13.3
Impact Factor
Theranostics 2021; 11(9):4050-4060. doi:10.7150/thno.56211 This issue Cite
Research Paper
Molecular imaging and biochemical response assessment after a single cycle of [225Ac]Ac-PSMA-617/[177Lu]Lu-PSMA-617 tandem therapy in mCRPC patients who have progressed on [177Lu]Lu-PSMA-617 monotherapy
1. Department of Nuclear Medicine, Saarland University - Medical Center, Homburg, Germany
2. Department of Urology, Saarland University - Medical Center, Homburg, Germany
Received 2020-11-20; Accepted 2021-1-22; Published 2021-2-16
Abstract
Rationale: Despite the promising results of prostate-specific membrane antigen (PSMA)-targeted 177Lu radioligand therapy in metastatic castration-resistant prostate carcinoma (mCRPC), some patients do not respond and other patients with initially good response develop resistance to this treatment. In this study, we investigated molecular imaging and biochemical responses after a single cycle of [225Ac]Ac-PSMA-617/[177Lu]Lu-PSMA-617 tandem therapy in patients who had progressed on [177Lu]Lu-PSMA-617 monotherapy.
Methods: Seventeen patients with mCRPC were included in a retrospective, monocenter study. Molecular imaging-based response was assessed by modified PERCIST criteria using the whole-body total lesion PSMA (TLP) and molecular tumour volume (MTV) derived from [68Ga]Ga-PSMA-11 PET/CT. Biochemical response was evaluated according to PCWG3 criteria using the prostate-specific antigen (PSA) serum value. Concordance and correlation statistics as well as survival analyses were performed.
Results: Based on the molecular imaging-based response assessment, 5 (29.4%) patients showed partial remission and 7 (41.2%) had stable disease. The remaining 5 (29.4%) patients had further progression, four with an increase in TLP/MTV of >30% and one with stable TLP/MTV but appearance of new metastases. Based on the biochemical response assessment, 5 (29.4%), 8 (47.1%), and 4 (23.5%) patients showed partial remission, stable disease, and progressive disease, respectively. A comparison of the response assessment methods showed a concordance of 100% (17/17) between TLP and MTV and 70.6% (12/17) between TLP/MTV and PSA. Patients with partial remission, independently assessed by each method, had better overall survival (OS) than patients with either stable or progressive disease. The difference in OS was statistically significant for the molecular imaging response assessment (median OS not reached vs. 8.3 m, p = 0.044), but not for the biochemical response assessment (median OS 18.1 m vs. 9.4 m, p = 0.468).
Conclusion: Based on both assessment methods, [225Ac]Ac-PSMA-617/[177Lu]Lu-PSMA-617 tandem therapy is an effective treatment for the highly challenging cohort of patients with mCRPC who have progressed on [177Lu]Lu-PSMA-617 monotherapy. Molecular imaging response and biochemical PSA response were mostly concordant, though a considerable number of cases (29.4%) were discordant. Molecular imaging response reflecting the change in total viable tumour burden appears to be superior to PSA change in estimating survival outcome after tandem therapy.
Keywords: 225Ac and 177Lu, PSMA radioligand therapy, Biochemical response, Molecular imaging response, Metastatic castration-resistant prostate cancer.
Introduction
Prostate carcinoma is the second most frequent malignancy in men [1], and a considerable number of patients ultimately progress to a metastatic castration-resistant stage (mCRPC) [2,3]. Apart from chemotherapy with taxanes (docetaxel, cabazitaxel) and next-generation androgen receptor signal inhibitors (abiraterone, enzalutamide) [4-6], radioligand therapy targeting prostate-specific membrane antigen (PSMA) is a promising therapy option, currently applied after failure of chemotherapy and hormone therapy [7]. PSMA radioligand therapy (PSMA-RLT) using PSMA ligands labelled with the beta emitter lutetium-177 (e.g. [177Lu]Lu-PSMA-617) has shown encouraging results in various retrospective studies [8-11] and in a few prospective clinical trials on patients with mCRPC [12,13]. Some patients, however, do not respond to PSMA-RLT with beta emitters and other patients with initially good response develop resistance [14-16]. This cohort of patients has limited remaining therapeutic options. Recently, PSMA-RLT with alpha emitters such as actinium-225 e.g. in form of [225Ac]Ac-PSMA-617 monotherapy [17-20] or [225Ac]Ac-PSMA-617/[177Lu]Lu-PSMA-617 tandem therapy, a concept first introduced by our group [21], has yielded impressive results. PSMA-RLT using alpha emitters may also be effective in patients who have progressed on [177Lu]Lu-PSMA-617 monotherapy due to the higher radiobiological impact of alpha radiation (20 times higher weighting factor than beta radiation) [22-24]. However, the higher radiobiological impact of alpha radiation also affects 'organs-at-risk', especially the salivary glands, which may lead to severe xerostomia and impair patients' quality of life. Tandem therapy combining alpha and beta emitters with adapted doses, instead of monotherapy with alpha emitter, may reduce the occurrence of these substantial adverse effects. There is little data, however, about the efficacy of alpha emitters in these highly challenging patients. Besides biochemical response assessment, molecular imaging-based response assessment could be of high importance and interest but has not been reported. In this study, we investigated the efficacy of a single cycle of [225Ac]Ac-PSMA-617/[177Lu]Lu-PSMA-617 tandem therapy in patients who had progressed on [177Lu]Lu-PSMA-617 monotherapy. We assessed molecular imaging response by [68Ga]Ga-PSMA-11 positron emission tomography (PET)/computed tomography (CT) of total tumour burden and biochemical response by serum prostate-specific antigen (PSA) values. Both response assessment methods were evaluated as potential predictors of outcome for this tandem therapy approach.
Methods
Study design
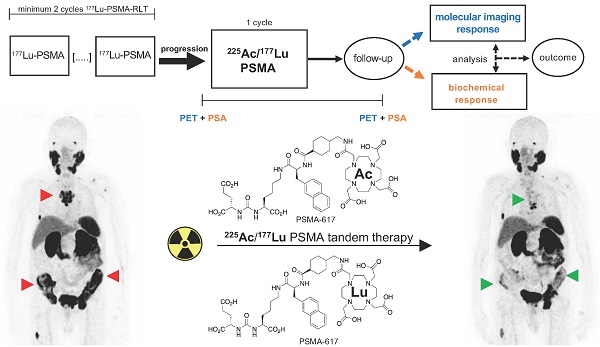
We performed a retrospective monocenter study of mCRPC patients treated with one cycle of [225Ac]Ac-PSMA-617/[177Lu]Lu-PSMA-617 tandem therapy after progression on [177Lu]Lu-PSMA-617 monotherapy. Progression of disease on [177Lu]Lu-PSMA-617 monotherapy was defined as a serum PSA increase of >25% in comparison to the previous cycle and an increased uptake (in target lesions) or appearance of new metastases by [68Ga]Ga-PSMA-11 PET/CT. Only patients with at least two cycles of [177Lu]Lu-PSMA-617 RLT were included to avoid the potential flare phenomenon of the initial cycle. Additionally, patients must have received [68Ga]Ga-PSMA-11 PET/CT within approximately one month before and another PET/CT scan within a few months after [225Ac]Ac-PSMA-617/[177Lu]Lu-PSMA-617 tandem therapy in order to assess response by molecular imaging. The study design is depicted schematically in Figure 1. Patients (n=6) who had progressed on [177Lu]Lu-PSMA-617 RLT but with known 'mismatch findings', meaning intense glucose metabolism on [18F]FDG PET/CT with missing or low PSMA expression, were excluded from the tandem therapy. In addition, patients with abnormal hematological blood parameters (hemoglobin < 8 mg/dL, platelets < 75,000 /μL, leukocytes < 3000 /μL), renal insufficiency (estimated glomerular filtration rate (eGFR) < 45 mL/min), or poor overall conditions (Eastern Cooperative Oncology Group (ECOG) performance status ≥ 3) did not receive tandem therapy.
Patients and ethics
Seventeen mCRPC patients were included in this retrospective study. All patients received several pre-treatments. Detailed information about the pre-treatments and patient characteristics are presented in Table 1. The patients received an average of 5 ± 2 cycles (range: 2-9 cycles) of [177Lu]Lu-PSMA-617 monotherapy with a cumulative mean 177Lu activity of 35 ± 13 GBq (range: 14-66 GBq) before the [225Ac]Ac-PSMA-617/[177Lu]Lu-PSMA-617 tandem therapy cycle. The mean time from the start of [177Lu]Lu-PSMA-617 monotherapy was 40 ± 25 weeks (range: 11-111 weeks). The mean PSA increase from the last cycle of [177Lu]Lu-PSMA-617 monotherapy to [225Ac]Ac-PSMA-617/[177Lu]Lu-PSMA-617 tandem therapy was 278 ± 547% (range: 26-2338%). [225Ac]Ac-PSMA-617/[177Lu]Lu-PSMA-617 tandem therapy and [68Ga]Ga-PSMA-11 PET/CT were performed on a compassionate use basis under the German Pharmaceutical Act §13 (2b). Patients gave written consent after being informed in detail about the risks and potential side effects of these interventions. Patients consented additionally to publication of any resulting data in accordance with the Declaration of Helsinki. The study was approved by the local institutional review board (ethics committee permission number 140/17).
Study design. Patients who had progressed on at least 2 cycles [177Lu]Lu-PSMA-617 RLT were treated with 1 cycle [225Ac]Ac-PSMA-617/[177Lu]Lu-PSMA-617 tandem RLT. Molecular imaging response and biochemical response were evaluated by PET and PSA serum value in follow-up. Both response assessment methods were compared and analysed as potential predictors of outcome for this tandem therapy approach.
Patient characteristics.
| Characteristics | |
|---|---|
| Age (y) | 69.4 ± 8.3 (57.0-89.0) |
| ECOG performance status | |
| 0 | 5 (29.4%) |
| 1 | 11 (64.7%) |
| 2 | 1 (5.9%) |
| Previous treatments | |
| Prostatectomy | 12 (70.6%) |
| Radiation | 12 (70.6%) |
| ADT | 17 (100%) |
| Enzalutamide or Abiraterone | 17 (100%) |
| Enzalutamide | 13 (76.5%) |
| Abiraterone | 15 (88.2%) |
| Enzalutamide and Abiraterone | 11 (64.7%) |
| Chemotherapy | 13 (76.5%) |
| Docetaxel | 13 (76.5%) |
| Cabazitaxel | 7 (41.2%) |
| Docetaxel and Cabazitaxel | 7 (41.2%) |
| 223Ra therapy | 5 (29.4%) |
| [177Lu]Lu-PSMA-617 RLT | 17 (100%) |
| Cycles | 5 ± 2 (2-9) |
| Cumulative 177Lu activity (GBq) | 35.3 ± 13.3 (13.7-66.1) |
| Sites of metastases | |
| Bone | 17 (100%) |
| Lymph node | 5 (29.4%) |
| Liver | 2 (11.8%) |
| Other | 1 (5.9%) |
[225Ac]Ac-PSMA-617/[177Lu]Lu-PSMA-617 tandem therapy
[225Ac]Ac-PSMA-617 and [177Lu]Lu-PSMA-617 were synthesized according to published procedures described in detail by Kratochwil et al. [14,25]. Both radioligands were administered simultaneously during an inpatient stay in accordance with German radiation protection regulations. The mean administered activity of [225Ac]Ac-PSMA-617 and [177Lu]Lu-PSMA-617 was 4 ± 2 MBq (range: 1.8-6.9 MBq), corresponding to 44 ± 18 kBq/kg body weight (BW) (range: 19-74 kBq/kg BW), and 6 ± 1 GBq (range: 3.8-8.2 GBq), respectively. Administered activities of 225Ac and 177Lu were primarily chosen with regard to total tumour burden, sites of metastases, and patient condition including ECOG performance status, organ function, and degree of existing xerostomia before tandem therapy. For example, patients with high tumour burden or patients who never responded to [177Lu]Lu-PSMA-617 monotherapy received higher 225Ac activities with high 225Ac/177Lu ratios (up to a ratio of 1 MBq 225Ac to 1 GBq 177Lu). Patients with low tumour burden or unfavorable patient conditions received lower total amounts of 225Ac and/or lower 225Ac/177Lu ratios. In order to prevent side effects, each patient received intravenous hydration (1000 mL 0.9% NaCl) 30 min before to 120 min after injection of the radioligand and cooling of the salivary glands.
[68Ga]Ga-PSMA-11 PET/CT
Each patient was imaged by [68Ga]Ga-PSMA-11 PET/CT on average 5 ± 8 d (range: 1-35 d) prior to and 53 ± 32 d (range: 21-119 d) after tandem therapy. A mean activity of 124 ± 17 MBq (range: 83-155 MBq) [68Ga]Ga-PSMA-11 was administered intravenously, followed by a 500 mL infusion of 0.9% NaCl. No diuretics were applied. Before administering the radioactivity, blood tests including PSA serum value, creatinine, hemoglobin, leukocytes, and platelets were performed. The mean time between injection and PET acquisition was ~60 min, in accordance with standard procedures for prostate cancer imaging [26]. The PET/CT scans were performed using a Biograph 40 mCT PET/CT (Siemens Medical Solutions, Knoxville, TN, USA) accredited by European Association of Nuclear Medicine (EANM) Research Ltd. PET acquisition was performed from vertex to mid-femur with an acquisition time of 3 min per bed position, which covers a 21.4 cm extended field-of-view (TrueV). The PET datasets were reconstructed using an iterative 3-dimensional ordered subset expectation maximization algorithm (3 iterations; 24 subsets) with gaussian filtering and a slice thickness of 5 mm. Random correction, decay correction, scatter correction, and attenuation correction were applied. CT was performed with a low-dose technique using an X-ray tube voltage of 120 keV and tube current modulated by CARE Dose4D with a maximal tube current-time product of 30 mAs.
Response assessment
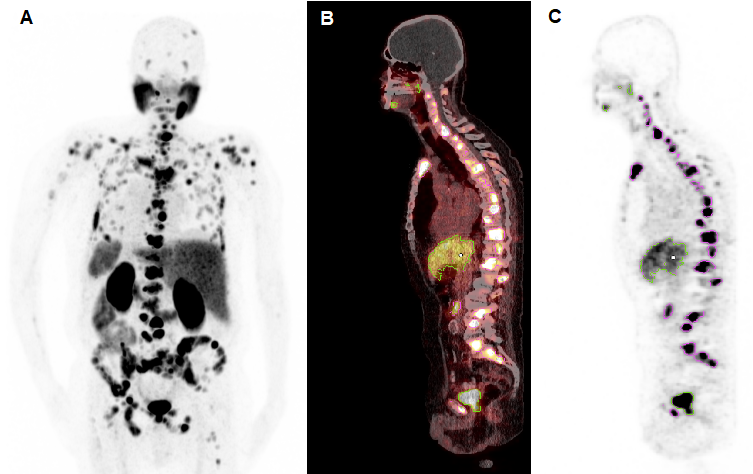
Molecular imaging-based response was assessed by calculating the whole-body total lesion PSMA (TLP) and molecular tumour volume (MTV) from [68Ga]Ga-PSMA-11 PET/CT collected before and after [225Ac]Ac-PSMA-617/[177Lu]Lu-PSMA-617 tandem therapy by applying a semi-automatic tumour segmentation using Syngo.Via (Enterprise VB 30, Siemens, Erlangen, Germany). For tumour segmentation, a standard uptake value (SUV) threshold of 3.0 was applied, in accordance with Ferdinandus et al. [27], except for liver metastases (in 2/17 patients), where an SUV threshold of 1.5 × SUVmean of the healthy liver tissue was applied. Physiological uptake in healthy organs was manually excluded. A representative example of the semi-automatic tumour segmentation is presented in Figure 2. TLP was calculated in analogy to total lesion glycolysis (TLG), which is an established parameter in [18F]FDG PET/CT for therapy monitoring [28], as the summed products of volume and uptake (SUVmean) of all lesions. TLP values are expressed as mL × SUV to distinguish them from MTV values, which are presented in mL. Between PET scans, androgen deprivation therapy (ADT) and therapy with next-generation androgen receptor signal inhibitors such as enzalutamide or abiraterone were continued unchanged to avoid altering PSMA expression [29] (ADT in 17/17, enzalutamide in 13/17, and abiraterone in 1/17 patients). As in other studies [30,31], the PET response criteria in solid tumours (PERCIST) version 1.0 [32] were slightly modified: partial remission was defined as a decrease in TLP or MTV of >30%, progression as an increase of >30% or development of new metastases, and stable disease as a change between ±30%.
Biochemical response was evaluated by the serum PSA value measured on the same day that the PET/CT scans were collected using the Prostate Cancer Working Group 3 criteria (PCWG3) [33]: partial remission was defined as a decrease in PSA of >50%, progression as an increase in PSA of >25%, and stable disease as a change between -50% and +25%. However, these changes were not confirmed by a second measurement as is recommended by the PCWG3.
Representative example of semi-automatic tumour segmentation using Syngo.Via. (A) Maximum intensity projection of [68Ga]Ga-PSMA-11 PET/CT. (B) Sagittal slice of PET/CT fusion. (C) PET data with semi-automatically drawn volumes of interest (violet, tumour lesions; green, physiological uptake).
Toxicity
Hematotoxicity, renal toxicity, and xerostomia were assessed after one cycle of [225Ac]Ac-PSMA-617/[177Lu]Lu-PSMA-617 tandem therapy. Toxicity was recorded using the Common Terminology Criteria for Adverse Events version 5.0 (CTCAE) on blood values of hemoglobin, leucocytes, and platelets, and eGFR. CTCAE grades of xerostomia were assessed based on patient reporting via a questionnaire.
Statistics and survival analysis
Descriptive and concordance analyses were performed on the molecular imaging (TLP and MTV) and biochemical (PSA) response assessments. In addition, several parameters such as patient characteristics, imaging parameters, pretherapeutic parameters, and therapeutic parameters were tested for correlation with changes in TLP, MTV, and PSA. Spearman's correlation was applied for this purpose using Prism 8 (GraphPad Software, San Diego, USA). A p-value of <0.05 was regarded as statistically significant. Overall survival (OS) was defined as the interval from the start of [225Ac]Ac-PSMA-617/[177Lu]Lu-PSMA-617 tandem therapy to the occurrence of any of the following: (1) death from any cause, (2) commencement of a different treatment such as chemotherapy, or (3) the last study visit. Patients were independently categorized by molecular imaging and biochemical response assessments into two groups: (a) patients with partial remission and (b) patients with stable or progressive disease. The cut-off follow-up date was October 30th, 2020. Median follow-up was determined, and OS was analyzed using the Kaplan-Meier method.
Results
Prior to [225Ac]Ac-PSMA-617/[177Lu]Lu-PSMA-617 tandem therapy, the median baseline values of TLP, MTV, and PSA were 3685 mL × SUV (range: 723-13679 mL × SUV), 453 mL (range: 153-2581 mL), and 152 ng/mL (range: 5.9-2570 ng/mL), respectively. There was no significant correlation between baseline TLP and PSA (r = 0.03, p = 0.90) or MTV and PSA (r = 0.11, p = 0.67), but there was a significantly high correlation between TLP and MTV (r = 0.99, p < 0.01). After one cycle of tandem therapy, the median values of TLP, MTV, and PSA were 2289 mL × SUV (range: 72-35819 mL × SUV), 421 mL (range: 14-4542 mL), and 135 ng/mL (range: 6.1-1251 ng/mL), respectively. The TLP, MTV, and PSA values for each patient are compiled in Table 2.
Molecular imaging response
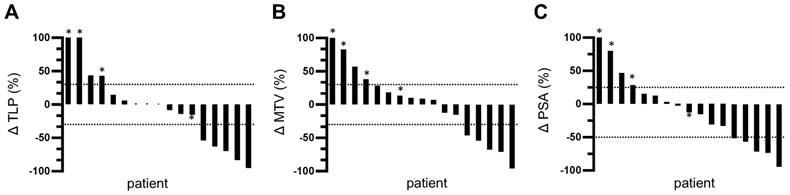
TLP and MTV responses after one cycle of tandem therapy were similar for each patient. 5/17 patients (29.4%) showed partial remission with a decrease in TLP/MTV of >30%. None of these patients developed new metastases. 8/17 patients showed a change in TLP and MTV between -30% and +30%. However, one of these patients (5.9%) developed new metastases and was classified as having progressive disease. Thus, stable disease was found in 41.2% of patients (7/17). The remaining 4 patients (23.5%) showed disease progression with an increase in TLP/MTV of >30% and three of these patients developed new metastases. The changes in TLP and MTV are visualized as waterfall plots in Figure 3A-B.
Absolute values of TLP, MTV, and PSA prior to and after tandem therapy (values over 15 were rounded to full values).
| Patient number | TLP prior to therapy (mL × SUV) | TLP after therapy (mL × SUV) | MTV prior to therapy (mL) | MTV after therapy (mL) | PSA prior to therapy (ng/mL) | PSA after therapy (ng/mL) |
|---|---|---|---|---|---|---|
| 1 | 934 | 1874 | 187 | 342 | 191 | 344 |
| 2 | 13679 | 5113 | 2581 | 1184 | 1277 | 367 |
| 3 | 5412 | 7750 | 838 | 1159 | 60 | 77 |
| 4 | 9717 | 9888 | 1197 | 1413 | 152 | 129 |
| 5 | 4290 | 4363 | 617 | 672 | 258 | 379 |
| 6 | 4365 | 2026 | 784 | 421 | 10.1 | 6.8 |
| 7 | 9070 | 35819 | 1263 | 4542 | 103 | 750 |
| 8 | 4282 | 726 | 588 | 171 | 123 | 7.0 |
| 9 | 1448 | 72 | 303 | 13 | 98 | 26 |
| 10 | 723 | 664 | 153 | 130 | 251 | 290 |
| 11 | 8547 | 9059 | 1348 | 1442 | 2570 | 1251 |
| 12 | 2142 | 2446 | 372 | 477 | 508 | 496 |
| 13 | 3685 | 3123 | 452 | 513 | 115 | 101 |
| 14 | 1447 | 1242 | 259 | 228 | 13.0 | 9.0 |
| 15 | 1959 | 596 | 368 | 119 | 311 | 135 |
| 16 | 1153 | 1166 | 265 | 293 | 968 | 1091 |
| 17 | 1594 | 2289 | 214 | 335 | 5.9 | 6.1 |
Biochemical response
5/17 patients (29.4%) showed partial remission based on a decrease in PSA of >50%. 8/17 patients (47.1%) exhibited stable disease with a PSA change between -50% and +25%. The remaining 4/17 patients (23.5%) showed disease progression with an increase in PSA of >25%. A waterfall plot of PSA changes is depicted in Figure 3C.
Concordance analyses
Comparison of the response assessment methods showed a concordance of 100% (17/17) between TLP and MTV and 70.6% (12/17) between TLP/MTV and PSA (Table 3). Concordant examples of partial remission, stable disease, and progression are shown in Figure 4. Of the discordant patients, two patients had stable disease by molecular imaging but one had partial remission and one had progressive disease by PSA, one patient had partial remission by molecular imaging and stable disease by PSA, and two patients had progressive disease according to molecular imaging but stable disease by PSA (Table 3). A discordant example is presented in Figure 5.
Changes in TLP, MTV, and PSA and their respective responses. PD, progressive disease; PR, partial remission; SD, stable disease. * indicates patients with new metastases by [68Ga]Ga-PSMA-11 PET/CT. Patients with discordant molecular imaging and biochemical responses are underlined.
| Patient number | ΔTLP (%) | ΔMTV (%) | ΔPSA (%) | TLP response | MTV response | PSA response |
|---|---|---|---|---|---|---|
| 1 | 100.7* | 82.7* | 80.1 | PD | PD | PD |
| 2 | -62.6 | -54.1 | -71.3 | PR | PR | PR |
| 3 | 43.2* | 38.3* | 28.3 | PD | PD | PD |
| 4 | 1.8 | 18.1 | -15.1 | SD | SD | SD |
| 5 | 1.7 | 8.9 | 46.9 | SD | SD | PD |
| 6 | -53.6 | -46.2 | -32.7 | PR | PR | SD |
| 7 | 294.9* | 259.8* | 628.2 | PD | PD | PD |
| 8 | -83.0 | -70.9 | -94.3 | PR | PR | PR |
| 9 | -95.1 | -95.5 | -73.5 | PR | PR | PR |
| 10 | -8.1 | -15.0 | 15.5 | SD | SD | SD |
| 11 | 6.0 | 7.0 | -51.3 | SD | SD | PR |
| 12 | 14.2 | 28.3 | -2.4 | SD | SD | SD |
| 13 | -15.3* | 13.3* | -12.2 | PD | PD | SD |
| 14 | -14.1 | -12.0 | -30.8 | SD | SD | SD |
| 15 | -69.6 | -67.6 | -56.6 | PR | PR | PR |
| 16 | 1.1 | 10.3 | 12.7 | SD | SD | SD |
| 17 | 43.6 | 56.8 | 3.2 | PD | PD | SD |
Correlation with pre-therapeutic parameters
Multiple pre-therapeutic parameters were tested for correlation with changes in TLP, MTV, and PSA. All pre-therapeutic parameters tested with their corresponding r- and p-values are summarized in Table 4. No significant correlation was noted for any of the parameters tested.
Toxicity
[225Ac]Ac-PSMA-617 and [177Lu]Lu-PSMA-617 were well-tolerated without any serious acute adverse events. No relevant change in eGFR was observed after the single cycle of tandem therapy. One patient had grade 3 thrombocytopenia but no other grade 3/4 hematotoxicities were observed. One patient experienced mild (grade 1) xerostomia attributed to the tandem therapy. In 4 patients with known mild xerostomia prior to tandem therapy, no change in xerostomia grade was recorded. Detailed CTCAE prior to and after tandem therapy are summarized in Table 5. In patients with stable disease or partial remission by both assessment methods, no relevant weight loss and no decrease in overall ECOG performance status was observed. Rather, reduction in pain, as assessed by the visual analogue scale, was noted in 3 patients.
Correlation between pre-therapeutic parameters and changes in TLP, MTV, and PSA. PFS, progression-free survival.
| ΔTLP | ΔMTV | ΔPSA | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| PSA prior to tandem therapy | -0.05 | 0.86 | -0.15 | 0.56 | -0.08 | 0.75 |
| PSA increase from previous cycle prior to tandem therapy | 0.08 | 0.75 | -0.04 | 0.89 | -0.25 | 0.32 |
| SUVmax of the most intense lesion prior to tandem therapy | 0.12 | 0.65 | 0.21 | 0.42 | 0.10 | 0.69 |
| SUVpeak of the most intense lesion prior to tandem therapy | 0.13 | 0.62 | 0.20 | 0.44 | 0.11 | 0.67 |
| TLP tumour load prior to tandem therapy | 0.08 | 0.77 | 0.06 | 0.81 | -0.17 | 0.51 |
| MTV tumour load prior to tandem therapy | 0.02 | 0.95 | -0.01 | 0.96 | -0.20 | 0.43 |
| Administered 225Ac activity | 0.12 | 0.65 | 0.11 | 0.67 | -0.21 | 0.41 |
| Administered 177Lu activity | -0.09 | 0.72 | -0.14 | 0.58 | -0.22 | 0.40 |
| Applied 225Ac/177Lu activity ratio | 0.18 | 0.49 | 0.20 | 0.45 | -0.05 | 0.86 |
| Cumulative 177Lu activity prior to tandem therapy | -0.10 | 0.71 | -0.21 | 0.43 | -0.12 | 0.66 |
| Time from initial diagnosis to tandem therapy | 0.03 | 0.90 | 0.19 | 0.47 | 0.26 | 0.31 |
| Time from initial diagnosis to castration resistance | 0.00 | 0.10 | 0.13 | 0.61 | 0.20 | 0.44 |
| PFS after chemotherapy (13/17 patients) | -0.26 | 0.40 | -0.31 | 0.30 | -0.16 | 0.61 |
| Time from start of 177Lu-PSMA-RLT to tandem therapy | -0.43 | 0.09 | -0.44 | 0.08 | -0.24 | 0.36 |
Waterfall plots of relative changes in (A) TLP, (B) MTV, and (C) PSA. * indicates appearance of new metastases. Values over 100% were cropped for simplification.
CTCAE before and after a single cycle of tandem therapy
| Before tandem therapy | After a single cycle tandem therapy | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| None | CTC 1° | CTC 2° | CTC 3° | CTC 4° | None | CTC 1° | CTC 2° | CTC 3° | CTC 4° | |
| Xerostomia | 13 (76.5%) | 4 (23.5%) | 0 (0%) | 0 (0%) | 0 (0%) | 12 (70.6%) | 5 (29.4%) | 0 (0%) | 0 (0%) | 0 (0%) |
| eGFR | 10 (58.8%) | 6 (35.3%) | 1 (5.9%) | 0 (0%) | 0 (0%) | 10 (58.8%) | 6 (35.3%) | 1 (5.9%) | 0 (0%) | 0 (0%) |
| Hemoglobin | 0 (0%) | 15 (88.2%) | 2 (11.8%) | 0 (0%) | 0 (0%) | 0 (0%) | 12 (70.6%) | 5 (29.4%) | 0 (0%) | 0 (0%) |
| Leucocytes | 11 (64.7%) | 3 (17.6%) | 3 (17.6%) | 0 (0%) | 0 (0%) | 12 (70.6%) | 3 (17.6%) | 2 (11.8%) | 0 (0%) | 0 (0%) |
| Platelets | 14 (82.4%) | 3 (17.6%) | 0 (0%) | 0 (0%) | 0 (0%) | 14 (82.4%) | 2 (11.8%) | 0 (0%) | 1 (5.9%) | 0 (0%) |
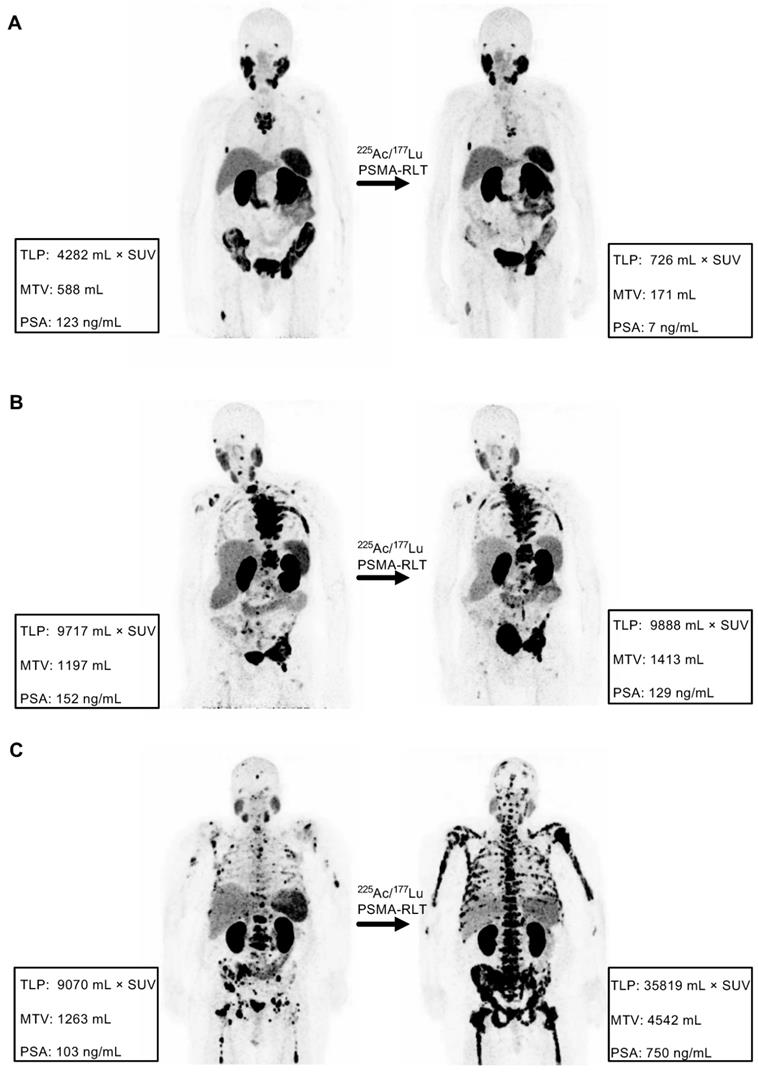
Examples of concordance between molecular imaging response assessment (TLP, MTV) and biochemical response assessment (PSA). (A) Partial remission in patient #8 (TLP -83%, MTV -71%, PSA -94%). (B) Stable disease in patient #4 (TLP +2%, MTV +18%, PSA -15%). (C) Progressive disease in patient #7 (TLP +295%, MTV +260%, PSA +628%).
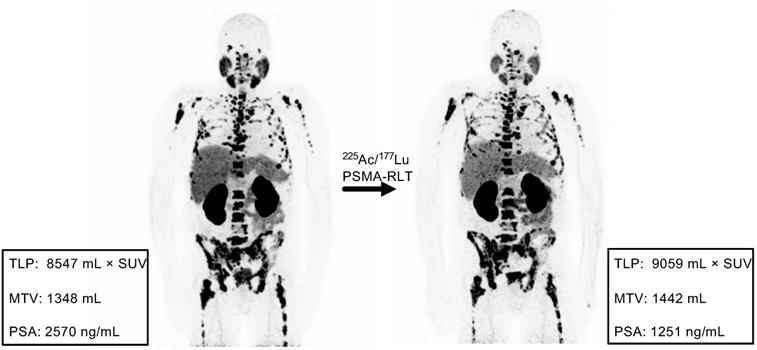
Example of discordance between molecular imaging response assessment (TLP, MTV) and biochemical response assessment (PSA). Patient #11 shows stable disease by TLP (+6%) and MTV (+7%) and partial remission by PSA (-51%).
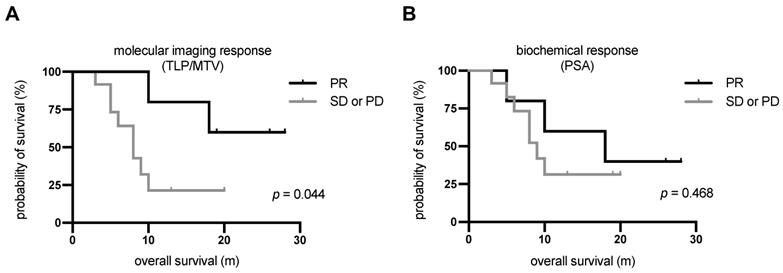
Kaplan-Meier curves for OS stratified by (A) molecular imaging response assessment and (B) biochemical response assessment. PD, progressive disease; PR, partial remission; SD, stable disease.
Survival analysis
The median follow-up time was 20 m (range: 3-28 m) from the date of [225Ac]Ac-PSMA-617/[177Lu]Lu-PSMA-617 tandem therapy. All patients showed disease progression in the follow-up time. The median PSA-based progression-free survival (PFS) was 3.7 m (95%CI: 3.0-4.4 m). Ten patients (58.8%) died by the end of the study. All deaths were mCRPC related. Patients showing partial remission by molecular imaging after one cycle of tandem therapy had not yet reached the median OS after a median follow-up time of 20 m. The median OS of patients showing either stable or progressive disease according to molecular imaging was 8.3 m (95%CI: 4.8-11.7 m). The difference in median OS between these patients and those showing partial remission was statistically significant (p = 0.044, log-rank test). The corresponding Kaplan-Meier curves are shown in Figure 6A. In contrast, there was no significant difference (p = 0.468) in OS between patients showing a PSA decline of >50% (median: 18.1 m, 95%CI: 1.1-35.1 m) and those with biochemically stable or progressive disease (median: 9.4 m, 95%CI: 7.3-11.4 m). The corresponding Kaplan-Meier curves are shown in Figure 6B. The 3 patients who showed disease progression by both response assessment methods did not receive further radioligand therapy. The remaining 14 patients continued radioligand therapy: 5 received [225Ac]Ac-PSMA-617/[177Lu]Lu-PSMA-617 tandem therapy cycles, 4 received [177Lu]Lu-PSMA-617 therapy cycles, and 5 received both.
Discussion
This is the first study investigating molecular imaging and biochemical response assessments after a single cycle of [225Ac]Ac-PSMA-617/[177Lu]Lu-PSMA-617 tandem therapy in patients with mCRPC (n = 17) who had progressed on [177Lu]Lu-PSMA-617 monotherapy. In this highly challenging patient cohort, 29.4% (5/17) of patients showed partial remission by either response assessment method after the single cycle of tandem therapy. Our study shows that a PSMA-targeted tandem therapy with alpha and beta emitters can be a successful treatment option for patients with mCRPC and resistance to monotherapy with beta emitters. The results are also in agreement with our previous study [21] in a different cohort of patients who had an insufficient response to monotherapy with [177Lu]Lu-PSMA-617. In the present study, the patients had progressive disease by both [68Ga]Ga-PSMA-11 PET/CT and serum PSA (PSA increase of >25% since last cycle), whereas patients in our previous study were only insufficient responders (defined by a PSA increase or decrease of <50%). These different inclusion criteria may explain why the PSA-based response rate after a median follow-up time of 2 m in this study is lower than that of the previous study (29.4% vs. 50%). All patients were heavily pre-treated with at least one line taxane (if not contraindicated) and next-generation androgen receptor signal inhibitors (enzalutamide and abiraterone), and showed resistance to [177Lu]Lu-PSMA-617 monotherapy. These patients, accordingly, had limited therapeutic options remaining.
Two different methods, molecular imaging assessment using [68Ga]Ga-PSMA-11 PET/CT to calculate TLP and MTV and biochemical assessment of serum PSA, were used to evaluate response. Comparing the TLP and MTV values before tandem therapy, a high correlation (r = 0.99 with p < 0.01) was observed as both reflect the total tumour burden. In assessing response, TLP and MTV provided a concordance of 100%, which is in line with the results of Hartrampf et al. [34]. No correlation between pre-therapeutic TLP or MTV and PSA was noted in our cohort (r = 0.03 with p = 0.90 and r = 0.11 with p = 0.67, respectively). Therefore, molecular imaging and PSA can be considered independent response assessment methods. For most patients, molecular imaging response was in accordance with biochemical response, which is currently the standard response method used in the clinic. A concordance of 70.6% (12/17) between molecular imaging and biochemical methods was noted in our study. Similar concordances of 65-87% have been observed in other studies with different cohorts of patients with prostate cancer [35-37]. Since 29.4% of patients had discordant results, a survival analysis was performed to evaluate which of these two methods better estimates the therapy outcome. Patients with partial remission, independently assessed by each method, had better OS than patients with either stable or progressive disease. The difference in OS was statistically significant for the molecular imaging response assessment (median OS not reached vs. 8.3 m, p = 0.044), but not for the biochemical response assessment (median OS 18.1 m vs. 9.4 m, p = 0.468). Accordingly, molecular imaging response can be considered a prognostic value for outcome of this tandem therapy. Despite the very small number of patients, this preliminary data suggests the need for treatment monitoring by [68Ga]Ga-PSMA-11 PET/CT in patients with mCRPC undergoing tandem RLT. Based on our results, the total tumour burden by [68Ga]Ga-PSMA-11 PET/CT (measured as either TLP or MTV) is an important parameter for monitoring PSMA-targeted tandem RLT and may be used to assess other treatments in patients with mCRPC. To confirm our findings, future studies in larger patient cohorts are recommended. No significant correlation was noted between several pre-therapeutic parameters and changes in TLP, MTV, or PSA (Table 4). No patient, therefore, should be excluded from tandem RLT based on any of these parameters. The statistical analysis may be biased by the small number of patients and further studies are needed.
One cycle of [225Ac]Ac-PSMA-617/[177Lu]Lu-PSMA-617 tandem therapy was safe and well-tolerated. The patient, who experienced grade 3 thrombocytopenia had extensive progression with disseminated bone metastases (the patient is depicted in Figure 4C). This thrombocytopenia seemed to be more related to progression of disease than to the tandem therapy. No other grade 3/4 hematotoxicities were observed. Only one patient experienced mild xerostomia attributed to the cycle of tandem therapy. This low rate of toxicity, especially of xerostomia, may be related to the lower administered activity of 225Ac (mean: 44 kBq/kg BW) in comparison to other studies applying [225Ac]Ac-PSMA-617 as monotherapy [19,20,22]. For example, Kratochwil et al. recommended 100 kBq/kg BW in [225Ac]Ac-PSMA-617 monotherapy to balance response and side effects [17]. Besides the expected low toxicity profile of the tandem therapy in comparison to 225Ac monotherapy, we also assume, as discussed in our previous study [21], that 177Lu may still have a contributing anti-tumour effect when augmented with low activities of 225Ac in patients somewhat resistant to 177Lu monotherapy. Alpha radiation even in lower quantities might be able to overcome beta resistance. From our clinical experience, this seems to be true to some extent and was observed in 4/9 patients that were re-challenged [177Lu]Lu-PSMA-617 therapy after tandem therapy (1 patient with partial remission, 3 patients with stable disease); however, this hypothesis remains completely unproven.
Our results provide preliminary evidence and a rational starting point for further studies. Ideally, prospective studies should be conducted to establish the tandem approach in mCRPC management. A dose-finding study that includes evaluation of the absolute activities of 225Ac and 177Lu as well as the 225Ac/177Lu ratio may be one of the next steps to improve response without impairing quality of life. In addition, evaluation of tandem therapy is also conceivable in patients without resistance to 177Lu monotherapy.
Limitations
The promising results reported herein should be considered in the light of some limitations. The study suffers from its retrospective nature and the limited number of patients. A further limitation is that the time for follow-up varied, which could impact the TLP, MTV, and PSA changes. The majority of patients received their follow-up ([68Ga]Ga-PSMA-11 PET/CT and serum PSA on the same day) about 4 to 8 weeks after the tandem therapy. In a few patients, this time window could not be met due to the patient's condition or organizational reasons. In these few outliers, however, the assessment methods showed concordant results and did not impact the survival analyses. Another major limitation of this study is that no fixed activity protocol was used. The 225Ac and 177Lu activities were individually chosen based on tumour and patient characteristics. Furthermore, the limited availability of 225Ac influenced the determination of the administered activities, resulting in a variable 225Ac/177Lu ratio. In addition, it has to be pointed out that there are several methods for calculating TLP and MTV [27,35,36]. Even though percentage-based thresholding (41% or 50%) is recommended by the EANM for TLG in [18F]FDG-PET/CT [28], this method is known to be adequate solely for non-heterogenous uptake distributions. In order to not underestimate the lesion volume in case of heterogeneous PSMA expression, which is often observed in disseminated and confluent disease after many therapies, we applied the method published by Ferdinandus et al. [27] with a fixed SUV threshold of 3.0. For calculating liver metastases, we applied a threshold of 1.5 × SUVmean of the healthy liver, which appeared to be appropriate compared to visual findings.
Conclusion
[225Ac]Ac-PSMA-617/[177Lu]Lu-PSMA-617 tandem therapy is an effective treatment for the highly challenging cohort of patients with mCRPC who have progressed on [177Lu]Lu-PSMA-617 monotherapy. Molecular imaging response (based on the [68Ga]Ga-PSMA-11 PET/CT-derived parameters TLP and MTV) and biochemical serum PSA response were mostly concordant, though a considerable number of cases (29.4%) were discordant. Molecular imaging response reflecting the change in viable total tumour burden appears to be superior to PSA change in estimating survival outcome after tandem therapy. Larger, and ideally prospective, studies are recommended to confirm and expand these findings.
Abbreviations
BW: body weight; CT: computed tomography; EANM: European Association of Nuclear Medicine; ECOG: Eastern Cooperative Oncology Group; eGFR: estimated glomerular filtration rate; mCRPC: metastatic castration-resistant prostate carcinoma; MTV: molecular tumour volume; PD: progressive disease; PET: positron emission tomography; PR: partial remission; PSA: prostate-specific antigen; PSMA: prostate-specific membrane antigen; PSMA-RLT: PSMA-targeted radioligand therapy; RLT: radioligand therapy; SD: stable disease; TLG: total lesion glycolysis; TLP: total lesion PSMA.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424
2. Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review: The Epidemiology of CRPC. Int J Clin Pract. 2011;65:1180-92
3. Watson PA, Arora VK, Sawyers CL. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer. 2015;15:701-11
4. Tannock IF, Horti J, Oudard S, James ND, Rosenthal MA. Docetaxel plus Prednisone or Mitoxantrone plus Prednisone for Advanced Prostate Cancer. N Engl J Med. 2004;351:1502-12
5. Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel Plus Prednisone or Mitoxantrone Plus Prednisone for Advanced Prostate Cancer: Updated Survival in the TAX 327 Study. J Clin Oncol. 2008;26:242-5
6. Cassinello J, Carballido Rodríguez J, Antón Aparicio L. Role of taxanes in advanced prostate cancer. Clin Transl Oncol. 2016;18:972-80
7. Kratochwil C, Fendler WP, Eiber M. et al. EANM procedure guidelines for radionuclide therapy with 177Lu-labelled PSMA-ligands (177Lu-PSMA-RLT). Eur J Nucl Med Mol Imaging. 2019;46:2536-44
8. Baum RP, Kulkarni HR, Schuchardt C. et al. 177Lu-Labeled Prostate-Specific Membrane Antigen Radioligand Therapy of Metastatic Castration-Resistant Prostate Cancer: Safety and Efficacy. J Nucl Med. 2016;57:1006-13
9. Rahbar K, Ahmadzadehfar H, Kratochwil C. et al. German Multicenter Study Investigating 177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J Nucl Med. 2017;58:85-90
10. Ahmadzadehfar H, Rahbar K, Baum RP. et al. Prior therapies as prognostic factors of overall survival in metastatic castration-resistant prostate cancer patients treated with [177Lu]Lu-PSMA-617. A WARMTH multicenter study (the 617 trial). Eur J Nucl Med Mol Imaging. 2020 doi 10.1007/s00259-020-04797-9. [Epub ahead of print]
11. Barber TW, Singh A, Kulkarni HR, Niepsch K, Billah B, Baum RP. Clinical Outcomes of 177Lu-PSMA Radioligand Therapy in Earlier and Later Phases of Metastatic Castration-Resistant Prostate Cancer Grouped by Previous Taxane Chemotherapy. J Nucl Med. 2019;60:955-62
12. Hofman MS, Violet J, Hicks RJ. et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825-33
13. Emmett L, Crumbaker M, Ho B. et al. Results of a Prospective Phase 2 Pilot Trial of 177Lu-PSMA-617 Therapy for Metastatic Castration-Resistant Prostate Cancer Including Imaging Predictors of Treatment Response and Patterns of Progression. Clin Genitourin Cancer. 2019;17:15-22
14. Kratochwil C, Giesel FL, Stefanova M. et al. PSMA-Targeted Radionuclide Therapy of Metastatic Castration-Resistant Prostate Cancer with 177Lu-Labeled PSMA-617. J Nucl Med. 2016;57:1170-6
15. Yadav MP, Ballal S, Sahoo RK, Dwivedi SN, Bal C. Radioligand Therapy With 177Lu-PSMA for Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. AJR Am J Roentgenol. 2019;213:275-85
16. Rahbar K, Bode A, Weckesser M. et al. Radioligand Therapy With 177Lu-PSMA-617 as A Novel Therapeutic Option in Patients With Metastatic Castration Resistant Prostate Cancer. Clin Nucl Med. 2016;41:522-8
17. Kratochwil C, Bruchertseifer F, Rathke H. et al. Targeted α-Therapy of Metastatic Castration-Resistant Prostate Cancer with 225Ac-PSMA-617: Dosimetry Estimate and Empiric Dose Finding. J Nucl Med. 2017;58:1624-31
18. Kratochwil C, Bruchertseifer F, Rathke H. et al. Targeted α-Therapy of Metastatic Castration-Resistant Prostate Cancer with 225Ac-PSMA-617: Swimmer-Plot Analysis Suggests Efficacy Regarding Duration of Tumor Control. J Nucl Med. 2018;59:795-802
19. Sathekge M, Bruchertseifer F, Knoesen O. et al. 225Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: a pilot study. Eur J Nucl Med Mol Imaging. 2019;46:129-38
20. Sathekge M, Bruchertseifer F, Vorster M. et al. Predictors of Overall and Disease-Free Survival in Metastatic Castration-Resistant Prostate Cancer Patients Receiving 225Ac-PSMA-617 Radioligand Therapy. J Nucl Med. 2020;61:62-9
21. Khreish F, Ebert N, Ries M. et al. 225Ac-PSMA-617/177Lu-PSMA-617 tandem therapy of metastatic castration-resistant prostate cancer: pilot experience. Eur J Nucl Med Mol Imaging. 2020;47:721-8
22. Yadav MP, Ballal S, Sahoo RK, Tripathi M, Seth A, Bal C. Efficacy and safety of 225Ac-PSMA-617 targeted alpha therapy in metastatic castration-resistant Prostate Cancer patients. Theranostics. 2020;10:9364-77
23. Ilhan H, Gosewisch A, Böning G. et al. Response to 225Ac-PSMA-I&T after failure of long-term 177Lu-PSMA RLT in mCRPC. Eur J Nucl Med Mol Imaging. 2020 doi 10.1007/s00259-020-05023-2. [Epub ahead of print]
24. Zacherl MJ, Gildehaus FJ, Mittlmeier L. et al. First clinical results for PSMA targeted alpha therapy using 225Ac-PSMA-I&T in advanced mCRPC patients. J Nucl Med. 2020 doi 10.2967/jnumed.120.251017. [Epub ahead of print]
25. Kratochwil C, Bruchertseifer F, Giesel FL. et al. 225Ac-PSMA-617 for PSMA-Targeted α-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J Nucl Med. 2016;57:1941-4
26. Fendler WP, Eiber M, Beheshti M. et al. 68Ga-PSMA PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2017;44:1014-24
27. Ferdinandus J, Violet J, Sandhu S. et al. Prognostic biomarkers in men with metastatic castration-resistant prostate cancer receiving [177Lu]-PSMA-617. Eur J Nucl Med Mol Imaging. 2020;47:2322-7
28. Boellaard R, Delgado-Bolton R, Oyen WJG. et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328-54
29. Rosar F, Dewes S, Ries M. et al. New insights in the paradigm of upregulation of tumoral PSMA expression by androgen receptor blockade: Enzalutamide induces PSMA upregulation in castration-resistant prostate cancer even in patients having previously progressed on enzalutamide. Eur J Nucl Med Mol Imaging. 2020;47:687-94
30. Grubmüller B, Rasul S, Baltzer P. et al. Response assessment using [68Ga]Ga-PSMA ligand PET in patients undergoing systemic therapy for metastatic castration-resistant prostate cancer. The Prostate. 2020;80:74-82
31. Grubmüller B, Senn D, Kramer G. et al. Response assessment using 68Ga-PSMA ligand PET in patients undergoing 177Lu-PSMA radioligand therapy for metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2019;46:1063-72
32. Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50:122S-50S
33. Scher HI, Morris MJ, Stadler WM. et al. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34:1402-18
34. Hartrampf PE, Heinrich M, Seitz AK. et al. Metabolic Tumour Volume from PSMA PET/CT Scans of Prostate Cancer Patients during Chemotherapy—Do Different Software Solutions Deliver Comparable Results? J Clin Med. 2020;9:1390
35. Michalski K, Mix M, Meyer PT, Ruf J. Determination of whole-body tumour burden on [68Ga]PSMA-11 PET/CT for response assessment of [177Lu]PSMA-617 radioligand therapy: a retrospective analysis of serum PSA level and imaging derived parameters before and after two cycles of therapy. Nuklearmedizin. 2019;58:443-50
36. Schmuck S, von Klot CA, Henkenberens C. et al. Initial Experience with Volumetric 68Ga-PSMA I&T PET/CT for Assessment of Whole-Body Tumor Burden as a Quantitative Imaging Biomarker in Patients with Prostate Cancer. J Nucl Med. 2017;58:1962-8
37. Schmidkonz C, Cordes M, Schmidt D. et al. 68Ga-PSMA-11 PET/CT-derived metabolic parameters for determination of whole-body tumor burden and treatment response in prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45:1862-72
Author contact
![]() Corresponding author: Florian Rosar, MD., Department of Nuclear Medicine, Saarland University - Medical Center, Kirrberger Str. 100, Geb. 50, D-66421 Homburg, Germany. Tel.: +49 6841 16 22201; Fax: +49 6841 16 24595; Email: florian.rosareu. ORCID ID: 0000-0002-2985-4099
Corresponding author: Florian Rosar, MD., Department of Nuclear Medicine, Saarland University - Medical Center, Kirrberger Str. 100, Geb. 50, D-66421 Homburg, Germany. Tel.: +49 6841 16 22201; Fax: +49 6841 16 24595; Email: florian.rosareu. ORCID ID: 0000-0002-2985-4099
 Global reach, higher impact
Global reach, higher impact