13.3
Impact Factor
Theranostics 2021; 11(7):3512-3526. doi:10.7150/thno.55241 This issue Cite
Review
Ovarian hormones-autophagy-immunity axis in menstruation and endometriosis
1. Laboratory for Reproductive Immunology, NHC Key Laboratory of Reproduction Regulation (Shanghai Institute of Planned Parenthood Research), Hospital of Obstetrics and Gynecology, Shanghai Medical School, Fudan University, Shanghai 200080, People's Republic of China.
2. Assisted Reproductive Technology Unit, Department of Obstetrics and Gynecology, Faculty of Medicine, Chinese University of Hong Kong, Hong Kong, People's Republic of China.
3. Center of Reproductive Medicine of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, People's Republic of China.
4. Reproductive Medicine Center, Department of Obstetrics and Gynecology, Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medicine School, Nanjing, 210000, People's Republic of China.
5. Center for Human Reproduction and Genetics, Suzhou Municipal Hospital, Suzhou 215008, People's Republic of China.
6. Wallace H. Coulter Department of Biomedical Engineering, Georgia Institute of Technology and Emory University, Atlanta 30332, Georgia, USA.
7. Division of Obstetrics and Gynecology, KK Women's and Children's Hospital, 229899, Singapore.
8. Shanghai Key Laboratory of Female Reproductive Endocrine Related Diseases, Shanghai, 200080, People's Republic of China.
#Co-first authors.
Abstract
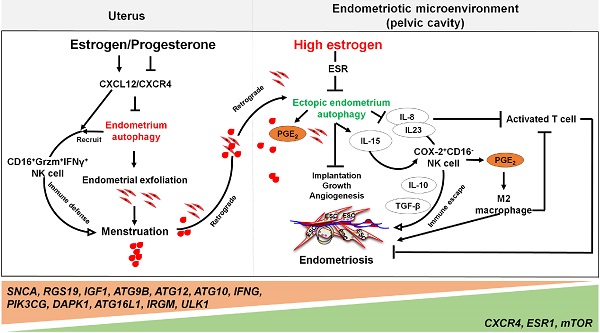
Menstruation occurs in few species and involves a cyclic process of proliferation, breakdown and regeneration under the control of ovarian hormones. Knowledge of normal endometrial physiology, as it pertains to the regulation of menstruation, is essential to understand disorders of menstruation. Accumulating evidence indicates that autophagy in the endometrium, under the regulation of ovarian hormones, can result in the infiltration of immune cells, which plays an indispensable role in the endometrium shedding, tissue repair and prevention of infections during menstruation. In addition, abnormal autophagy levels, together with resulting dysregulated immune system function, are associated with the pathogenesis and progression of endometriosis. Considering its potential value of autophagy as a target for the treatment of menstrual-related and endometrium-related disorders, we review the activity and function of autophagy during menstrual cycles. The role of the estrogen/progesterone-autophagy-immunity axis in endometriosis are also discussed.
Keywords: autophagy, endometrium, estrogen, macrophage, menstruation, neutrophil, NK cell, progesterone
 Global reach, higher impact
Global reach, higher impact