13.3
Impact Factor
Theranostics 2021; 11(7):3502-3511. doi:10.7150/thno.55014 This issue Cite
Research Paper
Cell membranes targeted unimolecular prodrug for programmatic photodynamic-chemo therapy
1. State Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha 410082, P. R China.
2. Department of Chemistry and Chemical Engineering, Beijing University of Technology, Beijing, 100124, P. R. China.
3. College of Physics and Microelectronics Science, Hunan University, Changsha 410082, P. R China.
4. College of Materials Science and Engineering, Hunan University, Changsha 410082, P. R China.
Abstract
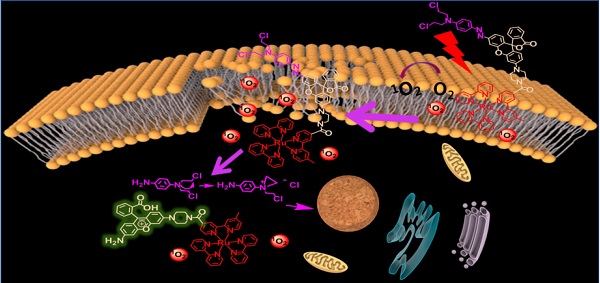
Photodynamic therapy (PDT) has emerged as one of the most up-and-coming non-invasive therapeutic modalities for cancer therapy in rencent years. However, its therapeutic effect was still hampered by the short life span, limited diffusion distance and ineluctable depletion of singlet oxygen (1O2), as well as the hypoxic microenvironment in the tumor tissue. Such problems have limited the application of PDT and appropriate solutions are highly demand.
Methods: Herein, a programmatic treatment strategy is proposed for the development of a smart molecular prodrug (D-bpy), which comprise a two-photon photosensitizer and a hypoxia-activated chemotherapeutic prodrug. A rhodamine dye was designed to connect them and track the drug release by the fluorescent signal generated through azo bond cleavage.
Results: The prodrug (D-bpy) can stay on the cell membrane and enrich at the tumor site. Upon light irradiation, the therapeutic effect was enhanced by a stepwise treatment: (i) direct generation of 1O2 on the cell membrane induced membrane destruction and promoted the D-bpy uptake; (ii) deep tumor hypoxia caused by two-photon PDT process further triggered the activation of the chemotherapy prodrug. Both in vitro and in vivo experiments, D-bpy have exhabited excellent tumor treatment effect.
Conclusion: The innovative programmatic treatment strategy provides new strategy for the design of follow-up anticancer drugs.
Keywords: cell membrane, singlet oxygen, glutathione, two-photon photodynamic therapy, combination therapy
 Global reach, higher impact
Global reach, higher impact