13.3
Impact Factor
Theranostics 2021; 11(18):8993-9008. doi:10.7150/thno.62302 This issue Cite
Research Paper
Activation of Hippo signaling pathway mediates mitochondria dysfunction and dilated cardiomyopathy in mice
1. Department of Physiology and Pathophysiology, School of Basic Medical Sciences, and Key Laboratory of Environment and Genes Related to Diseases, Ministry of Education, Xian Jiaotong University Health Science Center, Xian, China.
2. School of Life and Environmental Sciences, Deakin University, Geelong, Victoria, Australia.
3. Baker Heart and Diabetes Institute, Melbourne, Victoria, Australia.
4. Center of Electron Microscopy, Xian Jiaotong University Health Science Center, Xian, China.
5. New Jersey Medical School, Department of Cell Biology and Molecular Medicine Rutgers, New Jersey, United States of America.
*These authors contributed equally to this work.
Abstract
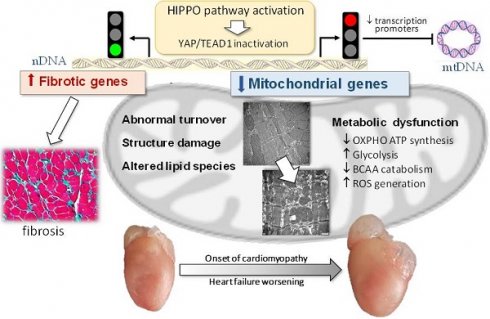
Rationale: Mitochondrial dysfunction facilitates heart failure development forming a therapeutic target, but the mechanism involved remains unclear. We studied whether the Hippo signaling pathway mediates mitochondrial abnormalities that results in onset of dilated cardiomyopathy (DCM).
Methods: Mice with DCM due to overexpression of Hippo pathway kinase Mst1 were studied. DCM phenotype was evident in adult animals but contractile dysfunction was identified as an early sign of DCM at 3 weeks postnatal. Electron microscopy, multi-omics and biochemical assays were employed.
Results: In 3-week and adult DCM mouse hearts, cardiomyocyte mitochondria exhibited overt structural abnormalities, smaller size and greater number. RNA sequencing revealed comprehensive suppression of nuclear-DNA (nDNA) encoded gene-sets involved in mitochondria turnover and all aspects of metabolism. Changes in cardiotranscriptome were confirmed by lower protein levels of multiple mitochondrial proteins in DCM heart of both ages. Mitochondrial DNA-encoded genes were also downregulated; due apparently to repression of nDNA-encoded transcriptional factors. Lipidomics identified remodeling in cardiolipin acyl-chains, increased acylcarnitine content but lower coenzyme Q10 level. Mitochondrial dysfunction was featured by lower ATP content and elevated levels of lactate, branched-chain amino acids and reactive oxidative species. Mechanistically, inhibitory YAP-phosphorylation was enhanced, which was associated with attenuated binding of transcription factor TEAD1. Numerous suppressed mitochondrial genes were identified as YAP-targets.
Conclusion: Hippo signaling activation mediates mitochondrial damage by repressing mitochondrial genes, which causally promotes the development of DCM. The Hippo pathway therefore represents a therapeutic target against mitochondrial dysfunction in cardiomyopathy.
Keywords: Hippo pathway, mitochondria, dilated cardiomyopathy, heart failure, transcriptome analysis
 Global reach, higher impact
Global reach, higher impact