13.3
Impact Factor
Theranostics 2021; 11(18):8964-8976. doi:10.7150/thno.64022 This issue Cite
Research Paper
Pre-therapeutic microglia activation and sex determine therapy effects of chronic immunomodulation
1. Dept. of Nuclear Medicine, University Hospital of Munich, LMU Munich, Munich, Germany.
2. DZNE - German Center for Neurodegenerative Diseases, Munich, Germany.
3. Institute of Neuroscience, Technical University of Munich, Munich, Germany.
4. Department of Diagnostic and Interventional Neuroradiology, Klinikum rechts der Isar, Technical University of Munich, Munich, Germany.
5. Metabolic Biochemistry, Biomedical Center (BMC), Faculty of Medicine, Ludwig-Maximilians-Universität München, Munich, Germany.
6. Institute for Stroke and Dementia Research, University Hospital of Munich, LMU Munich, Munich, Germany.
7. ISAR Bioscience GmbH, 82152 Planegg, Germany.
8. SyNergy, University of Munich, Munich, Germany.
9. Roche Pharma Research and Early Development, Neuroscience Discovery, Roche, Innovation Center Basel, F. Hoffmann-La Roche Ltd., Basel, Switzerland.
10. Center for Neuropathology and Prion Research, Ludwig-Maximilians-University of Munich, Munich, Germany.
11. Dept. of Nuclear Medicine, Inselspital Bern, Bern, Switzerland.
12. School of Psychology and Counselling, Queensland University of Technology, Brisbane, Australia.
Abstract
Modulation of the innate immune system is emerging as a promising therapeutic strategy against Alzheimer's disease (AD). However, determinants of a beneficial therapeutic effect are ill-understood. Thus, we investigated the potential of 18 kDa translocator protein positron-emission-tomography (TSPO-PET) for assessment of microglial activation in mouse brain before and during chronic immunomodulation.
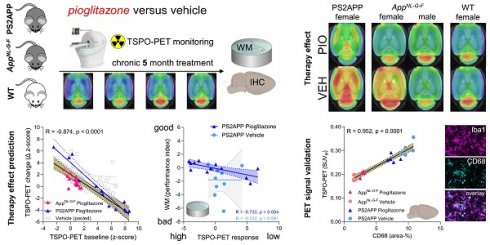
Methods: Serial TSPO-PET was performed during five months of chronic microglia modulation by stimulation of the peroxisome proliferator-activated receptor (PPAR)-γ with pioglitazone in two different mouse models of AD (PS2APP, AppNL-G-F). Using mixed statistical models on longitudinal TSPO-PET data, we tested for effects of therapy and sex on treatment response. We tested correlations of baseline with longitudinal measures of TSPO-PET, and correlations between PET results with spatial learning performance and β-amyloid accumulation of individual mice. Immunohistochemistry was used to determine the molecular source of the TSPO-PET signal.
Results: Pioglitazone-treated female PS2APP and AppNL-G-F mice showed attenuation of the longitudinal increases in TSPO-PET signal when compared to vehicle controls, whereas treated male AppNL-G-F mice showed the opposite effect. Baseline TSPO-PET strongly predicted changes in microglial activation in treated mice (R = -0.874, p < 0.0001) but not in vehicle controls (R = -0.356, p = 0.081). Reduced TSPO-PET signal upon pharmacological treatment was associated with better spatial learning despite higher fibrillar β-amyloid accumulation. Immunohistochemistry confirmed activated microglia to be the source of the TSPO-PET signal (R = 0.952, p < 0.0001).
Conclusion: TSPO-PET represents a sensitive biomarker for monitoring of immunomodulation and closely reflects activated microglia. Sex and pre-therapeutic assessment of baseline microglial activation predict individual immunomodulation effects and may serve for responder stratification.
Keywords: pioglitazone, TSPO-PET, AppNL-G-F mice, PS2APP mice, microglia, sex, prediction
 Global reach, higher impact
Global reach, higher impact