprotective effect in the sensorimotor cortex suffering from severe hypoxic injury, whereas KML29 treatment saved neurons in the striatum, including bundles of myelinated axons.
Conclusions: PET imaging allowed visualization of the different neuroprotective effects of minocycline and KML29, and indicated that combination pharmacotherapy using these drugs may be an effective therapy in acute ischemia.
Keywords: MAGL, TSPO, PET, Minocycline, KML29
 Global reach, higher impact
Global reach, higher impact