13.3
Impact Factor
Theranostics 2021; 11(16):7735-7754. doi:10.7150/thno.60757 This issue Cite
Research Paper
Orthogonal targeting of osteoclasts and myeloma cells for radionuclide stimulated dynamic therapy induces multidimensional cell death pathways
1. Department of Radiology, Washington University School of Medicine, St. Louis, MO 63110, USA.
2. Department of Medicine, Washington University School of Medicine, St. Louis, MO 63110, USA.
3. Department of Pharmaceutical Sciences, University of Maryland School of Pharmacy, Baltimore, MD 21201, USA.
4. Department of Biomedical Engineering, Washington University, St. Louis, MO 63105, USA.
5. Department of Biochemistry and Molecular Biophysics, Washington University School of Medicine, St. Louis, MO 63110, USA.
Abstract
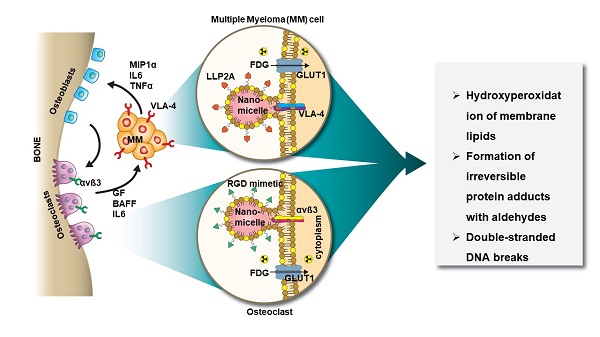
Rationale: Multiple myeloma (MM) is a multifocal malignancy of bone marrow plasma cells, characterized by vicious cycles of remission and relapse that eventually culminate in death. The disease remains mostly incurable largely due to the complex interactions between the bone microenvironment (BME) and MM cells (MMC). In the “vicious cycle” of bone disease, abnormal activation of osteoclasts (OCs) by MMC causes severe osteolysis, promotes immune evasion, and stimulates the growth of MMC. Disrupting these cancer-stroma interactions would enhance treatment response.
Methods: To disrupt this cycle, we orthogonally targeted nanomicelles (NM) loaded with non-therapeutic doses of a photosensitizer, titanocene (TC), to VLA-4 (α4ß1, CD49d/CD29) expressing MMC (MM1.S) and αvß3 (CD51/CD61) expressing OC. Concurrently, a non-lethal dose of a radiopharmaceutical, 18F-fluorodeoxyglucose ([18F]FDG) administered systemically interacted with TC (radionuclide stimulated therapy, RaST) to generate cytotoxic reactive oxygen species (ROS). The in vitro and in vivo effects of RaST were characterized in MM1.S cell line, as well as in xenograft and isograft MM animal models.
Results: Our data revealed that RaST induced non-enzymatic hydroperoxidation of cellular lipids culminating in mitochondrial dysfunction, DNA fragmentation, and caspase-dependent apoptosis of MMC using VLA-4 avid TC-NMs. RaST upregulated the expression of BAX, Bcl-2, and p53, highlighting the induction of apoptosis via the BAK-independent pathway. The enhancement of multicopper oxidase enzyme F5 expression, which inhibits lipid hydroperoxidation and Fenton reaction, was not sufficient to overcome RaST-induced increase in the accumulation of irreversible function-perturbing α,ß-aldehydes that exerted significant and long-lasting damage to both DNA and proteins. In vivo, either VLA-4-TC-NM or αvß3-TC-NMs RaST induced a significant therapeutic effect on immunocompromised but not immunocompetent MM-bearing mouse models. Combined treatment with both VLA-4-TC-NM and αvß3-TC-NMs synergistically inhibited osteolysis, reduced tumor burden, and prevented rapid relapse in both in vivo models of MM.
Conclusions: By targeting MM and bone cells simultaneously, combination RaST suppressed MM disease progression through a multi-prong action on the vicious cycle of bone cancer. Instead of using the standard multidrug approach, our work reveals a unique photophysical treatment paradigm that uses nontoxic doses of a single light-sensitive drug directed orthogonally to cancer and bone cells, followed by radionuclide-stimulated generation of ROS to inhibit tumor progression and minimize osteolysis in both immunocompetent murine and immunocompromised human MM models.
Keywords: multiple myeloma, bone marrow, tumor microenvironment, orthogonal drug delivery, nanomicelles, photosensitizer, Cerenkov radiation
 Global reach, higher impact
Global reach, higher impact