13.3
Impact Factor
Theranostics 2021; 11(9):4363-4380. doi:10.7150/thno.53624 This issue Cite
Research Paper
Hepatic miR-378 modulates serum cholesterol levels by regulating hepatic bile acid synthesis
1. CAS Key Laboratory of Nutrition, Metabolism and Food Safety, Shanghai Institute of Nutrition and Health, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai 200031, China.
2. Department of Endocrinology and Metabolism, Zhongshan Hospital, Fudan University, Shanghai 200032, China.
3. State Key Laboratory of Food Science and Technology, School of Food Science and Technology, Jiangnan University, Wuxi 214122, China.
4. Omics Core, Bio-Med Big Data Center, CAS-MPG Partner Institute for Computational Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai 200031, China.
5. Division of Pulmonary and Critical Care Medicine, Department of Medicine, Boston University Medical Campus, Boston, MA 02118, USA.
6. Shanghai Xuhui Central Hospital, Shanghai Clinical Center, Chinese Academy of Sciences, Shanghai 200031, China.
7. Department of Toxicology and Sanitary Chemistry, School of Public Health, Tianjin Medical University, Tianjin 300070, China.
8. Department of Physiology and Pathophysiology, School of Basic Medical Sciences, Peking University, and the Key Laboratory of Molecular Cardiovascular Science, Ministry of Education, Beijing 100191, China.
9. Key Laboratory of Food Safety Risk Assessment, Ministry of Health, Beijing 100021, China.
* These authors contributed equally to this work.
Abstract
Rationale: An improved understanding of thyroid hormone (TH) action on cholesterol metabolism will facilitate the identification of novel therapeutic targets for hypercholesterolemia. TH-regulated microRNAs (miRNAs) have been implicated in TH-controlled biological processes; however, whether and how TH-regulated miRNAs mediate the cholesterol-lowering effect of TH remains unclear. Our aim was to identify TH-regulated microRNAs that have cholesterol-lowering effects and explore the underlying mechanism.
Method: Microarray and RNA-seq were performed to identify TH-regulated microRNAs and the genes regulated by mmu-miR-378-3p (miR-378) in the liver of mice, respectively. Recombinant adenoviruses encoding miR-378, Mafg, and shRNA for Mafg, antagomiR-378, liver-specific miR-378 transgenic mice, and miR-378 knockout mice were employed to investigate the roles of hepatic miR-378 and MAFG in cholesterol and bile acid homeostasis. The levels of bile salt species were determined by using UFLC-Triple-time of flight/MS.
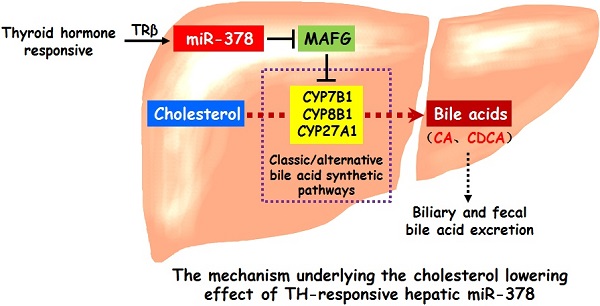
Results: Here, we show that hepatic miR-378 is positively regulated by TH. Transient overexpression of miR-378 in the liver of mice reduces serum cholesterol levels, accompanied with an increase in the expression of key enzymes in primary bile acid synthetic pathways and corresponding increases in biliary and fecal bile acid levels. Consistently, liver-specific miR-378 transgenic mice with moderate overexpression of hepatic miR-378 display decreased serum cholesterol levels and resistance to diet-induced hypercholesterolemia, while mice lacking miR-378 exhibit defects in bile acid and cholesterol homeostasis. Mechanistically, hepatic miR-378 regulates the expression of key enzymes in both classic and alternative bile acid synthetic pathways through MAFG, a transcriptional repressor, thereby modulating bile acid and cholesterol metabolism.
Conclusions: TH-responsive hepatic miR-378 is capable of modulating serum cholesterol levels by regulating both the classic and alternative BA synthetic pathways. Our study not only identifies a previously undescribed role of hepatic miR-378 but also provides new cholesterol-lowering approaches.
Keywords: mmu-miR-378-3p, cholesterol, bile acid, MAFG, thyroid hormone.
 Global reach, higher impact
Global reach, higher impact