13.3
Impact Factor
Theranostics 2021; 11(9):4436-4451. doi:10.7150/thno.54004 This issue Cite
Review
Exosome-inflammasome crosstalk and their roles in inflammatory responses
Medical Proteomics Unit, Office for Research and Development, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok 10700, Thailand.
Received 2020-10-2; Accepted 2021-2-9; Published 2021-3-4
Abstract
Inflammasome is a complex of multiple proteins found in cytoplasm of the cells activated by infectious and/or non-infectious stimuli. This complex involves caspase-1 activation, leading to unconventional secretion of interleukin-1β (IL-1β) and IL-18 and inflammatory cascade. Exosome is the nanoscale membrane-bound extracellular vesicle that plays significant roles in intercellular communications by carrying bioactive molecules, e.g., proteins, RNAs, microRNAs (miRNAs), DNAs, from one cell to the others. In this review, we provide the update information on the crosstalk between exosome and inflammasome and their roles in inflammatory responses. The effects of inflammasome activation on exosomal secretion are summarized. On the other hand, the (dual) effects of exosomes on inhibiting and promoting inflammasome activation are discussed. Finally, perspectives on therapeutic roles of exosomes in human diseases and future direction of the research on exosome-inflammasome crosstalk are provided.
Keywords: caspase-1, IL-1β, IL-18, inflammatory disease, miRNAs, NLRP1, proteins, therapeutics
Introduction
Inflammation is an immune response that can be triggered by infectious and/or non-infectious stimuli, e.g., cell damage, physical injury, toxins. This response is primarily triggered to cope with such harmful stimuli and to promote repairing process [1]. However, excessive or prolonged inflammation has deleterious effects that finally lead to tissue damage as observed in many inflammatory diseases, e.g., rheumatoid arthritis, colitis, asthma [2]. Inflammation is initiated after host proteins, collectively known as pattern recognition receptors (PRRs), bind to exogenous molecules called pathogen-associated molecular patterns (PAMPs) (leading to infection-related inflammation) or to endogenous ones called damage-associated molecular patterns (DAMPs) (leading to non-infection-induced inflammation). These bindings activate downstream intracellular signaling pathways, leading to production and secretion of inflammatory cytokines, which can exert pro-inflammatory or anti-inflammatory effects based on type of the stimuli and stage of inflammatory processes [1].
Known PRRs include: 1) Toll-like receptors (TLRs), 2) C-type lectin receptors (CLRs), 3) retinoic acid-inducible gene (RIG)-I-like receptors (RLRs), 4) nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), 5) absent in melanoma-2 (AIM2)-like receptors (ALRs), and 6) pyrin [3]. TLRs and CLRs are membrane proteins responsible for recognition of extracellular stimuli, whereas the rest are cytoplasmic receptors that recognize intracellular ligands. Pyrin protein and some members of NLRs and ALRs are responsible for activation of inflammasome, which is a cytoplasmic multi-protein complex that promotes caspase-1-dependent secretion of interleukin-1β (IL-1β) and IL-18 and pyroptosis [3].
The term “inflammasome” was first described by Martinon and colleagues in 2002 as a large protein complex assembled upon recognition of PAMPs or DAMPs by PRRs [4]. Among several proteins recruited into this complex, the last one is pro-caspase-1 that is then activated to caspase-1, resulting in unconventional secretion of IL-1β and IL-18, which are the cytokines lacking signal peptide [4, 5]. Interestingly, various PRRs cause generation of different inflammasome complexes [6]. One of the possible routes that IL-1β and IL-18 are unconventionally secreted from the cells is via releasing of endosomal vesicles, namely exosomes, because IL-1β and IL-18 are found in the released exosomes [5, 7-9].
Exosomes are the nanoscale membrane-bound extracellular vesicles (EVs) that are secreted by most of eukaryotic cells and contain bioactive molecules, e.g., proteins, RNAs, microRNAs (miRNAs), DNAs, metabolites, which can be transferred from one cell to the others [10-13]. Therefore, exosomes have important roles in the intercellular communications by transferring different messages between cells in the form of bioactive molecules they carry [14]. Depending on molecules packed into exosomes, they can perform various tasks, e.g., promoting cell proliferation and migration [15], lowering oxidative stress and apoptosis [16], activating cytokine secretion [17], and inhibiting or stimulating inflammasome activation (discussed later in this review).
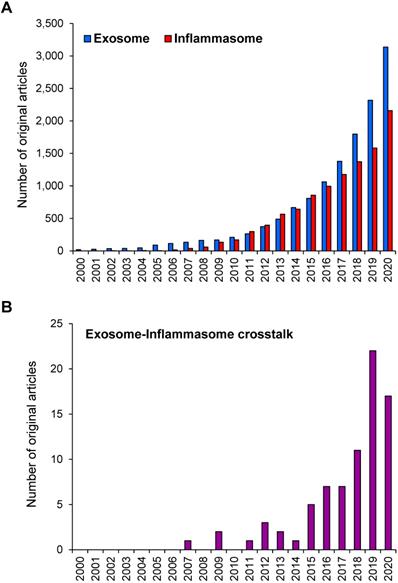
During the past two decades, research on exosome and inflammasome has gained a worldwide attention. Based on the PubMed search using the keyword “exosome” OR “inflammasome”, numbers of the original articles reported on the studies in these two areas have been increasing exponentially since 2000 (reviews and other non-original articles were excluded) (Figure 1A). Using the term “exosome AND inflammasome”, number of the original articles reporting the exosome-inflammasome crosstalk has been also increasing (Figure 1B). Although the latter number is relatively small compared with that of each field, these data indicate the emerging roles of exosome-inflammasome crosstalk in biomedical sciences. In this review, we provide the update information on the crosstalk between exosome and inflammasome as well as their roles in inflammatory responses. Some studies have demonstrated that inflammasome activation can regulate the release of exosomes. On the other hand, other lines of evidence have shown that exosomes are the upstream regulators for inflammasome activation.
An overview of inflammation
Common symptoms of local inflammation are swelling, redness, heat and pain resulting from pro-inflammatory cytokines released by immune cells in response to infection, toxicity, tissue injury, etc. These pro-inflammatory cytokines play crucial roles in recruiting immune cells to the injured sites, stimulating cell proliferation, and inducing apoptosis, all of which finally lead to destruction of the foreign bodies, toxins or microbes, elimination of the damaged cells, and restoration of homeostasis [2, 18]. Upon triggering of PRRs by PAMPs or DAMPs, intracellular inflammatory signaling pathways are activated accompanied with upregulation or downregulation of several genes/proteins and chemokine secretion [18, 19]. These inflammatory signaling pathways include nuclear factor kappa-B (NF-κB), Janus kinase (JAK)-signal transducer and activator of transcription (STAT), and mitogen-activated protein kinase (MAPK) pathways. Among them, NF-κB is the key pathway responding to PAMPs or DAMPs, while JAK-STAT and MAPK pathways respond primarily to cell stress, cytokines, or hormones [18, 20].
Number of the original articles on exosome and/or inflammasome in PubMed. (A) Number of the original articles using the keyword “exosome” OR “inflammasome”. (B) Number of the original articles using the keyword “exosome AND inflammasome”. Note that the reviews and other non-original articles were excluded.
Interestingly, NF-κB pathway has a direct link with inflammasome, because IL1B gene encoding IL-1β is one of the targets for NF-κB transcription factor [21]. However, activation of NF-κB pathway alone is not sufficient to stimulate inflammasome-dependent IL-1β secretion (discussed in more detail in the next section). Under unstimulated condition, NF-κB binds to its inhibitor (IκB) and remains inactive in the cytoplasm. Once stimulated, NF-κB is released from IκB and then activated, followed by translocation into nucleus to bind and modify its targets, e.g., tumor necrosis factor α (TNFα), IL-1β, IL-6, IL-8, IL-18, C-X-C motif chemokine 10 [1, 22, 23]. Receptors located upstream of NF-κB activation are TLRs, TNF receptor (TNFR), T-cell receptor, B-cell receptor, and IL-1 receptor (IL-1R) [22].
Inflammasome complex formation and activation
Inflammasome is a complex of multiple proteins found in cytoplasm of the activated cells. Formation of this complex is necessary for caspase-1 activation that leads to conversion of pro-IL-1β and pro-IL-18 to IL-1β and IL-18, respectively, both of which are the active forms that are then secreted out of the cells [24]. In addition, caspase-1 can mediate gasdermin D-induced pyroptosis, which is an inflammatory form of the programed cell death [25]. The inflammasome complex was initially identified by a study that aimed to investigate mechanism of pro-inflammatory caspase activation [4]. Components in this complex that were initially identified include NLRP1 (a protein in NLR family), apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), and caspase-1 [4]. The same research group later reported that NLRP2 and NLRP3 can also form the inflammasome complex with ASC and caspase-1 (but not caspase-5) [26]. Inflammasome is a part of innate immune system, which can be activated by infection or endogenous danger signals such as oxidative stress, self-DNA, and ATP [6, 27-31]. Since the output of inflammasome activation is the secretion of pro-inflammatory cytokines, inflammasome is thus associated with inflammation [1]. Dysregulation of inflammasome activation is the pathophysiologic mechanism underlying many inflammatory diseases, such as multiple sclerosis, atherosclerosis, Parkinson's disease, and Alzheimer's disease [27]. Inflammasome can be activated through two different pathways - canonical and non-canonical activation pathways.
Canonical inflammasome activation
Canonical activation of the inflammasome complex is initiated by cytoplasmic recognition of PAMPs or DAMPs by PRRs [25]. Such binding activates the receptors, resulting in a recruitment of ASC, which acts as an adaptor protein, to the complex. Pro-caspase-1 participates in the complex by binding to the caspase recruitment domain of ASC. Activation of pro-caspase-1 to caspase-1 leads to processing of pro‑IL‑1β and pro‑IL‑18, and then secretion of their mature forms. In addition, active caspase-1 cleaves gasdermin D to generate its N-terminal products, which then translocate to inner leaflet of the cell membrane [25]. The N-terminal fragments of gasdermin D can bind phosphatidylinositol 4-phosphate, phosphatidylinositol 4,5-bisphosphate, and/or phosphatidylserine to form an oligomer. Such oligomerization causes pore formation in the cell membrane, leading to pyroptosis, which is characterized by cell swelling and membrane rupture [32].
The common PRRs in the canonical inflammasome pathway include NLRP1 [4], NLRP3 [33, 34], NLR family apoptosis inhibitory protein (NAIP)-NLRC4 [35, 36], pyrin [37, 38], and AIM2 [28, 39]. These PRRs recognize different PAMPs and DAMPs. Among them, NLRP3 is most frequently investigated and has the most diverse activators [3, 6, 30, 40, 41]. Unlike other PRRs, NLRP3 and pyrin can be activated without a direct contact with their activators. NLRP3 activators, such as nigericin, ATP, gramicidin, and bacterial aerolysin and α-hemolysin, can induce NLRP3 activation through K+ efflux [42]. Amyloid-β, another NLRP3 activator, triggers the lysosomal release of cathepsin B, which then induces NLRP3 activation [43]. For pyrin, toxins released from the endocytosed bacteria can inactivate Rho GTPase, and such inactivation can induce the pyrin inflammasome activation [44].
Non-canonical inflammasome activation
For non-canonical pathway, activation of inflammasome involves function of species-specific caspases (human caspase-4, human caspase-5, or mouse caspase-11). In this pathway, lipopolysaccharide (LPS) from bacterial cells or outer membrane vesicles is endocytosed into the host cells. After LPS is released from the endosomes, it is recognized by human caspase-4, human caspase-5, or mouse caspase-11. These caspases then act as the cytosolic PRRs to bind the intracellular LPS to get activated [6, 45-47]. The activated human caspase-4, human caspase-5, or mouse caspase-11 then cleaves gasdermin D, resulting in pyroptosis [25, 47, 48]. In addition, by unknown mechanism, mouse caspase-11 can stimulate NLRP3 inflammasome formation, leading to secretion of the active forms of IL-1β and IL-18 [6, 49].
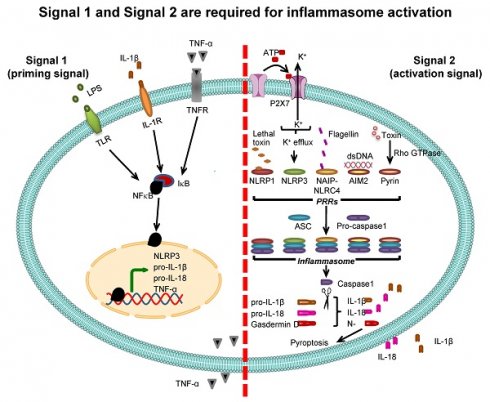
Inflammasome activation. Two signals are required for inflammasome activation. The first is “signal 1” or “priming signal”, which acts through NF-κB signaling pathway. The priming signal starts with binding of PAMPs (e.g., LPS) or DAMPs (e.g., heat shock proteins) to TLR, IL-1β to IL-1R, or TNF-α to TNFR (see more details in Table 1). Such receptor binding subsequently activates NF-κB signaling pathway by dissociating NF-κB from its inhibitor IκB and translocating NF-κB into the nucleus to upregulate expression of its target genes encoding NLRP3, pro-IL-1β, pro-IL-18, and TNF-α. The second is “signal 2” or “activation signal”, which acts through the induction of PRRs by various PAMPs or DAMPs (see more details in Table 2), resulting in recruitment of ASC and pro-caspase-1 to form inflammasome complex with such PRRs. Pro-caspase-1 is then cleaved to caspase-1 that subsequently converts pro-IL-1β and pro-IL-18 to IL-1β and IL-18, respectively, leading to secretion of IL-1β and IL-18. From both signals, the secreted IL-1β and TNF-α can potentiate activation of NF-κB signaling pathway and in turn increase their own production. In addition, caspase-1 cleaves gasdermin D and the N-terminal fragments of gasdermin D cause pyroptosis, an inflammatory form of cell death.
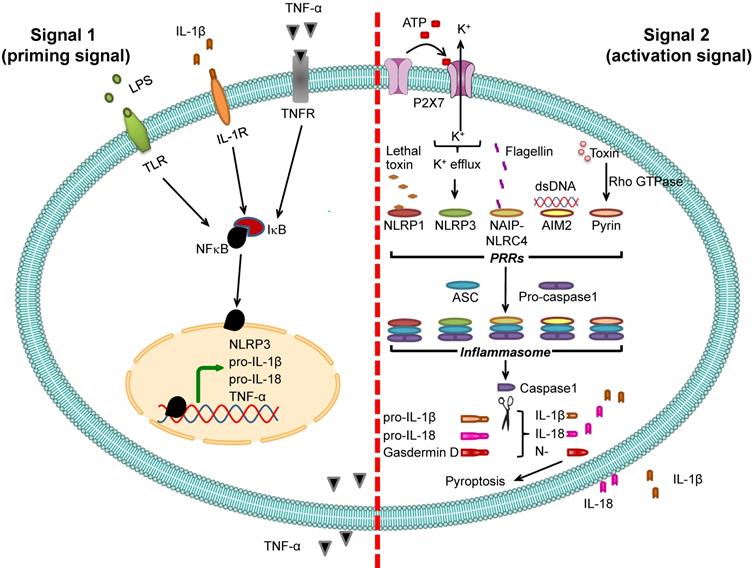
Two signals are required for inflammasome-dependent IL-1β secretion
Pro-IL-1β expression is relatively low under normal physiologic condition but is markedly increased by infection [50]. Promoter region of the gene encoding pro-IL-1β (IL1B) contains binding site for NF-κB transcription factor, and binding of NF-κB to this region activates pro-IL-1β transcription. Therefore, expression of pro-IL-1β is under regulation of NF-κB [21, 51]. Molecules that stimulate NF-κB binding to pro-IL-1β gene (IL1B) promoter are called “signal 1” or “priming signal” (Table 1). They are the extracellular molecules recognized by cell membrane PRRs such as TLRs. On the other hand, the molecules that subsequently activate pro-IL-1β after binding of NF-κB are called “signal 2” or “activation signal” and play essential roles in the inflammasome activation (Table 2). Therefore, inflammasome activation firstly requires signal 1 that activates NF-κB and its translocation into nucleus in order to turn on pro-IL-1β gene (IL1B) expression and secondly needs signal 2, such as ATP and K+ efflux as well as other several PAMPs and DAMPs, to stimulate assembly of inflammasome in order to convert pro-IL-1β to active IL-1β, and to induce its secretion [23, 52] (Figure 2).
Signal 1 of inflammasome activation system
| Pattern recognition receptors (PRRs) | Activators |
|---|---|
| TLR | PAMPs |
| - LPS [115, 116] | |
| - Peptidoglycan [116] | |
| - Zymosan [115, 117] | |
| - Lipoproteins [118] | |
| DAMPs | |
| - Heat shock proteins [119, 120] | |
| - Histones [121] | |
| - Defensins [122] | |
| IL-1R | IL-1β [123] |
| TNFR | TNF-α [124] |
As mentioned above that ligand-bound IL-1R causes NF-κB activation, the secreted IL-1β further enhances its own expression and inflammatory response through NF-κB pathway [23]. In the case of NLRP3-containing inflammasome, NF-κB is important not only for triggering pro-IL-1β expression but also for stimulating NLRP3 expression through binding of the first signal to TLR4 [53]. Stimulation of macrophages with only ATP or nigericin (signal 2) is adequate to initiate inflammasome assembly and to activate caspase-1, when macrophages are overexpressed with NLRP3 [54]. The requirement of signal 1-activated NF-κB to upregulate IL-1β, IL-18 and NLRP3 expression therefore indicates the coordination of inflammasome dependent and independent inflammation for proper inflammatory response.
Signal 2 of inflammasome activation system
| Pattern recognition receptors (PRRs) | Activators | |
|---|---|---|
| PAMPs | DAMPs | |
| NLRP1 | Bacillus anthracis lethal toxin [125] | NA |
| NLRP3 | - Aeromonas hydrophila aerolysin [42] | - ATP and K+ efflux [29, 42, 127] |
| - Gramicidin [42] | - Amyloid-β [43] | |
| - Influenza A virus [59] | - Calcium pyrophosphate dehydrate crystals [128] | |
| - Mycobacterium tuberculosis protein, ESAT-6 [126] | - Monosodium urate crystals [128] | |
| - Maitotoxin [127] | ||
| - Nigericin [42, 127] | ||
| - Staphylococcus aureus α-hemolysin [42] | ||
| - Viral RNA [59] | ||
| NAIP-NLRC4 | - Bacterial type III secretion systems [129] | NA |
| - Bacterial flagellin [35] | ||
| - Pseudomonas aeruginosa [130] | ||
| - Salmonella typhimurium [131] | ||
| AIM2 | Microbial cytoplasmic dsDNA [28, 39, 132] | Host cytoplasmic dsDNA [28, 39, 132] |
| Pyrin | Bacterial toxin# [44] | NA |
N/A: No available information.
#Pyrin does not directly bind to bacterial toxin, but requires inactivation of Rho GTPase caused by the toxin [133].
Any molecules that interfere with either signal 1 or signal 2 can alter inflammasome activation. An endogenous molecule GTPase Rab1a has been reported to play role in regulating plasma membrane localization of TLR4, and dysfunction of Rab1a prevents NF-κB activation, thereby inhibiting NLRP3 inflammasome activation [55]. M013 protein from Myxoma virus has been shown to prevent nuclear translocation of NF-κB and, as a result, IL-1β secretion is reduced [56]. In addition, treating the cells with a specific inhibitor of NF-κB can suppress pro-IL-1β transcription upon activation [57]. Moreover, betaine inhibits inflammasome activation by interrupting both NF-κB translocation and inflammasome assembly [58].
A remark here is that the mechanisms for inflammasome activation discussed above are just some of the common phenomena that generally occur. It should be noted that various types of the cells may express differential PRRs [59]. Moreover, different cells, e.g., macrophages, monocytes, neutrophils and endothelial cells, may use different PRRs and upstream signal pathways of NF-κB activation for the inflammasome activation (for more details please see [60]).
Exosome: biogenesis, biology, function and regulation
Exosome, with a size of approximately 40-150 nm, is one among the three types of membrane-bound EVs, whereas the other two are microvesicle (membrane shedding vesicle, with a size of 150-1,000 nm) and apoptotic body (with a size of 1-5 µm) [11, 61]. In this review, the term “EVs” is used when the cited references refer to all membrane-bound secreted vesicles including exosomes, whereas the term “exosomes” is used when the cited references really mean exosomes in their studies. Exosomes are originated from late endosomes of which membrane becomes inward budding to form intraluminal vesicles (ILVs) within endosomal compartment [61]. The late endosomes containing ILVs, namely multivesicular bodies (MVBs) (also called multivesicular endosomes), then traffic to and fuse with plasma membrane of the cells in order to release such ILVs [62]. Membrane trafficking of the MVBs is regulated by proteins such as small GTPase Rab27, Rab27b and Rab interacting lysosomal protein (RILP), which is a Rab7 trafficking adaptor protein [63, 64]. After release, these ILVs are called exosomes, which can be isolated by ultracentrifugation at 100,000-200,000 ×g. Several proteins have been reported to serve as the markers for exosomes, e.g., CD63, CD9, CD81, ALIX (apoptosis linked gene 2 (ALG-2) interacting protein X), and TSG101 (tumor susceptibility gene 101) [11, 61]. The presence of EVs and exosomes in biological fluids, e.g., blood [65, 66], cerebrospinal fluid [67], amniotic fluid [68], saliva [65, 68], urine [68], and breast milk [65], has been reported and their increase in these biological fluids or conditioned medium is correlated with inflammation [69-71].
As mentioned above, exosomes are composed of various proteins, RNAs, miRNAs, DNAs, lipids, and metabolites. Amounts of these bioactive molecules carried by exosomes vary, depending on type of the cells releasing them [11] and cell differentiation stage [72]. For example, muscle cells in various differentiation stages release exosomes containing differential protein contents (or proteome) [72]. Furthermore, the amounts of several molecules carried by exosomes are altered under specific conditions. For example, exosomes released from virus-infected cells contain high amount of the viral proteins [73, 74]. Cancer cells treated with chemotherapeutic compound secrete exosomes rich with DNA contents [10]. Exosomes derived from LPS-treated macrophages comprise greater amount of several proteins, especially cytokines, compared with the control [75]. Exosomes released from macrophages treated with calcium oxalate monohydrate (COM) contain higher levels of vimentin and annexin A2 but lower levels of heat shock protein 90β and calreticulin [76, 77]. Under abnormal conditions, EVs can carry a high level of inflammasome components such as those found in EVs derived from sera of stroke patients [66], traumatic brain injury patients [78], and reproductively senescent women [79]. In addition, hypoxic and starved mesenchymal stem cells (MSCs) can secrete exosomes containing a high level of metabolites with immunomodulatory activities to induce T-cell activation and macrophage polarization [9].
The molecules carried by exosomes play important roles in transferring messages from the releasing cells to the others. These messages allow the other cells to perceive biological events around them and also guide them how to respond. For example, once exosomes derived from macrophages collected from patients with glioblastoma multiforme (GBM) are taken up by the GBM cells, an miRNA (miR-21) residing in these exosomes can inhibit expression of a tumor suppressor gene PDCD4 (programmed cell death 4) in these cells, allowing the cells to grow further [80]. In addition, these miR-21-containing exosomes induce the recipient GBM cells to resist to a chemotherapy with temozolomide [80]. Transport of miR-1246 by exosomes from human umbilical cord blood mesenchymal stem cells (hUCBMSCs) to the liver reduces inflammation and liver damage caused by hepatic ischemia/reperfusion injury [81]. Exosomes containing miR-155 are able to promote macrophage recruitment into the lungs of naive mice and also increase macrophage proliferation in vitro [70]. Moreover, the non-infected cells can be affected by the virus infection via viral protein-carrying exosomes secreted by the nearby virus-infected cells [74, 82]. Specifically, Epstein‐Barr virus (EBV)-infected B-cells and nasopharyngeal carcinoma (NPC) cell line stably expressed an EBV protein namely latent membrane protein 1 (LMP1) can secrete EVs and exosomes carrying this viral protein. The uptake of these LMP1-containing exosomes by the non-infected B-cells and NPC cells enhances B-cell proliferation, tumor growth and radioresistance of the NPC cells [74, 82]. In a kidney stone model, exosomes derived from COM-treated macrophages enhance T-cell migration and macrophage phagocytic activity [76]. These enhancements are most likely due to the upregulation of vimentin in exosomes derived from the COM-treated macrophages because T-cell migration and macrophage phagocytic activity can be suppressed by knockdown of vimentin in the COM-treated macrophages [76].
Taken together, the aforementioned data indicate that the process, in which various biomolecules are packed into the exosomes, is considerably cell-specific and selective. A protein namely KRAS (Kirsten rat sarcoma viral oncogene homolog) has been reported to participate in sorting miRNAs into exosomes, because differences of miRNAs inside the exosomes secreted from different colorectal cancer cell lines are correlated with mutations of the KRAS gene [83]. Specific packing of miR-143 into exosomes of endothelial cells under shear stress has been shown to be regulated by GTPase Rab7a and Rab27b [84]. The packing of miR-155 into exosomes derive from hepatitis C virus (HCV)-infected hepatoma cells is controlled by RILP [64]. In addition, CD63 participates in loading of LMP1 into exosomes of the EBV-infected cells [85]. Furthermore, different isoforms of neutral sphingomyelinase play roles in packing normal soluble or misfolded forms of cellular prion protein into exosomes secreted from mouse hypothalamic neuronal cell line [86]. Although there are some reports on proteins responsible for selective packing of molecules into EVs, which also include exosomes, a mechanism underlining this process remains unclear [14]. Regarding the secretion of exosomes, various factors have been reported to stimulate exosomal secretion. Acidic environment has been demonstrated to enhance exosomal release from metastatic human melanoma cells [87]. Elevation of intracellular calcium level is another factor triggering the secretion of exosomes [88]. In concordance, binding of extracellular ATP to P2X7 receptor, which causes an increase of cytoplasmic calcium level, also enhances the secretion of exosomes and EVs [89]. Furthermore, hypoxic condition enhances the secretion of exosomes from breast cancer cell lines [90]. As mentioned above that exosomal amount in biological fluids is related to inflammatory condition, the activation of immune pathway, such as LPS-stimulated TLR4 [91] or inflammasome may also enhance exosomal secretion (discussed in more detail in the next section).
Exosome-inflammasome crosstalk
The crosstalk between exosome and inflammasome has been documented with increasing evidence during recent years. Interestingly, some studies have demonstrated that inflammasome activation can regulate the release of exosomes. On the other hand, exosomes are the upstream regulator for inflammasome activation. Most of the latter studies have elucidated the effects of exosomes on inflammasome activation by utilizing exosomes isolated from either biological fluids or cell culture media. The results have demonstrated that exosomes exert the dual effects. Several studies have shown their inhibitor effects on inflammasome activation, whereas other lines of evidence have shown the opposite data indicating the positive correlation between exosomal release and inflammasome activation. We thus summarize all the results obtained from these studies (Tables 3 & 4) and discuss below.
Summary for sources and responsible bioactive molecules of exosomes with inhibitory effects against inflammasome activation
| Sources of exosomes | Releasing cell type | Target cell(s)/tissue(s) | Effector molecule(s) | Reference |
|---|---|---|---|---|
| With paracrine effects | ||||
| Adipose tissue-derived mesenchymal stem cells (AMSCs) | Stem cell | Macrophage and Kupffer cell | miR-17 | [100] |
| Bone marrow derived stem cells (BMSCs) | Stem cell | Nucleus pulposus cell | Mitochondrial proteins | [16] |
| Embryonic stem cells (ESCs) | Stem cell | Cardiomyoblast | N/A | [96] |
| Human periodontal ligament stem cells (hPDLSCs) from patients with relapsing-remitting multiple sclerosis * | Stem cell | Spinal cord | N/A | [101] |
| Human umbilical cord-derived mesenchymal stem cells (hUCMSCs) | Stem cell | Retinal endothelial cell | miR-126 | [97] |
| hUCMSCs | Stem cell | Macrophage | N/A | [99] |
| hUCMSCs | Stem cell | Macrophage | miR-181c | [98] |
| Cyclic stretch-stimulated periodontal ligament (PDL) cells | Fibroblast-like cell | Macrophage | N/A | [102] |
| With paracrine and/or autocrine effects | ||||
| Fetal bovine serum # | N/A | Macrophage | N/A | [104] |
*The mixture of exosomes and microvesicles was used in the study.
#EVs were used in the study.
N/A: No available information.
Effects of inflammasome activation on exosomal secretion
Inflammasome activation had been previously assumed to be an enhancer for EVs secretion because several NLRP3 inflammasome activators can also stimulate EVs secretion [92]. Bone marrow-derived macrophages (BMDMs) primed with IFN-γ and LPS secrete exosomes and microvesicles containing major histocompatibility complex (MHC) class II after exposure to ATP [93]. Secretion of the MHC II-containing exosomes, but not the MHC II-containing microvesicles, has been shown to be inflammasome-dependent because BMDMs obtained from mice lacking ASC or NLRP3 fail to secrete these exosomes after IFN-γ/LPS priming followed by ATP treatment [93]. In addition, synovial fibroblasts treated with IL-1β secrete significantly greater amount of exosomes as compared with the untreated control cells [69]. IL-1β may stimulate exosomal secretion via activation of NF-κB because it is one of the NF-κB activators. NF-κB upregulates expression of LAMP-2 and Rab34, which are involved in membrane trafficking [94]. NF-κB also facilitates membrane trafficking of glucose transporter-1 via AKT activation [95]. Recently, a direct evidence for inflammasome-dependent exosomal secretion has been reported [64]. HCV infection or induction with LPS followed by ATP can trigger NLRP3 inflammasome activation in Huh-7.5 cells. Also, NLRP3 overexpression in the uninfected cells increases exosomal secretion. Interestingly, subsequent caspase-1-dependent cleavage of RILP is responsible for the increase in exosomal secretion and packaging of specific miRNAs inside [64]. In addition, fragile X mental retardation 1 (FMR1) protein together with endosomal sorting complex required for transport (ESCRT) plays roles in packaging of specific miRNAs with FMR1-binding motif into the exosomes [64].
By contrast, another study has reported that inflammasome activation may not be required for exosomal secretion. ATP is a known activator (signal 2) of NLRP3 inflammasome complex to induce NLRP3 inflammasome assembly [29] (Table 2). Together with pre-treatment of LPS, IL-1β secretion is enhanced [89]. Interestingly, ATP alone is inadequate to stimulate IL-1β secretion but can strongly induce EVs secretion [89]. This suggests that inflammasome activation may not be required for exosomal secretion.
Although most of the available references indicate that inflammasome activation induces exosomal secretion, the number of such studies is still limited. Due to the contradictory results, the direct effect of inflammasome activation on exosomal secretion remains inconclusive at this phase. Perhaps, types of PRRs, activators and the effector cells are important to determine the outcome. Further elucidations are required to address these issues.
Effects of exosomes on inflammasome activation: Exosomes hinder inflammasome activation
Many recent studies have shown that the release of exosomes is a strategy of the cells to ease inflammation and to prevent tissue damage, which is a common end-point of excessive inflammatory response, by inhibiting inflammasome activation. Interestingly, most of these studies investigated exosomes derived from various stem cells (Table 3). As mentioned above that oxidative stress is one of the stimuli for NLRP3 inflammasome activation, exosomes released from BMSCs can diminish H2O2-induced inflammation and cell death in nucleus pulposus cells by reducing expression levels of NLRP3, caspase-1 and IL-1β, and decreasing cleavages of pro-caspase-3 and pro-caspase-9 [16]. The BMSCs-derived exosomes can also attenuate mitochondrial damage induced by H2O2, and injection of these exosomes into a rabbit model of intervertebral disc degeneration (IVDD) can delay the progression of IVDD [16]. These inhibitory effects of BMSCs-derived exosomes may be driven by mitochondrial proteins carried by the exosomes. Because H2O2 causes oxidative stress and mitochondrial damage, providing the cells with fresh mitochondrial proteins by the exogenous exosomes therefore minimizes the damage and, as a consequence, reduces oxidative stress [16].
Summary for sources and responsible bioactive molecules of exosomes that induce inflammasome activation
| Sources of exosomes | Releasing cell type | Target cell(s)/tissue(s) | Effector molecule(s) | Reference |
|---|---|---|---|---|
| With paracrine effects | ||||
| Amniotic fluid and malignant ascites | N/A | Macrophage | N/A | [57] |
| IL-1β-treated osteoarthritic chondrocytes | Cartilage cell | Macrophage | miR-449a-5p | [107] |
| Lipopolysaccharide (LPS)-treated Raw264.7 macrophages | Immune cell | Hepatocyte | N/A | [75] |
| Palmitate-treated HepG2 cells# | Liver cancer cell | Macrophage | N/A | [108] |
| Plasma of HIV-infected patients | HIV-target cell (Immune cell) | Non-HIV infected macrophage | HIV protein Nef | [105] |
| Plasma of traumatic brain injury patients# | N/A | Pulmonary endothelial cells | Apoptosis associated speck-like protein containing a caspase recruitment domain (ASC) | [78] |
| With paracrine and/or autocrine effects | ||||
| Blue-light irradiated human adult retinal pigment epithelial (hARPE-19) cells | Epithelial cell | Non-irradiated retinal pigment epithelial cell | Active forms of IL-1β, IL-18, and caspase-1 | [9] |
| LPS/nigericin-treated bone marrow-derived macrophages (BMDMs) | Immune cell | Naive or nigericin-treated BMDMs | N/A | [106] |
| Manganese/LPS-treated microglial cells | Immune cell | Naive microglial cell | Apoptosis associated speck-like protein containing a caspase recruitment domain (ASC) | [71] |
#EVs were used in the study.
N/A: No available information.
Doxorubicin is commonly used for cancer treatment; however, it has been shown to stimulate inflammatory response by inducing expression of TLR4, NLRP3, caspase-1, caspase-11, gasdermin D and IL-1β in cardiomyoblasts [96]. By treating the cardiomyoblasts with exosomes derived from embryonic stem cells (ESCs), this inflammasome activating property of doxorubicin is minimized, whereas the exosomes derived from mouse embryonic fibroblasts have no effect on doxorubicin-induced upregulation of those inflammasome mediators [96]. These differential effects of exosomes derived from different cell types indicate cell-specific cargo of biomolecules carried by exosomes.
Retinal inflammation is one of the pathologic findings of diabetic retinopathy. High levels of IL-1β, IL-18 and caspase-1 are detected in vitreous body of diabetic rats [97]. In addition to the vitreous body, the inflammasome-related proteins, including NLRP3, IL-1β, IL-18 and caspase-1, increase in retina of diabetic rats and human retinal endothelial cells exposed to high glucose. This diabetes-induced inflammasome activation is abolished by treatment with exosomes secreted from hUCMSCs, but not the exosomes derived from dermal fibroblasts [97], again indicating the cell-specific exosomal cargo. Such injection of hUCMSCs-derived exosomes led to higher level of miR-126 in the retina, and pretreatment of the exosomes with miR-126 before injection results in dramatic induction of miR-126 in the retina [97]. Such pretreatment is more effective to suppress NLRP3 inflammasome activation induced by high glucose, suggesting that miR-126 is responsible for the inhibitory effects of exosomes against inflammasome activation [97].
Similarly, other studies have shown that hUCMSCs-derived exosomes can reduce expression levels of NLRP3 and caspase-1 in LPS-treated macrophages and prevent IL-1β secretion from these cells [98, 99]. These hUCMSCs-derived exosomes also decrease NLRP3 inflammasome in liver tissues of mice with acute liver injury [99]. The intravenous injection of hUCMSCs-derived exosomes into rats with severe burn can reduce serum IL-1β level, and this anti-inflammatory effect of hUCMSCs-derived exosomes is most likely driven by miR-181c carried by these exosomes [98].
Adipose tissue-derived mesenchymal stem cells (AMSCs) have been shown to release exosomes containing high level of miR-17 [100]. These miR-17-containing exosomes can decrease levels of NLRP3, caspase-1, IL-1β and IL-18 in liver tissues of mice with acute liver failure. Introducing these miR-17-containing exosomes to macrophages or primary Kupffer cells alleviates LPS-induced inflammasome-related protein expression and IL-1β and IL-18 secretion in these immune cells. Moreover, blocking the miR-17 function hampers such inhibitory effects of these exosomes on inflammasome activation [100]. A mixture of exosomes and microvesicles released from human periodontal ligament stem cells (hPDLSCs) obtained from relapsing-remitting multiple sclerosis patients are able to diminish TLR4, NF-κB, NLRP3, caspase-1 and IL-1β levels in spinal cords of mice with encephalomyelitis [101]. All of these data indicate that exosomes derived from stem cells can inhibit inflammasome activation via various mechanisms (mainly by microRNAs carried by the exosomes) (Figure 3).
Inhibition of inflammasome activation by stem cell-derived exosomes. Exosomes released from various stem cells can be uptaken by recipient cells and then interfere with inflammasome activation. These stem cell-derived exosomes carry bioactive molecules (mainly miRNAs) that block the effects of signal 1 by preventing NF-κB dissociation from IκB, leading to inhibition of NF-κB nuclear translocation and reduced expression of its target genes encoding NLRP3, pro- IL-1β, pro-IL-18 and TNF-α. In addition, the stem cell-derived exosomes inhibit formation of the inflammasome complex by PRRs, ASC and pro-caspase-1. As a result, conversion of pro-caspase-1 to caspase-1 and secretion of the pro-inflammatory cytokines, particularly IL-1β, IL-18 and TNF-α, are reduced.
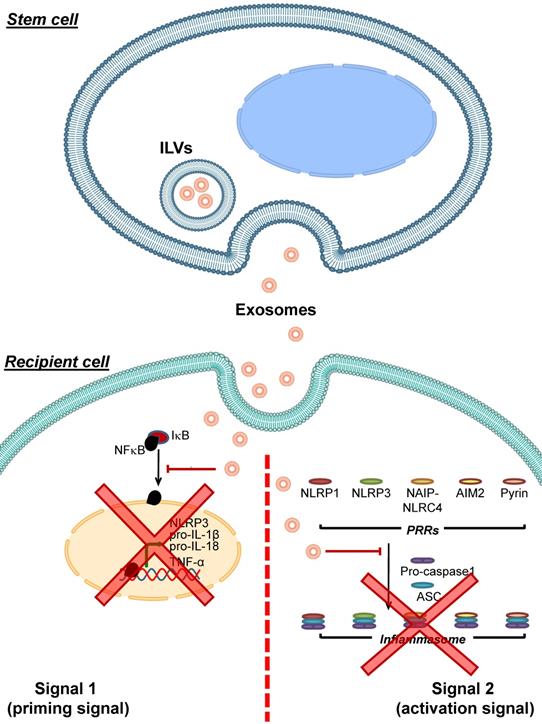
In addition to the MSCs-derived exosomes, periodontal ligament (PDL) cells, which are a component of periodontal tissue supporting teeth regularly exposed to mechanical force during mastication, are able to release exosomes [102]. These exosomes can suppress NRLP3 activation and IL-1β secretion from LPS-primed and nigericin-treated macrophages by preventing NF-κB to translocate into the nucleus, thereby blocking the expression of NLRP3 and pro-IL-1β [102]. EVs are also detected in fetal bovine serum (FBS), which is a common supplement in cell culture medium [103]. These FBS-derived EVs can be uptaken by A549 lung epithelial cells and enhance their migration [103]. Moreover, exosomes purified from FBS can diminish secretion of IL-1β from LPS-treated rat peritoneal macrophages [104]. These data underscore the inhibitory roles of exosomes derived from various cells other than MSCS in modulation of the inflammasome activation. Because of the global interest in research on this topic, much more data will come in the coming years.
Effects of exosomes on inflammasome activation: Exosomes enhance inflammasome activation
Unlike the exosomes derived from stem cells that negatively control inflammasome activation, the exosomes that activate inflammasome are originated from various cell types, including immune, cartilage, epithelial, and cancer cells (Table 4). Among these, immune cells-derived exosomes have the strongest evidence with the largest number of studies to support their roles in inflammasome activation (Figure 4).
Enhancement of inflammasome activation by immune cell-derived exosomes. Exosomes derived from immune cells can be uptaken by recipient cells and then enhance inflammasome activation. These immune cell-derived exosomes carry bioactive molecules that enhance the effects of signal 1 by inducing NF-κB dissociation from IκB, leading to NF-κB nuclear translocation and upregulation of its target genes encoding NLRP3, pro- IL-1β, pro-IL-18 and TNF-α. In addition, the immune cell-derived exosomes enhance formation of the inflammasome complex by PRRs, ASC and pro-caspase-1. As a result, conversion of pro-caspase-1 to caspase-1 and secretion of the pro-inflammatory cytokines, particularly IL-1β, IL-18 and TNF-α, increases.
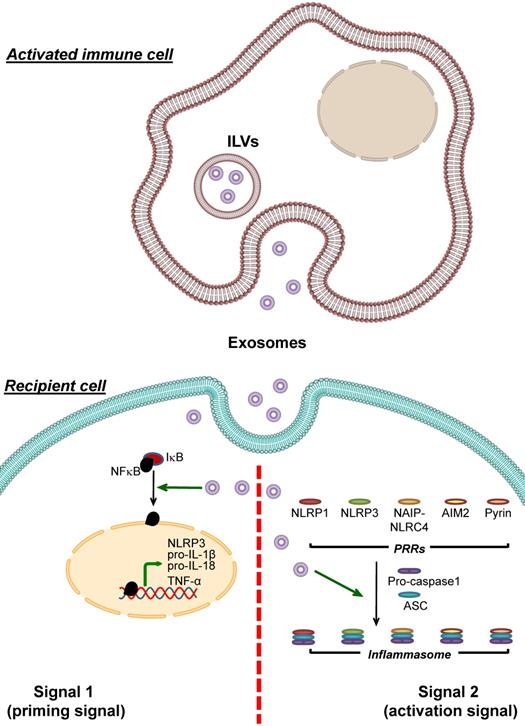
Peripheral blood lymphocytes transfected with provirus plasmid of human immunodeficiency virus (HIV) can release exosomes containing an HIV protein namely Nef [73]. Nef-containing exosomes purified from plasma of HIV-infected patients can be uptaken by the non-infected macrophages, resulting in enhancement of TLR4 translocation to the plasma membranes. As a consequence, these Nef-induced non-infected cells are prone to inflammatory stimuli and secrete more IL-1β and other inflammatory cytokines [105]. ASC-containing EVs isolated from sera of traumatic brain injury patients upregulate expression of inflammasome components and promote inflammasome activation in human pulmonary endothelial cells [78].
BMDMs can secrete exosomes under stimulated and unstimulated conditions. The exosomes derived from BMDMs treated with nigericin following LPS priming can induce expression of NLRP3 and pro-IL-1β in naive BMDMs, and activate cleavage of pro-caspase-1 and secretion of IL-1β [106]. Similarly, the resident immune cells like microglia are able to release exosomes, and the LPS-primed microglia exposed to manganese secrete greater amount of exosomes that possess ASC protein, a component of the inflammasome complex [71]. When naive microgial cells are exposed to these ASC-containing exosomes, ASC can be transferred to the naive cells, leading to increases of NLRP3 and pro-IL-1β in the cells. Moreover, treatment of LPS-primed microglial cells with exosomes purified from sera of manganese-administered mice potentiates IL-1β secretion from the LPS-primed cells [71].
Other than the primary cultured immune cells, macrophage cell line, such as Raw264.7, can also secrete exosomes. Interestingly, the exosomes derived from LPS-treated Raw264.7 cells are uptaken by AML-12 hepatocytes and then upregulate expression of NLRP3, ASC and caspase-1 in the hepatocytes [75]. Furthermore, injection of these exosomes derived from the LPS-treated Raw264.7 macrophages into mice can increase tissue levels of these inflammasome complex proteins in vivo [75].
In addition to the immune cells, other cell types can secrete exosomes that subsequently induce inflammasome activation. Exosomes derived from chondrocytes of patients with osteoarthritis (OA) are able to stimulate IL-1β secretion from LPS/ATP-treated phorbol-12-myristate-13-acetate (PMA)-induced THP1 macrophages [107]. The exosomes derived from OA chondrocytes treated with IL-1β can induce ASC specks in the cytoplasm of the LPS-treated PMA-induced THP1 cells, indicating inflammasome activation. In addition, intra-articular injection of these exosomes accelerates cartilage erosion in a murine model of OA. RNA sequencing and bioinformatics analysis of miRNAs found in the exosomes derived from IL-1β-treated versus untreated OA chondrocytes reveal miR-449a-5p as a candidate molecule with great potential to elicit stimulatory effect on inflammasome activation. As such, treatment with a miR-449a-5p inhibitor effectively blocks this stimulatory effect of exosomes derived from the IL-1β-treated OA chondrocytes [107].
Furthermore, human adult retinal pigment epithelial (hARPE-19) cells irradiated by blue-light have been shown to express high levels of IL-1β, IL-18 and caspase-1, and secrete exosomes containing active forms of these inflammasome-related proteins [9]. Incubation of the unirradiated hARPE-19 cells with these exosomes increase levels of NLRP3 mRNA/protein and active forms of IL-1β, IL-18 and caspase-1 [9]. A cancer cell line, such as HepG2, can secrete EVs in response to palmitate fatty acid treatment. The exosomes released from palmitate-treated HepG2 cells, but not those from vehicle-treated cells and HepG2 treated with palmitate plus lipid-lowering drug (ezetimibe), are able to increase pro-IL-1β expression and the release of mature IL-1β from THP-1 monocytic cells [108]. Moreover, exosomes isolated from malignant ascites and amniotic fluid can induce pro-IL-1β expression and IL-1β secretion in THP-1 monocytic cells [57]. These data obtained from both immune and non-immune cells highlight the important roles of exosomes for inflammasome activation.
Roles of exosomes for non-inflammasome-mediated inflammation
Exosomes not only have an impact on inflammasome-mediated inflammation but also play roles in secretion of non-inflammasome-mediated inflammatory cytokines. Exosomes secreted by hUCMSCs contain miR-1246 and/or miR-181c [81, 98]. These exosomes can reduce levels of pro-inflammatory cytokines, i.e., TNF-α, IL-6 and IL-17, in liver of mice with ischemia/reperfusion injury, liver of rats with severe burn, and LPS-treated macrophages [81, 98]. In consistent, these hUCMSCs-derived exosomes increase levels of the anti-inflammatory cytokines, i.e., IL-10 and transforming growth factor β [81, 98]. Exosomes derived from COM-treated macrophages are able to induce IL-8 secretion from renal tubular cells and monocytes [76, 77]. Exosomes isolated from sera of the LPS-treated mice increase expression levels of TNF-α and IL-6 pro-inflammatory cytokines in lungs of the naive mice injected with these exosomes. These pro-inflammatory properties have been proven to be driven by miR-155 packed into these exosomes [70]. Furthermore, exosomes purified from semen of the fertile men can stimulate secretion of pro-inflammatory cytokines, IL-6 and IL-8, from human endometrial stromal cells [17]. By contrast, exosomes purified from plasma of patients with esophageal squamous cell carcinoma induce anti-inflammatory IL-10 expression in CD19+ B-cells isolated from healthy donors and inhibit proliferation of these cells [109]. These findings indicate that exosomes also play significant roles in the non-inflammasome-mediated inflammation.
Therapeutic roles of exosomes
Since exosomes are able to carry bioactive molecules from one cell to the others and subsequently cause changes in the recipient cells, much wider attention and more extensive efforts have been made recently to implement exosomes as the new therapeutic tools for delivering therapeutic molecules to modify inflammation and related diseases. Several lines of recent evidence have shown that exosomes can be used for delivering short‐interfering RNAs (siRNA) and miRNAs in vivo. An advantage of using exosomes as the carrier for these RNAs is that exosomes prevent RNAs degradation by RNase, thereby increasing the efficiency of RNAs delivery to the target tissues [110]. For example, ASC siRNA can be loaded into exosomes prepared from culture medium of rat cortical neuronal cells [67]. The injection of these ASC siRNA-loaded exosomes into circulatory system of rats with spinal cord injury reduces ASC level in the spinal cords, thereby reducing caspase-1 activation and IL-1β level in the spinal tissue [67].
Similarly, miR-181c found in exosomes secreted from hUCMSCs can reduce TLR4 expression and NFκB activation both in vitro and in vivo [98]. As a consequence, secretion of pro-inflammatory cytokines decreases. Overexpression of miR-181c in hUCMSCs-derived exosomes strongly reduces white blood cell recruitment to the burn site and pro-inflammatory cytokine levels in the wound area in a rat model of severe burn [98]. Administration of hUCMSCs-derived exosomes rich in miR-146a alleviates inflammation by decreasing TNF-α pro-inflammatory cytokine and increasing IL-10 anti-inflammatory cytokine in LPS-treated BMDMs, and improves survival rate of mice with sepsis [111]. Moreover, exosomes derived from M2 polarized BMDMs containing miRNAs, particularly miR-690, rescue insulin sensitivity in Type 2 diabetic mice [112]. Mechanistic study to define the molecule responsible for such therapeutic effect reveals miR-690 that exerts the therapeutic function to improve the insulin sensitivity by interfering with mitochondrial NAD+ kinase level [112]. Similar approach reveals that exosomes with miR-22-3p upregulation can reduce LPS-induced acute lung injury by downregulating frizzled class receptor 6 (FZD6) protein [113]. These data strongly suggest that exosomes can serve as the new therapeutic tools to modulate inflammatory responses in various diseases.
Summary and outlook
Recent evidence has strongly indicated the crosstalk between exosome and inflammasome. On one hand, inflammasome activation can regulate the release of exosomes. Inflammasome can be activated by various stimuli and, as a result, exosomes released under different conditions, treatments or interventions may carry different components. However, precise mechanisms governing specific exosomal cargo loading are not fully understood. Moreover, number of the direct evidence for the effects of inflammasome activation on exosomal secretion is too small and the contradictory results make such effects inconclusive at this stage. Further elucidations on these aspects are therefore required.
On the other hand, exosomes are the upstream of inflammasome activation. Exosomes can either alleviate or enhance inflammasome activation. This discrepancy of exosomal effects on the inflammasome activation is likely affected by type of the cells producing exosomes and interventions or conditions that induce cells to release the exosomes. These two factors affect molecular contents of exosomes and hence determine the effects of exosomes on the target cells. Regardless of inhibitory or stimulatory effects of exosomes, miRNAs are the important molecules eliciting exosomal functions. According to the available references published to date, exosomes released from stem cells elicit inhibitory effects against inflammasome activation in the recipient cells, thereby reducing inflammatory response and preventing tissue damage caused by prolonged inflammation. By contrast, exosomes released from immune cells provoke inflammasome activation, leading to intensification of inflammation and inflammatory diseases. However, the information about the effects of exosomes on inflammasome function at molecular level is still insufficient. It is likely that exosomes released from different immune cells exert differential immunomodulatory activities via different compositions within the exosomal cargo.
During the past few years, a wide attention has been made to the therapeutic potential of exosomes. They can serve as the cargo to transfer the bioactive molecules that can be applied as the drug delivery system for treatment of various human diseases. However, number of the original investigations reporting the results is relatively small. Two questions raised here is the specificity of such therapeutic effects and adverse events of exosomes. A study in mice reveals distribution of the injected exosomes in liver, lung and spleen, but not in cardiac adipose tissue and skeleton muscle, suggesting differential abilities of different tissues to uptake the exogenous exosomes [114]. This study also demonstrates that the injected exosomes are likely to be uptaken by various other cells nonspecifically. Nevertheless, this point is still far from conclusive. As discussed earlier, exosomes derived from different sources/cell types or from different conditions/treatments have different cargo compositions and properties. These differences probably affect exosomal endocytosis by the target cells and may make the process selective to some extent. For this reason, selection of the source of exosomes for a treatment should be done more carefully. In addition, management of different diseases/disorders with various target tissues may require exosomes from different sources. Almost all the studies reported so far have focused only on the ability of cells in the tissue of interest to uptake such exosomes and on the effects of the exogenous exosomes on that particular tissue. However, they have not investigated whether exosomes also exert their effects in other tissues. Moreover, whether inflammasome manipulation affects the therapeutic effects of the administered exosomes remains unclear. Therefore, these aspects deserve further elucidations. Finally, large-scale prospective clinical trials are required to investigate the specific therapeutic effects and adverse events of the exogenous exosomes before they can be applied to clinical practice.
Acknowledgements
We are grateful to Dr. Paleerath Peerapen for her technical assistance on figure illustrations. This work was supported by The Office of National Higher Education Science Research and Innovation Policy Council (NXPO) through PMU-B and the Thailand Research Fund (IRN60W0004).
Author Contributions
All authors (CN and VT) drafted the manuscript, read and approved the final manuscript, and are responsible for all aspects of the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J. et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9:7204-18
2. Schett G, Neurath MF. Resolution of chronic inflammatory disease: universal and tissue-specific concepts. Nat Commun. 2018;9:3261
3. Komada T, Muruve DA. The role of inflammasomes in kidney disease. Nat Rev Nephrol. 2019;15:501-20
4. Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417-26
5. Kim J, Gee HY, Lee MG. Unconventional protein secretion - new insights into the pathogenesis and therapeutic targets of human diseases. J Cell Sci. 2018 131
6. Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. 2016;16:407-20
7. Dubyak GR. P2X7 receptor regulation of non-classical secretion from immune effector cells. Cell Microbiol. 2012;14:1697-706
8. Ansari MA, Singh VV, Dutta S, Veettil MV, Dutta D, Chikoti L. et al. Constitutive interferon-inducible protein 16-inflammasome activation during Epstein-Barr virus latency I, II, and III in B and epithelial cells. J Virol. 2013;87:8606-23
9. Zhang W, Ma Y, Zhang Y, Yang J, He G, Chen S. Photo-Oxidative Blue-Light Stimulation in Retinal Pigment Epithelium Cells Promotes Exosome Secretion and Increases the Activity of the NLRP3 Inflammasome. Curr Eye Res. 2019;44:67-75
10. Lian Q, Xu J, Yan S, Huang M, Ding H, Sun X. et al. Chemotherapy-induced intestinal inflammatory responses are mediated by exosome secretion of double-strand DNA via AIM2 inflammasome activation. Cell Res. 2017;27:784-800
11. Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ. et al. Reassessment of Exosome Composition. Cell. 2019;177:428-45 e18
12. Lee H, Groot M, Pinilla-Vera M, Fredenburgh LE, Jin Y. Identification of miRNA-rich vesicles in bronchoalveolar lavage fluid: Insights into the function and heterogeneity of extracellular vesicles. J Control Release. 2019;294:43-52
13. Showalter MR, Wancewicz B, Fiehn O, Archard JA, Clayton S, Wagner J. et al. Primed mesenchymal stem cells package exosomes with metabolites associated with immunomodulation. Biochem Biophys Res Commun. 2019;512:729-35
14. Raposo G, Stahl PD. Extracellular vesicles: a new communication paradigm? Nat Rev Mol Cell Biol. 2019;20:509-10
15. Johnson TK, Zhao L, Zhu D, Wang Y, Xiao Y, Oguljahan B. et al. Exosomes derived from induced vascular progenitor cells promote angiogenesis in vitro and in an in vivo rat hindlimb ischemia model. Am J Physiol Heart Circ Physiol. 2019;317:H765-H76
16. Xia C, Zeng Z, Fang B, Tao M, Gu C, Zheng L. et al. Mesenchymal stem cell-derived exosomes ameliorate intervertebral disc degeneration via anti-oxidant and anti-inflammatory effects. Free Radic Biol Med. 2019;143:1-15
17. Paktinat S, Hashemi SM, Ghaffari Novin M, Mohammadi-Yeganeh S, Salehpour S, Karamian A. et al. Seminal exosomes induce interleukin-6 and interleukin-8 secretion by human endometrial stromal cells. Eur J Obstet Gynecol Reprod Biol. 2019;235:71-6
18. Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805-20
19. Liu J, Cao X. Cellular and molecular regulation of innate inflammatory responses. Cell Mol Immunol. 2016;13:711-21
20. O'Shea JJ, Murray PJ. Cytokine signaling modules in inflammatory responses. Immunity. 2008;28:477-87
21. Hiscott J, Marois J, Garoufalis J, D'Addario M, Roulston A, Kwan I. et al. Characterization of a functional NF-kappa B site in the human interleukin 1 beta promoter: evidence for a positive autoregulatory loop. Mol Cell Biol. 1993;13:6231-40
22. Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195-224
23. Liu T, Zhang L, Joo D, Sun SC. NF-kappaB signaling in inflammation. Signal Transduct Target Ther. 2017 2
24. Zhang P, Tsuchiya K, Kinoshita T, Kushiyama H, Suidasari S, Hatakeyama M. et al. Vitamin B6 Prevents IL-1beta Protein Production by Inhibiting NLRP3 Inflammasome Activation. J Biol Chem. 2016;291:24517-27
25. Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660-5
26. Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. 2004;20:319-25
27. Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677-87
28. Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR. et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514-8
29. Duncan JA, Bergstralh DT, Wang Y, Willingham SB, Ye Z, Zimmermann AG. et al. Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proc Natl Acad Sci U S A. 2007;104:8041-6
30. Cheng Y, Cheng L, Gao X, Chen S, Wu P, Wang C. et al. Covalent modification of Keap1 at Cys77 and Cys434 by pubescenoside a suppresses oxidative stress-induced NLRP3 inflammasome activation in myocardial ischemia-reperfusion injury. Theranostics. 2021;11:861-77
31. Zhao Y, Qiu C, Wang W, Peng J, Cheng X, Shangguan Y. et al. Cortistatin protects against intervertebral disc degeneration through targeting mitochondrial ROS-dependent NLRP3 inflammasome activation. Theranostics. 2020;10:7015-33
32. Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H. et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153-8
33. Yang CC, Wu CH, Lin TC, Cheng YN, Chang CS, Lee KT. et al. Inhibitory effect of PPARgamma on NLRP3 inflammasome activation. Theranostics. 2021;11:2424-41
34. Yang X, Zhan N, Jin Y, Ling H, Xiao C, Xie Z. et al. Tofacitinib restores the balance of gammadeltaTreg/gammadeltaT17 cells in rheumatoid arthritis by inhibiting the NLRP3 inflammasome. Theranostics. 2021;11:1446-57
35. Lightfield KL, Persson J, Brubaker SW, Witte CE, von Moltke J, Dunipace EA. et al. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat Immunol. 2008;9:1171-8
36. Kofoed EM, Vance RE. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature. 2011;477:592-5
37. Yu JW, Wu J, Zhang Z, Datta P, Ibrahimi I, Taniguchi S. et al. Cryopyrin and pyrin activate caspase-1, but not NF-kappaB, via ASC oligomerization. Cell Death Differ. 2006;13:236-49
38. Seshadri S, Duncan MD, Hart JM, Gavrilin MA, Wewers MD. Pyrin levels in human monocytes and monocyte-derived macrophages regulate IL-1beta processing and release. J Immunol. 2007;179:1274-81
39. Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509-13
40. Lin Y, Wang S, Gao L, Zhou Z, Yang Z, Lin J. et al. Oscillating lncRNA Platr4 regulates NLRP3 inflammasome to ameliorate nonalcoholic steatohepatitis in mice. Theranostics. 2021;11:426-44
41. Kong Y, Feng W, Zhao X, Zhang P, Li S, Li Z. et al. Statins ameliorate cholesterol-induced inflammation and improve AQP2 expression by inhibiting NLRP3 activation in the kidney. Theranostics. 2020;10:10415-33
42. Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM, Nunez G. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142-53
43. Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T. et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857-65
44. Xu H, Yang J, Gao W, Li L, Li P, Zhang L. et al. Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature. 2014;513:237-41
45. Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S. et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246-9
46. Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P. et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187-92
47. Matikainen S, Nyman TA, Cypryk W. Function and Regulation of Noncanonical Caspase-4/5/11 Inflammasome. J Immunol. 2020;204:3063-9
48. Kayagaki N, Stowe IB, Lee BL, O'Rourke K, Anderson K, Warming S. et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666-71
49. Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J. et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117-21
50. Rausch UP, Jordan M, Rodel F, Aigner T, Otterness IG, Beuscher N. et al. Transcriptional and translational regulation of IL-1 alpha and IL-1 beta account for the control of IL-1 in experimental yersiniosis. Cytokine. 1994;6:504-11
51. Cogswell JP, Godlevski MM, Wisely GB, Clay WC, Leesnitzer LM, Ways JP. et al. NF-kappa B regulates IL-1 beta transcription through a consensus NF-kappa B binding site and a nonconsensus CRE-like site. J Immunol. 1994;153:712-23
52. Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013-22
53. Hu J, Wang H, Li X, Liu Y, Mi Y, Kong H. et al. Fibrinogen-like protein 2 aggravates nonalcoholic steatohepatitis via interaction with TLR4, eliciting inflammation in macrophages and inducing hepatic lipid metabolism disorder. Theranostics. 2020;10:9702-20
54. Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D. et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787-91
55. Zhang Y, Wang L, Lv Y, Jiang C, Wu G, Dull RO. et al. The GTPase Rab1 Is Required for NLRP3 Inflammasome Activation and Inflammatory Lung Injury. J Immunol. 2019;202:194-206
56. Rahman MM, Mohamed MR, Kim M, Smallwood S, McFadden G. Co-regulation of NF-kappaB and inflammasome-mediated inflammatory responses by myxoma virus pyrin domain-containing protein M013. PLoS Pathog. 2009;5:e1000635
57. Bretz NP, Ridinger J, Rupp AK, Rimbach K, Keller S, Rupp C. et al. Body fluid exosomes promote secretion of inflammatory cytokines in monocytic cells via Toll-like receptor signaling. J Biol Chem. 2013;288:36691-702
58. Xia Y, Chen S, Zhu G, Huang R, Yin Y, Ren W. Betaine Inhibits Interleukin-1beta Production and Release: Potential Mechanisms. Front Immunol. 2018;9:2670
59. Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ. et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556-65
60. Mussbacher M, Salzmann M, Brostjan C, Hoesel B, Schoergenhofer C, Datler H. et al. Cell Type-Specific Roles of NF-kappaB Linking Inflammation and Thrombosis. Front Immunol. 2019;10:85
61. Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9:19
62. Aheget H, Tristan-Manzano M, Mazini L, Cortijo-Gutierrez M, Galindo-Moreno P, Herrera C. et al. Exosome: A New Player in Translational Nanomedicine. J Clin Med. 2020 9
63. Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A. et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19-30 sup pp 1-13
64. Wozniak AL, Adams A, King KE, Dunn W, Christenson LK, Hung WT. et al. The RNA binding protein FMR1 controls selective exosomal miRNA cargo loading during inflammation. J Cell Biol. 2020 219
65. Lasser C, Alikhani VS, Ekstrom K, Eldh M, Paredes PT, Bossios A. et al. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J Transl Med. 2011;9:9
66. Kerr N, Garcia-Contreras M, Abbassi S, Mejias NH, Desousa BR, Ricordi C. et al. Inflammasome Proteins in Serum and Serum-Derived Extracellular Vesicles as Biomarkers of Stroke. Front Mol Neurosci. 2018;11:309
67. de Rivero Vaccari JP, Brand F 3rd, Adamczak S, Lee SW, Perez-Barcena J, Wang MY. et al. Exosome-mediated inflammasome signaling after central nervous system injury. J Neurochem. 2016;136(Suppl 1):39-48
68. Keller S, Ridinger J, Rupp AK, Janssen JW, Altevogt P. Body fluid derived exosomes as a novel template for clinical diagnostics. J Transl Med. 2011;9:86
69. Kato T, Miyaki S, Ishitobi H, Nakamura Y, Nakasa T, Lotz MK. et al. Exosomes from IL-1beta stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res Ther. 2014;16:R163
70. Jiang K, Yang J, Guo S, Zhao G, Wu H, Deng G. Peripheral Circulating Exosome-Mediated Delivery of miR-155 as a Novel Mechanism for Acute Lung Inflammation. Mol Ther. 2019;27:1758-71
71. Sarkar S, Rokad D, Malovic E, Luo J, Harischandra DS, Jin H. et al. Manganese activates NLRP3 inflammasome signaling and propagates exosomal release of ASC in microglial cells. Sci Signal. 2019 12
72. Romancino DP, Paterniti G, Campos Y, De Luca A, Di Felice V, d'Azzo A. et al. Identification and characterization of the nano-sized vesicles released by muscle cells. FEBS Lett. 2013;587:1379-84
73. Lenassi M, Cagney G, Liao M, Vaupotic T, Bartholomeeusen K, Cheng Y. et al. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic. 2010;11:110-22
74. Gutzeit C, Nagy N, Gentile M, Lyberg K, Gumz J, Vallhov H. et al. Exosomes derived from Burkitt's lymphoma cell lines induce proliferation, differentiation, and class-switch recombination in B cells. J Immunol. 2014;192:5852-62
75. Wang G, Jin S, Ling X, Li Y, Hu Y, Zhang Y. et al. Proteomic Profiling of LPS-Induced Macrophage-Derived Exosomes Indicates Their Involvement in Acute Liver Injury. Proteomics. 2019;19:e1800274
76. Singhto N, Kanlaya R, Nilnumkhum A, Thongboonkerd V. Roles of Macrophage Exosomes in Immune Response to Calcium Oxalate Monohydrate Crystals. Front Immunol. 2018;9:316
77. Singhto N, Thongboonkerd V. Exosomes derived from calcium oxalate-exposed macrophages enhance IL-8 production from renal cells, neutrophil migration and crystal invasion through extracellular matrix. J Proteomics. 2018;185:64-76
78. Kerr NA, de Rivero Vaccari JP, Umland O, Bullock MR, Conner GE, Dietrich WD. et al. Human Lung Cell Pyroptosis Following Traumatic Brain Injury. Cells. 2019 8
79. Raval AP, Martinez CC, Mejias NH, de Rivero Vaccari JP. Sexual dimorphism in inflammasome-containing extracellular vesicles and the regulation of innate immunity in the brain of reproductive senescent females. Neurochem Int. 2019;127:29-37
80. Chuang HY, Su YK, Liu HW, Chen CH, Chiu SC, Cho DY. et al. Preclinical Evidence of STAT3 Inhibitor Pacritinib Overcoming Temozolomide Resistance via Downregulating miR-21-Enriched Exosomes from M2 Glioblastoma-Associated Macrophages. J Clin Med. 2019 8
81. Xie K, Liu L, Chen J, Liu F. Exosomal miR-1246 derived from human umbilical cord blood mesenchymal stem cells attenuates hepatic ischemia reperfusion injury by modulating T helper 17/regulatory T balance. IUBMB Life. 2019;71:2020-30
82. Zhang Z, Yu X, Zhou Z, Li B, Peng J, Wu X. et al. LMP1-positive extracellular vesicles promote radioresistance in nasopharyngeal carcinoma cells through P38 MAPK signaling. Cancer Med. 2019;8:6082-94
83. Cha DJ, Franklin JL, Dou Y, Liu Q, Higginbotham JN, Demory Beckler M. et al. KRAS-dependent sorting of miRNA to exosomes. Elife. 2015;4:e07197
84. Jae N, McEwan DG, Manavski Y, Boon RA, Dimmeler S. Rab7a and Rab27b control secretion of endothelial microRNA through extracellular vesicles. FEBS Lett. 2015;589:3182-8
85. Hurwitz SN, Nkosi D, Conlon MM, York SB, Liu X, Tremblay DC. et al. CD63 Regulates Epstein-Barr Virus LMP1 Exosomal Packaging, Enhancement of Vesicle Production, and Noncanonical NF-kappaB Signaling. J Virol. 2017 91
86. Guo BB, Bellingham SA, Hill AF. The neutral sphingomyelinase pathway regulates packaging of the prion protein into exosomes. J Biol Chem. 2015;290:3455-67
87. Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A. et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284:34211-22
88. Savina A, Furlan M, Vidal M, Colombo MI. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem. 2003;278:20083-90
89. Valimaki E, Cypryk W, Virkanen J, Nurmi K, Turunen PM, Eklund KK. et al. Calpain Activity Is Essential for ATP-Driven Unconventional Vesicle-Mediated Protein Secretion and Inflammasome Activation in Human Macrophages. J Immunol. 2016;197:3315-25
90. King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421
91. Szul T, Bratcher PE, Fraser KB, Kong M, Tirouvanziam R, Ingersoll S. et al. Toll-Like Receptor 4 Engagement Mediates Prolyl Endopeptidase Release from Airway Epithelia via Exosomes. Am J Respir Cell Mol Biol. 2016;54:359-69
92. Cypryk W, Nyman TA, Matikainen S. From Inflammasome to Exosome-Does Extracellular Vesicle Secretion Constitute an Inflammasome-Dependent Immune Response? Front Immunol. 2018;9:2188
93. Qu Y, Ramachandra L, Mohr S, Franchi L, Harding CV, Nunez G. et al. P2X7 receptor-stimulated secretion of MHC class II-containing exosomes requires the ASC/NLRP3 inflammasome but is independent of caspase-1. J Immunol. 2009;182:5052-62
94. Gutierrez MG, Mishra BB, Jordao L, Elliott E, Anes E, Griffiths G. NF-kappa B activation controls phagolysosome fusion-mediated killing of mycobacteria by macrophages. J Immunol. 2008;181:2651-63
95. Sommermann TG, O'Neill K, Plas DR, Cahir-McFarland E. IKKbeta and NF-kappaB transcription govern lymphoma cell survival through AKT-induced plasma membrane trafficking of GLUT1. Cancer Res. 2011;71:7291-300
96. Tavakoli Dargani Z, Singla DK. Embryonic stem cell-derived exosomes inhibit doxorubicin-induced TLR4-NLRP3-mediated cell death-pyroptosis. Am J Physiol Heart Circ Physiol. 2019;317:H460-H71
97. Zhang W, Wang Y, Kong Y. Exosomes Derived From Mesenchymal Stem Cells Modulate miR-126 to Ameliorate Hyperglycemia-Induced Retinal Inflammation Via Targeting HMGB1. Invest Ophthalmol Vis Sci. 2019;60:294-303
98. Li X, Liu L, Yang J, Yu Y, Chai J, Wang L. et al. Exosome Derived From Human Umbilical Cord Mesenchymal Stem Cell Mediates MiR-181c Attenuating Burn-induced Excessive Inflammation. EBioMedicine. 2016;8:72-82
99. Jiang L, Zhang S, Hu H, Yang J, Wang X, Ma Y. et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate acute liver failure by reducing the activity of the NLRP3 inflammasome in macrophages. Biochem Biophys Res Commun. 2019;508:735-41
100. Liu Y, Lou G, Li A, Zhang T, Qi J, Ye D. et al. AMSC-derived exosomes alleviate lipopolysaccharide/d-galactosamine-induced acute liver failure by miR-17-mediated reduction of TXNIP/NLRP3 inflammasome activation in macrophages. EBioMedicine. 2018;36:140-50
101. Soundara Rajan T, Giacoppo S, Diomede F, Bramanti P, Trubiani O, Mazzon E. Human periodontal ligament stem cells secretome from multiple sclerosis patients suppresses NALP3 inflammasome activation in experimental autoimmune encephalomyelitis. Int J Immunopathol Pharmacol. 2017;30:238-52
102. Wang Z, Maruyama K, Sakisaka Y, Suzuki S, Tada H, Suto M. et al. Cyclic Stretch Force Induces Periodontal Ligament Cells to Secrete Exosomes That Suppress IL-1beta Production Through the Inhibition of the NF-kappaB Signaling Pathway in Macrophages. Front Immunol. 2019;10:1310
103. Shelke GV, Lasser C, Gho YS, Lotvall J. Importance of exosome depletion protocols to eliminate functional and RNA-containing extracellular vesicles from fetal bovine serum. J Extracell Vesicles. 2014 3
104. Beninson LA, Fleshner M. Exosomes in fetal bovine serum dampen primary macrophage IL-1beta response to lipopolysaccharide (LPS) challenge. Immunol Lett. 2015;163:187-92
105. Mukhamedova N, Hoang A, Dragoljevic D, Dubrovsky L, Pushkarsky T, Low H. et al. Exosomes containing HIV protein Nef reorganize lipid rafts potentiating inflammatory response in bystander cells. PLoS Pathog. 2019;15:e1007907
106. Zhang Y, Liu F, Yuan Y, Jin C, Chang C, Zhu Y. et al. Inflammasome-Derived Exosomes Activate NF-kappaB Signaling in Macrophages. J Proteome Res. 2017;16:170-8
107. Ni Z, Kuang L, Chen H, Xie Y, Zhang B, Ouyang J. et al. The exosome-like vesicles from osteoarthritic chondrocyte enhanced mature IL-1beta production of macrophages and aggravated synovitis in osteoarthritis. Cell Death Dis. 2019;10:522
108. Kim SH, Kim G, Han DH, Lee M, Kim I, Kim B. et al. Ezetimibe ameliorates steatohepatitis via AMP activated protein kinase-TFEB-mediated activation of autophagy and NLRP3 inflammasome inhibition. Autophagy. 2017;13:1767-81
109. Mao Y, Wang Y, Dong L, Zhang Q, Wang C, Zhang Y. et al. Circulating exosomes from esophageal squamous cell carcinoma mediate the generation of B10 and PD-1(high) Breg cells. Cancer Sci. 2019;110:2700-10
110. Brossa A, Tapparo M, Fonsato V, Papadimitriou E, Delena M, Camussi G. et al. Coincubation as miR-Loading Strategy to Improve the Anti-Tumor Effect of Stem Cell-Derived EVs. Pharmaceutics. 2021 13
111. Song Y, Dou H, Li X, Zhao X, Li Y, Liu D. et al. Exosomal miR-146a Contributes to the Enhanced Therapeutic Efficacy of Interleukin-1beta-Primed Mesenchymal Stem Cells Against Sepsis. Stem Cells. 2017;35:1208-21
112. Ying W, Gao H, Dos Reis FCG, Bandyopadhyay G, Ofrecio JM, Luo Z. et al. MiR-690, an exosomal-derived miRNA from M2-polarized macrophages, improves insulin sensitivity in obese mice. Cell Metab. 2021
113. Zheng Y, Liu J, Chen P, Lin L, Luo Y, Ma X. et al. Exosomal miR-22-3p from human umbilical cord blood-derived mesenchymal stem cells protects against lipopolysaccharid-induced acute lung injury. Life Sci. 2021;269:119004
114. Sun W, Li Z, Zhou X, Yang G, Yuan L. Efficient exosome delivery in refractory tissues assisted by ultrasound-targeted microbubble destruction. Drug Deliv. 2019;26:45-50
115. Boehmer ED, Meehan MJ, Cutro BT, Kovacs EJ. Aging negatively skews macrophage TLR2- and TLR4-mediated pro-inflammatory responses without affecting the IL-2-stimulated pathway. Mech Ageing Dev. 2005;126:1305-13
116. Qiao Y, Wang P, Qi J, Zhang L, Gao C. TLR-induced NF-kappaB activation regulates NLRP3 expression in murine macrophages. FEBS Lett. 2012;586:1022-6
117. Li DQ, Zhou N, Zhang L, Ma P, Pflugfelder SC. Suppressive effects of azithromycin on zymosan-induced production of proinflammatory mediators by human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2010;51:5623-9
118. Brightbill HD, Libraty DH, Krutzik SR, Yang RB, Belisle JT, Bleharski JR. et al. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732-6
119. Lee KJ, Kim YM, Kim DY, Jeoung D, Han K, Lee ST. et al. Release of heat shock protein 70 (Hsp70) and the effects of extracellular Hsp70 on matric metalloproteinase-9 expression in human monocytic U937 cells. Exp Mol Med. 2006;38:364-74
120. Giraldo E, Martin-Cordero L, Garcia JJ, Gehrmann M, Multhoff G, Ortega E. Exercise-induced extracellular 72 kDa heat shock protein (Hsp72) stimulates neutrophil phagocytic and fungicidal capacities via TLR-2. Eur J Appl Physiol. 2010;108:217-25
121. Yang X, Li L, Liu J, Lv B, Chen F. Extracellular histones induce tissue factor expression in vascular endothelial cells via TLR and activation of NF-kappaB and AP-1. Thromb Res. 2016;137:211-8
122. Funderburg N, Lederman MM, Feng Z, Drage MG, Jadlowsky J, Harding CV. et al. Human -defensin-3 activates professional antigen-presenting cells via Toll-like receptors 1 and 2. Proc Natl Acad Sci U S A. 2007;104:18631-5
123. Nadlonek N, Lee JH, Reece TB, Weyant MJ, Cleveland JC Jr, Meng X. et al. Interleukin-1 Beta induces an inflammatory phenotype in human aortic valve interstitial cells through nuclear factor kappa Beta. Ann Thorac Surg. 2013;96:155-62
124. Laegreid A, Medvedev A, Nonstad U, Bombara MP, Ranges G, Sundan A. et al. Tumor necrosis factor receptor p75 mediates cell-specific activation of nuclear factor kappa B and induction of human cytomegalovirus enhancer. J Biol Chem. 1994;269:7785-91
125. Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240-4
126. Mishra BB, Moura-Alves P, Sonawane A, Hacohen N, Griffiths G, Moita LF. et al. Mycobacterium tuberculosis protein ESAT-6 is a potent activator of the NLRP3/ASC inflammasome. Cell Microbiol. 2010;12:1046-63
127. Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M. et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228-32
128. Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237-41
129. Miao EA, Mao DP, Yudkovsky N, Bonneau R, Lorang CG, Warren SE. et al. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Natl Acad Sci U S A. 2010;107:3076-80
130. Sutterwala FS, Mijares LA, Li L, Ogura Y, Kazmierczak BI, Flavell RA. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J Exp Med. 2007;204:3235-45
131. Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP. et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213-8
132. Muruve DA, Petrilli V, Zaiss AK, White LR, Clark SA, Ross PJ. et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103-7
133. Schnappauf O, Chae JJ, Kastner DL, Aksentijevich I. The Pyrin Inflammasome in Health and Disease. Front Immunol. 2019;10:1745
Author contact
![]() Corresponding author: Prof. Visith Thongboonkerd, Head of Medical Proteomics Unit, Office for Research and Development, Siriraj Hospital, Mahidol University, 6th Floor-SiMR Building, 2 Wanglang Road, Bangkoknoi, Bangkok 10700, Thailand. Phone: +66-2-4192850, E-mail: thongboonkerdcom; vthongbocom.
Corresponding author: Prof. Visith Thongboonkerd, Head of Medical Proteomics Unit, Office for Research and Development, Siriraj Hospital, Mahidol University, 6th Floor-SiMR Building, 2 Wanglang Road, Bangkoknoi, Bangkok 10700, Thailand. Phone: +66-2-4192850, E-mail: thongboonkerdcom; vthongbocom.
 Global reach, higher impact
Global reach, higher impact