13.3
Impact Factor
Theranostics 2021; 11(19):9331-9341. doi:10.7150/thno.60385 This issue Cite
Research Paper
Pyruvate kinase M2 regulates fibrosis development and progression by controlling glycine auxotrophy in myofibroblasts
1. Department of Biology, Georgia State University, Atlanta, GA 30303, USA.
2. Department of Chemistry, Georgia State University, Atlanta, GA 30303, USA.
Abstract
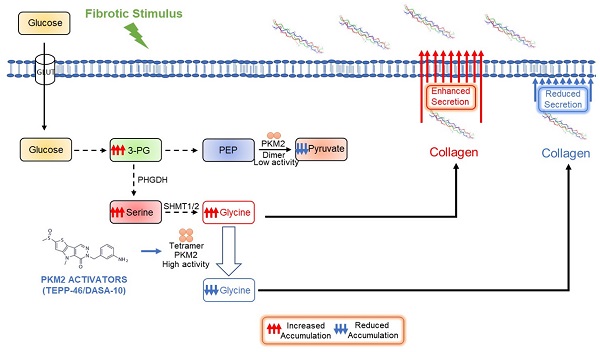
Rationale: Fibrosis is a pathologic condition of abnormal accumulation of collagen fibrils. Collagen is a major extracellular matrix (ECM) protein synthesized and secreted by myofibroblasts, composing mainly (Gly-X-Y)n triplet repeats with >30% Gly residue. During fibrosis progression, myofibroblasts must upregulate glycine metabolism to meet the high demands of amino acids for collagen synthesis.
Method: Expression of PKM2 in myofibroblasts was analyzed in cultured fibroblasts and fibrosis disease tissues. Functional roles of PKM2 and PKM2 activator in biosynthesis of serine → glycine and production of collagen from glycolysis intermediates were assayed in cultured activated LX-2 and human primary lung fibroblast cells. Mouse models of Liver, lung, and pancreas fibrosis were employed to analyze treatment effects of PKM2 activator in organ tissue fibrosis.
Results: We report here that myofibroblast differentiation upregulates pyruvate kinase M2 (PKM2) and promotes dimerization of PKM2. Dimer PKM2 slows the flow rate of glycolysis and channels glycolytic intermediates to de novo glycine synthesis, which facilitates collagen synthesis and secretion in myofibroblasts. Our results show that PKM2 activator that converts PKM2 dimer to tetramer, inhibits fibrosis progression in mouse models of liver, lung, and pancreatic fibrosis. Furthermore, metabolism alteration by dimer PKM2 increases NADPH production, which consequently protects myofibroblasts from apoptosis.
Conclusion: Our study uncovers a novel role of PKM2 in tissue/organ fibrosis, suggesting a possible strategy for treatment of fibrotic diseases using PKM2 activator.
Keywords: Pyruvate kinase M2, fibrosis, fibroblasts, collagen, Metabolism
 Global reach, higher impact
Global reach, higher impact