13.3
Impact Factor
Theranostics 2021; 11(16):7700-7714. doi:10.7150/thno.61459 This issue Cite
Research Paper
Low-dose total body irradiation facilitates antitumoral Th1 immune responses
1. Werner Siemens Imaging Center, Department of Preclinical Imaging and Radiopharmacy, Eberhard Karls University Tuebingen, Germany.
2. Department of Medical Oncology and Pneumology, Internal Medicine VIII, Eberhard Karls University Tuebingen, Germany.
3. Division of Radiobiology and Molecular Environmental Research, Department of Radiation Oncology, Eberhard Karls University Tuebingen, Germany.
4. Department of Dermatology, Eberhard Karls University Tuebingen, Germany.
5. Cluster of Excellence iFIT (EXC 2180) "Image-Guided and Functionally Instructed Tumor Therapies", Eberhard Karls University Tuebingen, Germany.
6. German Cancer Consortium (DKTK) and German Cancer Research Center (DKFZ), Heidelberg, Germany.
* These authors contributed equally
Abstract
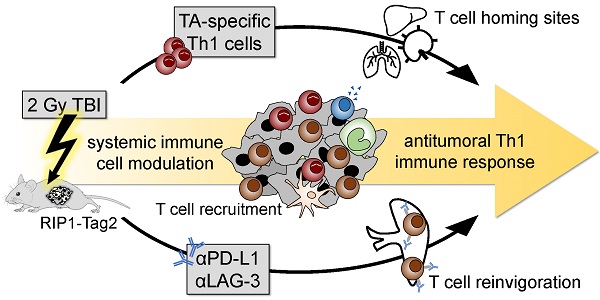
CD4+ T helper cells are capable of mediating long-term antitumoral immune responses. We developed a combined immunotherapy (COMBO) using tumor antigen-specific T helper 1 cells (Tag-Th1), dual PD-L1/LAG-3 immune checkpoint blockade, and a low-dose total body irradiation (TBI) of 2 Gy, that was highly efficient in controlling the tumor burden of non-immunogenic RIP1-Tag2 mice with late-stage endogenous pancreatic islet carcinomas. In this study, we aimed to explore the impact of 2 Gy TBI on the treatment efficacy and the underlying mechanisms to boost CD4+ T cell-based immunotherapies.
Methods: Heavily progressed RIP1-Tag2 mice underwent COMBO treatment and their survival was compared to a cohort without 2 Gy TBI. Positron emission tomography/computed tomography (PET/CT) with radiolabeled anti-CD3 monoclonal antibodies and flow cytometry were applied to investigate 2 Gy TBI-induced alterations in the biodistribution of endogenous T cells of healthy C3H mice. Migration and homing properties of Cy5-labeled adoptive Tag-Th1 cells were monitored by optical imaging and flow cytometric analyses in C3H and tumor-bearing RIP1-Tag2 mice. Splenectomy or sham-surgery of late-stage RIP1-Tag2 mice was performed before onset of COMBO treatment to elucidate the impact of the spleen on the therapy response.
Results: First, we determined a significant longer survival of RIP1-Tag2 mice and an increased CD4+ T cell tumor infiltrate when 2 Gy TBI was applied in addition to Tag-Th1 cell PD-L1/LAG-3 treatment. In non-tumor-bearing C3H mice, TBI induced a moderate host lymphodepletion and a tumor antigen-independent accumulation of Tag-Th1 cells in lymphoid and non-lymphoid organs. In RIP1-Tag2, we found increased numbers of effector memory-like Tag-Th1 and endogenous CD4+ T cells in the pancreatic tumor tissue after TBI, accompanied by a tumor-specific Th1-driven immune response. Furthermore, the spleen negatively regulated T cell effector function by upregulation PD-1/LAG-3/TIM-3 immune checkpoints, providing a further rationale for this combined treatment approach.
Conclusion: Low-dose TBI represents a powerful tool to foster CD4+ T cell-based cancer immunotherapies by favoring Th1-driven antitumoral immunity. As TBI is a clinically approved and well-established technique it might be an ideal addition for adoptive cell therapy with CD4+ T cells in the clinical setting.
Keywords: Total body irradiation, cancer immunology, T helper cells, combined immunotherapy, RIP1-Tag2
 Global reach, higher impact
Global reach, higher impact