13.3
Impact Factor
Theranostics 2021; 11(15):7570-7588. doi:10.7150/thno.58380 This issue Cite
Research Paper
Lactate dehydrogenase B regulates macrophage metabolism in the tumor microenvironment
1. Institute of Biochemistry I, Faculty of Medicine, Goethe-University Frankfurt, 60590 Frankfurt, Germany.
2. Department for Bioinformatics and Biochemistry, BRICS, Technische Universität Braunschweig, Rebenring 56, 38106 Braunschweig, Germany.
3. Institute of Clinical Chemistry and Clinical Pharmacology, University Hospital Bonn, 53127 Bonn, Germany.
4. Helmholtz Centre for Infection Research, Inhoffenstraße 7, 38124 Braunschweig, Germany.
5. German Cancer Consortium (DKTK), Partner Site Frankfurt, 60590 Frankfurt, Germany.
6. Frankfurt Cancer Institute, Goethe-University Frankfurt, 60596 Frankfurt, Germany.
7. Fraunhofer Institute for Translational Medicine and Pharmacology ITMP, 60596 Frankfurt, Germany.
* These authors jointly supervised the work.
Abstract
Background: Glucose metabolism in the tumor-microenvironment is a fundamental hallmark for tumor growth and intervention therein remains an attractive option for anti-tumor therapy. Whether tumor-derived factors such as microRNAs (miRs) regulate glucose metabolism in stromal cells, especially in tumor-associated macrophages (TAMs), to hijack them for trophic support, remains elusive.
Methods: Ago-RIP-Seq identified macrophage lactate dehydrogenase B (LDHB) as a target of tumor-derived miR-375 in both 2D/3D cocultures and in murine TAMs from a xenograft mouse model. The prognostic value was analyzed by ISH and multiplex IHC of breast cancer patient tissues. Functional consequences of the miR-375-LDHB axis in TAMs were investigated upon mimic/antagomir treatment by live metabolic flux assays, GC/MS, qPCR, Western blot, lentiviral knockdown and FACS. The therapeutic potential of a combinatorial miR-375-decoy/simvastatin treatment was validated by live cell imaging.
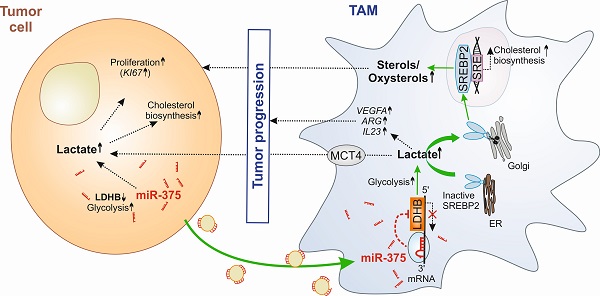
Results: Macrophage LDHB decreased in murine and human breast carcinoma. LDHB downregulation increase aerobic glycolysis and lactagenesis in TAMs in response to tumor-derived miR-375. Lactagenesis reduced fatty acid synthesis but activated SREBP2, which enhanced cholesterol biosynthesis in macrophages. LDHB downregulation skewed TAMs to function as a lactate and sterol/oxysterol source for the proliferation of tumor cells. Restoring of LDHB expression potentiated inhibitory effects of simvastatin on tumor cell proliferation.
Conclusion: Our findings identified a crucial role of LDHB in macrophages and established tumor-derived miR-375 as a novel regulator of macrophage metabolism in breast cancer, which might pave the way for strategies of combinatorial cancer cell/stroma cell interventions.
Keywords: Breast cancer, LDHB, RNA therapeutics, metabolism, tumor-associated macrophages.
 Global reach, higher impact
Global reach, higher impact